Abstract
α-synuclein is a key molecule in the pathogenesis of synucleinopathy including Parkinson's disease and multiple system atrophy. In this mini-review, we mainly focus on recent data obtained from cellular models of synucleinopathy and discuss the possible mechanisms of neurodegeneration. Recent progress suggests that the aggregate formation of α-synuclein is cytoprotective and that its precursor oligomer (protofibril) may be cytotoxic. The catechol-derived quinones are the candidate molecules that facilitate the oligomer formation of α-synuclein. Furthermore, the cellular membranes are shown to be the primary targets injured by mutant α-synucleins, and the mitochondrial dysfunction seems to be an initial step in the neuronal death.
INTRODUCTION
α-synuclein is a 140 amino acid brain protein, mainly localized in presynaptic terminals [1, 2]. Although the detailed physiological functions of α-synuclein are still elusive, recent studies suggest that it plays a key role in synaptic functions cooperated with cysteine-string protein-α (CSP α), which contains a typical domain for HSP40-type molecular cochaperones [3]. In the subgroup of neurodegenerative disorders termed “synucleinopathies,” α-synuclein is known to polymerize into fibrils and to accumulate in pathologic hallmark inclusions, such as lewy body (LB), lewy neuritis (LN), and glial cytoplasmic inclusions (GCIs). The LB and LN are characteristic of Parkinson's disease (PD), and point mutations or gene multiplications of α-synuclein are responsible for familial PD [4–6]. Moreover, transgenic flies overexpressing mutated human α-synuclein showed progressive locomotor disability with dopaminergic neuronal cell death with intracytoplasmic inclusions [7]. These findings suggest that abnormal α-synuclein metabolism plays a key role in neurodegenerative processes in PD and other synucleinopathies, but the precise underlying mechanisms still remain unknown [8]. To elucidate the possible roles of α-synuclein in neurodegeneration, we have developed cells that overexpress wild-type or mutant α-synucleins in dopaminergic or inducible catechol-quinone producing cell lines [9, 10].
Aggregate formation of α-synuclein and cell death
The inclusions in synucleinopathies were proved to be composed of β-sheet rich fibrils formed by nitrated species of α-synuclein [11]. Several lines of evidence suggested that reactive oxygen species (ROS) play a key role in the conformational change of α-synuclein and the following aggregate formation [12–15]. We developed human dopaminergic SH-SY5Y cells overexpressing wild-type or mutant α-synucleins, and established experimental models of intracellular aggregate formation following the exposure to various ROS [9]. The aggregates thus formed were immunopositive for ubiquitin, nitrotyrosine, and dityrosine, and positive for thioflavin S staining, which was in good agreement with the pathological features of inclusion bodies in synucleinopathies [9]. The γ-tubulin and molecular chaperones coexisted as well, suggesting that the aggregate formation was associated with the intracellular transport system for protein turnover responses against the toxic effects of misfolded proteins. Such mechanisms are called “aggresome” and are suggested to represent one of the cytoprotective responses [16–18]. Interestingly, the recent study on huntingtin showed that inclusion body formation reduced the risk of neuronal death [18]. However, it is still controversial whether the aggregate formation of α-synuclein has cytotoxicity in the neuronal cell or sequesters toxic species.
We established a cellular model in which intracellular α-synuclein aggregations were efficiently formed in response to various types of ROS exposure [9, 19]. Under these conditions, a significant number of cells showed caspase 3 activation [19]. To explore possible relationships between the aggregate formation and apoptosis, first we investigated whether α-synuclein aggregates colocalized with activated caspase-3 using a double immunostaining method. Following the combined exposure of the cells to a no donor and rotenone, α-synuclein aggregates were efficiently formed in the cytoplasm as previously reported [9]. Surprisingly, immunocytochemical analyses revealed that the aggregate positive cells did not show any caspase 3 activations and, conversely, that caspase 3 activated cells did not contain any α-synuclein aggregates [19]. Iron was able to induce α-synuclein aggregates more effectively than any other ROS inducers and no donors, suggesting the iron plays a key role in the aggregate formation [9]. When using both ROS and no inducers, the addition of ferric iron triggered further aggregate formation, but cells positive for activated caspase 3 were not coincident with aggregate positive cells. In quantification experiments, it was revealed that caspase 3-positive cells were decreased by the addition of ferric iron. On the other hand, by chelating ferric iron, the aggregate formation was decreased with concomitant increases of caspase 3 activation. These data suggest that the ferric iron plays a key role in the α-synuclein aggregation [19]. Furthermore, these data also imply that the aggregate formation may be cytoprotective against various cellular insults including oxidative stress [19, 20].
