Abstract
The molecular mechanisms underlying the effects of nitric oxide (NO) and carbon monoxide (CO), individually and collectively, on large-conductance calcium-activated K+ (KCa) channels were investigated in rat vascular smooth muscle cells (SMCs). Both NO and CO increased the activity of native KCa channels. Dehydrosoyasaponin-I, a specific agonist for β subunit of KCa channels, increased the open probability of native KCa channels only when it was delivered to the cytoplasmic surface of membrane. CO, but not NO, further increased the activity of native KCa channels that had been maximally stimulated by dehydrosoyasaponin-I. After treatment of SMCs with anti–KCa,β subunit antisense oligodeoxynucleotides, the stimulatory effect of NO, but not of CO, on KCa channels was nullified. CO, but not NO, enhanced the KCa current densities of heterologously expressed cloned KCa,α subunit, showing that the presence of KCa,β subunit is not a necessity for the effect of CO but essential for that of NO. Finally, pretreatment of SMCs with NO abolished the effects of subsequently applied CO or diethyl pyrocarbonate on KCa channels. In summary, the stimulatory effects of CO and NO on KCa channels rely on the specific interactions of these gases with KCa,α and KCa,β subunits.
Introduction
Nitric oxide (NO) is an endogenous vasorelaxant gas that is synthesized from the terminal guanidino nitrogen atoms of L-arginine by NO synthase (1). Carbon monoxide (CO) is another endogenously generated biological gas. The major route for the endogenous generation of CO involves heme oxygenase (HO), which works in concert with NADPH-cytochrome P450 reductase to cleave the heme ring in hemoproteins into biliverdin, CO, and iron (2). Results from our laboratory (3) and others (4–7) have demonstrated that endogenously generated CO can effectively relax vascular tissues. The physiological role of endogenous CO is thus indicated.
NO and CO are not redundant messenger molecules. For instance, NO potently dilated hepatic artery, but only slightly dilated the portal venous vascular bed. In contrast, CO had no effect on the hepatic artery, but relaxed the portal venous vascular bed (8). In some cases, NO and CO may even have opposite effects. CO inhibited, but NO increased, the release of IL-1β from rat hypothalamus stimulated by high K+ concentrations (9). NO and CO do have at least two common targets in vascular smooth muscle cells (SMCs), i.e., soluble guanylyl cyclase (sGC) and large-conductance calcium-activated K+ (KCa) channels (10–14). Although the debate has not been settled on whether NO and CO directly act on KCa channels or indirectly by altering intracellular second messengers, many single channel studies on the cell-free membrane patches have been supportive for a direct interaction of NO and CO with KCa channel proteins. Even for these single channel studies different mechanisms for the effects of NO and CO on KCa channels have been presented. The NO effect on KCa channels in rabbit aortic SMCs was related to the changes in sulfhydryl groups (10). Our previous study showed that CO acted on the histidine residue of KCa channel proteins in rat tail artery SMCs (13).
Large-conductance KCa channels are stimulated by increased intracellular calcium concentration and by membrane depolarization. These KCa channels are composed of two noncovalently linked subunits: the pore-forming α subunit and the accessory β subunit, which affects the electrophysiological and pharmacological properties of KCa channel complexes (15). The interactions of NO and CO with α and β subunits of KCa channels may be the determining factor for the selective modulation of KCa channels, which has not been addressed.
The interaction between NO and CO on the regulation of vascular contractility is another important but still largely uncharted issue. The activation of large-conductance KCa channels in SMCs accounts for a significant part of the relaxation induced by NO (16) or CO (20–50%) (3). The KCa channel–mediated vasorelaxing effect of CO was significantly decreased approximately 40% in the presence of sodium nitroprusside (SNP) (17). These results indicated that NO might desensitize KCa channels toward CO. Here is an intriguing situation. On one hand, we accept the argument that the vascular effects of NO and CO have different profiles. On the other hand, most studies, if not all, have been focused only on the individual vascular effects of these gases. Although physiologically more relevant, the integrated vascular effect of these gases and the underlying mechanisms have been unknown.
The present study was aimed at investigating the functional interaction of NO/CO with KCa,α and KCa,β subunits and the collective effect of NO and CO on large-conductance KCa channels in vascular SMCs. To accomplish these goals we used the patch-clamp technique to characterize the effects of NO and CO, individually and collectively, on KCa channel currents in vascular SMCs. The function of KCa,β subunit was pharmacologically modulated, and the expression of KCa,β subunit protein was suppressed by antisense oligonucleotide treatment. We also examined the effects of CO and NO on the heterologously expressed cloned KCa,α subunit. Our results indicate that the stimulatory effects of CO and NO on KCa channels rely on the specific interactions of these gases with KCa,α and KCa,β subunits. The collective acts of NO and CO constitute a feedback mechanism controlling the functions of large-conductance KCa channels in vascular SMCs.
Methods
Cell preparation.
Male Sprague-Dawley adult rats were anesthetized by intraperitoneal injection of sodium pentobarbital (60 mg/kg body weight). Single SMCs were dispersed from tail artery enzymatically following our established procedure (12, 13). The enzymatically dispersed cells were plated onto 35-mm Petri dishes in DMEM (Life Technologies Inc., Burlington, Ontario, Canada) containing penicillin (100 U/ml; Sigma-Aldrich, St. Louis, Missouri, USA) and streptomycin (0.1 mg/ml; Sigma-Aldrich) and maintained at 4°C for at least 4 hours. These freshly isolated cells were used in electrophysiological recording within 8–24 hours of isolation.
Animal use protocols were approved by the University Committee on Animal Care and Supply of the University of Saskatchewan.
Single channel and whole-cell recordings.
