Abstract
The interaction between β-catenin and LEF-1/TCF transcription factors plays a pivotal role in the Wnt-1 signaling pathway. The level of β-catenin is regulated by partner proteins, including glycogen synthase kinase-3β (GSK-3β) and the adenomatous polyposis coli (APC) tumor suppressor protein. Genetic defects in APC are responsible for a heritable predisposition to colon cancer. APC protein and GSK-3β bind β-catenin, retain it in the cytoplasm, and facilitate the proteolytic degradation of β-catenin. Abrogation of this negative regulation allows β-catenin to translocate to the nucleus and to form a transcriptional activator complex with the DNA-binding protein lymphoid-enhancing factor 1 (LEF-1). This complex is thought to be involved in tumorigenesis. Here we show that covalent linkage of LEF-1 to β-catenin and to transcriptional activation domains derived from the estrogen receptor or the herpes simplex virus protein VP16 generates transcriptional regulators that induce oncogenic transformation of chicken embryo fibroblasts. The chimeras between LEF-1 and β-catenin or VP16 are constitutively active, whereas fusions of LEF-1 to the estrogen receptor are regulatable by estrogen. These experiments document the oncogenicity of transactivating LEF-1 and show that the transactivation domain normally provided by β-catenin can be replaced by heterologous activation domains. These results suggest that the transactivating function of the LEF-1/β-catenin complex is critical for tumorigenesis and that this complex transforms cells by activating specific LEF-1 target genes.
Keywords: estrogen receptor, β-catenin, oncogenic transformation
Wnt signaling plays diverse and important roles in the development of vertebrates and of invertebrates. Among the best-studied effects of Wnt are the determination of segment polarity in Drosophila and specification of the body axis in Xenopus (reviewed in ref. 1). There is also increasing evidence that deregulated Wnt signaling is a significant factor in several human cancers (2, 3). A connection to cancer in animals goes back to the discovery of Wnt signaling; the first vertebrate Wnt growth factor was found as the product of a cellular oncogene, wnt-1 (then called int-1) that is activated by proviral insertion in murine mammary carcinomas (4–6).
Wnt growth factors are secreted glycoproteins. They function as ligands for members of the Frizzled family of seven-transmembrane-domain receptors (7, 8). An important component of the Wnt cascade downstream of Frizzled is β-catenin (9, 10). In the course of Wnt signaling, cytoplasmic β-catenin becomes translocated into the nucleus, where it binds to lymphoid-enhancing factor 1 (LEF-1)/T-cell transcription factor (TCF), thereby activating LEF-1/TCF-dependent transcription (11–17).
β-Catenin interacts with multiple other proteins. It links cadherins with the cytoskeleton, and it also binds the adenomatous polyposis coli (APC) tumor-suppressor protein and interacts with glycogen synthase kinase-3β (GSK-3β) (18–22). Recently discovered β-catenin-binding proteins are axin and conductin. These factors connect β-catenin and GSK-3β and thus facilitate the phosphorylation of β-catenin at sites near its amino terminus, marking the protein for rapid proteolytic degradation (23–28). Axin and conductin may also bind to APC protein, forming quaternary cytoplasmic complexes with GSK-3β and β-catenin (25–27). Cadherins, APC protein, GSK-3β, and axin function as negative regulators of β-catenin in Wnt signaling by sequestering β-catenin in the cytoplasm and by accelerating its proteolytic degradation.
Negative control of β-catenin can be abrogated by mutations in APC protein that abolish its interaction with β-catenin or mutations in β-catenin that prevent phosphorylation by GSK-3β (2, 3, 21). Debilitating mutations in APC protein are found in heritable APC and in sporadic colon cancers; mutations in β-catenin have been reported in several human cancers, including melanomas (2, 3, 29–33). A consequence of these mutations is increased β-catenin/LEF-1-dependent transcription (2, 17, 34). Aberrant regulation of gene expression by LEF-1 may play a part in the oncogenic transformation of the cell.
LEF-1 belongs to a family of transcription factors, all of which recognize the same DNA consensus motif through a high-mobility group (HMG) box DNA-binding domain (35, 36). LEF-1 lacks a conventional transactivation domain; nevertheless, it can transactivate gene expression (37, 38). It can facilitate the assembly of transcription complexes by introducing a sharp bend in the DNA as, for example, at the T-cell receptor α-chain enhancer (39–41). Alternatively, LEF-1 may activate target genes by forming bipartite transcription factor complexes in which LEF-1 provides the DNA-binding moiety, and β-catenin or another protein termed ALY contributes a transactivation domain (11, 13, 42).
