Abstract
Ethylene signal transduction involves ETR1, a two-component histidine protein kinase receptor. ETR1 functions upstream of the negative regulator CTR1. The similarity of CTR1 to members of the Raf family of mitogen-activated protein kinase kinase kinases (MAPKKKs) suggested that ethylene signaling in plants involves a MAPK pathway, but no direct evidence for this has been provided. Here we show that distinct MAPKs are activated by the ethylene precursor aminocyclopropane-1-carboxylic acid (ACC) in Medicago and Arabidopsis. In Medicago, the ACC-activated MAPKs were SIMK and MMK3, while in Arabidopsis MPK6 and another MAPK were identified. Medicago SIMKK specifically mediated ACC-induced activation of SIMK and MMK3. Transgenic Arabidopsis plants overexpressing SIMKK have constitutive MPK6 activation and ethylene-induced target gene expression. SIMKK overexpressor lines resemble ctr1 mutants in showing a triple response phenotype in the absence of ACC. Whereas MPK6 was not activated by ACC in etr1 mutants, ein2 and ein3 mutants showed normal activation profiles. In contrast, ctr1 mutants showed constitutive activation of MPK6. These data indicate that a MAPK cascade is part of the ethylene signal transduction pathway in plants.
Keywords: CTR1/ethylene/ETR1/gene expression/MAPK/plant
Introduction
Ethylene is a gas that functions as a plant hormone. Responses to ethylene include fruit ripening, leaf senescence and abscission, promotion or inhibition of seed germination, flowering and cell elongation (Abeles et al., 1992). Environmental stresses, such as chilling, flooding, wounding and pathogen attack, increase ethylene synthesis and thereby control gene expression (Abeles et al., 1992). Although the biosynthesis pathway of ethylene is well known, our understanding of the molecular mechanisms underlying ethylene perception and signaling is limited. Several components of the ethylene response pathway have been isolated in mutant screens in Arabidopsis (Chang, 1996; Woeste and Kieber, 1998). Isolation of these mutants was based on the so-called triple response of dark-grown seedlings which produce high rates of ethylene and show inhibition of elongation and radial swelling of roots and hypocotyls, as well as exaggeration of the curvature of the apical hook. The etr1 mutant encodes a two-component histidine kinase that is membrane located (Chen et al., 2002) and thought to be directly involved in ethylene sensing (Chang et al., 1993; Schaller and Bleecker, 1995). Most etr1 alleles are dominant and show ethylene insensitivity (Chang et al., 1993; Hua and Meyerowitz, 1998). In contrast, ctr1 mutants are recessive and show a constitutive triple response in the absence of ethylene (Kieber et al., 1993). ein2 and ein3 are additional ethylene-insensitive mutants that act downstream of ETR1 and CTR1. EIN2 encodes a transmembrane protein with partial homology to mammalian metal transporters (Alonso et al., 1999), whereas EIN3 is a nuclear protein with the structural and functional properties of a transcriptional activator (Chao et al., 1997; Solano et al., 1998). Epistasis analysis of the available mutants suggests a linear ethylene signaling pathway where CTR1 is downstream of ETR1. CTR1 is thought to be a negative regulator upstream of EIN2 and EIN3. At the downstream end of the pathway, EIN3 is assumed to activate a set of transcription factors called ERFs (ethylene response factors) or EREBPs (ethylene response element-binding proteins) either transcriptionally or post-transcriptionally, thereby regulating the expression of appropriate target genes in response to ethylene (Ohme-Takagi and Shinshi, 1995).
CTR1 encodes a protein kinase with homology to the class of mammalian Raf mitogen-activated protein kinase kinase kinases (MAPKKKs) that regulate a number of processes including cell differentiation, apoptosis and cell cycle transition (Morrison and Cutler, 1997). Raf kinases are involved in mediating signals of tyrosine kinase and seven-transmembrane receptors. Upon ligand binding, the GTP-bound small G protein Ras recruits Raf to the plasma membrane and initiates the MAPK phosphorylation cascade. CTR1 lacks the corresponding domain for Ras binding in Raf, suggesting a different mode of action in plants. Two-hybrid interaction assays suggest that CTR1 is directly regulated by ETR1 because CTR1 can physically interact with ETR1 in yeast (Clark et al., 1998).
To understand better the signaling events leading to the physiological responses and gene expression of plants to ethylene, we studied the potential involvement of a MAPK signaling cascade in Medicago and Arabidospis. The biochemical, reverse genetic and gene expression studies demonstrate the involvement of a MAPK pathway in ethylene signaling in plants.
