Abstract
The rearrangement of immunoglobulin (Ig) and T-cell receptor (TCR) genes in lymphocytes by V(D)J recombinase is essential for immunological diversity in humans. These DNA rearrangements involve cleavage by the RAG1 and RAG2 (RAG1/2) recombinase enzymes at recombination signal sequences (RSS). This reaction generates two products, cleaved signal ends and coding ends. Coding ends are ligated by non-homologous end-joining proteins to form a functional Ig or TCR gene product, while the signal ends form a signal joint. In vitro studies have demonstrated that RAG1/2 are capable of mediating the transposition of cleaved signal ends into non-specific sites of a target DNA molecule. However, to date, in vivo transposition of signal ends has not been demonstrated. We present evidence of in vivo inter-chromosomal transposition in humans mediated by V(D)J recombinase. T-cell isolates were shown to contain TCRα signal ends from chromosome 14 inserted into the X-linked hypo xanthine–guanine phosphoribosyl transferase locus, resulting in gene inactivation. These findings implicate V(D)J recombinase-mediated transposition as a mutagenic mechanism capable of deleterious genetic rearrangements in humans.
Keywords: HPRT/human T-cell receptor/RAG1/2/transposition/V(D)J recombination
Introduction
The genes responsible for the development of a mature and diverse immune system in vertebrates are unique, consisting of multiple germ-line gene sequences that undergo site-specific somatic DNA rearrangements. These recombination events rearrange variable (V), diversity (D) and joining (J) gene sequences to form the immunoglobulin (Ig) and T-cell receptor (TCR) gene products responsible for antigen and cell-specific immune diversity (Tonegawa, 1983). Specific V(D)J recombination signal sequences (RSS) are located at the borders of each rearranging gene segment. The RSS contains both a conserved heptamer (consensus 5′-CACAGTG-3′) and nonamer (consensus 5′-ACAAAAACC-3′), separated by 12 or 23 base pairs (Sakano et al., 1979). Normal V(D)J recombination occurs between an RSS with a 12 bp spacer and an RSS with a 23 bp spacer (Hiom and Gellert, 1998). RAG1/2 and high mobility DNA binding proteins HMG1 and HMG2 bind to and bring the two RSS gene segments into close proximity (Sawchuk et al., 1997; reviewed in van Gent et al., 1997; Fugmann et al., 2000). The DNA is subsequently cleaved, generating four free ends, which include the blunt 5′ phosphorylated signal ends containing the complete RSS, and the covalently joined hairpin coding ends (McBlane et al., 1995) (Figure 1). Ligation of the coding segments involves several non-homologous end-joining proteins that result in nucleotide nibbling (loss of bases) and insertions (bases added) at the coding junction (reviewed in Fugmann et al., 2000). This newly formed coding joint is retained in the chromosome to form a new gene product. The signal ends either form extra-chromosomal circularized DNA molecules that are lost from the cell during cell division, or become inverted and are retained in the chromosome, depending on their orientation (reviewed in Lewis, 1994).
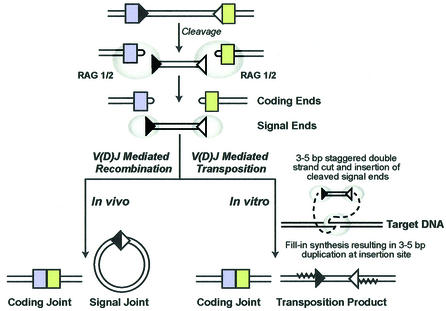
Fig. 1. V(D)J recombinase-mediated rearrangements. The steps involved in V(D)J recombination include site-specific recognition and cleavage at 12 bp RSS (black triangles) and 23 bp RSS (white triangles) sites by RAG1/2 (gray ovals), generating the hairpin coding ends (colored boxes) and the signal ends. Non-homologous end joining proteins process the coding ends. The result is a ligated coding joint that forms the functional gene product and a circularized signal joint. RAG1/2 are also capable of mediating transposition in vitro by strand transfer of the signal ends into a non-RSS target site. Transposition involves a staggered double-strand cut by RAG1/2, insertion of the signal ends, and fill-in synthesis resulting in a 3–5 bp target site duplication. This figure is adapted from Lewis and Wu (2000).
Several similarities have been identified between the mechanism of V(D)J recombination and the movements of transposable elements (reviewed in Thompson, 1995). RAG1 and RAG2 (RAG1/2) genes have a compact genomic organization and are convergently transcribed. This is consistent with components of a transposable element (Oettinger et al., 1990). RSS resemble inverted repeats found at the ends of the Tc1 family of invertebrate transposons (Dreyfus, 1992). V(D)J cleavage, retroviral integration and transposition also proceed via a common pathway that involves exposure of a 3′ hydroxyl group and a subsequent attack on a target phosphodiester bond (Mizuuchi, 1992; Craig, 1995; van Gent et al., 1996). In addition, the RAG proteins remain bound to the RSS sites after cleavage, another common feature seen in transposition events (Agrawal and Schatz, 1997; Hiom et al., 1998; Jones and Gellert, 2001). In vitro experiments have subsequently provided specific evidence that RAG1/2 can act as a transposase, mediating the transposition of cleaved signal ends into non-specific sites of a target DNA molecule (Agrawal et al., 1998; Hiom et al., 1998). However, there has been no evidence of RAG1/2- mediated transposition in vivo. In this report, we present evidence for transposition of cleaved TCRα signal ends into intron 1 of the hypoxanthine–guanine phosphoribosyl transferase (HPRT) gene in human peripheral T cells.
