Abstract
The COP9 signalosome (CSN) purified from human erythrocytes possesses kinase activity that phosphoryl ates proteins such as c-Jun and p53 with consequence for their ubiquitin (Ub)-dependent degradation. Here we show that protein kinase CK2 (CK2) and protein kinase D (PKD) co-purify with CSN. Immunoprecipi tation and far-western blots reveal that CK2 and PKD are in fact associated with CSN. As indicated by electron microscopy with gold-labeled ATP, at least 10% of CSN particles are associated with kinases. Kinase activity, most likely due to CK2 and PKD, co-immuno precipitates with CSN from HeLa cells. CK2 binds to ΔCSN3(111–403) and CSN7, whereas PKD interacts with full-length CSN3. CK2 phosphorylates CSN2 and CSN7, and PKD modifies CSN7. Both CK2 and PKD phosphorylate c-Jun as well as p53. CK2 phosphoryl ates Thr155, which targets p53 to degradation by the Ub system. Curcumin, emodin, DRB and resveratrol block CSN-associated kinases and induce degradation of c-Jun in HeLa cells. Curcumin treatment results in elevated amounts of c-Jun–Ub conjugates. We conclude that CK2 and PKD are recruited by CSN in order to regulate Ub conjugate formation.
Keywords: c-Jun/COP9 signalosome/protein kinase CK2/protein kinase D/p53
Introduction
The COP9 signalosome (CSN) is a multimeric complex that is conserved from yeast to man (Deng et al., 2000). Since its discovery in plant cells (Wei et al., 1994), a function in signaling and developmental processes has been implicated. In fact, the complex is a negative regulator of photomorphogenesis (for a review, see Wei and Deng, 1999) and is involved in development of Drosophila (Freilich et al., 1999). However, the exact function of the CSN has not been elucidated yet.
Purification and characterization of the CSN from mammalian cells revealed sequence homologies between CSN subunits and components of the 26S proteasome lid complex (Seeger et al., 1998; Wei et al., 1998), as well as components of the eukaryotic translation initiation factor 3 (eIF3) complex (Glickman et al., 1998). Structural similarities and co-purification (Kapelari et al., 2000) of the CSN and the lid suggested a functional relationship between the CSN and the ubiquitin (Ub)/26S proteasome system. Indeed, the CSN is involved in regulation of the stability of proteins such as p27 (Tomoda et al., 1999), c-Jun (Naumann et al., 1999), p53 (Bech-Otschir et al., 2001) and HY5 (Hardtke et al., 2000), which are substrates of the Ub/26S proteasome system.
Recently, an effect of the CSN on the activity of Ub ligases termed SCF (SKP1–CDC53–F-box protein) complexes has been shown. The SCF Ub ligases collaborate with specific Ub conjugating enzymes in the ubiquityl ation of different substrates. One component of the SCF complexes is a member of the cullin (Cul) protein family, which is covalently modified by the Ub-like protein NEDD8. The conjugation of NEDD8 to Cul1 enhances the recruitment of Ub conjugating enzyme Ubc4 to the SCF complex, which stimulates protein polyubiquitylation (Kawakami et al., 2001). It has been demonstrated in vitro and in vivo that the CSN removes NEDD8 from Cul1 (Lyapina et al., 2001; Schwechheimer et al., 2001; Zhou et al., 2001). The responsible deneddylation activity seems to be localized in the MPN domain of CSN5 (Cope et al., 2002). Although data on the effect of CSN-mediated deneddylation on SCF-dependent substrates are controversial, reduction of the SCF Ub ligase activity by NEDD8 removal is very likely, as it has been shown for pcu3/Cul-3 complexes in Schizosaccharomyces pombe (Zhou et al., 2001) and for the SCF complex involved in p27 ubiquitylation (Yang et al., 2002).
The CSN from human red blood cells co-purifies with kinase activity, which phosphorylates IκBα, c-Jun, p53 and interferon consensus sequence binding protein (ICSBP) (for a review, see Bech-Otschir et al., 2002). Because none of the CSN subunits possesses sequence homologies with protein kinases, an associated kinase activity has been assumed. Phosphorylation of p53 by the CSN-associated kinase activity targets the tumor suppressor to degradation by the Ub pathway. Inhibition of p53 phosphorylation by curcumin, an inhibitor of the CSN-associated kinases (Henke et al., 1999), leads to stabilization of the endogenous tumor suppressor in tumor cells (Bech-Otschir et al., 2001).
The transcription factor c-Jun is stabilized towards the Ub/26S proteasome system upon phosphorylation by CSN-associated kinase (Naumann et al., 1999). Overexpression of the CSN subunit 2 (CSN2) causes a significant increase of activation factor 1 (AP1) transactivation activity. Under these conditions c-Jun, a major component of AP1, is stabilized in HeLa cells, which is accompanied by de novo assembly of the CSN complex. The CSN-directed c-Jun signaling controls a major portion of vascular endothelial growth factor production in tumor cells (Pollmann et al., 2001).
The transcription factor HY5 is a positive regulator of light-regulated genes in plant cells. It is degraded in the dark by the Ub system. For this process the CSN as well as the autonomous repressor of photomorphogenesis COP1 is required (for a review, see Schwechheimer and Deng, 2001). COP1 has been suggested to be the responsible Ub ligase of HY5 (Osterlund et al., 2000). Binding of HY5 to COP1 is prevented by light-regulated phosphorylation of HY5 presumably by the protein kinase CK2 (CK2, formerly casein kinase II), which stabilizes the transcription factor in the light (Hardtke et al., 2000).
Recently, the co-purification of the inositol 1,3,4-trisphosphate 5/6-kinase (5/6-kinase) with the CSN (Wilson et al., 2001; Sun et al., 2002) has been reported. The enzyme is sensitive to curcumin and can act as a protein kinase that phosphorylates c-Jun and ATF-2. Here we show the co-purification and association of the protein kinase D (PKD) and CK2 with the CSN. We demonstrate that the two kinases phosphorylate c-Jun as well as p53. In addition, the two kinases are responsible for CSN subunit modification. We provide data indicating an impact of the kinases on ubiquitylation and degradation of c-Jun.
Results
CK2 and PKD co-purify with the CSN
To identify CSN-associated kinases we studied phosphopeptide analyses performed with c-Jun (Seeger et al., 1998), p53 (Bech-Otschir et al., 2001), ICSBP (Cohen et al., 2000) and IκBα. Experiments were perfomed with recombinant substrates of CSN-associated kinases, with purified CSN from human erythrocytes and with [γ-32P]ATP. Phosphorylated proteins were gel purified, digested with chymotrypsin and peptides were separated by high-performance liquid chromatography (HPLC). Radioactive, phosphorylated peptides identified by peptide sequencing and mass spectrometry are shown in Table I in order of their specific radioactivity. Most of the peptides contain serines and threonines as putative phosphorylation sites, suggesting that CSN is associated with Ser/Thr kinases.
Table I. Peptides phosphorylated by the CSN-associated kinases identified by phosphopeptide analyses with different proteins.
| Protein | Radioactive peptides | Putative P sites | Consensus |
|---|---|---|---|
| c-Jun | 58SDLLTSPDVGLLKLASPELERL79 | Ser63, Ser73 | JNK |
| Thr62 | CK2 | ||
| 115VRALAELHSQNTLPSVTSAAQ135 | ? | No | |
| 53LRAKNSDLLTSPDVGLL69 | Ser63 | JNK | |
| Thr62 | CK2 | ||
| 151GGSGSGGFSASLHSEPPVYANLSNF177 | ? | No | |
| 80IIQSSNGHITTTPTPTQF97 | ? | No | |
| 98LCPKNVTDEQEGFAEGF114 | ? | No | |
| 36QSMTLNLADPVGSLKPH52 | Ser48 | PKC | |
| p53 | 142PVQLWVDSTPPPGTRVRA159 | Thr155 | No |
| 94SSSVPSQKTY103 | Ser99 | PKC | |
| 384MFKTEGPDSD393 | Ser392 | CK2 | |
| ICSBP | 253PPADAIPSERQRQVTRK269 | Ser260 | PKC |
| 93EEVTDRSQLDISEPYK108 | Ser99 | CK2 | |
| IκBα | 271QLTLENLQMLPESEDEESYDTESEFT296 | Ser283 | CK2 |
| Thr291 | CK2 |
ICSBP, interferon consensus sequence binding protein; JNK, Jun-N-terminal kinase. Consensus sequences for CK2 or PKC phosphorylation are indicated in bold. Unknown phosphorylation sites are indicated by ‘?’.