The inside-out and outside-out configurations of the patch-clamp technique were used to record single KCa channel currents as described previously (12, 13). Pipettes with a resistance of 6–8 MΩ were used, and the seal resistance was usually greater than 10 GΩ. Membrane patches with no more than three channels were used for experiments. Single channel currents were filtered at 2 kHz (8-pole Bessel, –3 dB) and recorded with a 5-μs sampling interval in a gap-free mode. The channel open probability (Po) was calculated from the ratio between the open time and the total time. The presence of multiple channels (N) in one membrane patch was distinguished by simultaneous channel openings when exposing the inner surface of the membrane to 100 μM Ca2+ and depolarizing the patch to 50 mV. NPo values represented the Po of N channels. The unit amplitude of KCa channels and the channel open duration were determined from all point histograms using a Fetchan program (Axon Instruments Inc., Foster City, California, USA). A current level higher than 50% of the unit channel current was considered to reflect a channel opening. For each concentration of tested agent at least 60 seconds of channel activity was recorded directly on the hard disk of a computer. NPo of KCa channels was averaged over 2–5 minutes of recording to describe the changes in channel activity following different treatments. Membrane patches with unstable NPo over time were excluded from further analysis. The outer surface of membrane patches was bathed in a solution containing 145 mM KCl, 10 mM HEPES, and 10 mM glucose. The inner surface of membrane patches was exposed to a solution containing 145 mM KCl, 10 mM HEPES, 1.2 mM MgCl2, 10 mM glucose, 1 mM EGTA, and different amounts of CaCl2 to reach the desired final intracellular free Ca2+ concentrations, [Ca2+]i. Unless otherwise specified, [Ca2+]i was 0.5 μM, calculated using a computer program (EQCAL; Biosoft, Ferguson, Missouri, USA).
The whole-cell KCa currents were recorded as described previously with an Axopatch 1-D amplifier (Axon Instruments Inc.), controlled by a Digidata 1200 interface and a pClamp software program (version 6.02; Axon Instruments Inc.) (12). Membrane currents were filtered at 1 kHz with a 4-pole Bessel filter, digitized, and stored. At the beginning of each experiment, junctional potential between pipette solution and bath solution was electronically adjusted to zero. No leakage subtraction was performed to the original recordings, and all cells with visible changes in leakage currents during the course of study were excluded from further analysis. Test pulses were made at 10-mV increments from –80 to +60 mV. The holding potential was set at –80 mV. I-V curves were constructed using the current amplitude measured between the 300 to 600 ms of the test pulses. The bath solution contained 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES, 10 mM glucose (pH adjusted to 7.3 with NaOH). The pipette solution was composed of 145 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM HEPES, 5 mM glucose, 2 mM Na2-ATP (pH adjusted to 7.3 with KOH). [Ca2+] in the pipette solution was adjusted to 50 nM by adding adequate amount of CaCl2. Cells were continuously superfused with the bath solution containing tested chemicals. Osmolalities and pH of all solutions were adjusted to 290 mOsm/l and 7.4, respectively. All electrophysiological experiments were carried out at room temperature.
Transient transfection of COS-1 cells with KCa,α subunit.
The mSlo (BKCa,α subunit) cDNA was kindly provided by B. Ganetzky (University of Wisconsin, Madison, Wisconsin, USA) (GenBank accession no. U09383). Most of the 5′ noncoding region has been removed to improve expression. The cDNA was cleaved with the PmeI endonuclease from pcDNA3.1-mSlo construct and subcloned into a pIRES2-EGFP expression vector (CLONTECH Laboratories Inc., Palo Alto, California, USA) pretreated with SmaI endonuclease. After being transformed into competent Escherichia coli cells, colonies were screened for the clones containing the construct of right orientation using appropriate endonucleases.
COS-1 cells were purchased from American Type Culture Collection (Rockville, Maryland, USA). These cells were cultured at 37°C in a humidified incubator with 95% air and 5% CO2 in DMEM with 4 mM L-glutamine containing 4.5 g/l of glucose and 1.5 g/l of sodium bicarbonate and supplemented with 10% FBS and penicillin/streptomycin. The cells in 35-mm cell culture dishes were 70–80% confluent prior to the transfection experiment. The pIRES2-EGFP-mSlo construct was mixed with a FuGENE 6 transfection reagent (Roche Diagnostics, Laval, Quebec, Canada) in a ratio of 1 μg to 3 μl in 100 μl of FBS-free DMEM. After incubating for 45 minutes at room temperature, the mixture was added to COS-1 cells in 2 ml FBS-free DMEM (cell density: 8 × 104/35-mm dish). Mock transfection (vector only transfection) and nontransfected native COS-1 cells were included as negative controls for the experiment. The cells were allowed to express the transfected mSlo cDNA for 48–72 hours prior to electrophysiological experiments. The emitted green fluorescence served as the marker for selecting the transfected cells.
Antisense oligodeoxynucleotide treatment of cells.
The oligodeoxynucleotide (ODN) was designed to target the transcript segment encompassing the translation-initiation codon of rat KCa,β subunit (GenBank accession no. AF020712). The antisense ODN (5′-TCTTCCCCATGGCCACAGG-3′) and the scrambled ODN (5′-CTAGACATGCTGCCT CCGC-3′) were synthesized by Bio/Can Scientific (Mississauga, Ontario, Canada). The underlined nucleotides were the phosphorothioate backbone of ODN. The scrambled ODN contained the same base composition as the antisense sequence, but no complementary sequence to the scrambled ODN could be found in the entire GenBank database (using the basic local alignment search tool, or BLAST).
Freshly isolated rat tail artery SMCs were cultured at 37°C in an atmosphere of 5% CO2 in DMEM containing 10% FCS for 18–24 hours before ODN treatments. To increase the cellular uptake, antisense or scrambled ODN diluted in serum-free and antibiotic-free DMEM were first mixed with a cationic liposome solution (Lipofectin; Life Technologies Inc.) at room temperature for 10–20 minutes. After washing cultured cells twice with serum-free and antibiotic-free DMEM, the antisense or scrambled ODN-Lipofectin (final concentration 5 μM and 6 μM, respectively) complexes were added to 35-mm Petri dish in which SMCs were incubated with antibiotic-free DMEM containing 5% FCS. The ODN-Lipofectin–containing medium was replaced after an incubation period of 8–12 hours at 37°C in a CO2 incubator by normal culture medium (DMEM with 10% FCS). The cells were then continuously cultured at 37°C in a CO2 incubator in the absence of ODN-Lipofectin for 12–24 hours before the patch-clamp studies were conducted on these cells.
Chemicals and data analysis.