Results
Identification of mutants at the HPRT locus in human T cells
Analysis of mutations at the HPRT locus in peripheral T lymphocytes has been used to study in vivo somatic mutational events in humans (O’Neill et al., 1987). Selection of mutations that have occurred in vivo is accomplished by direct plating of mononuclear cells by limiting dilution in the presence of the cytotoxic purine analog, 6-thioguanine. T lymphocytes with a functional HPRT enzyme utilize 6-thioguanine and die, whereas HPRT mutant T lymphocytes proliferate. The assay thereby selects for individual T lymphocytes that have acquired in vivo mutations which result in a loss of HPRT function. HPRT mutant MFS6 M2 was isolated from the cord blood of a full-term healthy newborn female (Yoshioka et al., 2001), while mutant F1 was isolated from a healthy 45-year-old male (Hou, 1994). As previously reported, RT–PCR analysis of each mutant revealed cDNA products ∼350 bp larger than expected (Hou, 1994; Yoshioka et al., 2001). Sequence analysis revealed insertion of a 347 bp (MFS6 M2) or a 369 bp (F1) TCRα coding gene segment, consisting of V35 joined to J46, and V23 joined to J48 (Hou, 1994), respectively, between exons 1 and 2 in the HPRT cDNA. These TCRα rearrangements are non-functional due to a stop codon at the V-J junction (F1) or in the J region (MFS6 M2) (Table I).
Table I. Summary of HPRT cDNA containing transposed TCRα signal ends.
|
HPRT mutant |
||
|---|---|---|
| F1 | MFS6 M2 | |
| Subject | ||
| Sex | Male | Female |
| Age | 45 years | Newborn |
| Insert length (bp) | 369 | 347 |
| TCRα V | TCR V23 | TCRα V35 |
| Nibbled nucleotidesa | AGCA (91 267–91 270) | AG (226 402–226 403) |
| TCRα J | TCRα J48 | TCRα J46 |
| Nibbled nucleotidesa | TATCTAACTT (30 737–30 746) | AGAAGA (33 647–33 652) |
| N nucleotide insertions | TTATTGAG | GAAGAGG |
| TCR reading frame | TGA in V-J junction | TGA in J |
| HPRT reading frame | TGA at new codon 11 | TGA at new codon 16 |
Breakpoint analysis reveals transposition of TCRα signal ends
In light of these observations and current evidence of V(D)J-mediated transposition in vitro, we hypothesized that the non-functional TCRα V-J coding segments were excised via RAG1/2, generating signal ends that inserted into intron 1 of the HPRT gene. The non-functional V-J coding joints were detected due to splicing of the V-J coding joint as an exon into the HPRT mRNA. In addition to the non-functional coding joint in the cDNA, the proposed signal end insertions could include other V and J gene segments, and any intervening sequence flanking the non-functional coding joint. These segments would be detected by genomic analysis but would not be spliced into the HPRT message, and therefore would not be observed in the cDNA.
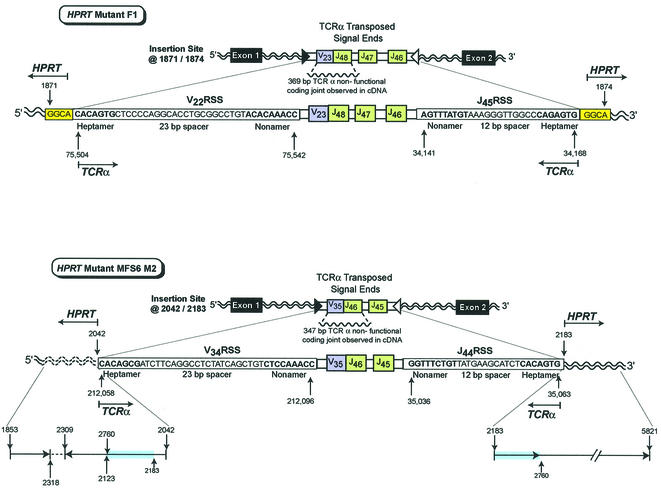
Primary PCR analysis focused on the 3′ breakpoint for each mutant, since a fragment containing multiple J regions could be more easily amplified given the smaller intervening regions between J coding sequences when compared with intervening regions of the V coding sequences. Initial PCR analysis of the 3′ breakpoint for mutant MFS6 M2 utilized a sense primer designed for the TCRα J46 region present in the cDNA product and an antisense HPRT primer located at position 5821 in intron 1 of HPRT (Table II). Each end of a unique 3.7 kbp PCR product was sequenced verifying capture of the breakpoint within the fragment. Sequence analysis of a nested 1.8 kbp PCR product generated using a sense primer for the TCRα J45 region and an antisense HPRT primer located at 4001 revealed TCRα sequence J44 RSS (nonamer–12 bp spacer–heptamer) GGTTTCTGT–12 bp–CACAGTG inserted at position 2183 in intron 1 of HPRT (Figure 2). Complete sequence analysis of the 3′ breakpoint fragment showed the expected HPRT sequence from position 2183 to 5821.