The c-Jun peptide with the highest specific radioactivity contains Ser63 and Ser73, known to be phosphorylation sites of the Jun-N-terminal kinase (JNK; Musti et al., 1997). However, immunoblots with purified CSN using different antibodies against different JNK isoforms revealed that these kinases were not detectable in our preparations (data not shown). Interestingly, the same c-Jun peptide 58–79 contains a consensus sequence for CK2 phosphorylation (see below). In addition, other radiolabeled peptides also exhibit putative CK2 phosphorylation sites. Immunoblots and immunoprecipitations revealed that CK2 is a CSN-associated kinase (see below).
A number of peptides contained protein kinase C (PKC) sites (see Table I). Antibodies against PKCα, β, γ and θ indicated that these PKC isoforms were not detectable in CSN preparations (data not shown). On the other hand, an antibody against PKCµ, also known as PKD, identified PKD in our CSN preparations.
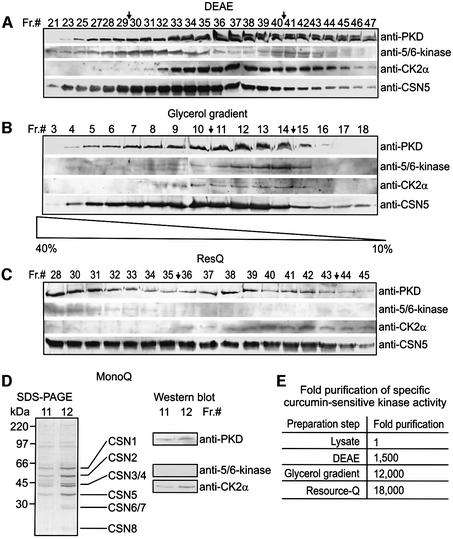
To investigate whether PKD and CK2 co-purify with the CSN, immunoblots on different CSN preparation steps were performed. The data are summarized in Figure 1. Figure 1A shows western blots with fractions of a DEAE column, a very early step of the preparation. Many fractions containing the CSN, as indicated by the anti-CSN5 antibody, also contained PKD and CK2α, a subunit of CK2. In addition, the 5/6-kinase co-eluted with CSN fractions. Fractions 30–40 were pooled for further CSN purification. The next step was a 10 to 40% glycerol gradient in which the CSN, PKD, CK2 and 5/6-kinase co-sedimented to similar fractions (Figure 1B). Western blots with fractions from a ResourceQ column are shown in Figure 1C. CK2 was detected in CSN fractions eluted with high salt. PKD was found in most of the CSN5-containing fractions. For further purification we used fractions 36–43. Fractions eluted with lower salt contained 5/6-kinase (Figure 1C). After the ResourceQ column the 5/6-kinase was not detectable in our CSN pool. The last step was a MonoQ ion exchange chromatography. Purified CSN, which was obtained in MonoQ fractions 11 and 12, is shown in Figure 1D. As demonstrated by immunoblotting, the same fractions contained PKD and CK2α, but not 5/6-kinase.
Fig. 1. CK2 and PKD co-purify with the CSN during its preparation from human erythrocytes. (A) Western blots with fractions 21–47 from DEAE column (Seeger et al., 1998). The blots were probed with anti-PKD, anti-5/6-kinase, anti-CK2α and anti-CSN5 antibodies. Fractions 30–40 indicated by arrows were pooled for determination of specific curcumin-sensitive kinase activity [see (E) and Materials and methods] and for further purification. (B) Western blots with fractions 3–18 from a 10 to 40% glycerol gradient using same antibodies as in (A). Fractions 11–14 (arrows) were pooled for further use. (C) Western blots with fractions 28–45 obtained from a RecourceQ column using the same antibodies as in (A). Fractions 36–43 were pooled. (D) SDS–PAGE of 20-µl aliquots of fractions 11 and 12 after MonoQ ion exchange chromatography. Core CSN subunits are indicated. Western blots with the same fractions using anti-PKD, anti-5/6-kinase and anti-CK2α antibodies revealed the presence of CK2α and PKD, but not of 5/6-kinase, in the final CSN preparation. (E) Curcumin-sensitive kinase activity co-purifies with the CSN. Fixing the kinase activity in the lysate to 1, a 18 000-fold co-purification of CSN-associated kinase activity was achieved by the final preparation steps.
During CSN preparation the specific curcumin-sensitive phosphorylation of c-Jun was measured in pooled fractions indicated by arrows (see Figure 1A–C). Curcumin is an inhibitor of CSN-associated kinases and the specific curcumin-sensitive kinase activity should, at least in part, reflect co-purification of associated kinase activity. The data are summarized in the table of Figure 1E. Compared with cell lysate, the curcumin-sensitive kinase activity per microgram of protein was 1500-fold higher in the pooled DEAE fractions. Further significant enrichment of specific curcumin-sensitive kinase in pooled CSN fractions was obtained by glycerol gradient centrifugation. Although specific curcumin-sensitive kinase activity increased from the glycerol gradient to ResourceQ column, 5/6-kinase disappeared from our CSN pool after ResourceQ. There was no further increase of specific curcumin-sensitive kinase activity by MonoQ.
CK2 and PKD are associated with the CSN
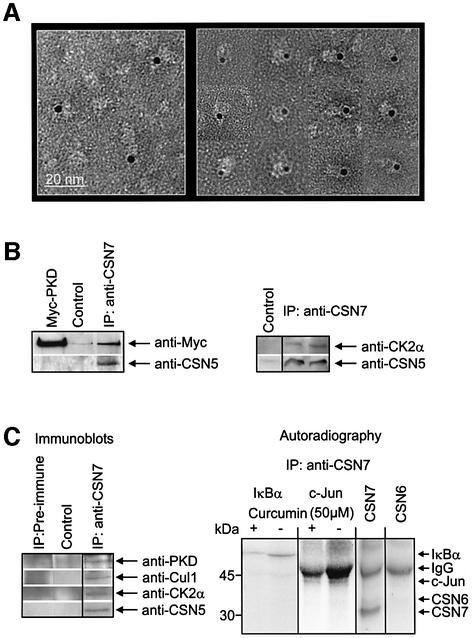
Because CSN subunits do not possess consensus sequences for ATP binding, a possible interaction of ATP with the CSN should be mediated by associated proteins, most likely by the associated kinases. Initial cross-linking experiments with perjodate-oxidized ATP indicated that ATP binds to the CSN (data not shown). Therefore, a gold-labeled non-cleavable ATP analog was incubated with the CSN and the mixture was analyzed by electron microscopy. The data are shown in Figure 2A. Approximately 10–15% of all CSN particles were estimated to be labeled with gold-ATP. As seen in a gallery of gold-ATP-labeled CSN particles (right panel), there was one gold particle associated with the periphery of one CSN complex. Because our CSN preparation did not contain detectable 5/6-kinase, ATP-binding cannot be explained by this enzyme.
Fig. 2. CK2 and PKD are associated with the CSN. (A) Electron microscopy of CSN particles that were incubated with gold-labeled uncleav able ATP analog. The gallery (right panel) shows typical positions of gold clusters mediated by kinases associated with CSN complexes. (B) Immunoprecipitation of the purified CSN was performed with anti-CSN7 antibody. In the left panel purified CSN and recombinant Myc-tagged PKD were mixed and the mixture was immunoprecipitated (IP: anti-CSN7). The western blot revealed the presence of CSN5 (anti-CSN5) and of Myc-tagged PKD in the precipitate. Myc-PKD is the recombinant PKD alone and for control immunoprecipitation protocol was performed without anti-CSN7 antibody, indicating unspecific binding to protein A–Sepharose. In the right panel, purified CSN shown in Figure 1D was immunoprecipitated (IP: anti-CSN7). The two lanes of western blots show two different immunoprecipitations and indicate the presence of CSN5 and of CK2α in the precipitate. The control was performed without anti-CSN7 antibody. (C) Immunoprecipitation of the CSN from HeLa cell lysate using the anti-CSN7 antibody. The left panel shows immunoblots of the precipitates (IP: anti-CSN7). The blot was probed with anti-PKD, anti-Cul1, anti-CK2α and anti-CSN5 antibodies. Control experiments were performed as in (B). Immuno precipitation with pre-immune serum (IP: pre-immune) indicates the specificity of immunoprecipitations. The right panel shows autoradiography of an SDS–PAGE carried out with the reaction mixtures of kinase assays. The anti-CSN7 immunoprecipitate was used as a source of kinase activity and was incubated in the presence of IκBα or c-Jun as a substrate, [γ-32P]ATP, and with or without curcumin. To demonstrate the specificity of the kinase reaction, the ability of anti-CSN7 immunoprecipitate to phosphorylate recombinant CSN7 or CSN6 was tested. Depending on the exposure time, phosphorylation of heavy chain rabbit IgG was seen (IgG).
