The thyrotropin receptor (TSHR), one of the primary antigens in autoimmune thyroid disease, is a target of both antigen-specific T cells and antibodies in patients with this condition (1). Autoantibodies to the TSHR (TSHR-Ab) act as thyroid stimulating factor (TSH) agonists in autoimmune hyperthyroidism (Robert Graves disease) but as TSH antagonists in autoimmune hypothyroidism (Hashimoto thyroiditis). The TSHR antigen is primarily expressed in the epithelial cells of the thyroid follicles, but TSHR mRNA and protein have been reported in a variety of cell types, some of which show evidence of receptor activity (Table 1).
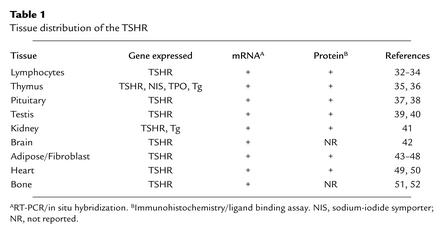
Table 1.
Tissue distribution of the TSHR
The TSHR gene, cloned in 1989 (2–5), maps to human chromosome 14q and encodes a predicted seven-transmembrane, G protein–coupled glycoprotein. Although it is similar to the luteinizing hormone receptor and the follicle-stimulating hormone receptor, the TSHR is the largest of the glycoprotein hormone receptors, due primarily to 8– and 50–amino acid insertions in its ectodomain (residues 38–45 and 317–367) (6). As predicted from its cDNA, the TSHR has an unglycosylated molecular weight of 84 kDa but the glycosylated holoreceptor runs on SDS-PAGE with an apparent molecular weight of 95–100 kDa. There are six potential N-linked glycosylation sites on the TSHR, and it was recently shown that the TSHR is also palmitoylated (7). The minimal 5′ promoter region required to confer thyroid-specific expression and cAMP autoregulation extends from 220 to 39 bp upstream of the transcription start site, but there are multiple transcription start sites between –89 and –68 bp (8).
TSHR signal transduction, regulation, and maturation
The TSHR, long known to signal via cAMP (9), can induce both the phospholipase C (PLC) and the protein kinase A signal transduction systems. Intracellular Ca2+ and PLC regulate iodide efflux, H2O2 production, and thyroglobulin iodination, while adenylate cyclase and cAMP regulate iodide uptake and transcription of thyroglobulin (Tg), thyroid peroxidase (TPO), and the sodium-iodide symporter (10, 11). Photoaffinity labeling of the TSHR with azido-GTP followed by immunoprecipitation suggests that all four Gα subtypes are involved (12), but only Gαs and Gαq have been shown to mediate TSHR signals.
TSHR levels are positively regulated by TSH in normal cells (13), secondary to prolongation of the mRNA half-life, while exposure to high concentrations of ligand causes the receptors to be downregulated (14). Posttranslational proteolysis clips the TSH receptor (TSHR) into two subunits (referred to as α, or A, and β, or B) (15, 16), linked to each other by disulfide bonds. These α and β subunits are formed by intramolecular cleavage, apparently at multiple sites, with removal of an intervening polypeptide segment of approximately 50 amino acids (amino acids 317–367) (17, 18). Disulfide bonds that link the two subunits are then reduced, presumably by protein disulfide isomerase leading to release of the α subunit from the membrane-bound receptor (17, 19). This phenomenon of releasing the α subunit is referred to as receptor shedding and explains the approximately 3:1 excess of β subunits found in thyroid membrane preparations (20). These observations also explain the early data indicating that the long-acting thyroid stimulator (LATS) activity in Graves patients’ sera, now attributed to activating TSHR-Ab’s autoantibodies, can be absorbed using the supernatant from frozen and thawed thyroid tissues (21). Moreover, such ectodomain shedding may also account for the puzzling size diversity of TSHRs detected by Western blot (20). Crucially, shedding also leads to a structural change in the α subunit once it is released from the receptor — a change whose consequences are considered by Chazenbalk et al. in this issue of the JCI (22).
Cleavage of TSHR and receptor interactions with pathogenic antibodies
Using modern techniques, Chazenbalk et al. (22) confirm the long-standing suspicion, raised by early work on LATS-absorbing activity, that the fractured TSHR has a higher affinity for TSHR-Ab’s than does the intact, membrane-bound receptor. Using both secreted and glycosidylphosphatidyl inositol-anchored (GPI) TSHR ectodomains, their elegant studies confirm that stimulating TSH receptor antibodies, which are directed primarily at the TSHR ectodomain, preferentially bind to these fragments rather than to intact membrane-bound receptor on the cell surface. This preferential binding distinguishes the agonistic TSHR-Ab’s found in individuals with Graves disease from the blocking antibody type found in Hashimoto thyroiditis patients. These observations are also consistent with stimulating TSHR-Ab’s being directed at a unique conformational epitope, which may be accessed more easily when the ectodomain is shed from the holoreceptor.