Results
ACC-induced activation of MAPKs
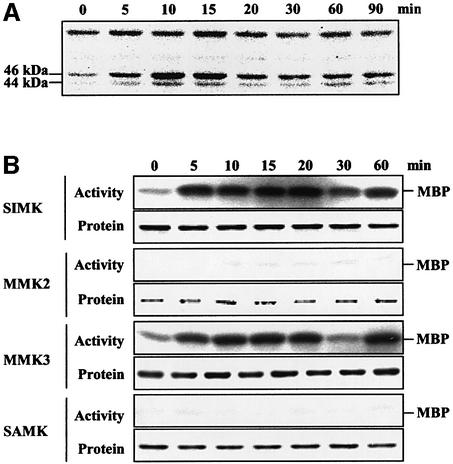
Although there exists no direct evidence that CTR1 functions as a MAPKKK in plants, recent work showed that a protein kinase with properties resembling those of MAPKs might be involved in ethylene signaling (Novikova et al., 2000). To investigate the possibility that MAP kinases are involved in ethylene signaling in plants, we performed in-gel kinase assays of protein extracts from Medicago and Arabidopsis cells before and after treatment with 1 mM aminocyclopropane-1-carboxylic acid (ACC), the rate-limiting precursor of ethylene synthesis. ACC is metabolized rapidly by the abundant enzyme ACC oxidase, and leads to a rapid increase in ethylene production (Cameron et al., 1979; Lurssen et al., 1979). As shown in Figure 1A, two bands corresponding to protein kinases of 46 and 44 kDa molecular mass were activated within 5 min of ACC treatment of suspension-cultured Medicago cells. Although maximal activity was observed at 10–15 min, the protein kinases showed a biphasic activation rising to significant levels at 60 and 90 min. To identify the protein kinases, we immunoprecipitated the cell extracts with antibodies specifically recognizing the four different Medicago MAPKs: SIMK, MMK2, MMK3 and SAMK (Cardinale et al., 2000). Thereafter, each immunopreciptated MAPK was tested for activation by in vitro kinase assays. Whereas MMK2 and SAMK were not activated in ACC-treated cells, SIMK and MMK3 showed strong activation (Figure 1B). SIMK and MMK3 encode 46 and 44 kDa protein kinases, respectively, and show activation profiles that closely resemble those seen in the in-gel kinase assays (Figure 1A). As can be seen by immunoblotting the protein extracts with the respective MAPK antibodies, the differences in MAPK activities in response to ACC were not due to differences in protein amounts (Figure 1B), suggesting that ethylene-induced activation of SIMK and MMK3 occurs by post-translational mechanisms.
Fig. 1. ACC induces the activation of two MAPKs in Medicago cell suspension culture. (A) In-gel protein kinase assay. Cell extracts containing 20 µg of total proteins per lane were separated by SDS–PAGE. MBP (0.5 mg/ml) was used as a substrate polymerized in the polyacrylamide gel. After protein renaturation, the kinase reactions were performed in the gel as described (Usami et al., 1995). (B) Identification of the ACC-activated MAPKs by immunokinase assay. A 100 µg aliquot of total protein extract was immunoprecipitated with 5 µg of protein A-purified M23, M11, M14 and M7 antibodies raised against synthetic peptides encoding the C-terminal seven, ten, ten and five amino acids of the Medicago SIMK, MMK2, MMK3 and SAMK MAPKs, respectively (Cardinale et al., 2000). Kinase reactions of the immunoprecipitated proteins were performed in 15 µl of kinase buffer containing 5 µg of MBP, 0.1 mM ATP and 2 µCi of [γ-32P]ATP. The protein kinase reactions were performed at room temperature for 30 min and the reactions were stopped by adding 4× SDS loading buffer. The phosphorylation of MBP was analyzed by autoradiography after SDS–PAGE. Western blotting was performed with equal amounts of protein extracts separated by SDS–PAGE, immunoblotted to PVDF membranes and probed with protein A-purified M23, M11, M14 and M7 antibodies. Alkaline phosphatase-conjugated goat anti-rabbit IgG was used as secondary antibody, and the reaction was visualized by fluorography.
As SIMK encodes the closest homolog of the Arabidopsis MAPK, MPK6, we also immunoprecipitated MPK6 from suspension-cultured Arabidopsis cells with the appropriate antibody (Nuhse et al., 2000). In vitro kinase assays of MPK6 showed strong activation in response to ACC treatment (data not shown, Figure 5A). Similar to Medicago, in-gel kinase assays of suspension-cultured Arabidopsis cells and leaves revealed activation of protein kinases of 46 and 44 kDa molecular mass in response to ACC (Supplementary figure 1 available at The EMBO Journal Online). The analysis of the MMK3 homolog in Arabidopsis is in progress, and preliminary results show that the 44 kDa band corresponds to MPK13 (our unpublished results).