To test whether CK2 and PKD are associated with the CSN, immunoprecipitations were carried out using an anti-CSN7 antibody. Purified CSN and recombinant Myc-tagged PKD were incubated at 37°C and after 30 min immunoprecipitation was performed (Figure 2B). The western blot with the anti-CSN5 antibody indicated that the CSN complex was in the precipitate. In addition, PKD co-immunoprecipitated under these conditions. In the case of CK2, purified CSN shown in Figure 1D (MonoQ fraction 12) was immunoprecipitated with anti-CSN7 antibody. Western blot analysis of the precipitate revealed that CK2α is associated with the CSN (Figure 2B, right panel). Immunoprecipitations were also performed with HeLa cell lysate (Figure 2C). HeLa cells were used because most of our cell experiments were performed with this cell line. As indicated by the anti-CSN5 antibody, endogenous HeLa cell CSN was successfully precipitated. The precipitate also contained Cul1, which has been shown to interact with the CSN (Lyapina et al., 2001; Schwechheimer et al., 2001). In addition, both PKD and CK2α were co-immunoprecipitated.
We were interested to determine whether the CSN from HeLa cells is associated with active kinases. Therefore, the anti-CSN7 immunoprecipitate was used in kinase assays with IκBα or c-Jun as substrate. As shown in Figure 2C (right panel), IκBα was phosphorylated under this condition. As estimated by densitometry, up to 80% of the IκBα phosphorylation was inhibited by curcumin. In addition, the anti-CSN7 immunoprecipitate phosphorylated c-Jun in a curcumin-sensitive manner. CSN subunits such as CSN7 are substrates of CSN-associated kinases, whereas others, such as CSN6, are not (see below). To evaluate the specificity of phosphorylation, CSN7 and CSN6 were used as substrates. As shown in Figure 2C (right panel), the anti-CSN7 immunoprecipitate phosphorylated CSN7, but not CSN6.
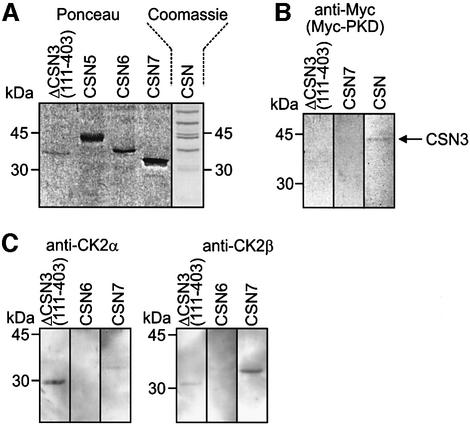
To identify CSN subunits, which interact with CK2 and/or PKD far-western blots with recombinant CSN subunits, the purified CSN complex and with recombinant kinases were carried out. Recombinant kinases were incubated with immobilized recombinant CSN subunits and CSN. After washing, blots were probed with anti-Myc, anti-CK2α or anti-CK2β antibodies. The data are summarized in Figure 3. Recombinant Myc-tagged PKD showed an interaction with full-length CSN3 subunit of the purified CSN. Under these conditions only very weak inter action with recombinant ΔCSN3(111–403) was detected (Figure 3B).
Fig. 3. Far-western blots indicate that CK2 and PKD bind to subunits of the CSN. (A) The Ponceau stain of nitrocellulose shows selected recombinant CSN subunits used in far-western blots. A Coomassie Blue-stained SDS–PAGE demonstrates the purified CSN, which was immobilized on nitrocellulose. (B) Recombinant N-terminal Myc-tagged PKD binds to full-length CSN3 of the purified CSN and very weak to ΔCSN3(111–403), as indicated by the anti-Myc antibody. Immobilized recombinant CSN7 is shown as negative control. (C) Immobilized recombinant CSN subunits were incubated with recombinant CK2 consisting of 2α2β. After washing the blots were probed with an anti-CK2α (left panel) or an anti-CK2β antibody (right panel). CSN6 is shown as a negative control.
In Figure 3C it is demonstrated that CK2 interacts with ΔCSN3(111–403) and CSN7. The anti-CK2α antibody shows a strong reaction with ΔCSN3(111–403) fragment, but weakly interacts with recombinant CSN7. In contrast, anti-CK2β antibody indicates strong interaction with CSN7. Its reaction with ΔCSN3(111–403), however, is very weak.
CK2 and PKD phosphorylate CSN subunits, p53 and c-Jun
To determine whether the associated kinases are responsible for protein modifications described previously (Bech-Otschir et al., 2002), in vitro kinase assays were performed with recombinant kinases and [γ-32P]ATP. It has been observed previously that CSN subunits are phosphorylated by the CSN-associated kinases (Kapelari et al., 2000) or by other kinases (Karniol et al., 1999). Therefore, recombinant CSN subunits and the purified CSN complex were used as substrates in kinase assays with recombinant CK2 and PKD. The data are summarized in Figure 4. CK2 modified CSN2 and CSN7 as recombinant proteins as well as in the purified complex (Figure 4B). PKD shows weak phosphorylation of recombinant CSN2, CSN5 and CSN7, but has a strong effect on CSN7 in the purified complex (Figure 4C). There are different effects of different proteins on the autophos phorylation of the recombinant kinases. To demonstrate that equal amounts of PKD were added to all samples, a Coomassie Blue-stained gel is shown in Figure 4C (lower panel). To determine whether phosphorylation of CSN subunits occurs in cells, lysate obtained from reticulocytes was incubated with [γ-32P]ATP. After incubation the CSN was immunoprecipitated. As shown in Figure 4D, autoradiography of immunoprecipitated CSN identified a radioactive band, which is most likely identical to CSN2. Under this condition significant phosphorylation of CSN7 was not observed. On the other hand, immunoprecipitated CSN from HeLa cells was able to phosphorylate CSN7 (Figure 2C, right panel).
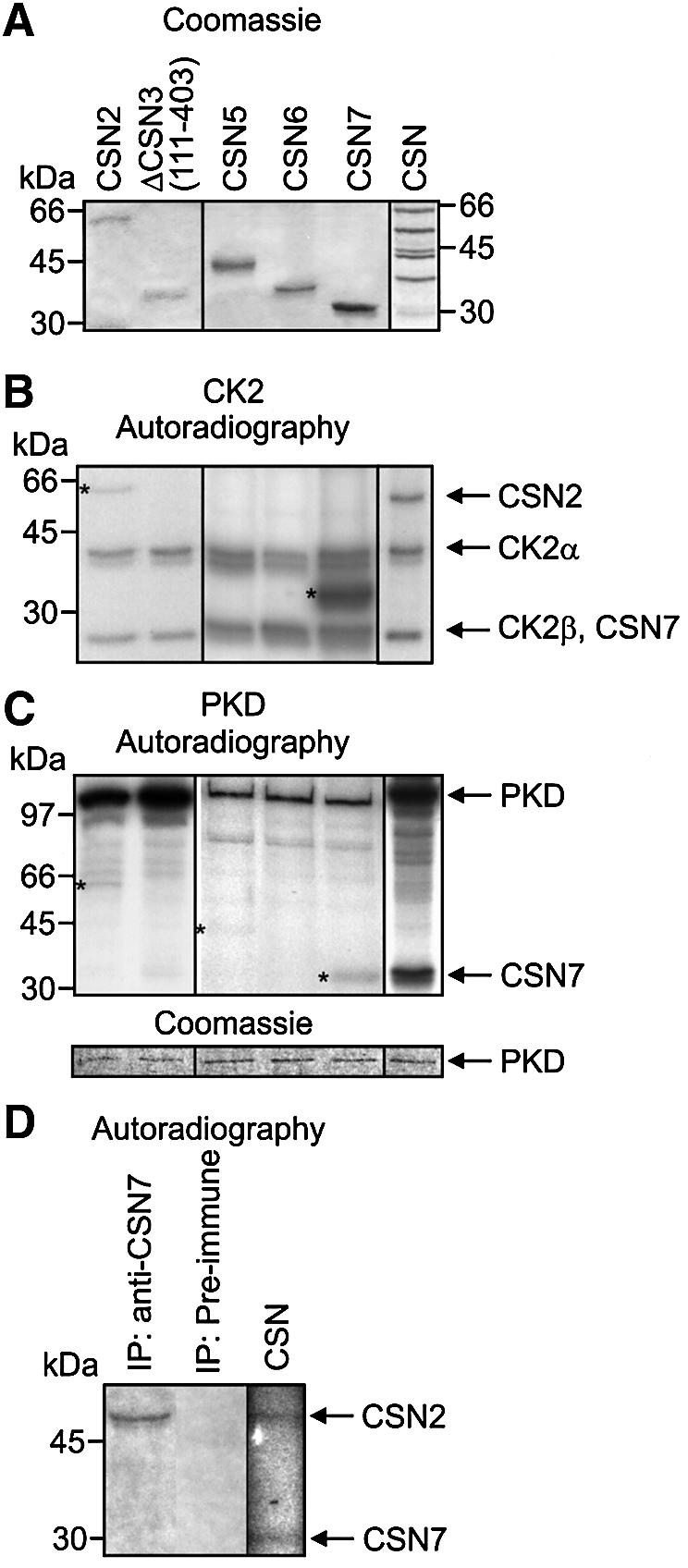
Fig. 4. CK2 and PKD phosphorylate subunits of the CSN. (A) In vitro kinase assays were performed with shown recombinant CSN subunits and purified CSN as substrates (Coomassie). (B) Each recombinant CSN subunit or purified CSN were incubated with recombinant CK2. Autoradiography shows phosphorylated recombinant CSN subunits labeled with stars. Autoradiography of the phosphorylated CSN complex reveals that phosphorylated CSN7 and autophosphorylated CK2β co-migrate in SDS–PAGE. Complex-bound subunits migrate faster in SDS–PAGE as compared with recombinant His6-tagged CSN subunits. (C) Kinase reactions were carried out as in (B) using recombinant PKD. Phosphorylated recombinant subunits are indicated by stars. Different proteins exert different effects on autophosphorylation of recombinant kinases. To demonstrate that equal amounts of PKD were added to each sample, Coomassie Blue-stained PKD is shown (lower panel). (D) Subunit CSN2 of endogenous CSN is phosphorylated in reticulocyte lysate. Reticulocyte lysate was incubated with [γ-32P]ATP. After incubation endogenous CSN was immunoprecipitated and the precipitate was analyzed by SDS–PAGE and autoradiography. Control (CSN): purified CSN was incubated with [γ-32P]ATP and then analyzed by SDS–PAGE and autoradiography.