Although LEF-1 and other TCF family members are clearly implicated in the development of cancer by their association with β-catenin, the dependence of LEF-1 on β-catenin as a transcriptional coactivator makes it difficult to study its oncogenic potential simply by overexpression. A clear prediction, however, would be, that LEF-1 acquires transforming properties if physically linked to β-catenin. On the other hand, β-catenin is involved in various cellular processes, not all of which are related to transcriptional activation. Therefore, it is still an open question whether the transactivating function of the LEF-1–β-catenin complex would be sufficient for oncogenic transformation.
Here we show that transactivating LEF-1 is indeed oncogenic and that it can utilize not only the transactivation domain supplied by β-catenin but also the hormone-inducible transactivation function 2 (TAF-2) domain of the human estrogen receptor or the acidic transactivation domain of the herpes simplex virus VP16 protein.
MATERIALS AND METHODS
Plasmids.
To generate the LEF-1-VP16 fusions, a BamHI/PvuII fragment from pGEX4T1-LEF-1 (13) coding for LEF-1 amino acids 1–394 was fused to a HindIII/XbaI fragment from pcDNA3-VP16 coding for amino acids 422–490 of the VP16 transactivation domain (43). The resulting fusion was inserted into the vector pCS2+, yielding LEF-1-VP16. From this construct we deleted a BspEI/Bsu36I fragment encompassing LEF-1 residues 7–264 to generate LEFΔN-VP16. For detection of the LEF-1-VP16 fusions in Western blots oligonucleotides encoding the influenza virus hemagglutinin (HA) tag were inserted into BglII/SacI restriction sites between the LEF-1 and VP16 portions. To construct the LEF-1-β-catenin fusions, the LEFΔN fragment was inserted into the HindIII/BamHI sites of the vector pCS2+ and oligonucleotides coding for two copies of the HA-tag were inserted into the EcoRI site such that this site was reconstituted only at the 3′ end of the HA tag. The resulting construct was designated LEFΔN. Next, an EcoRI/XhoI fragment with full-length β-catenin or a Bsu36I/XbaI fragment from pCS2+β-catenin (13) with β-catenin residues 53–781 was added to give LEFΔN-βcat and LEFΔN-βΔN, respectively. In some experiments a LEFΔN-βΔN construct without the HA tag was used without noticeable difference in the experimental outcome.
To obtain fusions of LEF-1 with the human estrogen receptor (ER), we first amplified a cDNA fragment coding for amino acids 281–595 of the ER with PCR primers adding EcoRI and XbaI sites to the ER sequences. The resulting PCR fragment was inserted into the vector pcDNA3 (pcDNA3-ER). A BamHI/PvuII fragment with LEF-1 sequences coding for residues 1–394 was then inserted into the BamHI/BspEI sites of pcDNA3-ER. A BamHI/Bsp120I fragment carrying the combined LEF-1-ER sequences was then inserted into the BamHI and StuI sites of the vector pCS2+ to yield LEF-1-ER. LEFΔN-ER was obtained from LEF-1-ER by deleting the BspEI/Bsu36I fragment of LEF-1 as before.
Retroviral expression vectors with the various fusion constructs were prepared as follows: Wild-type or mutant β-catenin from pGEX4T1.mmbc or pGEX4T1.mmbc(S33A) (44), LEFΔN-βcat, and LEFΔN-βΔN were cloned as BamHI fragments into the adapter plasmid pBSFI. LEF-1-VP16 and LEFΔN-VP16 were inserted as BamHI/SnaBI fragments into the BamHI/HincII sites of pBSFI. LEF-1-ER and LEFΔN-ER were cloned as BamHI/ApaI fragments into the BamHI and ApaI sites of pBSFI. LEF-1 was cloned into pBSFI by three-fragment ligation with NotI/BstXI fragment of pBSFI-LEF-1-VP16, BstXI/EcoRI fragment of pcIneo-LEF-1 and NotI/EcoRI fragment of pBSFI. Finally, SfiI restriction fragments carrying β-catenin, LEF-1, and the various LEF-1 fusions were excised from the pBSFI derivatives and directionally transferred into the replication-competent expression vector RCAS as described (45).
Cell Culture and Transient Transfection.
Human kidney cell line 293 (ATCC CRL-1573) and murine Neuro2a neuroblastoma cells (ATCC CCL-131) were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/10% fetal bovine serum containing 1 mM l-glutamine, 100 units/ml penicillin/streptomycin, 1 mM sodium pyruvate, MEM nonessential amino acids, and 10 mM Hepes/NaOH, pH 7.4, at 37°C under a 10% CO2/90% air atmosphere. For transient transfection, 5 × 105 cells were seeded into 35-mm tissue culture dishes. Six hours after plating, cells received 200 μl of a calcium phosphate coprecipitate containing 0.5 μg of luciferase reporter, 0.5 μg of pRSVLacZ (54) as internal control, and 0.2 μg of expression vector for the activator proteins. For transient transfection, we used the constructs based on the vector pCS2+ except for β-catenin, for which the RCAS derivatives were utilized. To monitor expression of the activator proteins, 5 μg of plasmid DNA was used for transfection (46). Where indicated, transfected cells were treated with 10−6 M 17β-estradiol dissolved in ethanol (stock solution 10−3 M). Control cells received a corresponding volume of ethanol only.