Sodium nitroprusside (SNP), S-nitroso-N-acetyl-penicillamine (SNAP), diethyl pyrocarbonate (DEPC), N-ethylmaleimide (NEM), L-lysine, and deferoxamine were from Sigma-Aldrich. Chromium mesoporphyrin (Cr-MP) was purchased from Porphyrin Products (Logan, Utah, USA). Heme-L-lysinate (HLL) (38 mM) was prepared as previously described by Tenhunen et al. (18), stored at –20°C, and protected from light until use. Iberiotoxin (IbTX) was from Alomone Labs (Jerusalem, Israel). Dehydrosoyasaponin I (DHS) was a gift from Merck Research Laboratories (Rahway, New Jersey, USA). Saturated CO solution was prepared fresh before each experiment and then diluted immediately to the desired concentrations with bath solution (3). DEPC was freshly diluted with anhydrous ethanol prior to each experiment and directly added to the bath or pipette solution at pH 7.0 in different experiments. NEM was dissolved in 1 M KOH as a 500-mM stock solution, dissolved in the bath solution immediately prior to the experiment, and the pH was readjusted to 7.4 (19). DHS stock solution (250 μM) was prepared in distilled water, dispensed into aliquots, and stored at –20°C.
Data were expressed as means plus or minus SE. Differences between treatments in the same cells were evaluated by paired Student t test or in conjunction with Newman-Keuls test where the same patches were treated by multiple agents. The significant level of difference was determined when P values were less than 0.05.
Results
Our previous studies have shown that exogenous CO increased the Po of KCa channels, but not the single channel conductance, in a concentration-dependent manner in rat tail artery SMCs (12, 13). The interaction of endogenous CO with KCa channels in this cell preparation was, to our knowledge, first examined in the present study. In the presence of Cr-MP, an inhibitor of HO, whole-cell KCa channel currents were significantly reduced (Figure 1a). To increase endogenous level of CO, we applied HLL, a substrate of HO (20), to tail artery SMCs. KCa channel currents were significantly increased 10 minutes after the application of HLL (Figure 1b). Whether the effect of HLL could be caused by free iron and biliverdin, another two end products of heme metabolism in addition to CO, was further investigated. Biliverdin failed to affect KCa currents, and HLL still significantly increased KCa currents in the presence of free iron scavenger deferoxamine (Figure 1c). In addition, L-lysinate, the vehicle of HLL, had no effect on KCa currents (Figure 1c). These results demonstrate that the KCa channels in rat tail artery SMCs are modulated by endogenous CO.
Figure 1.
The effect of endogenous CO on whole-cell KCa currents in rat tail artery SMCs. (a) KCa currents were suppressed by Cr-MP (30 μM). The I-V relationships of the KCa channels were constructed 10 minutes before and after the application of Cr-MP. n = 7. (b) KCa currents were increased by HLL (50 μM). The I-V relationships of the KCa channels were constructed 10 minutes before and after the application of HLL. n = 6. (c) KCa currents were not affected by treatment with biliverdin or L-lysine for 10 minutes. n = 6 in each group. In the presence of deferoxamine (DFX), HLL still increased KCa channel currents. Holding potential, –80 mV; test potential, +40 mV. *P < 0.05 vs. control. Data of each group in this figure were pooled from at least two batches of cells. I, current; V, membrane potential.
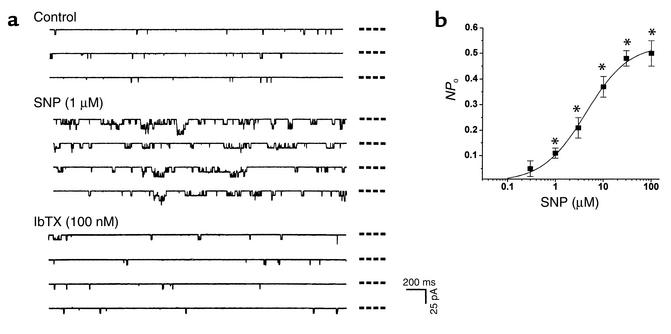
The effect of NO on KCa channels in rat tail artery SMCs was also examined in the present study. SNP increased the NPo of single KCa channels in both outside-out (Figure 2) and inside-out membrane patches (not shown). The increase in the NPo became significant with 1 μM SNP and reached the plateau with a concentration of SNP around 100 μM. The excitatory effect of SNP was sustained for the period of SNP application (5–20 minutes). The SNP-stimulated single channel activities were blocked by IbTX (100 nM) (Figure 2), corroborating the nature of large-conductance KCa channels under investigation. SNP (1–100 μM) had no effect, however, on single channel conductance (232 ± 11 pS and 241 ± 9 pS before and after the application of SNP; n = 6, P > 0.05).
Figure 2.
Effects of SNP on KCa currents in outside-out membrane patches of vascular SMCs. (a) SNP increased, but the subsequently applied IbTX blocked the single channels in the same membrane patch. The dashed lines beside each trace indicate the closed channel level. (b) The concentration-dependent excitatory effect of SNP on the NPo of KCa channels. n = 6 for each data point. A total of three batches of cells were used in this study. Membrane potential, –30 mV. *P < 0.05 vs. control. ms, millisecond; pA, picoampere.
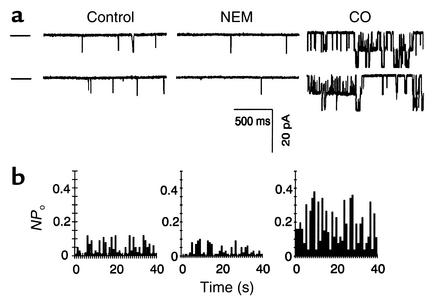
In order to identify different mechanisms underlying the effects of NO and CO on KCa channels in vascular SMCs, sulfhydryl groups of KCa channel complex were first modified with NEM. Treatment of inside-out patches with NEM (5 mM) led to a significant decrease in the open frequency of single KCa channels by 72% ± 3% (n = 5, P < 0.01), whereas the mean open time for each individual opening was not affected (not shown). Consequently, NPo of single KCa channels was significantly reduced (Figure 3). The stimulatory effect of SNP (10 μM) on KCa channels was diminished in the presence of NEM (n = 5, not shown). NEM treatment did not, however, alter the stimulatory effect of CO on the Po of single KCa channels (Figure 3).
Figure 3.
Effects of NEM (5 mM) and CO (30 μM) on single KCa channels recorded from inside-out membrane patches held at a membrane potential of –30 mV. (a) Original KCa current traces recorded in the absence and then the presence of NEM and CO, consecutively, in the same patch. The dashed lines beside the current traces denote the close state of the channels. (b) NEM decreased the Po of single KCa channels. However, the presence of NEM did not block CO-induced modification of single KCa channels. n = 6 from two batches of cell preparations.