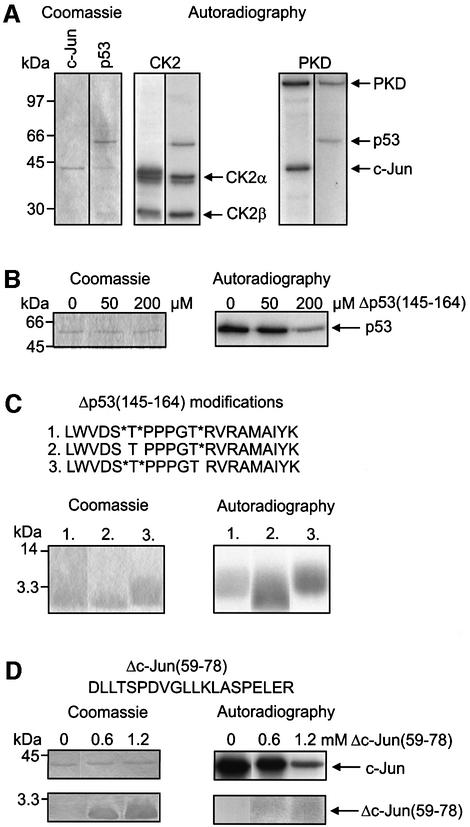
Next, p53 and c-Jun were used as substrates of recombinant PKD or CK2. Figure 5A shows that both CK2 and PKD phosphorylated c-Jun as well as p53. In addition, autophosphorylations of CK2α, CK2β and PKD were observed.
Fig. 5. CK2 and PKD phosphorylate c-Jun and p53. (A) Recombinant c-Jun and p53 shown in the Coomassie Blue-stained gel were used for in vitro kinase assays. CK2: recombinant c-Jun or p53 was incubated with recombinant CK2 in presence of [γ-32P]ATP. After 1 h at 37°C the reaction mix was separated by SDS–PAGE and the dried gel was autoradiographed. The autoradiography shows phosphorylation of c-Jun and p53 as well as autophosphorylation of CK2α and CK2β. PKD: the kinase reaction was carried out with recombinant PKD as described above. The autoradiography shows phosphorylation of c-Jun and p53 as well as autophosphorylation of PKD. (B) Inhibition of CK2-dependent p53 phosphorylation by phosphorylated Δp53(145–164) peptide. Recombinant p53 was incubated with recombinant CK2 as outlined in (A). Phosphorylated Δp53(145–164) was added to the reaction mix at indicated concentrations. (C) CK2 phosphorylates Thr155 of p53. Three different modifications of Δp53(145–164) shown in the upper panel were tested in kinase assays with recombinant CK2. Stars indicate phosphorylated serine (S*) or threonine (T*). After kinase reaction samples were analyzed by 15% SDS–PAGE and autoradiography. (D) Inhibition of CK2-dependent c-Jun phosphorylation by Δc-Jun(59–78). Recombinant c-Jun was incubated with recombinant CK2 as described in (A) and Δc-Jun(59–78) was added at indicated concentrations. Lower panel: Δc-Jun(59–78) was phosphorylated by CK2.
We then wished to determine which of the two kinases is responsible for p53 phosphorylation that targets the tumor suppressor to degradation by the Ub system. In previous experiments it has been shown that p53 is phosphorylated at Thr155 by the CSN-associated kinases and that this modification is crucial for p53 stability (Bech-Otschir et al., 2001). In addition, we have shown that the p53 peptide Δp53(145–164) acts as a specific competitor by inhibiting p53 phosphorylation, which stabilizes the tumor suppressor towards degradation by the Ub system in vitro and in vivo (Bech-Otschir et al., 2001). Therefore, the effect of Δp53(145–164) with phosphorylated Ser149, Thr150 and Thr155 on the phosphorylation of p53 by recombinant PKD and CK2 was tested. Phosphorylated Δp53(145–164) did not influence the modification of p53 by PKD (data not shown). In contrast, there was a significant inhibition of p53 phosphorylation by CK2, as shown in Figure 5B. Moreover, Δp53(145–164) was directly phosphorylated by CK2 (Figure 5C). To test whether Thr155 is a target residue of CK2, different modifications of Δp53(145–164) with phosphorylated serine (S*) and/or threonine (T*) were synthesized. Figure 5C demonstrates that peptide 3, which contained Thr155 as the only free acceptor of [32P]phosphate, was phosphorylated by CK2. In addition, Ser149 and Thr150 were also phosphorylated by CK2. These data confirm our earlier findings with recombinant p53 mutants p53(Ser149→Ala, Thr150→Val) or p53(Thr155→Val) (Bech-Otschir et al., 2001).
Another peptide derived from the phosphopeptides shown in Table I, Δc-Jun(59–78), containing a CK2 consensus phosphorylation site (Thr62), was synthesized and tested in kinase assay with recombinant CK2 and c-Jun as substrate. As shown in Figure 5D the peptide inhibited c-Jun phosphorylation by CK2. The peptide itself was phosphorylated, although with low affinity, indicating that Thr62 is a target of CK2. Interestingly, mutation of Thr62 to Ala did not change significantly degradation of a c-Jun(Thr62→Ala) mutant in HeLa cells as compared with wild-type c-Jun (data not shown).
Another important question was whether curcumin is able to block CK2 and PKD or whether known inhibitors of CK2 and PKD would affect the kinase activity associated with the CSN. The Ki values shown in Table II were obtained in kinase assays using c-Jun as substrate. Inhibitors were added in a concentration range from 0 to 100 µM and Ki values calculated using simple Michaelis–Menten kinetics. Curcumin inhibits the CSN-associated kinase activity as well as recombinant CK2 and PKD with comparable high affinity. Emodin and DRB, inhibitors of CK2 (Poele et al., 1999; Battistutta et al., 2000), as well as resveratrol, an inhibitor of PKD (Haworth and Avkiran, 2001), completely blocked the kinase activity of the purified complex. Interestingly, the CSN complex-bound kinases possessed higher affinities to the inhibitors compared with the recombinant kinases.
Table II. Ki values (µM) for the inhibition of CSN-associated kinases, recombinant CK2 and PKD by curcumin, emodin, DRB and resveratrol.
| Inhibitor | CSN | CK2 | PKD |
|---|---|---|---|
| Curcumin | 2.6 | 11.8 | 4.1 |
| Emodin | 4.4 | 22.7 | 94.5 |
| DRB | 12.0 | 97.9 | 75.5 |
| Resveratrol | 32.1 | 51.0 | 17.6 |
DRB, 5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole.
