Abstract
Multiple lines of evidence indicate that thrombopoietin (TPO) contributes to the development of hematopoietic stem cells (HSC), supporting their survival and proliferation in vitro. To determine whether TPO supports the impressive expansion of HSC observed following transplantation, we transplanted normal marrow cells into lethally irradiated Tpo–/– and Tpo+/+ mice and quantified HSC self-renewal and expansion and hematopoietic progenitor cell homing. Although essentially identical numbers of marrow-associated colony forming unit–culture (a surrogate measure of stem cell homing) were observed in each type of recipient 24 hours following transplantation, we found that a minimum of fourfold greater numbers of marrow cells were required to radioprotect Tpo-null mice than to radioprotect controls. To assess whether long-term repopulating (LTR) HSCs self-renew and expand in Tpo–/– recipients or controls, we performed limiting-dilution secondary transplants using donor cells from the Tpo–/– or Tpo+/+ recipients 5–7.5 weeks following primary transplantation. We found that LTR HSCs expand to levels 10–20 times greater within this time period in normal recipients than in Tpo-null mice and that physiologically relevant amounts of TPO administered to the Tpo–/– recipients could substantially correct this defect. Our results establish that TPO greatly promotes the self-renewal and expansion of HSCs in vivo following marrow transplantation.
Introduction
Thrombopoietin (TPO) was first cloned and its recombinant protein first produced and characterized in 1994 (1–4). Initial studies with the recombinant protein were directed toward demonstrating that the cytokine was the primary regulator of megakaryopoiesis and platelet production. In this regard it was rapidly appreciated that the cloned gene fulfilled all of the criteria historically set out for it (5, 6). For example, TPO affects nearly every stage of megakaryocyte (MK) development from the committed progenitor to the mature platelet. The hormone is the most potent cytokine to stimulate the development of MK colonies from marrow progenitors in semisolid cultures (7), and it induces the expression of glycoprotein IIb/IIIa, the platelet fibrinogen receptor, and the glycoprotein Ib/IX complex, the platelet vWF receptor, in immature MKs (5). In the presence of the cytokine, MKs grow in size, develop several types of specific granules, and form demarcation membranes, a precursor of platelet formation. And although TPO is not essential for the last stages of platelet formation (8), it does prime platelets to respond to subthreshold levels of classic agonists such as ADP, collagen, or thrombin (9, 10).
Based on results using partially purified preparations of the hormone, previous reports concluded that TPO is a specific growth factor for the MK and platelet lineage (11, 12). However, several lines of evidence using recombinant protein now indicate that the hormone exerts a profound influence on hematopoietic stem cells (HSCs). Using highly purified populations of marrow cells, two groups reported that TPO enhances the survival of murine HSCs in vitro and, in the presence of additional cytokines, could augment their proliferation (13, 14); this conclusion was quickly expanded to human cells (15). One of the first indications that these effects extended to in vivo hematopoiesis came with the discovery that TPO accelerates the recovery of all hematopoietic lineages following myelosuppressive therapies (16, 17). Moreover, mice genetically deficient in the TPO receptor, c-Mpl, display reduced levels of all types of hematopoietic progenitors (18), again suggesting the panhematopoietic effect of the cytokine. The profound effect of TPO on HSCs was then established when it was shown that all of the long-term repopulating (LTR) HSCs display c-Mpl, and that c-mpl–null mice display substantial reductions in the numbers of LTR HSCs (19, 20). This physiology was very recently extended to humans when it was discovered that many children with congenital amegakaryocytic thrombocytopenia, the majority of whom develop aplastic anemia within a few years of birth, display many different missense and nonsense mutations in the c-Mpl receptor (21).
Although experimental hematologists have tested several approaches to expand adult HSCs in vitro, there is little reproducible evidence that such strategies have been successful. In contrast, if a limited number of stem cells are transplanted into a lethally irradiated animal, within a few months the number of stem cells in the marrow and spleen of the recipient is in great excess of input numbers. For example, in one report of serial transplantation experiments, about ten times more LTR HSCs were found with each successive transfer of cells (22). Given the accumulating data indicating a role for TPO in stem cell biology, in the present work we sought to test whether the hormone is important for the self-renewal and expansion of HSCs that follow marrow transplantation. We report that, for both short-term radioprotection and long-term repopulation, TPO is vital, enhancing survival following lethal irradiation at least fivefold compared with that seen in the absence of the hormone, and accelerating the renewal and repopulation of the marrow HSC pool 13- to 20-fold in the first 2 months following transplantation.