Table II. PCR and breakpoint analysis of HPRT transposition mutantsa.
| HPRT mutant | PCR primer pair |
PCR product | Sequence results (5′→3′) | |
|---|---|---|---|---|
| Sense | Antisense | |||
| F1 |
|
|
|
|
| 3′ breakpoint | ||||
| 1st round | TCRα J46 (33 679–33 700) | HPRT 5821 (5844–5821) | 5 kbp | TCRα J46 seq. and HPRT (3127–5821) |
| 2nd round | TCRα J46 (33 679–33 700) | HPRT 3127 (3148–3127) | 2 kbp | TCRα J45RSS→HPRT (1871–3127) |
| 5′ breakpoint | ||||
| 1st round | HPRT A106 (1835–1851) | TCRα V21 (58 044–58 024) | 250 bp | HPRT (1851–1874)→TCRα V22RSS |
| |
|
V22 (75 717–75 697) |
|
|
| MFS6 M2 |
|
|
|
|
| 3′ breakpoint | ||||
| 1st round | TCRα J46 (33 679–33 700) | HPRT 5821 (5844–5821) | 3.7 kbp | TCRα J46 seq. and HPRT (4671–5821) |
| 2nd round | TCRα J46 (33 679–33 700) | HPRT 4001 (4021–4001) | 1.8 kbp | TCRα J44RSS→HPRT (2183–4000) |
| 5′ breakpoint | ||||
| 1st round | HPRT A106 (1835–1851) | TCRα V34 (212 419–212 400) | 2.5 kbp | HPRT (1851–1944) and TCRα V34 seq. |
| V26S2 (207 707–207 690) | ||||
| V33 (194 753–194 734) | ||||
| V32 (190 295–190 273) | ||||
| V31 (181 913–181 894) | ||||
| V30 (173 342–173 322) | ||||
| 2nd round | HPRT 2089 (2089–2112) | TCRα V34 (212 419–212 400) | 400 bp | HPRT (2052–2042)→TCRα V34RSS |
| 700 bp | HPRT (2089–2760) | |||
Fig. 2. In vivo transposition of TCRα V-J signal ends from chromosome 14 into the HPRT gene on the X chromosome. HPRT mutant F1 contains transposed TCRα signal ends containing ∼16.8 kbp of intervening sequence that includes the V22 RSS (heptamer–23 bp spacer–nonamer), a V23 joined to J48 non-functional coding joint, J47, J46, and the J45 RSS (nonamer–12 bp spacer–heptamer) inserted into HPRT at bases 1871/1874. The insertion site is located in intron 1 of the HPRT gene. A 4 bp target site duplication, GGCA (1871–1874), is shown at both sides of the insertion. HPRT mutant MFS6 M2 contains transposed TCRα signal ends containing ∼16.1 kbp of intervening sequence that includes the V34 RSS (heptamer–23 bp spacer–nonamer), a V35 joined to J46 non-functional coding joint, J45, and the J44 RSS (nonamer–12 bp spacer–heptamer) inserted into HPRT at 2042/2183. The insertion site is located in intron 1 of the HPRT gene. Reading 5′ to 3′, the 5′ insertion site includes bases 1853–2318 followed by the inverted sequence 2309–2123 and 2760–2042. There is normal sequence at the 3′ HPRT insertion site from 2183 to 5821 that includes the same duplication of bases from 2183 to 2760 observed at the 5′ insertion site. HPRT bases that are duplicated are highlighted in blue. The inverted segment is shown by an arrow in the 3′ to 5′ direction.
Analysis of the 3′ breakpoint for mutant F1 utilized the same sense primer (J46) located 3′ of the J48 coding region present in the cDNA product and the HPRT antisense primer located at position 5821 in intron 1 (Table II). Each end of a unique 5 kbp PCR product was sequenced, verifying capture of the breakpoint within the fragment. Sequence analysis of a nested 2 kbp PCR product, generated utilizing a sense TCRα primer for J46 and an antisense HPRT primer located at position 3127 in intron 1, revealed TCRα J45 RSS (nonamer–12 bp spacer– heptamer) AGTTTATGT–12 bp–CAGAGTG inserted at position 1871 in intron 1 of HPRT (Figure 2). Identification of the 3′ genomic breakpoint of both mutants revealed characteristic RSS signal ends of the TCRα sequence inserted at different sites within intron 1 of HPRT.