Reporter Gene Assays.
At harvest, cells were washed once with PBS, removed from the tissue culture dish in 0.5 ml of 40 mM Tris⋅HCl, pH 7.6/150 mM NaCl/1 mM EDTA (TEN) with a rubber policeman and transferred into a 1.5-ml Eppendorf tube. After pelleting, the cells were resuspended in 100 μl of 50 mM Tris–phosphate, pH 7.8/0.1% Nonidet P-40/10% (vol/vol) glycerol and lysed on ice for 20 min. Cell debris was removed by centrifugation at 14,000 × g for 10 min, and the cleared lysate was transferred into a fresh Eppendorf tube. To measure luciferase activity, 10 μl of cell lysate was placed into a 5-ml reaction tube, and luciferase activity was recorded in a Berthold Biolumat model LB953 (Perkin-Elmer) in relative light units (RLU) after injection of 300 μl of 25 mM Tris–phosphate, pH 7.8/10 mM MgCl2/2 mM ATP/0.05 mM luciferin. The activity of the β-galactosidase reporter gene was determined with 10 μl of cell lysate in a Berthold Biolumat LB953 after automated injection of 100 μl of 1% Galacton and 300 μl of 2% Emerald enhancer (Tropix, Bedford, MA) in 0.2 M NaOH 30 min later. Luciferase activities shown were normalized against the β-galactosidase activities and represent the average values from at least three independent transfection experiments.
Preparation of Whole Cell Extracts and Western Blot Analyses.
Transfected cells were scraped from the tissue culture dishes in 0.5 ml of TEN, transferred to 1.5-ml Eppendorf tubes, pelleted, and lysed in 200 μl of SDS/PAGE loading buffer. DNA was sheared by sonication, samples were boiled, and 10 μl of each lysate was applied to SDS/7.5% or 10% polyacrylamide gels. After transfer onto nitrocellulose membranes, the LEF-1-ER fusions were visualized with rabbit polyclonal anti-ER-(578–592) antibodies (Sigma) diluted 1:3000 in 10 mM Tris⋅HCl, pH 7.4/150 mM NaCl/0.1% Tween 20/2% nonfat dry milk and horseradish peroxidase-labeled secondary antibodies by using the ECL detection system (Amersham). LEF-1, LEF-1-VP16, and LEF-1-β-catenin fusions were detected with the rat monoclonal antibody 3F10 (Boehringer Mannheim) directed against the HA-epitope tag. Expression of β-catenin and LEF-1-β-catenin constructs was analyzed with a mouse monoclonal antibody (Transduction Laboratories, Lexington, KY).
Culture of Chicken Embryo Fibroblasts (CEF) for Transfection and Transformation Assays.
CEF cultures were prepared from White Leghorn embryos obtained from SPAFAS (Preston, CT). For focus assays, DNA was transfected into secondary CEF by using the dimethyl sulfoxide/Polybrene method (47). Focus assays and agar colony assays with virus stocks were performed as described (48). Foci were counted on day 20 after infection. Agar colony counts were obtained on day 17 after infection. Transformation experiments were performed at least four times. The results shown are from a representative experiment. For hormone treatment, 17β-estradiol (Sigma) was added to the agar overlays at 2 × 10−6 M and hydroxytamoxifen (Research Biochemicals, Natick, MA) at 1 × 10−7 M. After counting foci of transformed cells, the assay plates were stained with crystal violet and photographed.
RESULTS AND DISCUSSION
Construction of LEF-1 Chimeras.