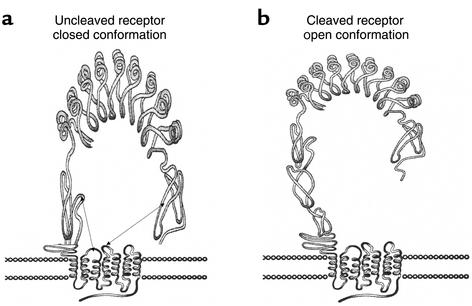
Apparently recognizing a conformational epitope on the receptor, activating TSHR-Ab’s from susceptible individuals bind to the shed receptor ectodomain, on the so-called open form of the holoreceptor. Accumulating data from mutational analyses indicate that this receptor can shift from a “closed” (inactive) to an “open” (active) form, which can induce signaling when bound by TSH agonists, including activating autoantibodies. While TSHR cleavage and reduction are not necessary for signal transduction (23), interactions between the ectodomain and the extracellular loops of the transmembrane domains are critical for the maintenance of an inactive state (Figure 1; see also ref. 24). Consistent with this model, removal of the TSHR ectodomain yields a constitutively active receptor, suggesting that its presence inhibits an otherwise constitutively active β subunit (25, 26). This model predicts that only the open form of the receptor should bind ligand and become activated. The observation (23) that a noncleavable TSHR mutant is able to signal could simply be explained by the mutated ectodomain forming an open structure.
Figure 1.
Two possible structural configurations of TSH receptor. (a) The uncleaved receptor is shown with a “buried” structure. The ectodomain and transmembrane interactions result in a “closed” conformation (connecting dotted lines). (b) Cleavage may induce an “open” conformation, a step that is thought to be a precondition for subsequent changes in receptor localization and activity, as described in the text and shown in Figure 2. This figure is adapted from ref. 25 with permission.
Recent work from our laboratory has demonstrated that the TSH receptor is constitutively oligomeric (27). Using fluorescent resonance energy transfer between two different fluorescently labeled TSHR constructs, we confirmed the presence of TSHR multimers in cell membranes, as we previously suggested based on molecular size (20). We have now also found that these oligomeric forms rapidly dissociate upon TSH binding (28) (Figure 2), indicating that ligand binding alters the shape of the receptor, promoting dissociation of the oligomeric receptors into active, open monomers. Moreover, murine monoclonal TSHR antibodies, which block TSH action, fail to mimic TSH or to promote TSHR monomer formation (R. Latif et al., unpublished data). If, as we suggest, the monomeric form of the TSHR can be recruited more efficiently into lipid rafts (29) than can the larger complexes, these observations may indicate a mechanism by which the monomer preferentially couples with G proteins (30) to initiate signaling (Figure 2).
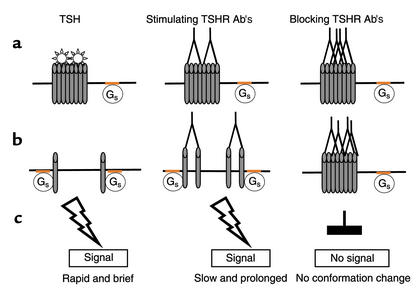
Figure 2.
A model for oligomerization in TSH receptor signaling. (a) TSH receptors initially exist in an open conformation as cleaved oligomers residing on the cell surface outside lipid rafts. Signal-transducing Gs proteins, on the other hand, are restricted to lipid rafts. (b) Following TSH binding, the oligomers break apart into monomers. Conversely, stimulating antibodies, as found in sera from patients with the autoimmune disorder Graves disease, are proposed to favor formation of TSH receptor dimers; blocking antibodies, are unable to bring about this conformational change. (c) Dissociated monomers, as well as dimers, move into the lipid rafts and bind to Gs proteins, thereby initiating the signaling cascade. In the case of TSH, the signal is rapid and brief because of faster movement of monomers into the lipid rafts, in contrast to the retarded movement of the dimers. Multivalent blocking TSHR antibodies may cross-link the oligomers, thereby preventing them from dissociating and impeding their entry into lipid rafts.