The 5′ breakpoint for mutant F1 was determined utilizing previously published Southern data (Hou, 1994) in conjunction with identification of the HPRT insertion site at the 3′ breakpoint. These data suggested that the breakpoint sequence would contain an RSS from either TCRα V21 or V22. A multiplex PCR was carried out using a sense HPRT primer located at position 1835 and two antisense TCRα V sequence primers located near the RSS for V21 and V22 (Table II). The PCR resulted in a unique 250 bp fragment containing the 5′ breakpoint that showed the V22 RSS sequence (heptamer–23 bp spacer–nonamer) CACAGTG–23 bp–ACACAAACC inserted at position 1874 of HPRT. These results support the transposition of TCRα signal ends containing ∼16.8 kbp of intervening sequence, consisting of TCRα V22RSS, V23 joined to J48, J47, J46, and J45RSS into intron 1 of the HPRT gene (Figure 2). The sequence GGCA (bases 1871–1874) was present at both the 5′ and 3′ insertion site of the TCRα transposed fragment, indicative of a staggered cut at the HPRT insertion site (Figure 2). These observations are in agreement with the 3–5 bp target site duplication observed in previous in vitro transposition studies (Agrawal et al., 1998; Hiom et al., 1998).
Analysis of the 5′ breakpoint for mutant MFS6 M2 used a similar multiplex approach. The PCR utilized a sense HPRT primer located at position 1835 and antisense TCRα primers designed for the next six V regions (V34, V33, V26s2, V32, V31, V30) 5′ of the V35 coding region contained in the HPRT cDNA (Table II). This reaction was followed by a semi-nested PCR using a sense HPRT primer located at position 2089 and each individual antisense V primer. The PCR that included the V34 primer resulted in a 400 bp fragment containing the 5′ breakpoint. Sequence analysis revealed V34 RSS sequence (heptamer–23 bp spacer–nonamer) CACAGCG–23 bp–CTCCAAACC inserted at position 2042 of HPRT. These results support the transposition of TCRα signal ends containing ∼16.1 kbp of intervening sequence, consisting of TCRα V34RSS, V35 joined to J46, J45, and J44RSS into intron 1 of HPRT at position 2042 at the 5′ breakpoint and position 2183 at the 3′ breakpoint (Figure 2). The PCR discussed above also generated additional products. Sequence analysis of these fragments confirmed the presence of an inverted duplication of the HPRT gene (Figure 2). Further analysis of intron 1 sequence 5′ of the insertion confirmed HPRT bases 1853–2318 following HPRT exon 1. This region is followed by a gap in confirmed sequence, then the inverted HPRT bases from position 2309 to 2123. This inverted sequence appears to be directly followed by an additional inverted region that includes HPRT bases from position 2760 to 2042, located directly adjacent to the inserted TCRα V34RSS at the 5′ insertion site (Figure 2). In addition, bases from position 2183 to 2760 are located at the 3′ end of the TCRα J44RSS insertion. This transposition event shows a more complex sequence surrounding the insertion site than did the first transposition event described in the male subject. Since we are observing a resolved in vivo transposition product, we can only speculate whether the inverted sequence was created during the transposition event, or occurred prior to the transposition, thereby targeting the signal ends to this site. Inverted repeats resulting in DNA hairpins have been shown to be preferential sites for transposition in vitro (Lee et al., 2002). Even though we have provided clear evidence of insertion of TCRα signal ends into HPRT for this mutant, an in vivo system is complicated by the fact that there are several pathways that could be involved in repair of double strand breaks (DSB) at the target site, due to nucleophilic attack by the signal ends. The observed inverted duplication at the 5′ insertion site with a loss of contiguous bases at the 3′ site suggests that other mechanisms were involved in the resolution of this event. It is possible that homologous recombination utilizing the additional X allele as template in the female subject was used in the resolution of this event. Alternatively, the exposed single-strand DNA at a DSB could be involved in snap-back replication (Morrical et al., 1991; Jackson, 2002). If this scenario took place at the time of insertion, the inverted duplication could be observed while the expected fill-in synthesis that creates the 3–5 bp duplication at the insertion site might not be. Lastly, if the inverted duplication formed prior to the transposition and was followed by insertion of the signal ends at two opposite hairpin ends, then one would observe the inverted duplication but might not observe a 3–5 bp duplication, as the two strands would be attacked in an uncoupled manner (Hiom et al., 1998; Lee et al., 2002). Inverted duplications were not observed in the products of the initial in vitro studies using RAG1/2 and HMG1/2 only (Agrawal et al., 1998; Hiom et al., 1998). However, inverted segments and duplications of DNA surrounding the insertion sites have been observed and described for other cut-and-paste type transposases, including Tn10, Mu and Tn7 (Foster et al., 1981; Mizuuchi et al., 1992; Kennedy et al., 1998).