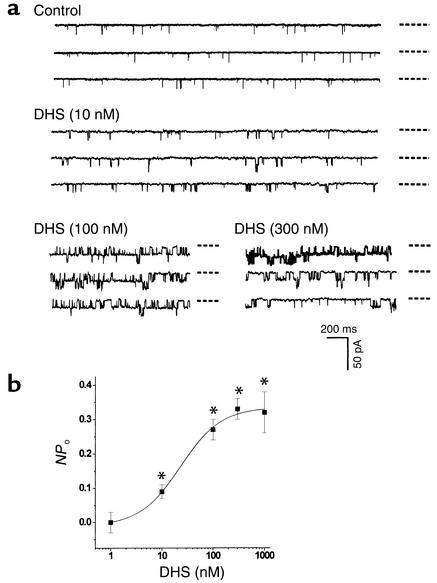
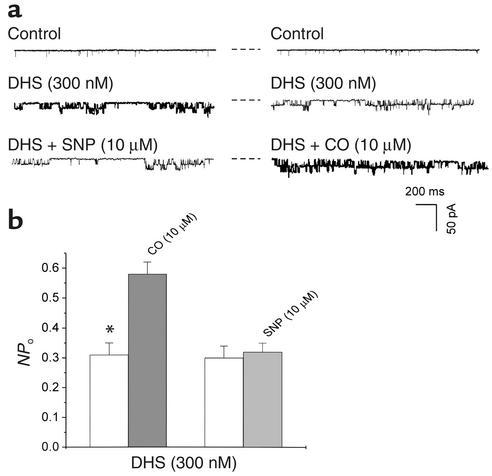
Both α and β subunits contribute to the functionality of native KCa channels. To identify which subunit of KCa channels was the potential target of CO or NO, we examined the effect of DHS on single KCa channel currents. DHS is a non-membrane permeable–specific probe for the functionality of β subunit of KCa channels. DHS (500 nM) did not significantly change the NPo of KCa channels in outside-out membrane patches (n = 5, not shown). However, DHS concentration dependently increased the NPo when applied to the inner membrane surface (Figure 4). After the maximum excitatory effect of DHS at 300 nM reached a stable level, the same membrane patches were exposed to either SNP or CO in different sets of experiments. The preconditioning with DHS abolished the excitatory effect of SNP on KCa channels, but not that of CO. These results summarized in Figure 5 indicated that the effects of DHS and SNP on native KCa channels might be mediated by the same β subunit–related mechanism, but CO might act on KCa channels in a β subunit–independent manner.
Figure 4.
The concentration-dependent effect of DHS on the NPo of KCa channels. (a) Actual single channel current traces recorded in inside-out membrane patches. The dashed lines beside each trace indicate the closed state of the channels. (b) Summary of the effects of DHS. n = 6 (from three batches of cells) for each data point. Membrane potential, –30 mV. *P < 0.05 vs. control.
Figure 5.
The activation of KCa channels in vascular SMCs. (a) Representative single channel current recordings showing two sets of experiments in which KCa channels were preconditioned with DHS, and either SNP (left panel) or CO (right panel) was subsequently applied. (b) The preconditioning with DHS abolished the effect of SNP on the NPo, but not that of CO. n = 6 (from two batches of cells) for each group. *P < 0.05. Inside-out patches. Membrane potential, –30 mV. The dashed lines beside each trace indicate the closed channel current level.
To abridge the functional coupling of β subunit with α subunit in KCa channel complexes, SMCs were treated with anti-KCa,β antisense ODN. Single channel conductance of KCa channels was not altered by the antisense ODN or scrambled ODN treatments (not shown). The decreased voltage dependence and calcium sensitivity after anti-KCa,β antisense ODN treatment were indicative of KCa channels that existed as the product of one gene that encodes α subunit. Anti-KCa,β antisense ODN treatment decreased the half-activation potential (V1/2) of KCa channels from –62.9 ± 5.7 mV to 20.4 ± 3.7 mV (n = 5 in each group of cells, P < 0.01) (21). The slopes of the voltage-dependence curves were 18.8 ± 5.8 mV and 19.3 ± 4.7 mV with or without anti-KCa,β antisense ODN treatment, respectively (P < 0.05). The presence of β subunit is known to give rise to different degrees of the significant shift in V1/2 of KCa channels, depending on the free calcium concentrations (21, 22). After anti-KCa,β antisense ODN treatment, the calcium concentration needed to activate 50% of KCa channels (Ca1/2) at +50 mV was increased from 5.7 ± 0.5 μM to 14.5 ± 1.1 μM (n = 5 in each group, P < 0.01). Similar values of Ca1/2 have been reported for the KCa channels composed of both α and β subunits or α subunit alone, respectively (21, 22). It is apparent from these results that the participation of KCa β subunit in the formation of KCa channel complexes had been effectively reduced by anti-KCa,β antisense ODN treatment.
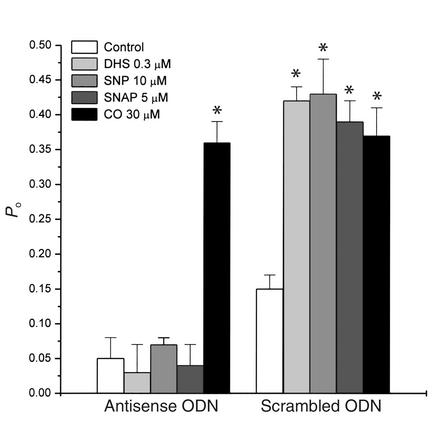
Should the effects on KCa channels of both DHS and NO rely on the presence of β subunit, the uncoupling of α and β subunits by anti-KCa,β antisense ODN treatment would render the insensitivity of KCa channels to DHS and NO. This scenario is portrayed in Figure 6, showing that the stimulatory effects of SNP and DHS on single KCa channels in inside-out patches were nullified by anti-KCa,β antisense ODN treatment. To further confirm the involvement of NO in the effect of SNP, another NO donor (SNAP) was used. SNAP increased the activity of KCa channels in the cells treated with scrambled ODN (control) but had no effect on KCa channels after anti-KCa,β antisense ODN treatment. In contrast, the stimulatory effect of CO on KCa channels was not affected by anti-KCa,β antisense ODN treatment (Figure 6).
Figure 6.