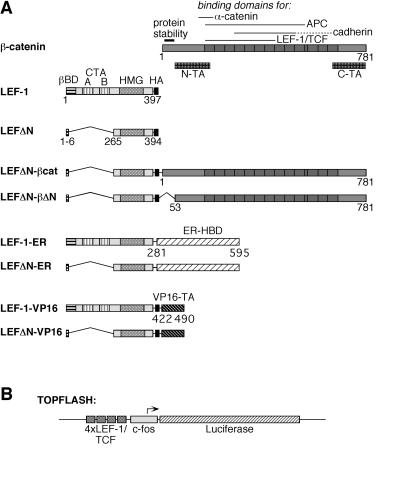
To analyze the oncogenic potential of transactivating LEF-1, we have generated fusion constructs covalently linking LEF-1 sequences with second protein domains that could substitute for the required cofactor in transcriptional regulation. These constructs are depicted in Fig. 1A. Two versions of LEF-1 were used, either full-length, wild-type LEF-1 or an amino-terminally truncated form, LEFΔN, which lacks the β-catenin-binding domain and the two context-dependent transactivation domains CTA-A and CTA-B (37). The β-catenin sequences either were full-length or carried a deletion of the amino-terminal 53 amino acids, which removes the destabilizing region from the molecule (44). As a control in some of the experiments, we used a mutated β-catenin, β-cat-mut, carrying four Ala substitutions at positions 33, 37, 41, and 47 of the β-catenin amino terminus. The mutations prevent phosphorylation by GSK-3β and result in a more stable protein (44). The ER component consisted of the hormone-binding domain (HBD), which also contains a ligand-controlled transactivation domain referred to as TAF-2. TAF-2 has previously been used to generate conditionally transforming versions of the Jun oncoprotein (49). TAF-2 can be activated by 17β-estradiol but not by tamoxifen (50). The VP16 segment encompassed amino acids 422–490 of that protein, representing a strong, constitutive transactivation domain (51). All constructs except those with the ER contained an influenza virus HA tag for immune detection (52).
Figure 1.
Structure of LEF-1 fusion proteins. (A) Schematic representation of β-catenin, LEF-1, and LEF-1-fusions. On the β-catenin molecule, several specific domains are marked: an amino-terminal region responsible for the instability of the protein and two transactivating domains, N-TA and C-TA. The armadillo repeats are represented as dark boxes. On the LEF-1 molecule, the β-catenin-binding domain (βBD), two context-dependent transactivation domains (CTA-A and CTA-B), and the HMG-box mediating DNA binding are marked. The HA-epitope tag on all constructs is represented by a black box. The hormone-binding domain (HBD) of the human ER and the carboxyl-terminal transactivation (TA) domain of the herpes simplex virus VP16 protein are shown as hatched boxes. Amino acid positions of the termini of the various protein domains are given. (B) Schematic representation of the Topflash reporter construct. Expression of the luciferase gene (hatched bar) in this plasmid is driven by the c-fos minimal promoter (gray box) and four copies of a LEF-1/TCF consensus binding motif (dark gray boxes).
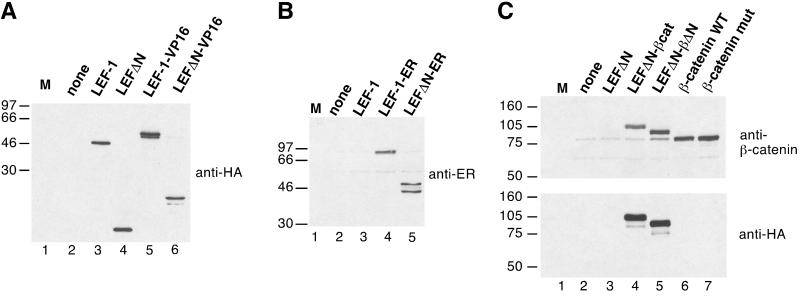
Protein expression directed by the constructs was verified in transient transfections. The transfections were carried out with line 293 human kidney cells except for the β-catenin constructs, for which the murine cell line Neuro2a was used because of its low endogenous β-catenin level. Extracts of the transfected cells were tested in immunoblots. All constructs were efficiently expressed (Fig. 2). The proteins migrated as sharp bands according to their expected sizes, and proteolytic degradation was minimal.
Figure 2.
(A) Expression of chimeric LEF-1 constructs. Human 293 cells were transfected with empty expression vector (lane 2) or expression vectors for LEF-1, LEFΔN, LEF-1-VP16, or LEFΔN-VP16. Whole cell lysates were prepared and resolved by electrophoresis in an SDS/10% polyacrylamide gel. Proteins were transferred onto nitrocellulose and probed with a monoclonal antibody recognizing the HA tag. (B) Cellular lysates from 293 cells transfected with empty expression vector (lane 2) or expression vectors for LEF-1, LEF-1-ER, or LEFΔN-ER were resolved by electrophoresis in an SDS/10% polyacrylamide gel and transferred onto nitrocellulose. The blot was probed with a polyclonal antibody directed against the hormone-binding domain of the human ER. (C) Cellular lysates from Neuro2a cells transfected with empty expression vector (lane 2) or expression vectors for LEFΔN, LEFΔNβ-cat, LEFΔN-βΔN, or wild-type (WT) and mutant β-catenin, respectively, as indicated were resolved by electrophoresis in SDS/7.5% polyacrylamide gels and transferred onto nitrocellulose. The upper blot was probed with a monoclonal antibody directed against β-catenin. The faint band visible in lanes 2–5 that migrates at the same position as the transfected β-catenin (lanes 6 and 7) represents endogenous protein of Neuro2a cells. The lower blot was probed with a monoclonal antibody against the HA tag. M lanes contain molecular size markers. The mass of the marker proteins in kDa is shown on the left.