Discussion
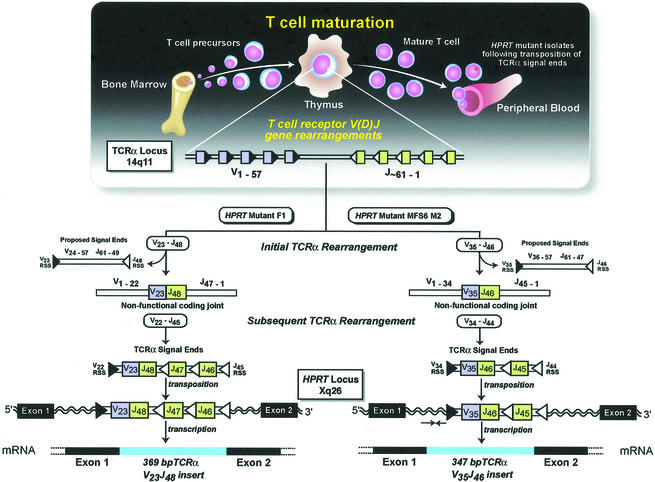
These data provide the first description of V(D)J recombinase-mediated inter-chromosomal transposition in humans. The presence of TCRα signal ends in both mutants is consistent with previous observations that the TCRα receptor locus can undergo several rearrangements, resulting in the generation of multiple signal ends (Livak and Schatz, 1996; McMahan and Fink, 1998). This would provide signal end substrates for transposition as a normal by-product of T-cell maturation. We propose that at least two TCRα V-J rearrangements occurred with the mutants described (Figure 3): the rearrangement that generated a non-functional coding joint and a subsequent rearrangement that formed a functional TCRα allowing for positive selection and release into the periphery. It was this second rearrangement that generated the signal ends that were inserted into the HPRT gene of these mutants. The fact that both insertions are located in intron 1 of the HPRT gene is of interest. The V(D)J enzyme complex cleaves at a cryptic RSS located at 2197 in intron 1 of HPRT (Fuscoe et al., 1991), providing evidence that this area of the intron is accessible to the enzyme system. In addition, it is possible that this intron provides some structure that is favorable for targeting of a transposition event.
Fig. 3. In vivo TCR events during maturation of T-cell clones F1 and MFS6 M2. During T-cell maturation, V(D)J-mediated rearrangements occur at the TCRα locus, generating V-J coding joints and cleaved signal ends. Both mutant clones underwent a rearrangement that resulted in the formation of a non-functional V-J coding joint V23 joined to J48 (F1) or V35 joined to J46 (MFS6 M2) (colored boxes), and their respective cleaved signal ends. A subsequent rearrangement occurred in an attempt to form a functional TCRα, generating cleaved signal ends that included the observed non-functional V-J coding joint. These TCRα signal ends were inserted via a transposition reaction into intron 1 of the HPRT gene. The non-functional V-J coding joints were observed in the cDNA due to correct splicing as an exon into the mRNA.
The previous lack of evidence supporting in vivo transposition has led to speculation that the V(D)J recombinase enzyme system once acted as a transposon capable of excision and reintegration of DNA, but has since diverged to provide only the site-specific reactions necessary to generate immunological diversity. Others have speculated that this enzyme system may be capable of transposition, but is closely regulated by additional elements that favor signal joint formation, leaving fewer signal ends for transposition (reviewed in Thompson, 1995; Lewis and Wu, 2000; Fugmann, 2001). Recent in vitro data have shown that signal joint formation does not prevent transposition because signal joints can be cleaved by a nick–nick mechanism, and are then capable of transposition (Neiditch et al., 2002).
Our data provide the first evidence that the V(D)J enzyme system is capable of in vivo transposition of cleaved signal ends. However, the frequency at which transposition occurs in vivo remains unclear. Even though it is not possible to determine the frequency of these events in the general population or address the frequency at loci other than HPRT, we can determine the frequency of these events at the HPRT locus in the groups that were studied. Subject MFS6 was one of 53 newborns studied where the mean HPRT mutant frequency (Mf) for the group was ∼1 × 10–6. A total of 138 mutants were analyzed in this study. Therefore, the frequency of the transposition event in this group was 7 × 10–9. In comparison, the frequency of the two most common mutations found in newborns, V(D)J recombinase mediated HPRT exon 2–3 deletions and transitions (C→T or G→A) at CpG dinucleotide sequences are 3.9 × 10–7 and 6.5 × 10–8, respectively (Yoshioka et al., 2001). Subject Hou F was one of 23 adults studied with a total of 109 mutants. The estimated Mf for the group is 15 × 10–6. Therefore, the frequency of the transposition event in this adult population is estimated to be 1 × 10–7. These frequency calculations are only for events that would result in a phenotypic loss of HPRT gene function allowing for mutant selection with the T-cell assay. There may be transposed signal ends that result in innocuous somatic mutations that are not accounted for in our frequency calculations. This suggests that transposition occurs more frequently than our calculations indicate.
It is of interest that in addition to the RSS sites located at the Ig and TCR loci, it has been estimated that there may be as many as 10 million cryptic RSS sites dispersed throughout the human genome (Lewis et al., 1997). Cleavage at these cryptic sites could generate additional signal ends, outside of the Ig and TCR loci, that might be transposed by RAG1/2. V(D)J recombinase-mediated cleavage at cryptic RSS sites does generate signal ends that result in large deletions at the HPRT locus in healthy individuals and at the sil/scl loci in individuals with T-cell leukemia (Aplan et al., 1990; Bernard et al., 1990; Macintyre et al., 1992; Breit et al., 1993; Finette et al., 1996; Yoshioka et al., 2001). These data suggest there may be large numbers of signal ends available for transposition. It is clear that additional observations which include other loci are needed to determine the overall frequency of transposition events in humans. However, our observation of in vivo inter-chromosomal transposition of signal ends demonstrates that RAG1/2-mediated transposition can occur in humans. In addition, there is increasing evidence that V(D)J recombinase mediates chromosomal translocations observed in neoplastic lymphoid cells (Tycko and Sklar, 1990; Rabbitts, 1994; Davila et al., 2001). Our observation of in vivo V(D)J transposition of signal ends to another chromosome supports the models that have been proposed for chromosomal translocation as a consequence of RAG1/2 processing of transposition intermediates (Hiom et al., 1998; Gellert et al., 1999; Lee et al., 2002). These data provide the first in vivo evidence that an essential process in lymphocyte development can result in mutagenic inter-chromosomal transposition, further supporting V(D)J-mediated transposition in the development of lymphoid malignancies.