Possible interaction between α-synuclein and dopamine-quinone derivatives
Since α-synuclein is ubiquitously expressed at high levels in all brain regions [21], the mechanisms responsible for the preferential and selective neurodegeneration of dopaminergic neurons in the substantia nigra remain to be determined. Previous studies suggested that the specific vulnerability of dopaminergic neurons may be linked to the cytotoxic oxidative potential of dopamine [22]. Highly reactive oxygen species (ROS) are generated not only in dopamine oxidation but also during the decay of catechol-derived orthoquinones which covalently incorporate into a variety of molecules including proteins and nucleic acids [23]. On the other hand, previous reports demonstrated that α-synuclein might regulate dopamine metabolism by direct interaction with the tyrosine hydroxylase [24], the dopamine transporter [25] and vesicular monoamine transporter (VMAT2), key proteins in the regulation of the dopamine content within nerve terminals [26, 27]. Therefore, the pathological metabolism of α-synuclein may be closely linked to the misregulation of dopamine, consequently leading to neuronal death. In support of this notion, catechol-derived orthoquinones (eg, dopamine-quinone or DOPA-quinone) accelerate and stabilize the formation of α-synuclein protofibrils by inhibiting the conversion of toxic protofibrils into fibrils [28, 29].
To shed light on the pathophysiological mechanisms underlying α-synuclein-mediated neurodegeneration in dopamine neurons, we developed novel neuronal cell lines coexpressing α-synuclein (wild-type or A53T) and tyrosinase that produces catecholamines and their oxidized metabolites [30, 31]. Investigating the effects of wild-type or mutant α-synuclein expression, we found that the coexpression of wild-type and A53T mutant α-synuclein in tyrosinase-overexpressing cells exacerbated DNA damage and successive apoptotic cell death compared to the cells overexpressing CAT or antisense α-synuclein. Both wild-type and A53T mutant α-synucleins coexpressed with tyrosinase resulted in the gradual accumulation of high-molecular weight complexes immunopositive for α-synuclein. This band, possibly representing oligomerized forms, corresponded to the size of α-synuclein tetramer and was also detected by the NBT/glycinate redox-cycling staining, suggesting that it was modified by quinones [32].
Moreover, during these processes, the mitochondrial membrane potential was specifically decreased without the activation of MAP kinases [32]. Although the underlying mechanism(s) of neuronal cell death following the coexpression of tyrosinase and α-synuclein are still elusive, it is likely that α-synuclein modified by the oxidized catechol metabolites forms cytotoxic intermediates, that is, “protofibrils”. Recent reports suggested that protofibrillar α-synuclein tightly binds to lipid bilayers and increases the membrane permeability by forming pore-like structures [33–35]. While the membranous structures damaged by protofibrils in dopaminergic nerve terminals remain unknown, intracellular organelles, such as synaptic vesicles and mitochondria, are possible candidates. In this regard, disruption of synaptic vesicle membranes would result in an increase of the cytoplasmic dopamine levels that would trigger the further accumulation of dopamine-quinone and dopamine-derived oxyradicals and thus lead to a vicious cycle. Likewise, mitochondrial enzymes in the electron transport chain and the functional permeability transition pores are impaired by dopamine oxidation products [36] making it plausible that the early damage of mitochondria observed in this cellular model reflects the actions of α-synuclein protofibrils and the subsequent increase of the membrane permeability in the presence of oxidized catecholamine metabolites [27].
Membrane injuries may trigger neurodegeneration
We further analyzed the resting membrane potential and whole-cell membrane conductance using the ramp voltage in the cell lines expressing wild-type or mutant α-synuclein [37]. Interestingly, the cells expressing A53T α-synuclein have the most depolarized membrane potential. By the application of the ramp voltage under the whole cell voltage-clamp condition, we obtained an almost linear current-voltage (I-V) relationship in each cell line. The slope of the I-V relationship in the cells expressing mutant α-synuclein was significantly steeper than that in the cells expressing the vector alone or wild-type α-synuclein, indicating that the expression of mutant α-synuclein results in higher ion permeability of the plasma membrane [37]. Because it has been suggested that abnormal intracellular calcium homeostasis plays a crucial role in the pathogenesis of neurodegenerative disorders [38], the intracellular free calcium concentrations in α-synuclein-transfected cells were quantified using a calcium indicator dye, fura-2 [39]. Notably, both the intracellular calcium concentrations under basal conditions and after depolarization induced by potassium chloride application were significantly higher in the mutant α-synuclein expressing cells than in cells expressing the empty vector or wild-type α-synuclein [37]. These results suggest that mutant α-synuclein is involved in the perturbation of the intracellular calcium homeostasis.