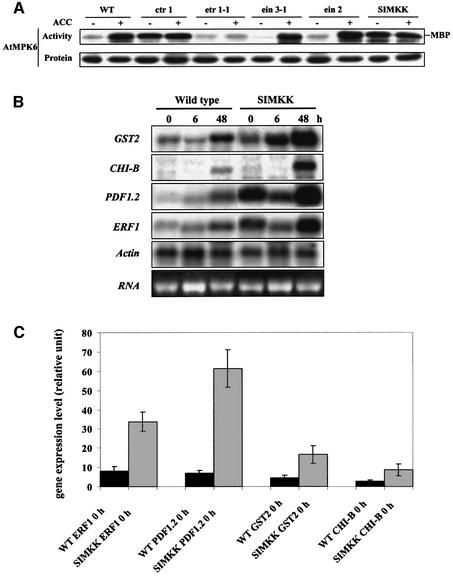
Fig. 5. (A) Comparison of MPK6 activity in Arabidopsis wild-type (WT), ctr1, etr1-1, ein2, ein3-1 and hyperactive SIMKK plants in the absence or presence of ACC. The A.thaliana Columbia lines were treated for 0 or 10 min with 1 mM ACC. Upper panel: MPK6 activity was detected by immunocomplex kinase assays using MPK6-specific antibody. Lower panel: MPK6 protein amounts were detected by immunoblotting the extracts used for the immunocomplex kinase assays with MPK6 antibody. (B) Ethylene-induced gene expression in wild-type and hyperactive SIMKK plants. Detached leaves were kept in culture medium for 2 h before treatment with 1 mM ACC. After 0, 6 and 48 h, total RNA was extracted from leaves, separated by denaturing agarose electrophoresis, blotted onto nylon filters and probed with 32P-labeled DNA fragments of ERF1, PDF1.2, GST2 and CHI-B. An actin gene fragment was used as a constitutive control. (C) Quantification of ERF1, PDF1.2, GST2 and CHI-B gene expression levels in untreated wild-type and hyperactive SIMKK plants. Gene expression levels were calculated from three independent RNA gel blots by PhosphorImager analysis and normalized to the respective actin expression levels.
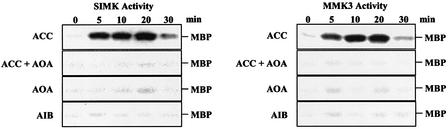
To prove that the Medicago MAPKs SIMK and MMK3 are activated specifically through ethylene synthesis, amino-oxyacetic acid (AOA), an inhibitor of the ACC oxidase, was used. AOA blocked ACC-induced MAPK activation (Figure 2), but did not activate SIMK or MMK3 by itself (Figure 2). Additional proof for the ethylene dependence of the MAPK activation was provided by treating cells with α-aminoisobutyric acid (AIB), an alternative substrate for ACC oxidase, which is converted into CO2, acetone and ammonia, but produces no ethylene. In contrast to ACC, treatment of cells with AIB did not result in activation of SIMK or MMK3 (Figure 2), indicating that ethylene production is necessary to activate the MAPKs.
Fig. 2. SIMK and MMK3 are activated specifically by ethylene production. Effect of α-aminoisobutyric acid (AIB), an inactive analog of ACC, or of the inhibition of ACC oxidase by amino-oxyacetic acid (AOA). After treatment with AIB (1 mM) or AOA (0.5 mM), SIMK and MMK3 activities were determinated at different times by immunocomplex kinase assay using 5 µg of protein A-purified M23- and M14-specific antibodies.
SIMKK mediates ACC-induced activation of MAPKs
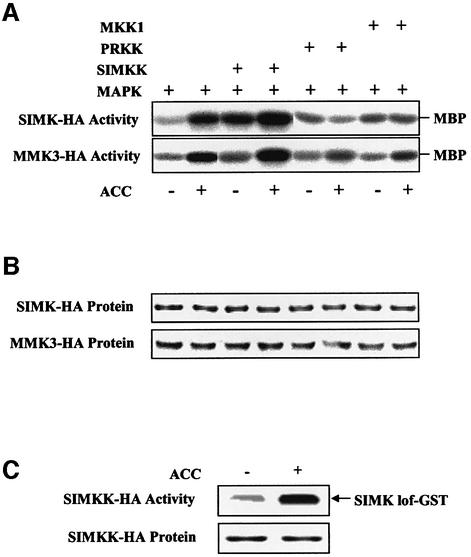
In order to identify the upstream component of the ethylene-activated MAPKs, different MAPKKs were tested for their ability to enhance ethylene signaling. For this purpose, we transiently expressed hemagglutinin (HA)-tagged versions of SIMK and MMK3 in Arabidopsis protoplasts in the presence or absence of several MAPKKs. We also tested whether SIMK and MMK3 could be activated in protoplasts by ACC through endogenous MAPK pathway components. Our studies showed that SIMK and MMK3 could be activated with kinetics similar to those found in Medicago cells. Maximum activation of both MAPKs was obtained at 10 min and was therefore taken as the reference point for further investigations (Figure 3). Of two MAPKKs that were shown to activate SIMK and MMK3 by pathogen-derived factors (Cardinale et al., 2000; Kiegerl et al., 2000), only SIMKK increased activation of both MAPKs upon ACC treatment (Figure 3A). In contrast, when compared with protoplasts expressing the MAPKs alone, PRKK resulted in a decrease of ACC-induced SIMK and MMK3 activities (Figure 3A). Similarly, co-expression of the MAPKs with the Medicago MAPKK MKK1 also resulted in decreased activation levels of ACC-induced SIMK and MMK3 (Figure 3A). The partial dominant-negative effect of the two MAPKKs PRKK and MKK1 on ACC-induced MAPK activation could be explained by the ability of these MAPKKs to bind to and thereby titrate out SIMK and MMK3 from endogenous complexes that are necessary for ethylene-mediated MAPK activation. Overall, these results suggest that SIMKK is an upstream activator of SIMK and MMK3 that mediates ethylene-induced activation of these MAPKs.