Materials and methods
Isolation of HPRT mutant T-cell clones
HPRT mutant clones were isolated using a T lymphocyte cloning assay utilizing 6-thioguanine as the selective agent as described previously (O’Neill et al., 1987). Mutant clones were expanded in vitro, pelleted and then frozen at –80°C for future analysis.
cDNA analysis
Cell pellets were used to generate cDNA by reverse transcription using a RNA PCR core kit (Perkin Elmer) utilizing M-MuLV reverse transcriptase with oligoDT(18) primers. RT and subsequent PCRs were performed using a Perkin Elmer 2400 thermocycler. The synthesized cDNA was amplified using primers flanking the coding region of HPRT. The first round reaction used sense primer 5′-CCTCTGCTCCGCCACCG-3′ and antisense primer 5′-CGCCCAAAGGGAACTGATACTCTATAGGC-3′, with a PCR profile of 94°C for 5 min followed by 30 cycles of 94°C (1 min), 65°C (1 min), 72°C (2 min) and a final extension at 72°C for 7 min. A second round nested PCR used the internal sense primer 5′-CCTGAGCAGTCAGCCCGCGC-3′ and antisense primer 5′-GCAAAAAGCTCTACTAAGCAGATGGCCACAG-3′ using a similar profile, with the exception of an annealing temperature of 55°C. Primers were purchased from Gibco-BRL. PCR products were purified using the QIAquick gel extraction kit (Qiagen), followed by cycle sequencing on an ABI 373 sequencer (Applied Biosystems).
Genomic analysis
Mutant F1: PCR amplification of the 3′ breakpoint utilized a sense primer TCRα J46, 5′-GGGACCGGGACTCGTTTAGCAG-3′; and antisense HPRT primer 5821, 5′-GGAATGGGCAGAAATTGCTAGTT-3′. This reaction was followed by a semi-nested PCR using sense primer TCRα J46; and antisense HPRT primer 3127, 5′-GTATGTCTGTTAGCCT CTCTGA-3′. PCR amplification of the 5′ breakpoint utilized a sense HPRT primer A106, 5′-CAGTTTCCCGGGTTCGG-3′; and antisense primers TCRα V21, 5′-AAGATAGGCAGAGGAGTAGGG-3′ and TCRα V22, 5′-GGAGGTGTGTATTGAAAAGGG-3′.
Mutant MFS6 M2: PCR amplification of the 3′ breakpoint utilized a sense primer TCRα J46 and antisense HPRT primer 5821. This reaction was followed by a semi-nested PCR using sense primer TCRα J46; and antisense HPRT primer 4001, 5′-GGCAGGCATCACACCCCAAAG-3′. PCR amplification of the 5′ breakpoint utilized sense HPRT primer A106; and antisense primers TCRα V34, 5′-GGTGGGTAAATAGCAAAGGG-3′, V26s2, 5′-GGGGCTAGATGAAGAAATG-3′, V33, 5′-GTGCCTTCTTCCTGTCTGTG-3′, V32, 5′-GGATCTCAATGAAACAACCTCAG3′, V31, 5′-GCTGCTAACAAATGCCTGGG-3′ and V30, 5′-GTCCTATTGTCAAGCTGTGGG-3′. This reaction was followed by a semi-nested reaction using HPRT sense primer 2089, 5′-TTGGGGTGCGATGGTGAGGTTCTC-3′; and the antisense primer TCRα V34. PCRs were performed using a Perkin Elmer 480 thermocycler with the Expand Long Template System (Roche Biosciences) 1× buffer system 2 (2.25 mM MgCl2, 500 µM each dNTP, 300 nM primer, 100–200 ng genomic DNA). When multiple V primers were used, primer concentrations were adjusted to 150 nM each with two V primers, or 100 nM each with six V primers. PCRs were carried out in a final volume of 25 µl. First round reactions were performed at 94°C for 5 min followed by the addition of 2 U of Expand Long Template enzyme mixture (Taq DNA and Pwo DNA polymerase) with 40 cycles of 94°C (20 s), annealing and elongation at 63°C (8 min), with a final elongation at 68°C (8 min). Semi-nested PCRs were performed at 94°C (20 s), annealing at 63°C (1 min), elongation at 68°C (2 min) for 30 cycles, with a final elongation at 68°C (5 min). PCR products were purified using QIAquick-spin columns (Qiagen) and sequenced using Taq DyeDeoxy terminator cycle sequencing (ABI). For sequencing we used one round of 25 cycles at 96°C (30 s), 50°C (15 s) and 60°C (4 min). The products were purified with Sephadex G-50 (Pharmacia) using micro-spin columns (Promega) and then electrophoresed and read on an ABI automated sequencer model 373A (Applied Biosystems). Nucleotides in the TCRα region are numbered based on TCRα V21–35 sequence submitted to the DDBJ/EMBL/GenBank database under accession No. AE000660; TCRα J sequence submitted to the DDBJ/EMBL/GenBank database under accession No. M94081; and HPRT sequence submitted to the DDBJ/EMBL/GenBank database under accession No. M26434. Primers were obtained from Operon.