The effects of DHS, SNP, SNAP, and CO on the normalized Po of KCa channels in inside-out patches of vascular SMCs after anti-KCa,β antisense ODN treatment. Membrane potential, +30 mV. [Ca2+]i, 1 μM. n = 7 (from at least two batches of cells) for each group. *P < 0.01 vs. control.
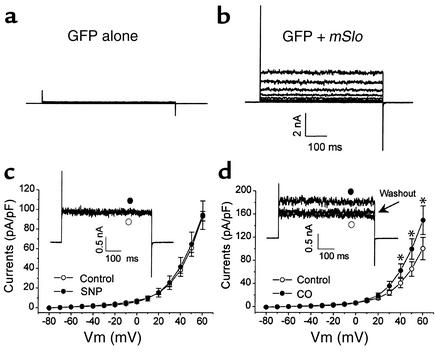
COS-1 cells transfected with cDNAs encoding green fluorescent protein (GFP) alone did not yield KCa currents in the presence of 50 nM cytoplasmic free Ca2+ (Figure 7a) (n = 11). In contrast, the cells cotransfected with GFP and mSlo cDNAs showed a big current that appeared noisy under the same recording conditions (Figure 7b). SNP (10 μM) did not affect the current density of the expressed KCa channels (60.8 ± 4.5 and 64.7 ± 12.2 picoamperes/picofarads [pA/pF] before and after the application of SNP; +50 mV, n = 8) (Figure 7c). CO (150 μM), on the other hand, significantly increased KCa current density from 65.4 ± 12.6 to 100.1 ± 17.7 pA/pF (+50 mV; n = 5). This effect became obvious at membrane potentials positive to +20 mV and was reversible by the washout procedure (Figure 7d).
Figure 7.
Effects of SNP and CO on the whole-cell KCa currents generated by heterologous expression of KCa,α subunit in COS-1 cells. Membrane potential was stepped with 600-ms duration from –80 to +60 mV in 10-mV increments from a holding potential of –80 mV at 0.1 Hz. (a) The cells transfected with GFP cDNA alone did not yield KCa currents. (b) KCa currents were recorded in COS-1 cells transiently cotransfected with GFP and mSlo cDNAs. (c) SNP had no effect on the mSlo-encoded KCa currents (n = 8 from three batches of cell preparations). The inset shows the original current traces at +50 mV. (d) CO significantly increased the mSlo-encoded KCa currents (n = 5 from three batches of cell preparations). The inset shows the original current traces at +50 mV. *P < 0.05 vs. control. nA, nanoampere.
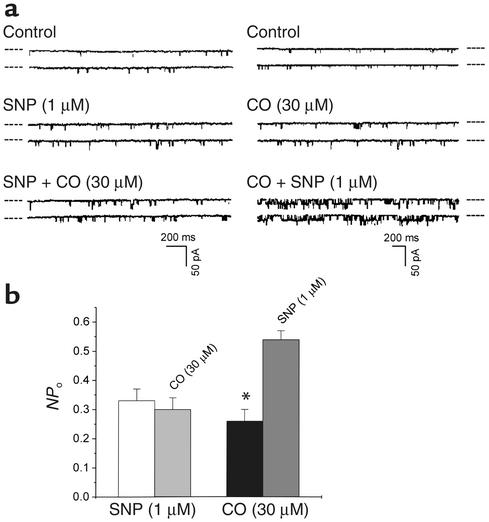
The collective effect of NO and CO on native KCa channels in SMCs was not a simple algebraic addition of their individual effects. After the cell membranes were pretreated with SNP for 10 minutes, subsequent application of CO failed to further increase the NPo of KCa channels in outside-out patches. However, pretreatment of the cell membranes with CO for 10 minutes did not prevent SNP from further increasing the NPo (Figure 8). The possibility was thus raised that the CO-sensitive α subunit of KCa channels might be modified by NO so that the α subunit became desensitized to CO. In contrast, the NO-sensitive β subunit of KCa channels was likely resistant to CO, and the effects of CO and the subsequently applied NO on β subunit would become additive.
Figure 8.
The interaction of NO and CO on KCa channels in vascular SMCs. (a) Representative recordings showing the modulation of KCa currents by the sequential application of SNP and CO (left panel) or CO and then SNP (right panel) to different outside-out membrane patches. The dashed lines beside each trace indicate the current level of closed channels. (b) Pretreatment of cell membrane with SNP for 10 minutes abolished the effect of subsequently applied CO on KCa currents. However, pretreatment of cell membrane with CO for 10 minutes did not eliminate the excitatory effect of SNP on KCa currents. Membrane potential, –30 mV. n = 5 (from at least two batches of cells) for each group. *P < 0.05.
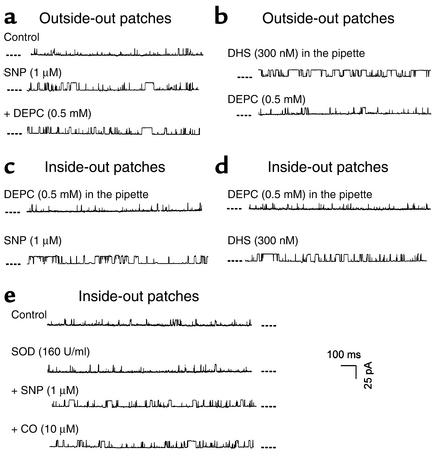
We demonstrated previously that CO increased KCa channel activities by activating a histidine residue of KCa channel proteins located on the outer surface of the cell membrane (13). To explore the molecular basis of the interaction of NO and CO, we set out to examine whether NO modified the histidine-related reactivity of KCa channels. Because the application of DEPC to the inner membrane surface did not affect KCa channel activity or the CO effect on KCa channels (13), DEPC in the present study was always applied to the outer membrane surface. After the SNP-induced increase in the NPo became stable, the outside-out membrane patches were superfused with the bath solution containing 0.5 mM DEPC for at least 5 minutes. During this period of time, no reduction in the SNP-enhanced KCa channel activity was spotted (Figure 9a). The NPo were 0.22 ± 0.04 and 0.19 ± 0.07 before and after the application of DEPC (n = 6 from two batches of cell preparations, P > 0.05). Since DHS affected KCa channels only when it was given intracellularly (Figure 4), in the next set of outside-out single-channel recording experiments we included DHS (300 nM) in the pipette solution. The exposure of the same patches to DEPC (0.5 mM) led to a significant decrease in the mean NPo of KCa channels (0.32 ± 0.07 vs. 0.15 ± 0.04; n = 5, P < 0.05) (Figure 9b). These results suggested that in addition to the stimulation of KCa,β subunit, NO might also somehow act on the histidine of α subunit so that DEPC could not chemically modify this residue. After the outer surface–located histidine residue of KCa channels was modified by DEPC, the subsequent bath-applied SNP (n = 6 from two batches of cells) or DHS (n = 5 from two batches of cells) still significantly increased KCa channel activities in inside-out membrane patches (Figure 9, c and d). Finally, in the presence of superoxide dismutase (SOD), SNP still inhibited the excitatory effect of CO on single KCa channels (n = 6 from two batches of cells) (Figure 9e).