Transcriptional Activation by LEF-1 Fusion Constructs.
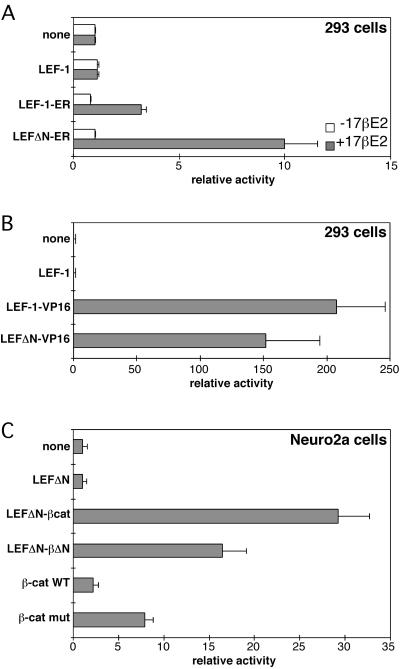
Stimulation of gene expression by LEF-1 and the LEF-1 fusions was determined with the luciferase reporter plasmid Topflash (Fig. 1B) that contained four LEF-1–binding sites in front of the fos promoter. The results of the transactivation experiments are summarized in Fig. 3. The LEF-1–ER constructs (Fig. 3A) activated transcription of the reporter 3-fold and 10-fold in the presence of estrogen but not in its absence. Tamoxifen did not activate the LEF-1-ER constructs (data not shown). No transactivation was observed with LEF-1 alone. The two fusion proteins LEF-1-ER and LEFΔN-ER are therefore hormone-regulatable transactivators without detectable background activity in the absence of hormone. The construct LEFΔN-ER carrying an amino-terminal deletion was a more potent transactivator than the construct with the full-length LEF-1. Possible reasons for this difference are a competition or interference of the TAF-2 and LEF-1 transactivation domains with components of the general transcriptional apparatus. In contrast to the conditional activation induced by the ER fusions, the activity of the LEF-1-VP16 and LEF-1-β-catenin constructs was constitutive (Fig. 3 B and C) and 2 to 20 times higher than that induced by LEF-1-ER. β-Catenin alone induced a very low level of reporter transcription, whereas the stable variant, β-cat-mut, was more active but did not reach the levels of the LEF-1-β-catenin fusion constructs. These data show that fusion of LEF-1 with an inducible or constitutive transactivation domain generates functional transcriptional regulators. Only one of the fusion partners, β-catenin, forms a functional unit with LEF-1 under physiological conditions. Nevertheless, the heterologous ER-TAF2 and the VP-16 transactivation domains, which are not known to interact with LEF-1, proved effective in activating the Topflash reporter gene. Fusions with the amino-terminal deletion mutant of LEF-1 are active. The β-catenin-binding domain and the CTA-A and CTA-B transactivation domains missing from these constructs are therefore not required, and endogenous β-catenin probably does not play a role in transactivation by these constructs. The genetic fusion has made the protein–protein interactions mediated by the β-catenin-binding and context-dependent transactivation domains dispensable. The fusions underline the modular nature of transcription factors. They use the LEF-1 portion as DNA-binding domain and add, by covalent linkage, sequences that function as transactivation domains.
Figure 3.
Activation of the Topflash reporter by LEF-1-fusion constructs. (A) Human 293 cells were transfected with the Topflash reporter plasmid and expression plasmids for LEF-1, LEF-1-ER, or LEFΔN-ER. Cells were treated with 10−6 M 17β-estradiol (+17βE2) in ethanol or solvent only (−17βE2) for 24 hr. Luciferase activities were determined as described in Materials and Methods and are expressed as relative activity. Reporter gene activity in control cells transfected with Topflash and empty expression vector were arbitrarily set as 1. Values given are derived from at least three independent experiments. (B) Human 293 cells were transfected with the Topflash reporter plasmid and expression plasmids for LEF-1, LEF-1-VP16, or LEFΔN-VP16 as indicated. Luciferase activities were determined as in A. (C) Mouse Neuro2a cells were transfected with the Topflash reporter plasmid and expression plasmids for LEFΔN, LEFΔN-βcat, LEFΔN-βΔN, and wild-type (WT) or mutant β-catenin as indicated. Luciferase activities were determined as in A. Note the different scales in A, B, and C.