Fig. 3. SIMK activation is mediated by SIMKK activation. (A) Transient expression in Arabidopsis protoplasts. HA-tagged SIMK and MMK3 were prepared as described (Kiegerl et al., 2000). The open reading frames of MKK1, PRKK and SIMKK were cloned into the plant expression vector pRT101 (Kiegerl et al., 2000). Transient expression experiments were performed with protoplasts from Arabidopsis cells using PEG for transformation. A 5 µg aliquot of each plasmid DNA was used for co-transformation. After treatment, extracts were prepared from protoplasts 12–16 h after transformation (Kiegerl et al., 2000) and used for immunocomplex kinase assay using antibodies raised against HA. (B) Protein amounts of SIMK-HA and MMK3-HA. Immunoblotting was performed with equal amounts of protein extracts separated by SDS–PAGE, immunoblotted to PVDF membranes and probed with HA antibody. Alkaline phosphatase- conjugated goat anti-mouse IgG was used as secondary antibody, and the reaction was visualized by fluorography. (C) ACC activation of SIMKK. After transient expression of HA-tagged SIMKK, Arabidopsis protoplasts were treated with ACC for 0 or 10 min, and SIMK loss of function fused to GST (SIMK-lof–GST) was used as a substrate for SIMKK.
To prove that SIMKK itself is activated by ethylene, HA-tagged SIMKK (SIMKK-HA) was immunoprecipitated from protoplasts expressing the MAPKK before and after treatment with ACC. Kinase activity of SIMKK-HA was assessed by in vitro kinase assays using recombinant kinase-dead SIMK (SIMK-lof–GST) as a substrate. As shown in Figure 3C, SIMKK-HA was strongly activated upon treatment of protoplasts with ACC.
Hyperactive SIMKK plants show a ctr1-like phenotype in the absence of ACC
To investigate further the function of SIMKK in the context of ethylene signaling, transgenic Arabidopsis plants were produced that overexpress SIMKK under the control of the cauliflower mosaic virus (CaMV) 35S promoter. Out of 10 homozygous transgenic lines, two showed hyperactive SIMKK and constitutive MPK6 activity (Figure 5A, –ACC). Inspection of dark-grown seedlings of these hyperactive lines revealed strong inhibition of root growth, exaggerated curvature of the apical hook and excessive radial swelling of the hypocotyl in the absence of ACC (Figure 4, –ACC). These features resembled the triple response of ctr1 mutant plants (Figure 4, –ACC) that can otherwise be observed in wild-type plants only in response to ethylene or ACC treatment (Figure 4, +ACC). Similar to ctr1 mutants, the two hyperactive SIMKK lines could not be stimulated further by ACC treatment (Figure 4, +ACC).
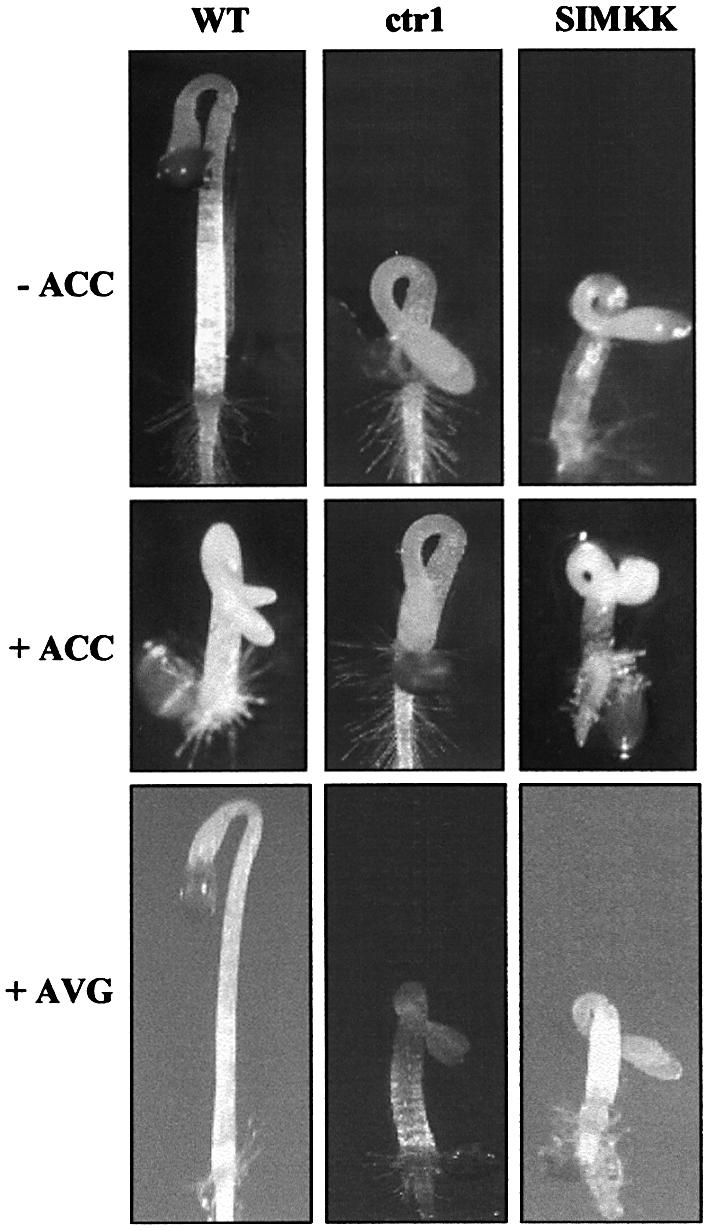
Fig. 4. Triple response phenotype of hyperactive SIMKK Arabidopsis plants. Arabidopsis thaliana Columbia wild-type (WT), ctr1 and hyperactive SIMKK lines were grown for 3 days in the dark on 15% agar plates in the absence or presence of 1 mM ACC (±ACC) or 50 mM AVG (+AVG).