Although stimulating TSHR-Ab’s are unique to patients with Graves disease, their serum has been shown to most commonly contain a mixture of both blocking and stimulating TSHR-Ab’s (1), and the bioactivity of the serum reflects a balance between the two. Unfortunately, the surprising failure of many investigators to isolate high-affinity monoclonal stimulating TSHR antibodies represents a major impediment to understanding the basis of this response. Nevertheless, we hypothesize that stimulating, but not blocking, TSHR-Ab’s lead to the formation of TSHR dimers, which may still be able to move into the lipid rafts and initiate signal transduction (Figure 2). While monomers can move quickly within the lipid rafts, as shown by fluorescence recovery after photobleaching experiments, movement of dimeric receptors within the rafts is predicted to be slower, perhaps accounting for the relatively weak but prolonged signaling that results from exposure to LATS sera (31).
The failure to obtain monoclonal stimulating antibodies of high affinity to the TSHR remains a great enigma in modern thyroid research. While blame has always been assigned to insensitive detection systems combined with a small repertoire of TSHR-Ab–secreting B cells, it is also possible that the state of the TSHR, not just the nature of the antibody, determines whether antibody binding can activate signaling. Combinations of TSHR-Ab’s, which on their own may not activate the receptor, may be required to shift the receptor into an activated form — perhaps by promoting TSHR monomer formation. This hypothesis is eminently testable and promises more excitement in this rapidly developing field.
Acknowledgments
This work was supported in part by NIH grants DK-52464, DK-35764, and DK-45011 (to T. Davies), and the David Owen Segal Endowment (to R. Latif).
Footnotes
See the related article beginning on page 209.
References
- 1.Davies, T.F. 2000. Werner & Ingbar’s the thyroid: a fundamental and clinical text. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 518–530.
- 2.Parmentier M, et al. Molecular cloning of the thyrotropin receptor. Science. 1989;246:1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- 3.Misrahi M, et al. Cloning, sequencing and expression of human TSH receptor. Biochem Biophys Res Commun. 1990;166:394–403. doi: 10.1016/0006-291x(90)91958-u. [DOI] [PubMed] [Google Scholar]
- 4.Nagayama Y, Kaufman KD, Seto P, Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun. 1989;165:1184–1190. doi: 10.1016/0006-291x(89)92727-7. [DOI] [PubMed] [Google Scholar]
- 5.Gross B, Misrahi M, Sar S, Milgrom E. Composite structure of the human thyrotropin receptor gene. Biochem Biophys Res Commun. 1991;177:679–687. doi: 10.1016/0006-291x(91)91842-z. [DOI] [PubMed] [Google Scholar]
- 6.Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- 7.Kosugi S, Mori T. Cysteine-699, a possible palmitoylation site of the thyrotropin receptor, is not crucial for cAMP or phosphoinositide signaling but is necessary for full surface expression. Biochem Biophys Res Commun. 1996;221:636–640. doi: 10.1006/bbrc.1996.0648. [DOI] [PubMed] [Google Scholar]
- 8.Ikuyama S, Niller HH, Shimura H, Akamizu T, Kohn LD. Characterization of the 5′-flanking region of the rat thyrotropin receptor gene. Mol Endocrinol. 1992;6:793–804. doi: 10.1210/mend.6.5.1318504. [DOI] [PubMed] [Google Scholar]
- 9.Wilson B, Raghupathy E, Tonoue T, Tong W. TSH-like actions of dibutyryl-cAMP on isolated bovine thyroid cells. Endocrinology. 1968;83:877–884. doi: 10.1210/endo-83-4-877. [DOI] [PubMed] [Google Scholar]
- 10.Field JB, Ealey PA, Marshall NJ, Cockcroft S. Thyroid-stimulating hormone stimulates increases in inositol phosphates as well as cyclic AMP in the FRTL-5 rat thyroid cell line. Biochem J. 1987;247:519–524. doi: 10.1042/bj2470519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem. 2001;276:21458–21463. doi: 10.1074/jbc.M100561200. [DOI] [PubMed] [Google Scholar]