Figure 9.
The mechanisms underlying the inhibition of CO effect on KCa channels by NO. The dashed lines beside each trace indicate the closed channel current level. Membrane potential, +30 mV. [Ca2+]i in these experiments was 1 μM. (a) Pretreatment of the outside-out membrane patch with SNP rendered the KCa channels insensitive to DEPC modification. (b) Inclusion of DHS in the pipette solution increased the NPo of the outside-out patches but did not prevent KCa channels from inhibition by the subsequent bath-applied DEPC. (c) Inclusion of DEPC in the pipette solution did not interfere with the excitatory effect of the bath-applied SNP on the NPo in the inside-out patches. (d) Inclusion of DEPC in the pipette solution did not interfere with the excitatory effect of the bath-applied DHS on the NPo in the inside-out patches. (e) The presence of SOD in the bath solution did not remove the inhibition of CO effect–by SNP.
Discussion
Large-conductance KCa channels in vascular SMCs are one of the important targets of NO and CO actions. Our present study addressed the molecular mechanisms for the individual as well as integrated effects of NO and CO on KCa channels. Our novel discoveries are summarized below. First, not only exogenous CO but also endogenous CO increased KCa channel currents in rat tail artery SMCs. Second, NO increased the NPo of large-conductance KCa channels in rat tail artery SMCs by a mechanism related to the modification of the sulfhydryl group of the KCa channel complex. Third, the excitatory effects of CO and NO on KCa channels were likely mediated, respectively, by α and β subunits of KCa channel complex. Fourth, the interaction of CO and histidine residue of KCa channels did not interfere with the excitatory effect of NO on KCa channels. Fifth, although NO had no effect on KCa,α subunit in the absence of KCa,β subunit, the interaction of KCa channel complex with NO significantly inhibited the excitatory effect of CO. And sixth, the inhibition of CO effect on KCa channels by NO likely resulted from a repulsive effect of NO on, instead of oxidation of, histidine residue. Taken together, our results provided molecular basis for understanding the acute interaction of NO and CO, two endogenous vasoactive gases, on the regulation of vascular contractility.
A higher concentration of CO was needed to act on the KCa channel complex to achieve the similar excitation as induced by a lower concentration of NO, consistent with different vasorelaxant potencies of these two gases. This could be partially explained by the difference in the chemical interactions of CO and NO with KCa,α and KCa,β subunits, respectively. A relatively weak reaction between CO and the imidazole group of histidine occurs by the formation of a hydrogen bond, which could be largely responsible for the effect of CO on KCa channels (13). On the other hand, the modification of sulfhydryl groups by NO, named s-nitrosylation, is likely related to the formation of a disulfide bond between NO and the affected protein, including KCa channels (23). A much weaker hydrogen bond, as compared with the disulfide bond, may result in a greater dissociation rate between CO and its target than between NO and KCa channels. This speculation merits further investigation.
Physiologically, vascular tone may be under the tonic influence of CO (ascribed to the long life span and low vasorelaxant potency of the gas) and regulated by the phasic effect of NO that has a short half life and greater vasorelaxant potency. It can be perceived, based on our results, that the persistent presence of CO in the surroundings of vascular SMCs would not affect the sensitivity of KCa channels to NO so that the vascular contractility could be acutely and precisely regulated by NO surge. In case a prolonged NO exposure at normal or higher concentrations is encountered, as in ischemia-reperfusion damage, septicemia, or other pathophysiological situations, the tonic relaxant influence of CO on vascular tone would be reduced due to its decreased effect on KCa channels in vascular SMCs. Thus, the interaction between NO and CO would provide a feedback mechanism to help regain the control of the disturbed vascular contractility. Elucidating the interaction of NO and CO on the function of KCa channels in vascular SMCs was the first endeavor and provided important progress leading to a better understanding of the integrated fine-tuning of cardiovascular functions by gaseous vasoactive factors.
Since our results are largely derived from cell-free patch-clamp studies in which the involvement of second messengers has been minimized, a direct action of CO and NO on KCa channel complex in rat tail artery SMCs under our experimental conditions is envisioned. It is worth noting that our conclusion by no means excludes the possibility that NO and CO may still affect KCa channels indirectly in other types of vascular SMCs. Mounting evidence shows that NO (10, 16, 24) and CO (11, 12) can directly act on KCa channel proteins to alter the channel gating. Other studies show that the activation of KCa channels by NO is mediated by cGMP-dependent mechanisms (25, 26). No study has reported a cGMP-dependent effect of CO on KCa channels, to our knowledge. The inconsistency in the cGMP dependency of the effect of NO on KCa channels cannot yet be readily explained. One putative explanation is the difference in cell types from different vascular beds, which have been used under different experimental conditions (26).