Acknowledgments
Acknowledgements
We thank members of the University of Vermont Cancer Center DNA Analysis Facility for their technical support, and M.Gellert for valuable discussions during the preparation of this manuscript. Research funding was provided by NICHD, NCI and the Leukemia and Lymphoma Society.
References
- Agrawal A. and Schatz,D.G. (1997) RAG1 and RAG2 form a stable post cleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell, 89, 43–53. [DOI] [PubMed] [Google Scholar]
- Agrawal A., Eastman,Q.M. and Schatz,D.G. (1998) Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature, 394, 744–751. [DOI] [PubMed] [Google Scholar]
- Aplan P.D., Lombardi,D.P., Ginsberg,A.M., Cossman,J., Bertness,V.L. and Kirsch,I.R. (1990) Disruption of the SCL locus by ‘illegitimate’ V-(D)-J recombinase activity. Science, 250, 1426–1429. [DOI] [PubMed] [Google Scholar]
- Bernard O., Guglielmi,P., Jonveaux,P., Cherif,D., Gisselbrecht,S., Mauchauffe,M., Berger,R., Larsen,C.J. and Mathieu-Mahul,D. (1990) Two distinct mechanisms for the SCL gene activation in the t(1;14) translocation of T-cell leukemias. Genes Chromosome Cancer, 1, 194–208. [DOI] [PubMed] [Google Scholar]
- Breit T.M., Mol,E.J., Wolvers-Tettero,I.L., Ludwig,W.D., van Wering,E.R. and van Dongen,J.J. (1993) Site-specific deletions involving the tal-1 and sil genes are restricted to cells of the T-cell receptor alpha/beta lineage: T-cell receptor delta gene deletion mechanism affects multiple genes. J. Exp. Med., 177, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N.L. (1995) Unity in transposition reactions. Science, 270, 253–254. [DOI] [PubMed] [Google Scholar]
- Davila M., Foster,S., Kelsoe,G. and Yang,K. (2001) A role for secondary V(D)J recombination in oncogenic chromosomal translocations? Adv. Cancer Res., 81, 61–92. [DOI] [PubMed] [Google Scholar]
- Dreyfus D.H. (1992) Evidence suggesting an evolutionary relationship between transposable elements and immune system recombination sequences. Mol. Immunol., 29, 807–810. [DOI] [PubMed] [Google Scholar]
- Finette B.A., Poseno,T. and Albertini,R.J. (1996) V(D)J recombinase-mediated HPRT mutations in peripheral blood lymphocytes of normal children. Cancer Res., 56, 1405–1412. [PubMed] [Google Scholar]
- Foster T.J., Lundblad,V., Hanley-Way,S., Halling,S.M. and Kleckner,N. (1981) Three Tn10-associated excision events: relationship to transposition and role of direct and inverted repeats. Cell, 23, 215–227. [DOI] [PubMed] [Google Scholar]
- Fugmann S.D. (2001) RAG1 and RAG2 in V(D)J recombination and transposition. Immunol. Res., 23, 23–39. [DOI] [PubMed] [Google Scholar]
- Fugmann S.D., Lee,A.I., Shockett,P.E., Villey,I.J. and Schatz,D.G. (2000) The RAG proteins and V(D)J recombination: complexes, ends and transposition. Annu. Rev. Immunol., 18, 495–527. [DOI] [PubMed] [Google Scholar]
- Fuscoe J.C., Zimmerman,L.J., Lippert,M.J., Nicklas,J.A., O’Neill,J.P. and Albertini,R.J. (1991) V(D)J recombinase-like activity mediates hprt gene deletion in human fetal T-lymphocytes. Cancer Res., 51, 6001–6005. [PubMed] [Google Scholar]
- Gellert M., Hesse,J.E., Hiom,K., Melek,M., Modesti,M., Paull,T.T., Ramsden,D.A. and van Gent,D.C. (1999) V(D)J recombination: links to transposition and double-strand break repair. Cold Spring Harb. Symp. Quant. Biol., 64, 161–167. [DOI] [PubMed] [Google Scholar]
- Hiom K. and Gellert,M. (1998) Assembly of a 12/23 paired signal complex: a critical control point in V(D)J recombination. Mol. Cell, 1, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Hiom K., Melek,M. and Gellert,M. (1998) DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocation. Cell, 94, 463–470. [DOI] [PubMed] [Google Scholar]
- Hou S.-M. (1994) Novel types of mutation identified at the hprt locus of human T-lymphocytes. Mutat. Res., 308, 23–31. [DOI] [PubMed] [Google Scholar]
- Jackson S.P. (2002) Sensing and repairing DNA double-strand breaks. Carcinogenesis, 23, 687–696. [DOI] [PubMed] [Google Scholar]
- Jones J.M. and Gellert,M. (2001) Intermediates in V(D)J recombination: a stable RAG1/2 complex sequesters cleaved RSS ends. Proc. Natl Acad. Sci. USA, 98, 12926–12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A.K., Guhathakurta,A., Kleckner,N. and Haniford,D.B. (1998) Tn10 transposition via a DNA hairpin intermediate. Cell, 95, 125–134. [DOI] [PubMed] [Google Scholar]