Inhibitors of the CSN-associated kinases CK2 and PKD stimulate Ub-dependent degradation of c-Jun in HeLa cells
We have shown previously that phosphorylation by the CSN-associated kinases leads to degradation of p53 by and to stabilization of c-Jun towards the Ub system (Naumann et al., 1999; Bech-Otschir et al., 2001). Degradation of endogenous c-Jun in HeLa cells occurred in the presence of curcumin (Pollmann et al., 2001). If the effect of curcumin is due to inhibition of CK2 and PKD, other inhibitors shown in Table II should exert similar effects on c-Jun degradation in HeLa cells. In Figure 6A it is demonstrated that 50 µM of curcumin significantly reduced ectopically expressed c-Jun levels in HeLa cells after 4 h of treatment compared with the control. Near disappearance of c-Jun was also observed in the presence of emodin (200 µM). Less effect on overexpressed c-Jun levels was obtained with 200 µM of DRB or with 100 µM of resveratrol. The difference might be due in part to lower affinities of DRB and resveratrol towards the CSN-associated kinases compared with curcumin and emodin (see Table II). The proteasome inhibitor MG 132 had only little effect on the stability of Flag-tagged c-Jun supporting the fact that in the absence of kinase inhibitors c-Jun is stable towards the proteasome system (Pollmann et al., 2001). Figure 6B shows degradation of Flag-tagged c-Jun and formation of high molecular weight (HMW)-c-Jun species, depending on curcumin concentration. Treatment of HeLa cells with 25 or 50 µM curcumin led to a significant decrease of c-Jun accompanied by formation of HMW-c-Jun species recognized by the anti-Flag antibody. Treatment of HeLa cells with both 50 µM curcumin and 10 µM MG 132 resulted in partial stabilization of Flag-tagged c-Jun and in dramatic accumulation of HMW-c-Jun species. Probing the same samples shown in Figure 6B with an anti-Ub antibody revealed that Ub conjugate formation increased concomitantly with the formation of HMW-c-Jun species in dependence on curcumin concentration (Figure 6C). The highest amounts of Ub conjugates were detected when both curcumin and MG 132 were added to HeLa cells.
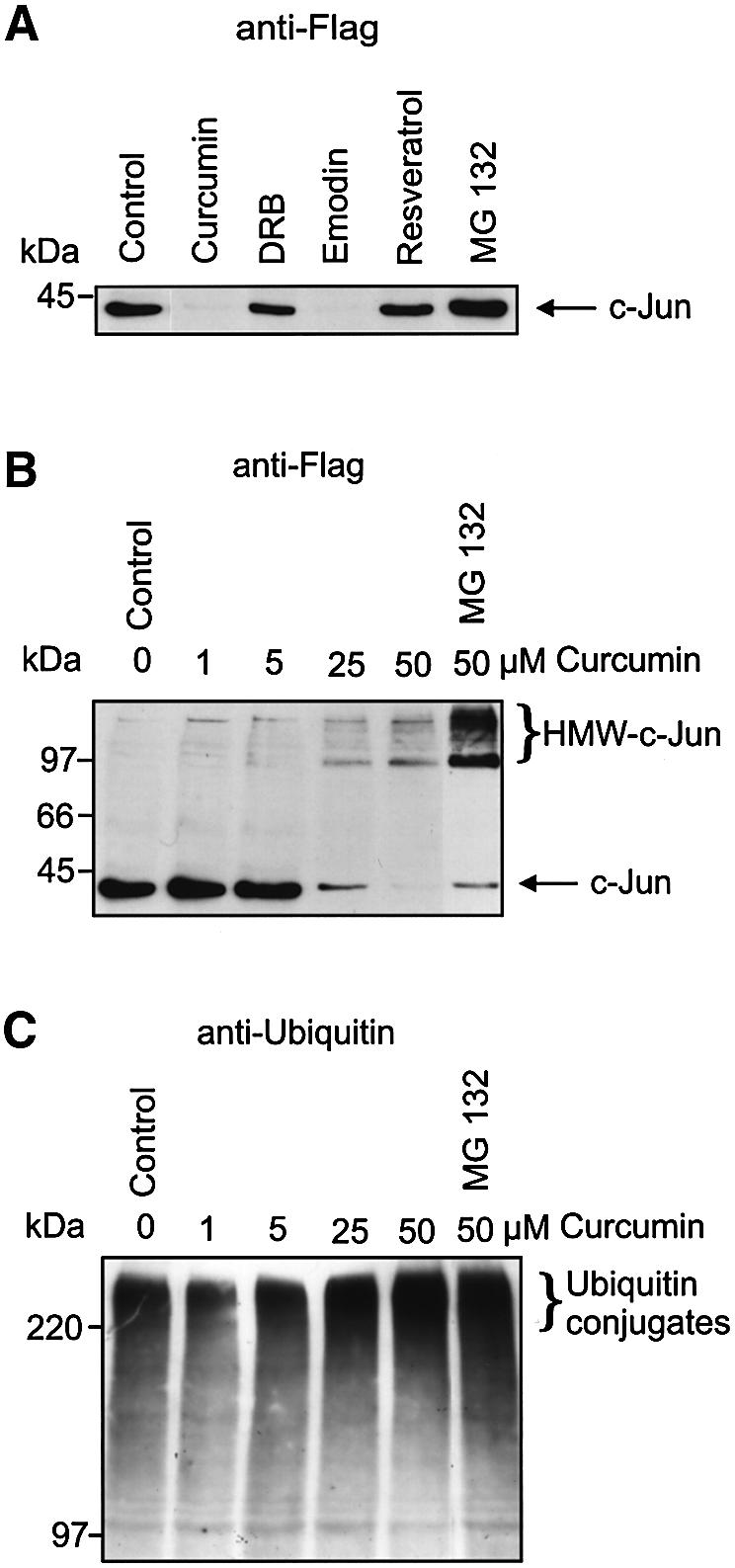
Fig. 6. Ub-dependent degradation of c-Jun is stimulated by inhibitors of CSN-associated kinases. (A) HeLa cells were transiently transfected with c-Jun cDNA in pcDNA3.1 encoding an N-terminal Flag tag. Twenty-four hours after transfection cells were treated with curcumin, DRB, emodin, resveratrol or MG 132. HeLa cell lysate was immunoblotted using anti-Flag antibody. Control: cells treated with 0.25% DMSO. (B) HeLa cells transiently transfected with c-Jun as in (A) were treated with indicated concentrations of curcumin or curcumin (50 µM) and MG 132 (10 µM). Ten percent SDS–PAGE and immunoblots with anti-Flag antibody revealed formation of HMW-c-Jun species. (C) The same samples as in (B) were separated by 7.5% SDS–PAGE, blotted to nitrocellulose and tested with anti-Ub antibody.
Discussion
CK2 and PKD are associated with the CSN
The objective of this study was to identify kinases associated with the CSN. We show that curcumin-sensitive kinase activity, as well as the protein kinases CK2 and PKD, co-purify with the complex during its preparation from human erythrocytes. Moreover, the data presented demonstrate that CK2 and PKD are in fact associated with the CSN, as indicated by immunoprecipitation and far-western blots.
Electron microscopy analysis of CSN particles that were incubated with gold-labeled uncleavable ATP analog indicates that at least 10% of CSN particles are associated with kinases most likely mediating ATP binding. Because it is not clear whether all ATP-binding sites were saturated under the conditions used and few ATP molecules are linked to each gold particle, there might be a higher portion of complexes associated with kinases. In addition, kinases might dissociate from the complex during purification procedures. Observation of only one gold cluster per CSN particle might be due to steric hindrance caused by the 3 nm gold clusters, and does not necessarily mean that only one kinase binds to one particle. The molecular weight of purified CSN has been estimated to be 450 kDa (Seeger et al., 1998). Summing up the molecular weights of the eight core CSN subunits a total of 320 kDa can be calculated. The CK2 holoenzyme has ∼140 kDa and the PKD 100 kDa, suggesting that only one kinase is associated. In glycerol gradients, however, the CSN complex smears up to 700 kDa, indicating that binding of the two kinases at the same time is possible.
CSN purification and immunoprecipitations demonstrate that CK2 and PKD are associated with the CSN in red blood cells and in HeLa cells. Immunoprecipitated particles possess kinase activity, most likely due to CK2 and/or PKD, that phosphorylates IκBα, c-Jun and CSN7, but not CSN6. At the moment it is not clear whether kinase activity of the immunoprecipitated CSN is due to CK2 and/or PKD.