Antisense ODN treatment introduces DNA complementary in sequence to a portion of the target mRNA to block its translation. The use of antisense ODN in combination with electrophysiology measurement offers a useful approach for evaluating the contribution of different genes to the native ionic currents. The present study used a specific phosphorothioate antisense ODN to hybridize the translation initiation codon of KCa,β subunit mRNA to eliminate the participation of β subunit in the assembly of native KCa channel complex in rat tail artery SMCs. Due to the unavailability of commercial Ab against KCa,β subunit, we were not able to confirm the diminished expression of KCa,β subunit at protein levels after anti-KCa,β antisense ODN treatment. However, the decreased calcium sensitivity and voltage dependence of KCa channels as well as the lack of effect of DHS on KCa channels after anti-KCa,β antisense ODN treatment speak strongly for the reduction of β subunit as part of the KCa channel complex (27). Since the effect of NO on KCa channels was abolished after anti-KCa,β antisense ODN treatment, it is reasoned that the effect of NO may rely on the presence of β subunit. The specificity of anti-KCa,β antisense ODN was further demonstrated by the scrambled ODN that did not alter the sensitivities of KCa channels whatsoever to NO, CO, and DHS. On the other hand, that CO still increased KCa channel activity after anti-KCa,β antisense ODN treatment claims KCa,α subunit as the primary target of this gas. The cell membranes were not treated with anti-KCa,α antisense ODN in the present study since no KCa currents could be conducted if the translation of the pore-forming element KCa,α subunit were blocked. Importantly, the conclusion derived from the antisense experiments was supported by our study of the heterologous expression of KCa,α subunit. CO, but not NO, significantly increased the KCa currents generated by KCa,α subunits in COS-1 cells. Although the possibility that NO also reacts with KCa,α subunit still remains, our results leave little doubt that the KCa,β subunit is irreplaceable for NO to affect KCa channel activities.
A pharmacological approach in our study to investigate the subunit-dependent effects of NO and CO on KCa channels was to examine the interaction of DHS with NO/CO. DHS is a glycosylated triterpene that acts on KCa,β subunit in smooth muscles (21, 22). The inhibition of IbTX binding by DHS has also been used as the evidence for the presence of KCa,β subunit in rat uterus smooth muscle and in HEK293 cells expressing KCa,α and KCa,β subunits (28). By the same token, the absence of KCa,β subunit in rat brain and in HEK293 cells expressing only KCa,α subunit was concluded based on the failure of DHS to inhibit the IbTX binding to plasma membranes. In our study, after the saturated excitatory effect of DHS on KCa channels was achieved, the stimulatory effect of NO on KCa channels was nullified, but CO was still capable of increasing the NPo of KCa channels. Obviously, the NO effect was related to the functional status of KCa,β subunit and CO effect was not.
Multiple subtypes or isoforms of large-conductance KCa channels with large variation in calcium sensitivity have been observed (29). One of the explanations for this diversity is the different coupling affinity or ratio between KCa,α and KCa,β subunits (30). There are several lines of evidence corroborating this notion. First, the calcium sensitivity of KCa channels is greatly affected by the presence of β subunit. Second, although the α/β complex form of KCa channels was predominant, KCa channels comprising α subunit alone existed (21). Third, tissue distribution patterns of α subunit and β subunit did not always match, and in certain types of tissues they might not even couple (31). Combining the importance of α/β subunit coupling to the functionality of KCa channels with our present results may help to elucidate the mechanisms for the different effects of NO or CO on KCa channels in different types of vascular SMCs. For instance, with the presence of KCa,β subunit as the functional necessity for the NO effect, NO might not have a significant effect on KCa channels in certain types of vascular SMCs if the coupling of KCa,β subunit to KCa,α subunit is lacking or weakened.
As shown in our previous study, DEPC induced a relatively irreversible covalent modification of histidine residue and decreased KCa channel activity. After the chemical modification with DEPC, KCa channels lost their responses to CO stimulation (13). Our present study showed that pretreatment of KCa channels with SNP significantly inhibited the effect of the subsequently applied CO on KCa channels. It was speculated that SNP might act on the CO-sensitive histidine to desensitize KCa channels. If this were the case, not only CO but also DEPC would have no effect on KCa channels after the NO treatment. Our results shown in Figure 9a support this theory. Moreover, the saturated intracellular DHS effect on KCa channels did not prevent CO from increasing (Figure 5), or DEPC from inhibiting (Figure 9b), KCa channels though it abolished the effect of NO. It seems that NO has two distinct acting sites. By acting on the inner surface–located DHS-sensitive site (likely on β subunit), NO increased KCa channel openings. By acting on the outer surface–located DEPC-sensitive histidine residue, NO reduced KCa channel sensitivity to the agents that targeted on histidine. Interestingly, after the outer membrane surface was first treated with DEPC, the subsequently applied NO or DHS (inner surface) still increased the NPo of KCa channels (Figure 9, c and d). These results suggested that the DEPC–sensitive histidine is likely located on KCa,α subunit and its structural change would not affect the KCa,β subunit–dependent excitatory effects of NO or DHS.
The nature of the interaction between NO and CO on KCa channels is unknown. In reaction with superoxide anions, NO would transform to peroxynitrite, which leads to the oxidation of many proteins. However, this mechanism could not explain the observed NO effect since the inclusion of SOD did not alter the effect of NO on KCa channels, nor change the inhibitory effect of NO on the CO-induced increase in NPo. Alternatively, the action of NO on KCa,β subunit may exert an allosteric effect on the neighboring KCa,α subunit. The noncovalent association of α and β subunits of KCa channels is tight, with a distance in between of less than 12 Å (32). The conformational changes of KCa,β subunit would prevent the formation of hydrogen bonds between CO and the imidazole group of histidine of KCa,α subunit (13). This putative “repulsive” mechanism is reminiscent of the effect of NO on the activation of sGC. Upon binding to sGC, NO induces the displacement of the iron atom, and this repulsive trans effect prevents the access to and binding of the proximal histidine of sGC to the heme iron (33).
Acknowledgments
This study was supported by Heart and Stroke Foundation of Saskatchewan, Canada. L. Wu was a postdoctoral fellow of Heart and Stroke Foundation of Canada/Canadian Institutes of Health Research (CIHR). K. Cao was a postdoctoral fellow of Health Services Utilization and Research Commission of Saskatchewan. R. Wang is a CIHR Investigator.
Footnotes
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: heme oxygenase (HO); smooth muscle cells (SMCs); soluble guanylyl cyclase (sGC); calcium-activated K+ (KCa); sodium nitroprusside (SNP); open probability (Po); number of multiple channels (N); intracellular free Ca2+ ([Ca2+]i); oligodeoxynucleotide (ODN); S-nitroso-N-acetyl-penicillamine (SNAP), diethyl pyrocarbonate (DEPC), N-ethylmaleimide (NEM); chromium mesoporphyrin (Cr-MP); heme-l-lysinate (HLL); iberiotoxin (IbTX); dehydrosoyasaponin I (DHS); half-activation potential (V1/2); green fluorescent protein (GFP); picoamperes/picofarads (pA/pF); superoxide dismutase (SOD).