- Lee G.S., Neiditch,M.B., Sinden,R.R. and Roth,D.B. (2002) Targeted transposition by the V(D)J recombinase. Mol. Cell. Biol., 22, 2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S.M. (1994) The mechanism of V(D)J joining: lessons from molecular, immunological and comparative analyses. Adv. Immunol., 56, 27–150. [DOI] [PubMed] [Google Scholar]
- Lewis S.M. and Wu,G.E. (2000) The old and the restless. J. Exp. Med., 191, 1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S.M., Agard,E., Suh,S. and Czyzyk,L. (1997) Cryptic signals and the fidelity of V(D)J joining. Mol. Cell. Biol., 17, 3125–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak F. and Schatz,D.G. (1996) T-cell receptor alpha locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol. Cell. Biol., 16, 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre E.A., Smit,L., Ritz,J., Kirsch,I.R. and Strominger,J.L. (1992) Disruption of the SCL locus in T-lymphoid malignancies correlates with commitment to the T-cell receptor alpha beta lineage. Blood, 80, 1511–1520. [PubMed] [Google Scholar]
- McBlane J.F., van Gent,D.C., Ramsden,D.A., Romeo,C., Cuomo,C.A., Gellert,M. and Oettinger,M.A. (1995) Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell, 83, 387–395. [DOI] [PubMed] [Google Scholar]
- McMahan C.J. and Fink,P.J. (1998) RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity, 9, 637–647. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K. (1992) Transpositional recombination: mechanistic insights from studies of Mu and other elements. Annu. Rev. Biochem., 61, 1011–1051. [DOI] [PubMed] [Google Scholar]
- Morrical S.W., Wong,M.L. and Alberts,B.M. (1991) Amplification of snap-back DNA synthesis reactions by the uvsX recombinase of bacteriophage T4. J. Biol. Chem., 266, 14031–14038. [PubMed] [Google Scholar]
- Neiditch M.B., Lee,G.S., Huye,L.E., Brandt,V.L. and Roth D.B. (2002) The V(D)J recombinase efficiently cleaves and transposes signal joints. Mol. Cell, 4, 871–878. [DOI] [PubMed] [Google Scholar]
- Oettinger M.A., Schatz,D.G., Gorka,C. and Baltimore,D. (1990) RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science, 248, 1517–1523. [DOI] [PubMed] [Google Scholar]
- O’Neill J.P., McGinniss,M.J., Berman,J.K., Sullivan,L.M., Nicklas,J.A. and Albertini,R.J. (1987) Refinement of a T-lymphocyte cloning assay to quantify the in vivo thioguanine-resistant mutant frequency in humans. Mutagenesis, 2, 87–94. [DOI] [PubMed] [Google Scholar]
- Rabbitts T.H. (1994) Chromosomal translocations in human cancer. Nature, 372, 143–149. [DOI] [PubMed] [Google Scholar]
- Sakano H., Huppi,K., Heinrich,G. and Tonegawa,S. (1979) Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature, 280, 288–294. [DOI] [PubMed] [Google Scholar]
- Sawchuk D.J., Weis-Garcia,F., Malik,S., Besmer,E., Bustin,M., Nussenzweig,M.C. and Cortes,P. (1997) V(D)J recombination: Modulation of RAG1 and RAG2 cleavage activity on 12/23 substrate by whole cell extract and DNA-bending proteins. J. Exp. Med., 185, 2025–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.B. (1995) New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity, 3, 531–539. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. (1983) Somatic generation of antibody diversity. Nature, 302, 575–581. [DOI] [PubMed] [Google Scholar]
- Tycko B. and Sklar,J. (1990) Chromosomal translocations in lymphoid neoplasia: a reappraisal of the recombinase model. Cancer Cells, 2, 1–8. [PubMed] [Google Scholar]
- van Gent D.C., Mizuuchi,K. and Gellert,M. (1996) Similarities between initiation of V(D)J recombination and retroviral integration. Science, 271, 1592–1594. [DOI] [PubMed] [Google Scholar]
- van Gent D.C., Hiom,K., Paull,T.T. and Gellert,M. (1997) Stimulation of V(D)J cleavage by high mobility group proteins. EMBO J., 16, 2665–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M., O’Neill,J.P., Vacek,P.M. and Finette,B.A. (2001) Gestational age and gender specific in utero V(D)J recombinase mediated deletions. Cancer Res., 61, 3432–3438. [PubMed] [Google Scholar]