Binding of CK2 and PKD to the complex seems to be mediated by CSN3. Interestingly, Rpn3/S3, the homologous subunit of CSN3 in the 26S proteasome lid complex, also interacts with CK2 in yeast (for a review, see Ferrell et al., 2000). In addition, CK2 interacts with the CSN subunit CSN7. The exact CSN3 and CSN7 regions involved in kinase binding have to be elucidated in future studies.
Kinase binding is connected with phosphorylation of CSN2 and CSN7 by CK2 and of CSN7 by PKD of the purified CSN complex. It is noteworthy that all CSN subunits contain several consensus sequences for CK2 and PKD phosphorylation, but only CSN2 and CSN7 are specifically modified. Interestingly, experiments with reticulocyte lysate revealed that only CSN2 was phosphorylated. On the other hand, immunoprecipitated CSN from HeLa cells phosphorylated CSN7. Perhaps specific signaling is necessary to regulate CSN subunit phosphorylation. Although we do not know the exact physiological function of CSN subunit phosphorylation, the data imply a functional relationship between the two kinases and the CSN. It has been shown that CSN7 (Karniol et al., 1999) and CSN2 (Kapelari et al., 2000) are phosphorylated. However, to our knowledge this is the first time that the kinases responsible for CSN subunit phosphorylation have been identified.
Binding of CK2 and PKD to the CSN does not exclude the possibility that CSN particles can also interact with other kinases, such as 5/6-kinase. Our observations support in part the results obtained by Majerus and co-workers (Wilson et al., 2001; Sun et al., 2002). In our preparations the 5/6-kinase co-purifies with the CSN until ResourceQ column, after which we selected a CSN pool that did not contain detectable 5/6-kinase. Therefore, the 5/6-kinase cannot be responsible for the activities shown in this study.
The CSN-associated kinases CK2 and PKD are involved in the regulation of c-Jun degradation by the Ub pathway
The CK2 has been described previously as a pleiotropic, ubiquitous and constitutive active protein kinase involved in cell cycle regulation, signal transduction, development, apoptosis and cancer (for a review, see Guerra and Issinger, 1999). It is an anti-apoptotic agent that triggers cell growth and proliferation (for a review, see Ahmed et al., 2002). It phosphorylates more than 160 proteins and is localized almost everywhere in the cell. Cellular functions of CK2 still remain unclear (for a review, see Faust and Montenarh, 2000). In human cells the holo-enzyme consists of two catalytic α and/or α′ and of two regulatory β subunits. The recombinant CK2 used in our binding studies had the composition 2α2β. At the moment we do not know whether a kinase consisting of 2α′2β can interact with the CSN. Here we show for the first time that CK2 phosphorylation of p53 is most likely responsible for the instability of the tumor suppressor under normal cell growth conditions. CK2 phosphorylates Δp53(145–164) at Thr155. In previous experiments we have shown that modification at Thr155 is crucial for p53 stability towards the Ub system. Δp53(145–164) reduces phosphorylation of p53 and stabilizes the tumor suppressor in vitro and in vivo (Bech-Otschir et al., 2001). This is in line with results demonstrating that CK2 activity is elevated in human solid tumors and leukemia (for a review, see Guerra and Issinger, 1999) and that CK2 inhibition by DRB triggers p53-dependent apoptosis in human colon carcinoma cells (Poele et al., 1999).
CK2 also phosphorylates c-Jun. There is a significant inhibition of CK2-dependent c-Jun phosphorylation by Δc-Jun(59–78). Phosphorylation of Δc-Jun(59–78) revealed that Thr62 might be a target of CK2. The function of Thr62 phosphorylation by CK2 is not known.
The PKD belongs to a new family of serine/threonine kinases, which differ from the PKC family members by their pleckstrin homology domain and substrate specificity. It can be located in the cytosol, in the Golgi, in mitochondria, at the plasma membrane and in the nucleus, and has a role in Golgi function, in cell proliferation, signal transduction, apoptosis and cancer (for a review, see Van Lint et al., 2002). PKD is activated by PKC signaling (Waldron et al., 2001) and has an inhibitory effect on JNK signaling (Hurd and Rozengurt, 2001). Here we show that PKD phosphorylates c-Jun as well as p53. At the moment we do not know the exact function of c-Jun and p53 phosphorylation by PKD.
Our inhibitor studies are in line with other results showing that CK2 and PKD are recruited by the CSN. Curcumin, DRB, emodin and resveratrol inhibit CK2 and PKD, as well as CSN-associated kinase activity. Whether higher affinities towards inhibitors of complex-bound kinases versus those of free kinases reflect modification of kinase activity or specificity by CSN binding and whether free kinases have similar effects on protein stability remain to be shown in future studies.
Curcumin, DRB, emodin and resveratrol induced degradation of transiently expressed c-Jun in HeLa cells. Specificity of the inhibitors used is not very high. However, because all tested inhibitors of CSN-associated kinases have similar effects on c-Jun levels, the conclusion that c-Jun is stabilized by CSN-mediated phosphorylation is very likely. Most of the HMW-c-Jun species formed in the presence of curcumin seem to be Ub conjugates; they significantly accumulate in the presence of MG 132 and cross-react with the anti-Ub antibody. We speculate that c-Jun phosphorylation by CSN-associated kinases prevents binding of the transcription factor to the responsible, as yet unknown, Ub ligase and inhibitors of CSN-associated kinases target c-Jun to ubiquitylation and proteasome-dependent degradation.
Models of the CSN as a platform that regulates Ub conjugate formation
Recently, it has been shown that the CSN interacts with SCF Ub ligases and regulates ubiquitylating activity by deneddylation of cullins (Lyapina et al., 2001; Schwechheimer et al., 2001; Zhou et al., 2001; Cope et al., 2002). In addition, the CSN seems to be of general importance for the activity of different Ub ligases as it has been described for plant cells (Schwechheimer et al., 2002). Here we show that the CSN can recruit CK2 and/or PKD. CK2 phosphorylates p53 at Thr155, which induces a p53 conformation recognized by the Ub ligase Mdm2 (Bech-Otschir et al., 2001). Interestingly, Mdm2 is also phosphorylated by CK2 (Hjerrild et al., 2001) and might also bind to the CSN (S.Uhle and W.Dubiel, unpublished data).
In the case of c-Jun, CSN-mediated phosphorylation stabilizes the transcription factor and inhibition of CSN-associated kinases triggers Ub conjugate formation and degradation. Perhaps the mechanism of c-Jun degradation is similar to that of the plant transcription factor HY5. CK2 phosphorylates HY5, which prevents binding of the transcription factor to its putative Ub ligase COP1 (Hardtke et al., 2000). Based on these results we hypothesize that the CSN is a platform, which recruits kinases for regulating Ub conjugate formation by different E3s.
Many questions remain open: what is the mechanism that regulates binding and activity of CSN-associated kinases? Which of the CSN-associated kinases phosphorylate which substrates, under what circumstances, and what are the consequences? What is the molecular basis for stabilization/destabilization towards the Ub system?
Materials and methods
Purification of human CSN and kinase assay
The CSN was isolated from human erythrocytes and kinase activity was determined with [γ-32P]ATP (ICN) as outlined previously (Seeger et al., 1998; Henke et al., 1999). Recombinant CK2 (2α2β) and recombinant N-terminal Myc-tagged PKD were obtained from Calbiochem. Recombinant c-Jun (Pollmann et al., 2001), CSN subunits (Kapelari et al., 2000) and p53 (Bech-Otschir et al., 2001) were produced as described previously. Recombinant IκBα was from Santa Cruz. The protein kinase inhibitors emodin and resveratrol were purchased from Calbiochem, curcumin was from Sigma and MG 132 from Affiniti. Specific curcumin-sensitive kinase activity was determined in erythrocyte lysate and in pooled fractions from different preparation steps using 1 µg of c-Jun as substrate in a final reaction volume of 20 µl with and without 50 µM curcumin. After SDS–PAGE and autoradiography the data were evaluated by densitometry. The relative kinase activity in the presence of curcumin was subtracted from that in the absence of curcumin. The difference was related to the protein content of the samples (Bradford; Bio-Rad) and is called the relative specific curcumin-sensitive kinase activity. By fixing the relative specific curcumin-sensitive kinase activity in the lysate to one, the fold co-purification of curcumin-sensitive kinase activity with the CSN was calculated.
To determine Ki values, emodin, DRB, resveratrol or curcumin was added to kinase assays with c-Jun as substrate at final concentrations of 0, 10, 20, 50 and 100 µM. After SDS–PAGE and autoradiography the results were evaluated by densitometry. Ki values were calculated using Michaelis–Menten kinetics.