Oncogenic Transformation Induced by LEF-1 Chimeras.
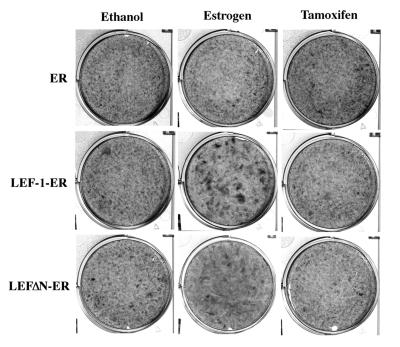
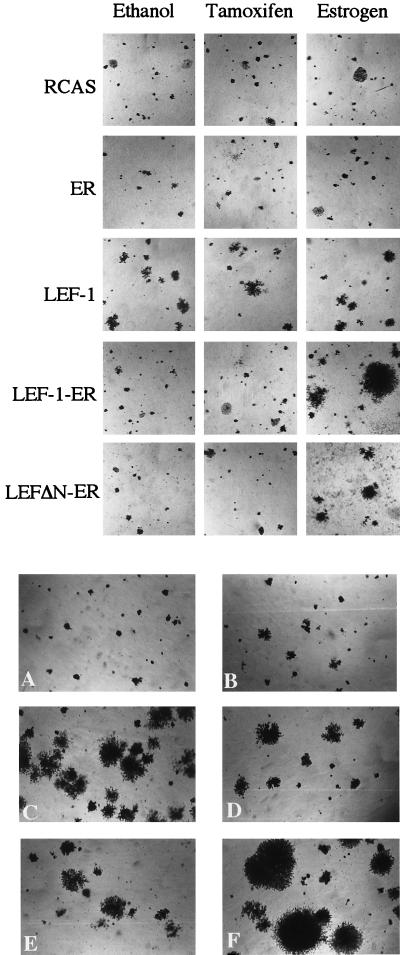
The chimeric proteins shown in Fig. 1 were expressed in CEF with the avian retroviral vector RCAS. The LEF-1-ER and LEFΔN-ER fusions induced foci of transformed cells only when the cultures were incubated in estrogen-containing medium (Table 1). Transformed cell foci appeared 10–14 days after infection. They were characterized by cells that grew in multiple layers, readily distinguishable from the background monolayer of nontransformed fibroblasts (Fig. 4). A construct expressing the ER HBD alone and the empty RCAS vector did not cause transformation. Expression of LEF-1 by itself in the RCAS vector also failed to induce foci in fibroblast cultures derived from most chicken embryos tested. Occasionally, some embryos yielded cultures in which LEF-1 could induce foci of transformation (data not shown). The cause for this unusual susceptibility of some chicken embryos to LEF-1-induced transformation requires further investigation. The ER fusions also induced anchorage-independent growth (Table 2 and Fig. 5 Upper). Both LEF-1-ER and LEFΔN-ER are transforming, although the latter was less effective in inducing anchorage-independent growth. The agar colonies induced by LEFΔN-ER were smaller, but their numbers were comparable to the ones induced by LEF-1-ER. Again, this oncogenic activity depended on the presence of estrogen in the nutrient medium. RCAS vector alone and RCAS expressing the ER HBD did not induce growth in agar. Expression of the ER fusions in the presence of tamoxifen failed to induce transformation because tamoxifen does not activate TAF-2. These results show that the LEF-1 fusions with the ER HBD function as hormone-regulatable oncogenes.
Table 1.
Focus formation on CEF infected with LEF-1-ER fusion constructs
| Infecting construct | FFU/ml
|
||
|---|---|---|---|
| No additive | Estrogen | Tamoxifen | |
| Vector (RCAS) | 0 | 0 | 0 |
| LEF-1-ER | 20 | 4 × 105 | 0 |
| LEFΔN-ER | 0 | 2 × 106 | 0 |
| ER | 0 | 0 | 0 |
FFU, focus-forming units.
Figure 4.
Estrogen-dependent formation of transformed cell foci by LEF-1-ER fusion products. CEF were infected with the constructs indicated on the left and then overlaid with nutrient agar containing estrogen (2 × 10−6 M), tamoxifen (1 × 10−7 M), or ethanol (vehicle). On day 20 after infection the cells were fixed and stained with crystal violet and photographed.
Table 2.