Methods
Mice and animal care.
All mice used in this study were C57BL/6. The initial stem cell donors were CD45.1 and were obtained from Taconic (Germantown, New York, USA); all others were of the CD45.2 genotype (The Jackson Laboratory, Bar Harbor, Maine, USA). TPO-null mice bred onto the C57BL/6 background were kindly provided by Fred de Sauvage (Genentech Inc., South San Francisco, California, USA) and have been previously described (23). Mice were housed in a specific pathogen–free environment, and the Animal Care Committee of the University of Washington approved all protocols involving mice.
Reagents.
Recombinant murine TPO was kindly provided by Akihiro Shimosaka (Kirin Pharmaceuticals Inc., Gumma, Japan). It was administered to some recipient animals by subcutaneous injection once before transplantation and then thrice weekly for a month at a dose of 80 ng, a dose chosen based on preliminary experiments in which the capacity to restore a normal platelet count in Tpo–/– mice was evaluated.
Marrow cell transplantation.
Marrow cell transplantation was performed using standard protocols; mice were subjected to 1150 cGy whole-body irradiation from a 137Cs source, and then infused with donor cells by tail vein injection. Mice were fed standard mouse chow, and antibiotics were administered in the drinking water for 1 week prior to and 3 weeks following transplantation. For secondary transplants, 5–7.5 weeks following primary marrow cell infusion, mice were sacrificed; then single-cell suspensions of femoral, tibial, and pelvic marrow were prepared and infused into normal CD45.2+ recipients that were treated with antibiotics for 1 week prior to and 3 weeks following transplantation. Blood was sampled for CD45 analysis at 12 and 22 weeks; a minimum of four mice at each time point were analyzed, and the results are reported as mean percentage CD45.2+ cells ± SEM.
Marrow and spleen colony-forming cell homing experiments.
Donor mice were sacrificed by cervical dislocation and marrow cells prepared. Three groups of recipient mice were irradiated with 1150 cGy and infused with marrow cells containing one of two doses of colony forming unit–culture (CFU-C). One day later the mice were sacrificed and single-cell suspensions of marrow and spleen were prepared and cultured in triplicate for colony forming unit–granulocyte macrophage (CFU-GM), CFU-Mix, and burst forming unit–erythroid (BFU-E) by standard methods (16). The total number of CFU-C recovered after 24 hours was expressed as a percentage of infused CFU-C. In a second test of the capacity of marrow cells to home, we labeled cells for 10 minutes at 37°C in 0.5 μM 5,6 carboxyfluorescein diacetate succinimidyl ester (CFSE), washed and infused them into wild-type and Tpo–/– mice, and then evaluated marrow of the recipients for CFSE+ cells 24 hours later.
Flow cytometry analysis.
To determine the origin of the circulating hematopoietic cells following transplantation, blood was obtained from transplanted mice 3 and 5 months following the procedure and analyzed by flow cytometry using a phycoerythrin-labeled mAb to murine CD45.1 (Pharmingen, La Jolla, California, USA).
Results
TPO enhances the capacity of transplanted marrow cells to radioprotect.