The triple response phenotype of the hyperactive SIMKK lines was also compared with ctr1 and wild-type plants under light conditions. In contrast to wild-type plants, hyperactive SIMKK lines showed a ctr1-like phenotype in the light (data not shown). Although some deviations from growth rates and leaf sizes of wild-type plants were also observed, the phenotype of adult hyperactive SIMKK lines was not studied in detail in this work.
To quantify the effect of ACC on wild-type, ctr1 and hyperactive SIMKK lines, the hypocotyl lengths were measured from five seedlings of each genotype. As indicated in table I of the Supplementary data, ACC treatment reduced wild-type hypocotyl lengths from 6.7 to 3.2 mm. In contrast, both ctr1 and hyperactive SIMKK lines hardly responded to treatment by ACC.
The above results suggested that SIMKK induces the triple response in a manner similar to ctr1. However, an equally compatible possibility was that hyperactive SIMKK lines were overproducing ethylene and therefore showed a triple response and increased MAPK activity in the absence of ACC (Figure 5A, –ACC). To test this hypothesis, ethylene biosynthesis was blocked in wild-type, ctr1 and hyperactive SIMKK lines by treatment with aminoethoxyvinylglycine (AVG), an inhibitor of ACC synthase. Under these conditions, ctr1 and hyperactive SIMKK still showed a triple response phenotype (Figure 4, +AVG). Moreover, AVG treatment did not inhibit the constitutive activation of MPK6 in ctr1 and hyperactive SIMKK lines (data not shown). These results argue that hyperactive SIMKK is responsible for MAPK activation and the triple response phenotype.
ETR1 and CTR1 are upstream regulators of the ethylene-induced MAPK pathway
CTR1 is supposed to be a negative regulator of ethylene signaling. Accordingly, ctr1 knockout mutants behave as if ethylene was constantly present. To test whether CTR1 is an upstream component of the MAPK signaling pathway, MPK6 activity was determined by immuno kinase assays in wild-type and ctr1 mutant plants. Compared with wild-type plants, high MPK6 activity levels were found in ctr1 mutants (Figure 5A, –ACC), and ACC could not increase activation levels of MPK6 in ctr1 mutants (Figure 5A, +ACC). These data indicate that CTR1 acts as an upstream negative regulator of the MPK6 pathway and confirm the genetic model that CTR1 is a negative regulator of ethylene signaling. We also investigated the behavior of MPK6 activity in the hyperactive SIMKK lines. Similar to ctr1 mutants, the hyperactive SIMKK lines showed constitutive MPK6 activity (Figure 5A, –ACC) that could not be stimulated further by external ACC (Figure 5A, +ACC). These data are compatible with the notion that SIMKK is an activator of MPK6 that is downstream of CTR1. To place the MAPK components more precisely into the context of the ethylene pathway, we also analyzed ethylene-insensitive mutants of the receptor ETR1 (Figure 5A). Untreated homozygous lines of etr1-1 showed background MPK6 activity levels that were comparable with wild-type plants in the absence of ACC treatment (Figure 5A, –ACC). However, ACC treatment of etr1-1 did not result in activation of MPK6 (Figure 5A, +ACC), showing that ETR1 is an upstream regulator of the ethylene-responsive MAPK pathway.
The ethylene-induced MAPK pathway functions upstream or independently of EIN2 and EIN3
According to the genetic model, EIN2 and EIN3 are downstream of CTR1. If MPK6 was downstream of EIN2 and EIN3, the ethylene-insensitive ein2 and ein3 mutants should abrogate the ability of ACC to activate MPK6. Treatment of ein2 and ein3 mutant plants with ACC resulted in MPK6 activation that was indistinguishable from wild-type plants (Figure 5A, +ACC), indicating that MPK6 is not downstream but functions upstream or independently of EIN2 and EIN3.
Different levels of MPK6 protein in the different lines might be responsible for the observed differences in MPK6 activity levels. To test this possibility, the protein extracts used for the immunokinase assays shown in Figure 5A were immunoblotted with MPK6 antibody. As can be seen in the lower panel of Figure 5A, similar protein amounts of MPK6 were present in wild-type, ctr1, etr1-1, ein3-1, ein2 and hyperactive SIMKK lines, suggesting that post-translational modifications were the major cause of the observed differences in MPK6 activities.