To detect CSN subunit phosphorylation in reticulocyte lysate, 50 µl of lysate (Promega) were incubated with 40 µCi of [γ-32P]ATP for 30 min at 37°C. After incubation, CSN was immunoprecipitated (see below) and examined by SDS–PAGE and autoradiography.
Chymotryptic phosphopeptide analyses of His-tagged recombinant proteins were performed as outlined previously for c-Jun (Seeger et al., 1998).
Phosphorylated Δp53(145–164) and unphosphorylated Δc-Jun(59–78) peptides were synthesized using the FMOC strategy on a 433A peptide synthesizer (ABI). Phosphorylated Ser and Thr were obtained from Novabiochem.
Preparation of gold-labeled AMP-PCP
N6-[(6-aminohexyl)carbamoylmethyl]-AMP-PCP was synthesized as described previously for ATP (Gebeyehu et al., 1987). N6-[(6-aminohexyl)carbamoylmethyl]-AMP-PCP (25 mg) was incubated for 15 h at 4°C with 2-iminothiolane (50 mg; Sigma) in 0.5 ml of 1 M triethanolamine pH 8.4, 0.25 M KCl and 25 mM Mg(OAc)2. Thiol-AMP-PCP was purified by anion exchange chromatography on Sephadex A-25 using a linear gradient of 0.15–0.7 M TEAB. The product eluted at 0.5–0.55 M salt and was recovered by lyophilization.
The preparation of 3.5 nm colloidal gold was adapted from the method of Slot and Geuze (1985), with minor modifications. In brief, 80 ml of 0.012% NaAuCl4 in water and 20 ml of 0.25% tannic acid, 0.2% sodium citrate, 1 mM potassium carbonate were brought to 60°C and rapidly mixed together. The gold colloids were formed within seconds and no additional purification was needed. Ten microliters of 10 mM thiol-AMP-PCP were used to stabilize the 3.5 nm coloidal gold contained in 100 µl.
Electron microscopy of negatively stained CSN particles
A 2 µl drop of CSN was incubated with 1 µl of gold-labeled AMP-PCP for 7 min. Next, the 5 µl solution was applied to 100 × 400 mesh copper grids that had been coated with carbon and glow discharged in a plasma cleaner for 45 s. After blotting, the sample was negatively stained with 2% aqueous solution of uranyl acetate for 45 s. Electron microscopy was carried out using a CM12 transmission electron microscope (Philips) operating at 120 kV accelerating voltage. Images were recorded digitally (Photometrix slow scan CCD; 1024 × 1024 pixel) at a total magnification of 45 700× and 2 µm defocus.
Immunoprecipitation
The anti-CSN7 antibody used for immunoprecipitations was produced in rabbits by standard methods using full-length recombinant CSN7 for immunization. Five microliters of the anti-CSN7 antibody were incubated with 40 µl (∼10 µg protein) of purified CSN fraction or with HeLa cell lysate obtained from 2–5 × 106 cells or with 50 µl of reticulocyte lysate for 2 h on ice. After incubation, 50 µl of protein A–Sepharose (Amersham) were added to the mixture and incubation was continued on a rotary plate at 4°C for 2 h. After washing three times with lysis buffer [20 mM Tris pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.05% Triton X-100, complemented with fresh solution of 20 mM NaF, 2 mM Na2VO4 and a protein inhibitor cocktail (Roche)], the pellet was used for SDS–PAGE or for kinase assay.
In the case of PKD co-immunoprecipitation, 40 µl of purified CSN were pre-incubated with 0.4 µg of N-terminal Myc-tagged PKD for 30 min at 37°C.
Controls were performed by carrying out the protocol in the absence of anti-CSN7 antibody and in experiments with the pre-immune serum the anti-CSN7 antibody was substituted by pre-immune serum.
Far-western and western blots
Far-western blots with recombinant CSN subunits were performed as outlined previously (Kapelari et al., 2000). In brief, recombinant CSN subunits and purified CSN were immobilized on nitrocellulose and incubated for 2 h at room temperature with 0.5 µg/ml recombinant Myc-tagged PKD or with 1.5 µg/ml recombinant CK2 (New England Biolabs) in phosphate-buffered saline. After washing, the blots were probed with a monoclonal anti-Myc (Calbiochem) or with monoclonal anti-CK2α or anti-CK2β (Calbiochem) antibodies.
Western blot analysis was performed using the same monoclonal antibodies against CK2. For PKD detection, an anti-PKD antibody was employed, which was produced in rabbits by standard methods using the C-terminal PKD peptide EEREMKALSERVSIL for immunization. All western blots shown in Figure 1 were performed with the anti-PKD C-20 antibody from Santa Cruz. The anti-5/6-kinase antibody was a gift from M.Wilson. The anti-CSN5 antibody was obtained from B.Christy and the anti-Cul1 from Calbiochem. All blots were developed by ECL or ECL-Plus technique (Amersham).
Cell culture
HeLa cells were cultured in RPMI 1640 medium containing 10% (v/v) fetal calf serum, 2 mM glutamine (Life Technologies, Inc.), penicillin (100 U/ml) and streptomycin (100 µg/ml) in a humidified 5% CO2 atmosphere.
For inhibitor treatment, 5 × 106 HeLa cells were incubated with 200 µM emodin, 100 µM resveratrol, 200 µM DRB, 50 µM curcumin and/or 10 µM MG 132 in 0.25% dimethyl sulfoxide (DMSO) for 4 h. Control cells were treated with 0.25% DMSO. After inhibitor treatment, cells were harvested and lysed using ice-cold triple-detergent lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, 1 µg/ml aprotinin, 1% NP-40, 0.5% sodium deoxycholate) as described previously (Bech-Otschir et al., 2001). In western blots, HeLa cell lysates were probed with anti-Flag antibody (Stratagene) or with anti-Ub antibody (Daco).
For immunoprecipitation, HeLa cells were lysed with lysis buffer (see Immunoprecipitation).
Transient transfections of HeLa cells with Flag-tagged c-Jun
Wild-type c-Jun cDNA was obtained by PCR and cloned into pcDNA3.1 vector encoding an N-terminal Flag tag. Electroporation was performed with 300 µl of cell suspension (1.5 × 107 cells/ml) and 10 µg of the c-Jun expression construct. Twenty-four hours after transfection, cells were treated with curcumin, emodin, DRB, resveratrol and/or MG 132 in 0.25% DMSO for 4 h. Controls were incubated with 0.25% DMSO. After incubation, cells were harvested and lysed with lysis buffer [20 mM Tris pH 8.0, 137 mM NaCl, 2 mM EDTA, 0.1% Triton X-100, supplemented with 20 mM NaF, 2 mM Na3VO4 and protease inhibitors (Roche)]. Aliquots of HeLa cell lysate (∼5 µg of total protein) were probed with anti-Flag or anti-Ub antibodies.
Acknowledgments
Acknowledgements
We are grateful to W.Henke for calculating the Ki values. We thank B.Christy for providing us with the anti-CSN5 antibody and M.Wilson for the anti-5/6-kinase antibody. This work was supported by grants DU 229/5-2 and 229/6-1 from the Deutsche Forschungsgemeinschaft to W.D.