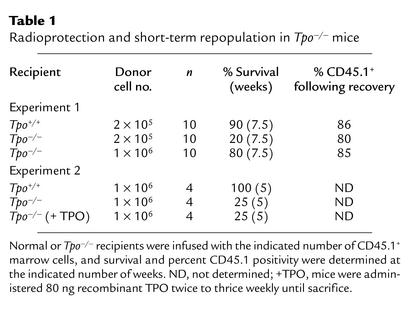
In order to determine whether TPO was important for the ability of short-term repopulating stem cells to radioprotect recipient mice, we transplanted 2 × 105 normal CD45.1 marrow cells into normal CD45.2 recipients and compared post-transplant survival to that of CD45.2 Tpo–/– mice transplanted with either 2 × 105 or 1 × 106 normal CD45.1 marrow cells. As shown in Table 1, we found that nine of ten normal recipients of 2 × 105 normal marrow cells survived; a mean of 86% of the hematopoietic cells were CD45.1+ in the recipients. In contrast, only two of ten Tpo–/– recipients of the same number of normal marrow cells survived, with 80% of those hematopoietic cells CD45.1+. The infusion of 1 × 106 normal marrow cells was required to rescue eight of ten transplanted Tpo–/– mice. In a second experiment, 1 × 106 normal marrow cells were injected into four Tpo+/+ recipients, four Tpo–/– recipients that were given saline thrice weekly, and four Tpo–/– recipients given thrice-weekly injections of 80 ng recombinant murine TPO. This dose and dosing schedule of TPO was determined to be the most physiologically relevant, as it returned the platelet count of Tpo–/– mice to normal levels and maintained it at those levels. All four of the Tpo+/+ recipients survived more than 5 weeks; in contrast, only one of four of both Tpo–/– recipient groups survived more than 5 weeks. Thus, since we saw little or no radiation-induced death in the control groups, the four- to fivefold enhanced radioprotection afforded by normal marrow cells in lethally irradiated normal mice compared with Tpo–/– mice is a minimal estimation.
Table 1.
Radioprotection and short-term repopulation in Tpo–/– mice
TPO does not affect the homing of hematopoietic cells to marrow or spleen.
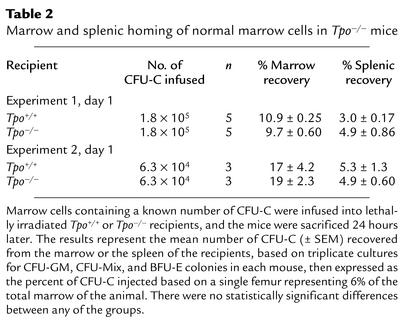
The previous results assessing radioprotection and short-term repopulation could have been due either to reduced stem cell homing to marrow in Tpo–/– mice or to defective expansion of the stem cells lodged in the Tpo–/– marrow. To distinguish between these possibilities, in two sets of experiments we transplanted normal marrow containing either 1.8 × 105 or 6.3 × 104 CFU-C into groups of three to five lethally irradiated Tpo–/– or normal mice and quantitated CFU-C recovered in the marrow 24 hours later. Previous studies have indicated that CFU-C homing is an accurate surrogate for stem cell homing (24–26). As shown in Table 2, we found that in both sets of experiments cells homed as efficiently in Tpo–/– recipients as in wild-type mice, the values in Tpo–/– mice being 89% and 112% of normal for homing to the marrow, and 163% and 94% of normal for lodgment in the spleen, respectively. In a second type of analysis, we labeled normal donor marrow cells with the membrane-soluble dye CFSE and assessed the percentage of marrow cells that were CFSE+ 24 hours following their infusion into lethally irradiated recipients. We found a mean of 1.4% CFSE+ marrow cells in Tpo+/+ recipients and 1.3% CFSE+ in the Tpo–/– recipients. Thus, the difference in radioprotection and short-term repopulation following transplantation of normal marrow cells into Tpo+/+ and Tpo–/– mice was not due to abnormal hematopoietic cell homing in the latter group of mice.
Table 2.
Marrow and splenic homing of normal marrow cells in Tpo–/– mice
TPO enhances the self-renewal and expansion of LTR HSCs.