The ethylene-induced MAPK pathway activates ethylene-induced target genes
In order to test whether the MPK6 pathway acts upstream or independently of EIN2 and EIN3, ACC-induced expression of ERF1, PDF1.2, GST2 and CHI-B, four downstream marker genes of the ethylene pathway (Alonso et al., 1999), were analyzed in hyperactive SIMKK and wild-type plants. Hyperactive SIMKK plants showed several fold enhanced expression of all four genes in the absence of ACC (Figure 5B and C). Compared with wild-type plants and somewhat surprisingly, hyperactive SIMKK lines showed higher expression levels of all four genes upon ACC treatment (Figure 5B), suggesting that some as yet little understood feed-forward mechanism might be functioning during ethylene signaling. In summary, these data demonstrate that a MAPK pathway is involved in the induction of several ethylene target genes.
Discussion
Ethylene is one of the five classical plant hormones and is involved in a multitude of physiological and developmental processes that have been studied in great depth over the last century. During the last decade, the signal transduction of ethylene has moved into the focus of research. Based primarily on genetic screens of triple response mutants in Arabidopsis, various components of the ethylene signaling pathway could be identified. Epistasis, genetic and biochemical analyses of these mutants suggest that ETR1, encoding a two-component histidine protein kinase, functions as the ethylene receptor. ETR1 functions directly upstream of CTR1. The close similarity of CTR1 to members of the Raf family of mammalian MAPKKKs was taken as evidence to suggest that a MAPK pathway is involved in ethylene signaling. So far, however, no direct evidence for this hypothesis has been provided. In this report, we show that two MAPKs are activated by the ethylene precursor ACC in Medicago and Arabidopsis. The MAPKK SIMKK was found specifically to mediate ACC-induced activation of the MAPKs in Medicago. Transgenic Arabidopsis plants with hyperactive SIMKK showed constitutive MAPK activation, enhanced ethylene-induced target gene expression and a triple response phenotype in the absence of ACC treatment. These data indicate that a MAPK pathway is part of the ethylene signal transduction pathway in plants.
Identification of the MAPKs that respond to and mediate ethylene signaling is important. However, it is equally interesting to know whether the identified MAPK pathway functions independently or as part of the genetically defined ethylene signaling cascade. For this purpose, mutants of all four identified components of the ethylene pathway, ETR1, CTR1, EIN2 and EIN3, were analyzed biochemically for their ethylene-inducible MPK6 MAPK activity profile in the absence of and after treatment with ACC. Dominant ethylene-insensitive etr1-1 mutants showed no MPK6 activity in the absence or presence of ACC (Figure 5A), indicating that ETR1 is an upstream component of the MAPK pathway. In contrast, ctr1 mutants revealed constitutive activation of the MPK6 kinase (Figure 5A), demonstrating that CTR1 functions as an upstream negative regulator of the MAPK pathway. These results support the concept that ETR1 is an upstream regulator of CTR1 and the MAPK pathway. In wild-type plants, ETR1 becomes inactivated in response to ethylene. However, in the dominant insensitive etr1-1 mutant, etr1 cannot be inactivated by ethylene any more and therefore CTR1 stays constitutively active, abrogating the ability of ethylene to activate the MAPK pathway.
Although consistent with the genetic model that CTR1 functions as a negative regulator of ethylene signaling, the result that a protein kinase loss-of-function CTR1 mutant should result in activation of its downstream MAPKs is surprising. However, EDR1, encoding another Arabidopsis MAPKKK, was also identified as a negative regulator of pathogen defense responses (Frye et al., 2001). Moreover, complementation assays of CTR1 in yeast and functional analyses of mammalian Raf-1 raise the possibility that these MAPKKKs might not be functioning in MAPKK activation but in that of other targets (Pan and Chang, 1999; Huser et al., 2001; Mikula et al., 2001). In this context, it is worth noting that in contrast to other MAPKKs, SIMKK shows high autoactivity that is likely to be due to the unique feature of carrying a negatively charged amino acid in its phosphorylation loop (Kiegerl et al., 2000). This feature could allow SIMKK regulation through direct physical interaction with CTR1 or as yet unknown intermediate factor(s). In this scenario, ethylene-induced MAPK activation would be relayed through CTR1-mediated relief of SIMKK inhibition.
Biochemical analysis of the ethylene-insensitive ein2 and ein3 mutants showed no enhanced MPK6 activity in untreated plants but normal activation upon ACC treatment (Figure 5A). These data are consistent with the notion that the MAPK pathway functions either upstream or independently of EIN2 and EIN3. Since SIMKK transgenic lines with constitutively active MPK6 show enhanced expression of ethylene-inducible marker genes, it is likely that EIN2 and EIN3 are downstream targets of the MAPK pathway.