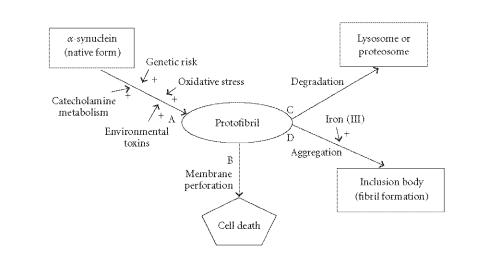
Taken together, our data from cellular models of synucleinopathy suggest that oligomer or protofibril, but not aggregate or fibril, formation of α-synuclein plays a key role in the pathomechanisms of synucleinopathy (Figure 1). The iron specifically triggers the aggregate formation of α-synuclein, but this seems to be a cytoprotective process [19]. The cytotoxic protofibril formation may be facilitated by not only gene mutations, but also the modification of α-synuclein by catechol-derived quinones [28, 32]. The cellular membranes are the primary targets injured by protofibrils [37], and the mitochondrial dysfunction seems to be an initial step in the neurodegeneration of synucleinopathy [32]. Obviously, protofibrils or oligomers of α-synuclein are heterogeneous in size and stability and exist as mixtures in the cytosol. Therefore, at present it is difficult to specify the detailed molecular structures that may be responsible for the cellular injuries. However, from these data, it is plausible that the reduction of the protofibril pool may rescue neurons from death (Figure 1). If this is the case, not only the acceleration of degradation (C in Figure 1) but also the facilitation of aggregate formation (D in Figure 1), may be a novel strategy for the treatment of synucleinopathy. Of course, the most important way would be to decrease the input (A in Figure 1) into the protofibril pool.
Figure 1.
Possible mechanisms of neurodegeneration in synucleinopathy.
References
- 1.Eriksen JL, Dawson TM, Dickson DW, Petrucelli L. Caught in the act: α-synuclein is the culprit in Parkinson's disease. Neuron. 2003;40(3):453–456. doi: 10.1016/s0896-6273(03)00684-6. [DOI] [PubMed] [Google Scholar]
- 2.Dev KK, Hofele K, Barbieri S, Buchman VL, Van der Putten H. Part II: α-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45(1):14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 3.Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. α-synuclein cooperates with CSPα in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Munoz E, Oliva R, Obach V, et al. Identification of Spanish familial Parkinson's disease and screening for the Ala53Thr mutation of the α-synuclein gene in early onset patients. Neuroscience Letters. 1997;235(1-2):57–60. doi: 10.1016/s0304-3940(97)00710-6. [DOI] [PubMed] [Google Scholar]
- 5.Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nature Genetics. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 6.Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Annals of Neurology. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 7.Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 8.Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Archives of Neurology. 2005;62(3):353–357. doi: 10.1001/archneur.62.3.353. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki M, Hasegawa T, Takeda A, et al. Histochemical features of stress-induced aggregates in α-synuclein overexpressing cells. Brain Research. 2004;1004(1-2):83–90. doi: 10.1016/j.brainres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa T, Matsuzaki M, Takeda A, et al. Accelerated α-synuclein aggregation after differentiation of SH-SY5Y neuroblastoma cells. Brain Research. 2004;1013(1):51–59. doi: 10.1016/j.brainres.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Giasson BI, Duda JE, Murray IV, et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 12.Paik SR, Shin HJ, Lee JH. Metal-catalyzed oxidation of α-synuclein in the presence of Copper(II) and hydrogen peroxide. Archives of Biochemistry and Biophysics. 2000;378(2):269–277. doi: 10.1006/abbi.2000.1822. [DOI] [PubMed] [Google Scholar]
- 13.Paxinou E, Chen Q, Weisse M, et al. Induction of α-synuclein aggregation by intracellular nitrative insult. The Journal of Neuroscience. 2001;21(20):8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in α-synuclein fibril formation. The Journal of Biological Chemistry. 2001;276(14):10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi A, Takeda A, Onodera H. Systemic increase of oxidative nucleic acid damage in Parkinson's disease and multiple system atrophy. Neurobiology of Disease. 2002;9(2):244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 16.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. The Journal of Cell Biology. 1998;143(7):1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM. Aggresomes formed by α-synuclein and synphilin-1 are cytoprotective. The Journal of Biological Chemistry. 2004;279(6):4625–4631. doi: 10.1074/jbc.M310994200. [DOI] [PubMed] [Google Scholar]