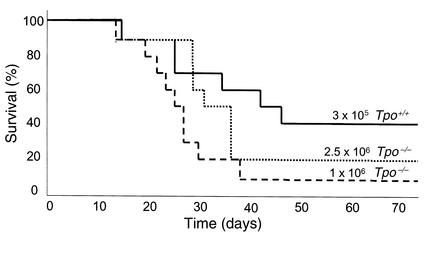
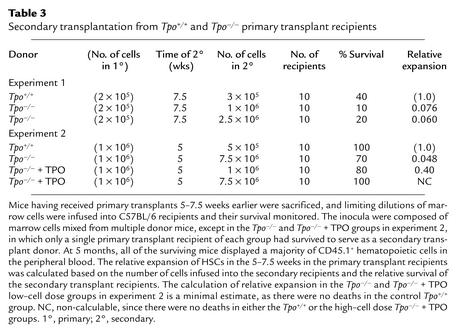
Previous studies demonstrated that upon infusion into lethally irradiated mice, HSCs expand within the first few months following the procedure. To determine whether TPO affects this aspect of stem cell physiology and to quantitate the effect, limiting-dilution secondary transplants were performed using marrow cells from the Tpo+/+ or Tpo–/– primary transplant recipients from the two experiments reported upon in Table 1, 5 or 7.5 weeks following primary transplantation. In the first experiment, the Tpo+/+ and Tpo–/– mice transplanted with 2 × 105 normal marrow cells 7.5 weeks earlier were sacrificed, and 3 × 105 marrow cells from the normal primary transplant recipients and either 1 × 106 or 2.5 × 106 marrow cells from the Tpo–/– primary transplant recipients were infused into normal, lethally irradiated C57BL/6 secondary recipients. In the second experiment, the primary transplant recipients were sacrificed at 5 weeks, and limiting-dilution secondary transplants were performed to calculate HSC expansion during the primary transplant. In the second experiment we also studied whether the administration of TPO during the primary transplant period could correct for the genetic deficiency. The survival curves from the first set of secondary transplants are shown in Figure 1, and the results from both experiments are tabulated and the relative stem cell expansion calculated in Table 3.
Figure 1.
Survival of mice transplanted with marrow cells from previously transplanted Tpo+/+ or Tpo–/– mice. The curves shown are survival as a function of time for: normal recipient mice transplanted with 3 × 105 marrow cells from normal recipient mice transplanted 7.5 weeks earlier with 2 × 105 normal marrow cells (solid line); 1 × 106 marrow cells from Tpo–/– mice transplanted 7.5 weeks earlier with 2 × 105 normal marrow cells (dashed line); and 2.5 × 106 marrow cells from Tpo–/– mice transplanted 7.5 weeks earlier with 2 × 105 normal marrow cells (dotted line).
Table 3.
Secondary transplantation from Tpo+/+ and Tpo–/– primary transplant recipients
If one assumes that the successful re-engraftment of, for example, four of ten mice represents the presence of twice as many HSCs in the transplant inoculum as if two of ten mice were engrafted, then one can calculate the relative numbers of stem cells present in the primary transplant recipients. As illustrated in Table 3, the number of HSCs present in the marrow of mice transplanted 5 weeks earlier with normal HSCs was approximately 20-fold greater when the transplantation inoculum was derived from transplanted normal mice than when the transplanted cells had come from Tpo–/– mice transplanted with normal HSCs. At 7.5 weeks, there were 13- to 16-fold more HSCs in the normal primary recipients than in their Tpo–/– counterparts. Flow cytometric analysis for CD45.1 indicated that 81% ± 2% (mean ± SEM) of peripheral blood cells were of donor type 12 weeks after transplantation, and 76% ± 5.5% were of donor origin 22 weeks after secondary transplantation, demonstrating that hematological recovery was due to the transplanted cells, not to endogenous (CD45.2+) recovery. As seen in the second experiment, the administration of a small dose of recombinant TPO to the Tpo–/– recipients substantially corrected (i.e., to 40% of normal) the defect in HSC expansion seen in the null mice.
Discussion
The purpose of the present study was to determine whether the effects of TPO on steady-state numbers of HSCs also extend to the self-renewal and early expansion that these cells undergo following marrow transplantation. If so, then the use of the recombinant protein following marrow or stem cell transplantation, especially in recipients in which limiting numbers of cells are available, such as in patients who are difficult to mobilize, or from small-volume cord blood transplant products, might prove of therapeutic value. Although we didn’t test that hypothesis directly in this study, our results clearly indicate that TPO plays an important role in the initial stem cell renewal and expansion seen in mice transplanted with limiting numbers of HSCs.