References
- Ahmed K., Gerber,D.A. and Cochet,C. (2002) Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol., 12, 226–230. [DOI] [PubMed] [Google Scholar]
- Battistutta R., Sarno,S., De Moliner,E., Papinutto,E., Zanotti,G. and Pinna,L.A. (2000) The replacement of ATP by the competitive inhibitor emodin induces conformational modifications in the catalytic site of protein kinase CK2. J. Biol. Chem., 275, 29618–29622. [DOI] [PubMed] [Google Scholar]
- Bech-Otschir D., Kraft,R., Huang,X., Henklein,P., Kapelari,B., Pollmann,C. and Dubiel,W. (2001) COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J., 20, 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Otschir D., Seeger,M. and Dubiel,W. (2002) The COP9 signalosome: at the interface between signal transduction and ubiquitin-dependent proteolysis. J. Cell Sci., 115, 467–473. [DOI] [PubMed] [Google Scholar]
- Cohen H., Azriel,A., Cohen,T., Meraro,D., Hashmueli,S., Bech-Otschir,D., Kraft,R., Dubiel,W. and Levi,B.Z. (2000) Interaction between interferon consensus sequence-binding protein and COP9/signalosome subunit CSN2 (Trip15). A possible link between interferon regulatory factor signaling and the COP9/signalosome. J. Biol. Chem., 275, 39081–39089. [DOI] [PubMed] [Google Scholar]
- Cope G.A., Suh,G.S., Aravind,L., Schwarz,S.E., Zipursky,S.L., Koonin,E.V. and Deshaies,R.J. (2002) Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science, 298, 608–611. [DOI] [PubMed] [Google Scholar]
- Deng X.-W. et al. (2000) Unified nomenclature for the COP9 signalosome and its subunits: an essential regulator of development. Trends Genet., 16, 202–203. [DOI] [PubMed] [Google Scholar]
- Faust M. and Montenarh,M. (2000) Subcellular localization of protein kinase CK2. Cell Tissue Res., 301, 329–340. [DOI] [PubMed] [Google Scholar]
- Ferrell K., Wilkinson,C.R.M., Dubiel,W. and Gordon,C. (2000) Regulatory subunit interactions of the 26S proteasome, a complex problem. Trends Biochem. Sci., 25, 83–88. [DOI] [PubMed] [Google Scholar]
- Freilich S., Oron,E., Kapp,Y., Nevo-Caspi,Y., Orgad,S., Segal,D. and Chamovitz,D.A. (1999) The COP9 signalosome is essential for development of Drosophila melanogaster. Curr. Biol., 9, 1187–1190. [DOI] [PubMed] [Google Scholar]
- Gebeyehu G., Rao,P.Y., SooChan,P., Simms,D.A. and Klevan,L. (1987) Novel biotinylated nucleotide analogs for labeling and colorimetric detection of DNA. Nucleic Acids Res., 15, 4513–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman M.H., Rubin,D.M., Coux,O., Wefes,I., Pfeifer,G., Cjeka,Z., Baumeister,W., Fried,V.A. and Finley,D. (1998) A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell, 94, 615–623. [DOI] [PubMed] [Google Scholar]
- Guerra B. and Issinger,O.-G. (1999) Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis, 20, 391–408. [DOI] [PubMed] [Google Scholar]
- Hardtke Ch.S., Gohda,K., Osterlund,M.T., Oyama,T., Okada,K. and Deng,X.-W. (2000) HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J., 19, 4997–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth R.S. and Avkiran,M. (2001) Inhibition of protein kinase D by resveratrol. Biochem. Pharmacol., 62, 1647–1651. [DOI] [PubMed] [Google Scholar]
- Henke W., Ferrell,K., Bech-Otschir,D., Seeger,M., Schade,R., Jungblut,P., Naumann,M. and Dubiel,W. (1999) Comparison of human COP9 signalsome and 26S proteasome lid. Mol. Biol. Rep., 26, 29–34. [DOI] [PubMed] [Google Scholar]
- Hjerrild M., Milne,D., Dumaz,N., Hay,T., Issinger,O.-G. and Meek,D. (2001) Phosphorylation of murine double minute clone 2 (MDM2) protein at serine-267 by protein kinase CK2 in vitro and in cultured cells. Biochem. J., 355, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd C. and Rozengurt,E. (2001) Protein kinase D is sufficient to suppress EGF-induced c-Jun Ser63 phosphorylation. Biochem. Biophys. Res. Commun., 282, 404–408. [DOI] [PubMed] [Google Scholar]
- Kapelari B., Bech-Otschir,D., Hegerl,R., Schade,R., Dumdey,R. and Dubiel,W. (2000) Electron microscopy and subunit–subunit interaction studies reveal a first architecture of COP9 signalosome. J. Mol. Biol., 300, 1169–1178. [DOI] [PubMed] [Google Scholar]
- Karniol B., Malec,P. and Chamovitz,D.A. (1999) Arabidopsis FUSCA5 encodes a novel phosphoprotein that is a component of the COP9 complex. Plant Cell, 11, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T. et al. (2001) NEDD8 recruits E2-ubiquitin to SCF E3 ligase. EMBO J., 20, 4003–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapina S. et al. (2001) Promotion of NEDD–CUL1 conjugate cleavage by COP9 signalosome. Science, 292, 1382–1385. [DOI] [PubMed] [Google Scholar]
- Musti A.M., Treier,M. and Bohmann,D. (1997) Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science, 275, 400–402. [DOI] [PubMed] [Google Scholar]
- Naumann M., Bech-Otschir,D., Huang,X., Ferrell,K. and Dubiel,W. (1999) COP9 signalosome-directed c-Jun activation/stabilization is independent of JNK. J. Biol. Chem., 274, 35297–35300. [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke,C.S., Wei,N. and Deng,X.-W. (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature, 405, 462–466. [DOI] [PubMed] [Google Scholar]
- Poele R.H., Okorokov,A.L. and Joel,S.P. (1999) RNA synthesis block by 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) triggers p53-dependent apoptosis in human colon carcinoma cells. Oncogene, 18, 5765–5772. [DOI] [PubMed] [Google Scholar]
- Pollmann C., Huang,X., Mall,J., Bech-Otschir,D., Naumann,M. and Dubiel,W. (2001) The COP9 signalosome directs VEGF production in tumor cells. Cancer Res., 61, 8416–8421. [PubMed] [Google Scholar]
- Schwechheimer C. and Deng,X.-W. (2001) COP9 signalosome revisited: a novel mediator of protein degradation. Trends Cell Biol., 11, 420–425. [DOI] [PubMed] [Google Scholar]
- Schwechheimer C., Serino,G., Callis,J., Crosby,W.L., Lyapina,S., Deshaies,R.J., Gray,W.M., Estelle,M. and Deng,X.-W. (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response. Science, 292, 1379–1382. [DOI] [PubMed] [Google Scholar]
- Schwechheimer C., Serino,G. and Deng,X.-W. (2002) Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell, 14, 2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M., Kraft,R., Ferrell,K., Bech-Otschir,D., Dumdey,R., Schade,R., Gordon,C., Naumann,M. and Dubiel,W. (1998) A novel protein complex involved in signal transduction possessing similarities to 26S proteasome subunits. FASEB J., 12, 469–478. [PubMed] [Google Scholar]
- Slot J.W. and Geuze,H.J. (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur. J. Cell Biol., 38, 87–93. [PubMed] [Google Scholar]
- Sun Y., Wilson,M.P. and Majerus,P.W. (2002) Inositol 1,3,4-trisphosphate associates with the COP9 signalosome by binding to CSN1. J. Biol. Chem., 277, 45759–45764. [DOI] [PubMed] [Google Scholar]
- Tomoda K., Kubota,Y. and Kato,J. (1999) Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature, 398, 160–165. [DOI] [PubMed] [Google Scholar]
- Van Lint J., Rykx,A., Maeda,Y., Vantus,T., Sturany,S., Malhotra,V., Vandenheede,J.R. and Seufferlein,T. (2002) Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol., 12, 193–200. [DOI] [PubMed] [Google Scholar]
- Waldron R.T., Rey,O., Iglesias,T., Tugal,T., Cantrell,D. and Rozengurt,E. (2001) Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J. Biol. Chem., 276, 32606–32615. [DOI] [PubMed] [Google Scholar]
- Wei N. and Deng,X.W. (1999) Making sense of the COP9 signalosome. A regulatory protein complex conserved from Arabidopsis to human. Trends Genet., 15, 98–103. [DOI] [PubMed] [Google Scholar]
- Wei N., Chamovitz,D.A. and Deng,X.W. (1994) Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell, 78, 117–124. [DOI] [PubMed] [Google Scholar]
- Wei N., Tsuge,T., Serino,G., Dohmae,N., Takio,K., Matsui,M. and Deng,X.W. (1998) The COP9 complex is conserved between plants and mammals and is related to the 26S proteasome regulatory complex. Curr. Biol., 8, 919–922. [DOI] [PubMed] [Google Scholar]
- Wilson M.P., Sun,Y., Cao,L. and Majerus,P.W. (2001) Inositol 1,3,4-trisphosphate 5/6-kinase is a protein kinase that phosphorylates the transcription factors c-Jun and ATP-2. J. Biol. Chem., 276, 40998–41004. [DOI] [PubMed] [Google Scholar]
- Yang X., Menon,S., Lykke-Anderson,K., Tsuge,T., Xiao,D., Wang,X., Rodriguez-Suarez,R.J., Zhang,H. and Wei,N. (2002) The COP9 signalosome inhibits p27kip1 degradation and impedes G1–S phase progression via deneddylation of SCF Cul1. Curr. Biol., 12, 667–672. [DOI] [PubMed] [Google Scholar]
- Zhou C., Seibert,V., Geyer,R., Rhee,E., Lyapina,S., Cope,G., Deshaies,R.J. and Wolf,D.A. (2001) The fission yeast COP9/signalosome is involved in cullin modification by ubiquitin related Ned8p. BMC Biochem., 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]