- 12.Laugwitz KL, et al. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci USA. 1996;93:116–120. doi: 10.1073/pnas.93.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber GK, Weinstein SP, Graves PN, Davies TF. The positive regulation of human thyrotropin (TSH) receptor messenger ribonucleic acid by recombinant human TSH is at the intranuclear level. Endocrinology. 1992;130:2858–2864. doi: 10.1210/endo.130.5.1572298. [DOI] [PubMed] [Google Scholar]
- 14.Akamizu T, et al. Cloning, chromosomal assignment, and regulation of the rat thyrotropin receptor: expression of the gene is regulated by thyrotropin, agents that increase cAMP levels, and thyroid autoantibodies. Proc Natl Acad Sci USA. 1990;87:5677–5681. doi: 10.1073/pnas.87.15.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bernard S, et al. Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J Biol Chem. 1999;274:101–107. doi: 10.1074/jbc.274.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Buckland PR, Rees SB. A structural comparison of guinea pig thyroid and fat TSH receptors by photoaffinity labelling. FEBS Lett. 1984;166:109–114. doi: 10.1016/0014-5793(84)80054-x. [DOI] [PubMed] [Google Scholar]
- 17.Misrahi M, Milgrom E. Cleavage and shedding of the TSH receptor. Eur J Endocrinol. 1997;137:599–602. doi: 10.1530/eje.0.1370599. [DOI] [PubMed] [Google Scholar]
- 18.Chazenbalk GD, et al. Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology. 1997;138:2893–2899. doi: 10.1210/endo.138.7.5259. [DOI] [PubMed] [Google Scholar]
- 19.Couet J, et al. Shedding of human thyrotropin receptor ectodomain. Involvement of a matrix metalloprotease. J Biol Chem. 1996;271:4545–4552. doi: 10.1074/jbc.271.8.4545. [DOI] [PubMed] [Google Scholar]
- 20.Graves PN, Vlase H, Bobovnikova Y, Davies TF. Multimeric complex formation by the thyrotropin receptor in solubilized thyroid membranes. Endocrinology. 1996;137:3915–3920. doi: 10.1210/endo.137.9.8756566. [DOI] [PubMed] [Google Scholar]
- 21.Smith BR. Binding of long-acting thyroid stimulator to 4S thyroid protein coupled to sepharose. J Endocrinol. 1972;52:229–237. doi: 10.1677/joe.0.0520229. [DOI] [PubMed] [Google Scholar]
- 22.Chazenbalk GD, et al. Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest. 2002;110:209–217. doi:10.1172/JCI200215745. doi: 10.1172/JCI15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chazenbalk GD, Tanaka K, McLachlan SM, Rapoport B. On the functional importance of thyrotropin receptor intramolecular cleavage. Endocrinology. 1999;140:4516–4520. doi: 10.1210/endo.140.10.7031. [DOI] [PubMed] [Google Scholar]
- 24.Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol. 2002;16:736–746. doi: 10.1210/mend.16.4.0816. [DOI] [PubMed] [Google Scholar]
- 25.Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, et al. The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology. 2000;141:3514–3517. doi: 10.1210/endo.141.9.7790. [DOI] [PubMed] [Google Scholar]
- 27.Latif R, Graves P, Davies TF. Oligomerization of the human TSH receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J Biol Chem. 2001;276:45217–45224. doi: 10.1074/jbc.M103727200. [DOI] [PubMed] [Google Scholar]
- 28.Latif R, Graves PN, Davies TF. A new action for TSH: the regulation of thyrotropin receptor oligomerization. Program Abstr Endocr Soc Annu Meet. 2002;83:135. (Abstr.) [Google Scholar]
- 29.Miura Y, Hanada K, Jones TL. G(s) signaling is intact after disruption of lipid rafts. Biochemistry. 2001;40:15418–15423. doi: 10.1021/bi015574a. [DOI] [PubMed] [Google Scholar]
- 30.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 31.Adams DD. Thyroid-stimulating autoantibodies. Vitam Horm. 1980;38:119–203. doi: 10.1016/s0083-6729(08)60485-9. [DOI] [PubMed] [Google Scholar]
- 32.Chabaud O, Lissitzky S. Thyrotropin-specific binding to human peripheral blood monocytes and polymorphonuclear leukocytes. Mol Cell Endocrinol. 1977;7:79–87. doi: 10.1016/0303-7207(77)90077-6. [DOI] [PubMed] [Google Scholar]
- 33.Davies TF, Teng CS, McLachlan SM, Smith BR, Hall R. Thyrotropin receptors in adipose tissue, retro-orbital tissue and lymphocytes. Mol Cell Endocrinol. 1978;9:303–310. doi: 10.1016/0303-7207(78)90072-2. [DOI] [PubMed] [Google Scholar]