In the initial stage of each experiment, the level of radioprotection provided by the transplantation of the normal HSCs in Tpo+/+ and Tpo–/– recipient mice was compared; in both experiments, survival in the wild-type recipient was improved four to five times over survival in null mice. It is possible that this is an underestimate of the Tpo effect, as the survival in the control arm was 90% and 100% in the two experiments, indicating that there might have been even more short-term repopulating activity in the 2 × 105 or 1 × 106 cell inoculum measurable in the wild-type recipients. Additional experiments using lower cell doses could sort this issue out.
The improved survival afforded by the TPO-replete host could have been due to an enhanced capacity of HSCs to home to and lodge in the marrow microenvironment rather than to any favorable effect of the hormone on the initial survival or proliferation of the short-term repopulating cells that provide radioprotection. Although stromal cells are not thought to be targets of TPO, it is clear that endothelial cells express the TPO receptor c-Mpl (27). Along with potential TPO effects on stem cells while they traffic in the circulation, these considerations raise the theoretical possibility that HSC homing might be affected in the Tpo-null mouse. Thus, to be certain that the effects of TPO were on the self-renewal and expansion of HSCs, we injected marrow cells containing two different numbers of CFU-C into normal and Tpo-null mice and determined the number of such progenitors that lodged in the primary hematopoietic organs of mice 24 hours later. We chose this time point to avoid confusing the effects of CFU-C lodging and the proliferation of more primitive, CFU-C–generating progenitors that had homed to the marrow or spleen. We found virtually identical numbers of CFU-C in the marrow and spleens of the recipients, strongly suggesting that differential homing did not account for our radioprotection results.
We next studied whether TPO affected expansion of LTR HSCs by performing limiting-dilution secondary transplant experiments, using our primary Tpo+/+ and Tpo–/– recipients as donors. We found a profound reduction in the capacity of HSCs to expand in Tpo–/– recipients, from a minimum of 13-fold to a maximum of 21-fold less expansion than in the wild-type mice. Although in one experiment 100% survival in the Tpo+/+ control group made it impossible to gauge whether the difference in relative HSC expansion might have been even greater if we had used a smaller inoculum of cells, we doubt that this is the case, as the two experiments yielded quite similar levels of LTR HSC expansion 5 and 7.5 weeks following primary transplantation. Studies performed by Iscove and Nawa demonstrated that serial transplantation experiments of the type performed in this report lead to about tenfold increases in LTR HSC numbers over input levels (22), suggesting that TPO might account for the maximal level of stem cell expansion possible under these conditions. Moreover, experiments performed by Eaves’s group indicate that genetic alteration of the c-Kit/Steel Factor system imposes a similar level of HSC deficiency (28). In that study, transplantation of W41/W41 stem cells into normal recipients reduced HSC expansion 17-fold compared with wild-type donor cells. Although the W41 allele is a hypomorph and not a complete null, taken together the similar quantitative analyses suggest that TPO and Steel Factor might act synergistically on expansion of HSCs in vivo, as they do in vitro (7, 13, 14).
In the second set of our experiments we also tested whether exogenous TPO could correct the HSC expansion defect seen in Tpo-null mice. We first tested for the amount of TPO needed to provide a “replacement dose” for Tpo-null mice, as the use of pharmacological concentrations of a hormone might exert effects not seen with the physiological level. By administering different doses of recombinant TPO to Tpo-null mice, we found in preliminary experiments that 80 ng administered thrice weekly led to normalization of the platelet count after about 1 month. Using this dose of TPO in Tpo–/– recipients, we found that HSCs expand about 40% as well as in wild-type recipients. However, like the quantitative considerations raised above, this calculation is the maximal correction that could have occurred in this experiment, as all the control animals survived and repopulated. It is possible that if we had used lower numbers of marrow cells for the secondary transplants we might have seen a less impressive normalization of HSC expansion. This argument is of more than academic interest: if exogenous TPO administered to Tpo-null mice does not fully correct the stem cell defect of these animals, it is possible that the local effect of stromal cell–derived hormone is greater than that coming from the circulation. In this regard, debate remains in the field as to whether marrow stromal cell production of TPO is of importance for stress hematopoiesis.