- 18.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431(7010):805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Takeda A, Itoyama Y. Role of iron in the intracellular aggregation of α-synuclein. Movement Disorders. 2004;19(sup9):S36. [Google Scholar]
- 20.Lee HG, Petersen RB, Zhu X, et al. Will preventing protein aggregates live up to its promise as prophylaxis against neurodegenerative diseases? Brain Pathology. 2003;13(4):630–638. doi: 10.1111/j.1750-3639.2003.tb00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda S, Sakakibara S, Watanabe E, Yoshimoto K, Koibuchi N. Vulnerability of monoaminergic neurons in the brainstem of the zitter rat in oxidative stress. Progress in Brain Research. 2002;136:293–302. doi: 10.1016/s0079-6123(02)36025-4. [DOI] [PubMed] [Google Scholar]
- 22.Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. Journal of Neuroscience Research. 1999;55(6):659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chemical Research in Toxicology. 2000;13(3):135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 24.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for α-synuclein in the regulation of dopamine biosynthesis. The Journal of Neuroscience. 2002;22(8):3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of α-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB Journal. 2001;15(6):916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 26.Perez RG, Hastings TG. Could a loss of α-synuclein function put dopaminergic neurons at risk? Journal of Neurochemistry. 2004;89(6):1318–1324. doi: 10.1111/j.1471-4159.2004.02423.x. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu A, Wersinger C, Vernier P. α-synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Letters. 2004;565(1–3):1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 28.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science. 2001;294(5545):1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 29.Li HT, Lin DH, Luo XY, et al. Inhibition of α-synuclein fibrillization by dopamine analogs via reaction with the amino groups of α-synuclein: implication for dopaminergic neurodegeneration. FEBS Journal. 2005;272(14):3661–3672. doi: 10.1111/j.1742-4658.2005.04792.x. [DOI] [PubMed] [Google Scholar]
- 30.Takeda A, Tomita Y, Okinaga S, Tagami H, Shibahara S. Functional analysis of the cDNA encoding human tyrosinase precursor. Biochemical and Biophysical Research Communications. 1989;162(3):984–990. doi: 10.1016/0006-291x(89)90770-5. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa T, Matsuzaki M, Takeda A, et al. Increased dopamine and its metabolites in SH-SY5Y neuroblastoma cells that express tyrosinase. Journal of Neurochemistry. 2003;87(2):470–475. doi: 10.1046/j.1471-4159.2003.02008.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa T, Matsuzaki-Kobayashi M, Takeda A, et al. Synergistic interaction between α-synuclein and oxidized catechol metabolites produced by tyrosinase: implications for selective neurodegeneration in Parkinson's disease. FEBS Letters. 2006;580:2147–2152. doi: 10.1016/j.febslet.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418(6895):291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 34.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson's diseaselinked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41(14):4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 35.Kayed R, Sokolov Y, Edmonds B, et al. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. The Journal of Biological Chemistry. 2004;279(45):46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 36.Gluck MR, Zeevalk GD. Inhibition of brain mitochondrial respiration by dopamine and its metabolites: implications for Parkinson's disease and catecholamine-associated diseases. Journal of Neurochemistry. 2004;91(4):788–795. doi: 10.1111/j.1471-4159.2004.02747.x. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, et al. High ion permeability of plasma membrane caused by the α-synuclein mutations contributes to cellular degeneration. Journal of Neurochemistry. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 38.Mattson MP, Chan SL. Dysregulation of cellular calcium homeostasis in Alzheimer's disease: bad genes and bad habits. Journal of Molecular Neuroscience. 2001;17(2):205–224. doi: 10.1385/JMN:17:2:205. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa K, Wang Y, Yao PJ, et al. Alteration in calcium channel properties is responsible for the neurotoxic action of a familial frontotemporal dementia tau mutation. Journal of Neurochemistry. 2003;87(2):427–436. doi: 10.1046/j.1471-4159.2003.02020.x. [DOI] [PubMed] [Google Scholar]