- 34.Bagriacik EU, Klein JR. The thyrotropin (thyroid-stimulating hormone) receptor is expressed on murine dendritic cells and on a subset of CD45RBhigh lymph node T cells: functional role for thyroid-stimulating hormone during immune activation. J Immunol. 2000;164:6158–6165. doi: 10.4049/jimmunol.164.12.6158. [DOI] [PubMed] [Google Scholar]
- 35.Murakami M, et al. Thymic hyperplasia in patients with Graves’ disease. Identification of thyrotropin receptors in human thymus. J Clin Invest. 1996;98:2228–2234. doi: 10.1172/JCI119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzweg C, Joba W, Heufelder AE. Expression of thyroid-related genes in human thymus. Thyroid. 1999;9:133–141. doi: 10.1089/thy.1999.9.133. [DOI] [PubMed] [Google Scholar]
- 37.Prummel MF, et al. Expression of the thyroid-stimulating hormone receptor in the folliculo-stellate cells of the human anterior pituitary. J Clin Endocrinol Metab. 2000;85:4347–4353. doi: 10.1210/jcem.85.11.6991. [DOI] [PubMed] [Google Scholar]
- 38.Brokken LJ, Scheenhart JW, Wiersinga WM, Prummel MF. Suppression of serum TSH by Graves’ Ig: evidence for a functional pituitary TSH receptor. J Clin Endocrinol Metab. 2001;86:4814–4817. doi: 10.1210/jcem.86.10.7922. [DOI] [PubMed] [Google Scholar]
- 39.Davies TF, Smith BR, Hall R. Binding of thyroid stimulators to guinea pig testis and thyroid. Endocrinology. 1978;103:6–10. doi: 10.1210/endo-103-1-6. [DOI] [PubMed] [Google Scholar]
- 40.Kumar RS, et al. Cloning and functional expression of a thyrotropin receptor from the gonads of a vertebrate (bony fish): potential thyroid-independent role for thyrotropin in reproduction. Mol Cell Endocrinol. 2000;167:1–9. doi: 10.1016/s0303-7207(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 41.Sellitti DF, et al. Renal expression of two ‘thyroid-specific’ genes: thyrotropin receptor and thyroglobulin. Exp Nephrol. 2000;8:235–243. doi: 10.1159/000020674. [DOI] [PubMed] [Google Scholar]
- 42.Crisanti P, et al. The expression of thyrotropin receptor in the brain. Endocrinology. 2001;142:812–822. doi: 10.1210/endo.142.2.7943. [DOI] [PubMed] [Google Scholar]
- 43.Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 44.Haraguchi K, Shimura H, Lin L, Endo T, Onaya T. Differentiation of rat preadipocytes is accompanied by expression of thyrotropin receptors. Endocrinology. 1996;137:3200–3205. doi: 10.1210/endo.137.8.8754740. [DOI] [PubMed] [Google Scholar]
- 45.Endo T, Ohta K, Haraguchi K, Onaya T. Cloning and functional expression of a thyrotropin receptor cDNA from rat fat cells. J Biol Chem. 1995;270:10833–10837. doi: 10.1074/jbc.270.18.10833. [DOI] [PubMed] [Google Scholar]
- 46.Roselli-Rehfuss L, Robbins LS, Cone RD. Thyrotropin receptor messenger ribonucleic acid is expressed in most brown and white adipose tissues in the guinea pig. Endocrinology. 1992;130:1857–1861. doi: 10.1210/endo.130.4.1547715. [DOI] [PubMed] [Google Scholar]
- 47.Bahn RS, et al. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 48.Valyasevi RW, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–2562. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 49.Drvota V, et al. Evidence for the presence of functional thyrotropin receptor in cardiac muscle. Biochem Biophys Res Commun. 1995;211:426–431. doi: 10.1006/bbrc.1995.1831. [DOI] [PubMed] [Google Scholar]
- 50.Sellitti DF, et al. Differential expression of thyrotropin receptor mRNA in the porcine heart. Thyroid. 1997;7:641–646. doi: 10.1089/thy.1997.7.641. [DOI] [PubMed] [Google Scholar]
- 51.Inoue M, Tawata M, Yokomori N, Endo T, Onaya T. Expression of thyrotropin receptor on clonal osteoblast-like rat osteosarcoma cells. Thyroid. 1998;8:1059–1064. doi: 10.1089/thy.1998.8.1059. [DOI] [PubMed] [Google Scholar]
- 52.Marians RC, et al. TSH serves as a negative regulator of osteoblast and osteoclast development and function as demonstrated in the TSH receptor knock-out mouse. American Thyroid Association 2001 Abstract Guide. 2001;2:37. (Abstr.) [Google Scholar]