Based on the effect of TPO on HSC self-renewal and expansion, one might predict that the administration of exogenous TPO to transplant recipients would enhance engraftment kinetics. In fact, this has been tested in both preclinical and clinical settings, and the results have been disappointing. For example, although the administration of TPO to murine stem cell transplant recipients was reported to accelerate hematological recovery in one study, the effect was quite modest (29) and did not occur in another model of marrow transplantation if TPO was given only to the recipient (30). Similarly disappointing results were reported following peripheral blood HSC transplantation into patients (31), although a modest effect was seen following the use of bone marrow stem cells (32). Moreover, we found that the administration of TPO in the short term, during the primary transplant experiments illustrated in Table 1, failed to radioprotect Tpo–/– mice given a standard number of marrow cells. These results do not necessarily cast doubt on the conclusions of the present study, as even the levels of TPO that are normally present in mice were sufficient to at least partially correct the LTR HSC expansion defect in Tpo–/– mice when administered exogenously in our experiments. Moreover, given the higher-than-normal levels of TPO that occur in animals and patients following myelosuppressive therapy, such as the conditioning therapy for transplantation, it is very likely that endogenous TPO is sufficient to provide for HSC self-renewal and expansion following transplantation. In our studies we failed to find an effect of exogenous TPO on short-term radioprotection, in contrast to the results with LTR HSCs. It is possible that the effect of TPO on stem cell expansion requires more time than is required to radioprotect a mouse, or that a minor effect could have been revealed if additional dose-finding experiments had been performed. Thus, whether exogenously administered TPO is useful for clinical transplantation will require additional study.
Finally, although we use the term “expansion” throughout this report to indicate that the numbers of HSCs found 5 or 7.5 weeks following transplantation are greater than the numbers of input cells, it is possible that TPO affects both HSC survival and proliferation, or that it only acts on the former. In this regard, using p53-null mice, Pestina and colleagues recently reported that TPO accelerates blood cell recovery following the administration of otherwise lethal chemoradiotherapy by preventing programmed cell death of hematopoietic cells (33). As recovery from such toxic insults is thought to be dependent on primitive marrow hematopoietic cells, it is possible that the effect of TPO on HSC expansion might be dependent on the same effect. The relative importance of these two fundamental processes on TPO-induced HSC expansion following transplantation deserves further study.
Acknowledgments
The authors wish to acknowledge the support of the NIH (grants R01 CA31615, R01 DK44855, and R01 HL58734).
Footnotes
See the related Commentary beginning on page 303.
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: thrombopoietin (TPO); megakaryocyte (MK); hematopoietic stem cell (HSC); long-term repopulating (LTR); colony forming unit–culture (CFU-C); colony forming unit–granulocyte macrophage (CFU-GM); burst forming unit–erythroid (BFU-E).
References
- 1.de Sauvage FJ, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 2.Lok S, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 3.Bartley TD, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77:1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 4.Sohma Y, et al. Molecular cloning and chromosomal localization of the human thrombopoietin gene. FEBS Lett. 1994;353:57–61. doi: 10.1016/0014-5793(94)01008-0. [DOI] [PubMed] [Google Scholar]
- 5.Kaushansky K, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 6.Kaushansky K. Thrombopoietin: the primary regulator of platelet production. Blood. 1995;86:419–431. [PubMed] [Google Scholar]
- 7.Broudy VC, Lin NL, Kaushansky K. Thrombopoietin (c-mplligand) acts synergistically with erythropoietin, stem cell factor, and IL-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85:1719–1726. [PubMed] [Google Scholar]
- 8.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85:402–413. [PubMed] [Google Scholar]