Taken together, this work shows that a MAPK pathway is involved in ethylene signaling in Medicago and Arabidopsis plants. In accordance with previous genetic and biochemical analyses, the following model for ethylene signal transduction is proposed (Figure 6). ETR1 acts upstream of the MAPK module, being composed of the MAPKKK CTR1, the MAPKK SIMKK, and the MAPKs SIMK/MMK3 or MPK6/13. In agreement with the genetic model, our data indicate that CTR1 acts as a negative regulator of the downstream MAPKs which are upstream regulators of ethylene target genes. How the MAPKs activate ethylene target genes through EIN2 and EIN3 presently is unclear, but physical interaction or phosphorylation are obvious options that are under investigation.
Fig. 6. Proposed model of the MAPK pathway mediating ethylene signaling in plants. The histidine kinase ETR1 functions as an ethylene receptor and activates CTR1 in the absence of ethylene. CTR1 is a negative regulator of the MAPKK SIMKK and the MAPKs SIMK/MMK3 in Medicago, and MPK6/13 in Arabidopsis. In the presence of ethylene, ETR1 and CTR1 become inactivated, relieving SIMKK from inhibition. Subsequent activation of the MAPKs activates gene expression of ethylene-responsive genes via direct activation of EIN2 and EIN3 or through other factors.
Materials and methods
Transient expression in Arabidopsis protoplasts
HA-tagged SIMK and MMK3 were prepared as described (Kiegerl et al., 2000). The open reading frames of MKK1, PRKK and SIMKK were cloned into the plant expression vector pRT101 (Kiegerl et al., 2000). Transient expression experiments were performed with protoplasts from Arabidopsis cells using PEG for transformation. A 5 µg aliquot of each plasmid DNA was used for co-transformation. After treatment, extracts were prepared from protoplasts 12–16 h after transformation (Kiegerl et al., 2000) and used for immunocomplex kinase assay with antibodies raised against HA.
In-gel protein kinase assays
Cell extracts were prepared at different times after treatment in extraction buffer [25 mM Tris–HCl pH 7.8, 15 mM EGTA, 75 mM NaCl, 1 mM dithiothreitol (DTT), 10 mM MgCl2, 1 mM NaF, 0.5 mM NaVO3, 15 mM β-glycerophosphate, 15 mM 4-nitrophenylphosphate, 0.1% Tween-20, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 5 µg/ml leupeptin, 5 µg/ml aprotinin]. After centrifugation at 20 000 g for 45 min, the cleared supernatant was used.
For in-gel protein kinase reactions, cell extracts containing 20 µg of total protein per lane were separated by SDS–PAGE. Myelin basic protein (MBP; 0.5 mg/ml) was used as a substrate polymerized in the polyacrylamide gel. After protein renaturation, the kinase reactions were performed in the gel as described (Usami et al., 1995).
Immunocomplex kinase assays
Protoplast or cell suspension extracts (prepared as described above) containing equal protein amounts were subjected to a 2 h pre-incubation in the presence of 20 µl of mixed protein A– and G–Sepharose beads (1:1). The supernatant was then immunoprecipitated with either 5 µg of protein A-purified M23, M11, M14 and M7 antibodies raised against synthetic peptides encoding the C-terminal seven, ten, ten and five amino acids of the Medicago SIMK (and AtMPK6), MMK2, MMK3 and SAMK MAPKs, respectively (Cardinale et al., 2000), or 5 µl of HA antibody (BABCO, Richmond, CA) and 20 µl of protein A– (for SIMK, MMK2, MMK3 and SAMK) or protein G–Sepharose beads (for HA-tagged SIMK and MMK3) for 2 h at 4°C. The beads were washed three times with wash buffer (50 mM Tris pH 7.4, 250 mM NaCl, 5 mM EGTA, 5 mM EDTA, 0.1% Tween-20) and once with kinase buffer (20 mM HEPES pH 7.4, 10 mM MgCl2, 5 mM EGTA and 1 mM DTT). Kinase reactions of the immunoprecipitated proteins were performed in 15 µl of kinase buffer containing 5 µg of MBP, 0.1 mM ATP and 2 µCi of [γ-32P]ATP. The protein kinase reactions were performed at room temperature for 30 min and the reactions were stopped by adding 4× SDS loading buffer. The phosphorylation of MBP (substrate for MAPKs) or of SIMK loss of function fused to GST (SIMK-lof–GST, substrate for SIMKK-HA) was analyzed by autoradiography after SDS–PAGE.
Immunoblotting
Immunoblotting was performed with equal amounts of protein separated by SDS–PAGE, immunoblotted to PVDF membranes (Millipore) and probed with protein A-purified M23, M11, M14 and M7 antibodies or with HA antibody as recommended by the manufacturer (BABCO). Alkaline phosphatase-conjugated goat anti-rabbit IgG (for protein A-purified antibodies) or anti-mouse IgG (for HA antibodies) (Sigma) were used as secondary antibodies, and the reaction was visualized by fluorography using CDP-Star (Amersham Life Sciences) as a substrate.