Agar colony formation by CEF infected with LEF-1-ER constructs
| Infecting construct | Colonies per 104 cells
|
||
|---|---|---|---|
| No additive | Estrogen | Tamoxifen | |
| Vector (RCAS) | 0.68 | 0.68 | 0.55 |
| LEF-1 | (180)* | (180)* | (180)* |
| LEF-1-ER | 2.0 | 83 | 1.7 |
| LEFΔN-ER | 0.63 | 31 | 0.38 |
| ER | 1.3 | 1.7 | 1.0 |
Colonies induced by LEF-1 remained very small.
Figure 5.
(Upper) Estrogen-dependent agar colony formation by LEF-1-ER fusion products. CEF transfected with the indicated constructs were seeded in soft nutrient agar containing estrogen (2 × 10−6 M), tamoxifen (1 × 10−7 M), or ethanol (vehicle). Photographs of the agar colonies were taken on day 17 after infection. (Lower) Agar colony formation by various LEF-1 constructs. CEF transfected with RCAS (A), LEF-1 (B), LEF-1-VP16 (C), LEFΔN-VP16 (D), LEFΔN-βcat (E), and LEFΔN-βΔN (F) were seeded in soft nutrient agar. Photographed 17 days after infection.
The LEF-1 chimeras with β-catenin and with VP16 were also transforming, as demonstrated by the induction of anchorage-independent growth and of morphologically distinct foci characterized by multiple layers of cells (Table 3 and Fig. 5 Lower). The LEFΔN-βΔN construct induced the most rapidly growing agar colonies. This chimera lacks the β-catenin interacting sequences and context-dependent transactivation domains of LEF-1 as well as the destabilizing region of β-catenin. LEF-1 alone induced very small colonies; CEF infected by the RCAS vector did not grow in agar.
Table 3.
Agar colony formation by CEF infected with LEF-1 fusion constructs
| Infecting construct | Colonies per 104 cells
|
|
|---|---|---|
| Exp. 1 | Exp. 2 | |
| Vector (RCAS) | 0.4 | 1.7 |
| LEF-1 | (70)* | (70)* |
| LEF-1-VP16 | 79 | 200 |
| LEFΔN-VP16 | 8.3 | 15 |
| LEFΔN-βcat | 64 | 60 |
| LEFΔN-βΔN | 53 | 53 |
Colonies induced by LEF-1 remained very small (see Fig. 5 Lower).
The results of the in vitro transformation assays reveal the oncogenic potential of LEF-1. The fusion constructs with diverse activation domains derived from heterologous transcription factors support the hypothesis that aberrant transcription of LEF-1/TCF target genes may be the underlying cause of human cancers carrying mutations in APC protein or β-catenin. All transforming constructs also function as transcriptional activators. However, there is no quantitative correlation between the strength of transactivation in vitro and transformation efficiency in CEF. For instance, LEFΔN-ER is a better transactivator than LEF-1-ER but it is a weaker transformer. LEF-1-VP16 and LEFΔN-VP16 do not differ significantly as transactivators, but LEFΔN-VP16 is an order of magnitude less effective in agar colony formation. In general, the fusions with LEFΔN are less potent transformers than fusions with full-length LEF-1. The latter may bind endogenous β-catenin and thereby acquire additional transactivation domains. Yet in transient transfection assays the LEF-1 fusions did not show enhanced transactivation as compared with LEFΔN fusions. Whether the full-length LEF-1 fusions interact with endogenous β-catenin and whether the stabilized, transforming versions of β-catenin, βΔN, and β-cat-mut also form an oncogenic complex with endogenous LEF-1 is not yet known. A tight, quantitative correlation between transformation and transactivation, as measured by reporter gene assays, would not be expected because of the different cell systems used in transformation and transactivation assays and because downstream targets of LEF-1 in transformed cells will have properties that differ significantly from those of the reporter. Some of these targets are likely to be important in determining the oncogenic phenotype of LEF-1-transformed cells (53). These targets must now be identified and characterized. The LEF-1 chimeras, especially the regulatable ER fusions described in this report, will facilitate this task.
Acknowledgments
We thank K. Rose and Ossie Batista for excellent technical assistance, and Linda Miller and Judy Robertson for help with the manuscript. We thank Dr. R. Grosschedl for (a gift of) epitope-tagged LEF-1, Dr. H. Clevers for the Topflash luciferase reporter, Dr. P. Chambon for the human ER cDNA construct (HEO), Dr. K.-H. Klempnauer for pCDNA3-VP16, and Dr. R. Rupp for pCS2+. This work was supported by National Institutes of Health Research Grants CA42564 and CA78230 and by the Deutsche Forschungsgemeinschaft (DFG).