- 9.Chen J, Herceg-Harjacek L, Groopman JE, Grabarek J. Regulation of platelet activation in vitro by the c-Mpl ligand, thrombopoietin. Blood. 1995;86:4054–4062. [PubMed] [Google Scholar]
- 10.Kojima H, et al. Modulation of platelet activation in vitro by thrombopoietin. Thromb Haemost. 1995;74:1541–1545. [PubMed] [Google Scholar]
- 11.Hill RJ, Levin J. Regulators of thrombopoiesis: their biochemistry and physiology. Blood Cells. 1989;15:141–166. [PubMed] [Google Scholar]
- 12.McDonald TP. Thrombopoietin: its biology, purification, and characterization. Exp Hematol. 1988;16:201–205. [PubMed] [Google Scholar]
- 13.Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early-acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87:4544–4551. [PubMed] [Google Scholar]
- 14.Sitnicka E, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- 15.Kobayashi M, Laver JH, Kato T, Miyazaki H, Ogawa M. Thrombopoietin supports proliferation of human primitive hematopoietic cells in synergy with steel factor and/or interleukin-3. Blood. 1996;88:429–436. [PubMed] [Google Scholar]
- 16.Kaushansky K, et al. Thrombopoietin expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy. J Clin Invest. 1995;96:1683–1687. doi: 10.1172/JCI118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neelis KJ, et al. Prevention of thrombocytopenia by thrombopoietin in myelosuppressed rhesus monkeys accompanied by prominent erythropoietic stimulation and iron depletion. Blood. 1997;90:58–63. [PubMed] [Google Scholar]
- 18.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietin receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 19.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solar GP, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92:4–10. [PubMed] [Google Scholar]
- 21.Ballmaier M, et al. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97:139–146. doi: 10.1182/blood.v97.1.139. [DOI] [PubMed] [Google Scholar]
- 22.Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol. 1997;7:805–808. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 23.Moore MW, deSauvage FJ. Low levels of erythroid and myeloid progenitors in thrombopoietin- and c-mpl-deficient mice. Blood. 1996;88:803–808. [PubMed] [Google Scholar]
- 24.Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta Haematol. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- 25.Cui J, et al. Bone marrow cell trafficking following intravenous administration. Br J Haematol. 1999;107:895–902. doi: 10.1046/j.1365-2141.1999.01779.x. [DOI] [PubMed] [Google Scholar]
- 26.Oostendorp RA, Ghaffari S, Eaves CJ. Kinetics of in vivo homing and recruitment into cycle of hematopoietic cells are organ-specific but CD44-independent. Bone Marrow Transplant. 2000;26:559–566. doi: 10.1038/sj.bmt.1702536. [DOI] [PubMed] [Google Scholar]
- 27.Vigon I, et al. Molecular cloning and characterization of MPL, the human homolog of the v-mploncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci USA. 1992;89:5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller CL, et al. Studies of W mutant mice provide evidence for alternate mechanisms capable of activating hematopoietic stem cells. Exp Hematol. 1996;24:185–194. [PubMed] [Google Scholar]
- 29.Molineux G, Hartley CA, McElroy P, McCrea C, McNiece IK. Megakaryocyte growth and development factor stimulates enhanced platelet recovery in mice after bone marrow transplantation. Blood. 1996;88:1509–1514. [PubMed] [Google Scholar]
- 30.Fibbe WE, et al. Accelerated reconstitution of platelets and erythrocytes following syngeneic transplantation of bone marrow cells derived from thrombopoietin pretreated donor mice. Blood. 1995;86:3308–3313. [PubMed] [Google Scholar]
- 31.Bolwell B, et al. Phase 1 study of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) in breast cancer patients after autologous peripheral blood progenitor cell transplantation (PBPC) Bone Marrow Transplant. 2000;26:141–145. doi: 10.1038/sj.bmt.1702465. [DOI] [PubMed] [Google Scholar]
- 32.Beveridge R, et al. Randomized, double-blind, placebo-controlled trial of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) in breast cancer patients (Pts) following autologous bone marrow transplantation (ABMT) Blood. 1997;90(Suppl. 1):580a . (Abstr.) [Google Scholar]
- 33.Pestina TI, Cleveland JL, Yang C, Zambetti GP, Jackson CW. Mpl ligand prevents lethal myelosuppression by inhibiting p53-dependent apoptosis. Blood. 2001;98:2084–2090. doi: 10.1182/blood.v98.7.2084. [DOI] [PubMed] [Google Scholar]