RNA gel blot analysis
Total RNA was isolated from detached leaves using Trizol reagent (Sigma) as described by the supplier. The northern blot analysis was performed using 10 µg of total RNA per lane, separated on 1.5% agarose gels containing 1.1% formaldehyde. The gel was blotted to a nylon membrane (Hybond-N, Amersham) and cross-linked by UV. The probes for hybridization were labeled by random priming using the Ready-To-Go™ DNA Labelling Beads (–dCTP) kit from Amersham. The membrane was hybridized to the probes at 65°C, and washed for 5 min with 2× SSC at room temperature, once for 30 min at 65°C with 0.5% SDS and 2× SSC, and subsequently with 0.1× SSC once for 30 min at room temperature. The membrane was exposed to a PhosphorImager screen and analyzed with a PhosphorImager (Molecular Dynamics), and then exposed to Biomax MR films (Kodak).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
The authors thank Claudia Jonak and Hirofumi Nakagami for their critical reading of the manuscript. The work was supported by grants from the Austrian Science Foundation and the IHRP program of the European Union.
References
- Abeles F., Morgan,P. and Saltveit,M. (1992) Ethylene in Plant Biology. Academic Press, San Diego, CA.
- Alonso J.M., Hirayama,T., Roman,G., Nourizadeh,S. and Ecker,J.R. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science, 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Cameron A.C., Fenton,C.A.L., Yu,Y., Adams,D.O. and Yang,S.F. (1979) Increased production of ethylene by plant tissues treated with 1-aminocyclopropane-1-carboxylic acid. HortScience, 14, 178–180. [Google Scholar]
- Cardinale F., Jonak,C., Ligterink,W., Niehaus,K., Boller,T. and Hirt,H. (2000) Differential activation of four specific MAPK pathways by distinct elicitors. J. Biol. Chem., 275, 36734–36740. [DOI] [PubMed] [Google Scholar]
- Chang C. (1996) The ethylene signal transduction pathway in Arabidopsis: an emerging paradigm? Trends Biochem. Sci., 21, 129–133. [PubMed] [Google Scholar]
- Chang C., Kwok,S.F., Bleecker,A.B. and Meyerowitz,E.M. (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science, 262, 539–544. [DOI] [PubMed] [Google Scholar]
- Chao Q., Rothenberg,M., Solano,R., Roman,G., Terzaghi,W. and Ecker,J.R. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell, 89, 1133–1144. [DOI] [PubMed] [Google Scholar]
- Chen Y.F., Randlett,M.D., Findell,J.L. and Schaller,G.E. (2002) Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J. Biol. Chem., 277, 19861–19866. [DOI] [PubMed] [Google Scholar]
- Clark K.L., Larsen,P.B., Wang,X. and Chang,C. (1998) Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc. Natl Acad. Sci. USA, 95, 5401–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C.A., Tang,D. and Innes,R.W. (2001) Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc. Natl Acad. Sci. USA, 98, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J. and Meyerowitz,E.M. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell, 94, 261–271. [DOI] [PubMed] [Google Scholar]
- Huser M. et al. (2001) MEK kinase activity is not necessary for Raf-1 function. EMBO J., 20, 1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber J.J., Rothenberg,M., Roman,G., Feldmann,K.A. and Ecker,J.R. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell, 72, 427–441. [DOI] [PubMed] [Google Scholar]
- Kiegerl S. et al. (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell, 12, 2247–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurssen K., Naumann,K. and Shroder,R. (1979) 1-aminocyclopropane-1-carboxylic acid: an intermediate of ethylene biosynthesis in higher plants. Z. Pflanzenphysiol., 92, 285–294. [Google Scholar]
- Mikula M. et al. (2001) Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J., 20, 1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D.K. and Cutler,R.E. (1997) The complexity of Raf-1 regulation. Curr. Opin. Cell Biol., 9, 174–179. [DOI] [PubMed] [Google Scholar]
- Novikova G.V., Moshkov,I.E., Smith,A.R. and Hall,M.A. (2000) The effect of ethylene on MAPKinase-like activity in Arabidopsis thaliana. FEBS Lett., 474, 29–32. [DOI] [PubMed] [Google Scholar]
- Nuhse T.S., Peck,S.C., Hirt,H. and Boller,T. (2000) Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J. Biol. Chem., 275, 7521–7526. [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi M. and Shinshi,H. (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell, 7, 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. and Chang,C. (1999) Functional complementation of the Schizosaccharomyces pombe wis1 mutant by Arabidopsis MEK1 and non-catalytic enhancement by CTR1. FEBS Lett., 459, 405–410. [DOI] [PubMed] [Google Scholar]
- Schaller G.E. and Bleecker,A.B. (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science, 270, 1809–1811. [DOI] [PubMed] [Google Scholar]
- Solano R., Stepanova,A., Chao,Q. and Ecker,J.R. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev., 12, 3703–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S., Banno,H., Ito,Y., Nishihama,R. and Machida,Y. (1995) Cutting activates a 46-kiloDalton protein kinase in plants. Proc. Natl Acad. Sci. USA, 92, 8660–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste K. and Kieber,J.J. (1998) The molecular basis of ethylene signalling in Arabidopsis. Philos. Trans. R. Soc. Lond. B Biol. Sci., 353, 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]