ABBREVIATIONS
- LEF-1
lymphoid-enhancing factor 1
- APC
adenomatous polyposis coli
- GSK-3β
glycogen synthase kinase-3β
- CEF
chicken embryo fibroblast(s)
- ER
estrogen receptor
- TAF-2
transactivation function 2
- N-TA
amino-terminal transactivation domain
- C-TA
carboxyl-terminal transactivation domain
- TCF
T-cell factor
- βBD
β-catenin binding domain
- CTA-A
context-dependent transactivation domain A
- CTA-B
context-dependent transactivation domain B
- HMG
high-mobility group
- HA
hemagglutinin
- HBD
hormone-binding domain
References
- 1.Cadigan K M, Nusse R. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 2.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 3.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 4.Nusse R, Varmus H E. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 5.Nusse R, van Ooyen A, Cox D, Fung Y K, Varmus H. Nature (London) 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- 6.Nusse R, Varmus H E. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 7.Bhanot P, Brink M, Samos C H, Hsieh J C, Wang Y, Macke J P, Andrew D, Nathans J, Nusse R. Nature (London) 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 8.Yang-Snyder J, Miller J R, Brown J D, Lai C J, Moon R T. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 9.Nusse R. Cell. 1997;89:321–323. doi: 10.1016/s0092-8674(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 10.Miller J R, Moon R T. J Cell Biol. 1997;139:229–243. doi: 10.1083/jcb.139.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens J, von Kries J P, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Nature (London) 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 12.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 13.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann B G, Kemler R. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 14.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 15.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S C, Grosschedl R, Bienz M. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 16.Brunner E, Peter O, Schweizer L, Basler K. Nature (London) 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 17.Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- 18.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 19.Kemler R. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 20.Aberle H, Schwartz H, Kemler R. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Munemitsu S, Albert I, Rubinfeld B, Polakis P. Mol Cell Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papkoff J, Rubinfeld B, Schryver B, Polakis P. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakanaka C, Weiss J B, Williams L T. Proc Natl Acad Sci USA. 1998;95:3020–3023. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart M J, de los Santos R, Albert I N, Rubinfeld B, Polakis P. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 27.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 28.Behrens J, Jerchow B A, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y, Mori T, Utsunomiya J, Baba S, Petersen G, et al. Proc Natl Acad Sci USA. 1992;89:4452–4456. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, et al. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 32.Kinzler K W, Nilbert M C, Vogelstein B, Bryan T M, Levy D B, Smith K J, Preisinger A C, Hamilton S R, Hedge P, Markham A, et al. Science. 1991;251:1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- 33.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 34.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 35.Travis A, Amsterdam A, Belanger C, Grosschedl R. Genes Dev. 1991;5:880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- 36.Waterman M L, Fischer W H, Jones K A. Genes Dev. 1991;5:656–669. doi: 10.1101/gad.5.4.656. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson P, Waterman M L, Jones K A. Genes Dev. 1993;7:2418–2430. doi: 10.1101/gad.7.12a.2418. [DOI] [PubMed] [Google Scholar]
- 38.Giese K, Grosschedl R. EMBO J. 1993;12:4667–4676. doi: 10.1002/j.1460-2075.1993.tb06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giese K, Cox J, Grosschedl R. Cell. 1992;69:185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- 40.Love J J, Li X, Case D A, Giese K, Grosschedl R, Wright P E. Nature (London) 1995;376:791–795. doi: 10.1038/376791a0. [DOI] [PubMed] [Google Scholar]
- 41.Giese K, Kingsley C, Kirshner J R, Grosschedl R. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 42.Bruhn L, Munnerlyn A, Grosschedl R. Genes Dev. 1997;11:640–653. doi: 10.1101/gad.11.5.640. [DOI] [PubMed] [Google Scholar]
- 43.Mink S, Haenig B, Klempnauer K H. Mol Cell Biol. 1997;17:6609–6617. doi: 10.1128/mcb.17.11.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt P K. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 47.Kawai S, Nishizawa M. Mol Cell Biol. 1984;4:1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bos T J, Monteclaro F S, Mitsunobu F, Ball A R, Jr, Chang C H, Nishimura T, Vogt P K. Genes Dev. 1990;4:1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- 49.Kruse U, Iacovoni J S, Goller M E, Vogt P K. Proc Natl Acad Sci USA. 1997;94:12396–12400. doi: 10.1073/pnas.94.23.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster N J, Green S, Jin J R, Chambon P. Cell. 1988;54:199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- 51.Triezenberg S J, Kingsbury R C, McKnight S L. Genes Dev. 1988;2:718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- 52.Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson I A, Lerner R A, Wigler M. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He T C, Sparks A B, Rago C, Hermeking H, Zawel L, da Costa L T, Morin P J, Vogelstein B, Kinzler K W. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 54.Edlund T, Walker M D, Barr P J, Rutter W J. Science. 1985;230:912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]