References
- 1.Furchgott RF, Zawadski JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;228:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can J Physiol Pharmacol. 1998;76:1–15. doi: 10.1139/cjpp-76-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang R, Wang ZZ, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durante W, Peyton KJ, Schafer AI. Platelet-derived growth factor stimulates heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2666–2672. doi: 10.1161/01.atv.19.11.2666. [DOI] [PubMed] [Google Scholar]
- 5.Leffler CW, Nasjletti A, Johnson RA, Fedinec AL. Contributions of prostacyclin and nitric oxide to carbon monoxide-induced cerebrovascular dilation in piglets. Am J Physiol. 2001;280:H1490–H1495. doi: 10.1152/ajpheart.2001.280.4.H1490. [DOI] [PubMed] [Google Scholar]
- 6.Motterlini R, et al. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 7.Sabaawy HE, et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 8.Pannen BHJ, Bauer M. Differential regulation of hepatic arterial and portal venous vascular resistance by nitric oxide and carbon monoxide in rats. Life Sci. 1998;62:2025–2033. doi: 10.1016/s0024-3205(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 9.Mancuso C, Tringali G, Grossman A, Preziosi P, Navarra P. The generation of nitric oxide and carbon monoxide produces opposite effects on the release of immunoreactive interleukin-1beta from the rat hypothalamus in vitro: evidence for the involvement of different signaling pathways. Endocrinology. 1998;139:1031–1037. doi: 10.1210/endo.139.3.5822. [DOI] [PubMed] [Google Scholar]
- 10.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 11.Kaide JI, et al. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J Clin Invest. 2001;107:1163–1171. doi: 10.1172/JCI11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Wu L, Wang ZZ. The direct effect of carbon monoxide on KCachannels in vascular smooth muscle cells. Pflügers Arch. 1997;434:285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Wu L. The chemical modification of KCachannels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- 14.Werkstrom V, Ny L, Persson K, Andersson KE. Carbon monoxide-induced relaxation and distribution of haem oxygenase isoenzymes in the pig urethra and lower oesophagogastric junction. Br J Pharmacol. 1997;120:312–318. doi: 10.1038/sj.bjp.0700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munujos P, Knaus H, Kaczorowski GJ, Garcia ML. Cross-linking of charybdotoxin to high-conductance calcium-activated potassium channels: identification of the covalently modified toxin residue. Biochemistry. 1995;34:10771–10776. doi: 10.1021/bi00034a009. [DOI] [PubMed] [Google Scholar]
- 16.Abderrahmane A, et al. Direct activation of KCa channel in airway smooth muscle by nitric oxide: involvement of a nitrothiosylation mechanism? Am J Respir Cell Mol Biol. 1998;19:485–497. doi: 10.1165/ajrcmb.19.3.2996. [DOI] [PubMed] [Google Scholar]
- 17.Wang, R., and Zhao, W. 1999. The integrated regulation of vascular tone by carbon monoxide and nitric oxide. Proceedings of the 8th International Symposium of the Society of Chinese Bioscientists in America. Hong Kong, China. POS-W31. (Abstract).
- 18.Tenhunen R, Tokola O, Linden IB. Haem arginate: a new stable haem compound. J Pharm Pharmacol. 1987;39:78–786. doi: 10.1111/j.2042-7158.1987.tb05119.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Ozanne SE, Hales CN, Ashford MLJ. Effects of chemical modification of amino and sulfhydryl groups on KATPchannel function and sulfonylurea binding in CRI-G1 insulin-secreting cells. J Membr Biol. 1994;139:167–181. doi: 10.1007/BF00232621. [DOI] [PubMed] [Google Scholar]
- 20.Leffler CW, et al. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Meera P, Song M, Knaus H-G, Toro L. Molecular constituents of maxi KCachannels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997;502:545–559. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McManus OB, et al. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 23.Xian M, Chen X, Liu Z, Wang K, Wang PG. Inhibition of papain by S-nitrosothiols. Formation of mixed disulfides. J Biol Chem. 2000;275:20467–20473. doi: 10.1074/jbc.M001054200. [DOI] [PubMed] [Google Scholar]
- 24.Koh SD, Campbell JD, Carl A, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle cells. J Physiol. 1995;489:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CH, et al. Nitric oxide activates Ca2+-activated K+channels in cultured bovine adrenal chromaffin cells. Neuroscience Lett. 1998;248:127–129. doi: 10.1016/s0304-3940(98)00316-4. [DOI] [PubMed] [Google Scholar]
- 26.Carrier GO, Fuchs LC, Winecoff AP, Giulumian AD, White RE. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. Am J Physiol. 1997;273:H76–H84. doi: 10.1152/ajpheart.1997.273.1.H76. [DOI] [PubMed] [Google Scholar]
- 27.Ahring PK, Strobaek D, Christophersen P, Olesen SP, Johansen TE. Stable expression of the human large-conductance Ca2+-activated K+channel alpha- and beta-subunits in HEK293 cells. FEBS Lett. 1997;415:67–70. doi: 10.1016/s0014-5793(97)01096-x. [DOI] [PubMed] [Google Scholar]
- 28.Wanner SG, et al. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochemistry. 1999;38:5392–5400. doi: 10.1021/bi983040c. [DOI] [PubMed] [Google Scholar]
- 29.Mistry DK, Garland CJ. Characteristics of single, large-conductance calcium-dependent potassium channels (BKCa) from smooth muscle cells isolated from the rabbit mesenteric artery. J Membr Biol. 1998;164:125–138. doi: 10.1007/s002329900399. [DOI] [PubMed] [Google Scholar]
- 30.Wu JV, Shuttleworth TJ, Stampe P. Clustered distribution of calcium sensitivities: an indication of hetero-tetrameric gating components in Ca2+-activated K+channels reconstituted from avian nasal gland cells. J Membr Biol. 1996;154:275–282. doi: 10.1007/s002329900152. [DOI] [PubMed] [Google Scholar]
- 31.Tseng-Crank J, et al. Cloning, expression, and distribution of a Ca2+-activated K+channel beta-subunit from human brain. Proc Natl Acad Sci USA. 1996;93:9200–9205. doi: 10.1073/pnas.93.17.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269:3921–3924. [PubMed] [Google Scholar]
- 33.Traylor TG, Sharma VS. Why NO? Biochemistry. 1992;31:2847–2849. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]