Abstract
Two ATP-binding cassette (ABC) transporters, ABCG5 and ABCG8, have been proposed to limit sterol absorption and to promote biliary sterol excretion in humans. To test this hypothesis, a P1 clone containing the human ABCG5 and ABCG8 genes was used to generate transgenic mice. The transgenes were expressed primarily in the liver and small intestine, mirroring the expression pattern of the endogenous genes. Transgene expression only modestly affected plasma and liver cholesterol levels but profoundly altered cholesterol transport. The fractional absorption of dietary cholesterol was reduced by about 50%, and biliary cholesterol levels were increased more than fivefold. Fecal neutral sterol excretion was increased three- to sixfold and hepatic cholesterol synthesis increased two- to fourfold in the transgenic mice. No significant changes in the pool size, composition, and fecal excretion of bile acids were observed in the transgenic mice. Transgene expression attenuated the increase in hepatic cholesterol content induced by consumption of a high cholesterol diet. These results demonstrate that increased expression of ABCG5 and ABCG8 selectively drives biliary neutral sterol secretion and reduces intestinal cholesterol absorption, leading to a selective increase in neutral sterol excretion and a compensatory increase in cholesterol synthesis.
Introduction
Multiple physiological mechanisms limit the entry of dietary sterols into the bloodstream. Excess cholesterol is removed from the body either by direct secretion into the bile or after conversion to bile acids. Although the mechanisms by which dietary sterols are absorbed by the intestine and secreted into the bile have been well characterized physiologically, the machinery responsible for these two processes has not been molecularly defined. The most abundant sterols in the human diet are cholesterol, the principal animal-derived sterol, and sitosterol, the major plant sterol. These two sterols, although structurally very similar, are handled quite differently by the intestine and liver of normal mammals. The absorption of sitosterol is significantly more limited than that of cholesterol. Humans absorb less than 5% of dietary sitosterol (1), and the small amount of sitosterol that reaches the liver is preferentially secreted into the bile (2). Consequently, plasma levels of sitosterol are very low (<1 mg/dl) in normal individuals. A much higher proportion (45–55%) of dietary cholesterol is absorbed by the proximal small intestine in humans, and the fractional excretion of sterols into the bile is lower for cholesterol than for sitosterol (3).
Recently, a critical component of the transport machinery for dietary sterols was revealed by the finding that mutations in the genes encoding the ATP-binding cassette (ABC) half-transporters ABCG5 and ABCG8 cause sitosterolemia, a rare autosomal recessive disorder of sterol metabolism (4, 5). Patients with sitosterolemia have increased fractional absorption and decreased biliary secretion of all dietary neutral sterols (3, 6–8), which invariably leads to dramatically elevated plasma levels of sitosterol and other plant sterols. Most sitosterolemic individuals are also hypercholesterolemic (9). The disease phenotypically resembles homozygous familial hypercholesterolemia in that both diseases are characterized by the development of xanthomas in childhood accompanied by premature coronary atherosclerosis (6, 9, 10). ABCG5 and ABCG8 are expressed predominantly in hepatocytes and enterocytes in the proximal small intestine of humans and mice (11). The expression levels of both genes are coordinately increased in these cells in mice consuming a high-cholesterol diet (4). LXRα, a nuclear receptor that plays a key role in regulating genes involved in cholesterol trafficking (12), is required for the increased expression of mouse Abcg5 and Abcg8 in response to dietary cholesterol (11). These findings implicate both proteins as key components of the pathways responsible for trafficking of dietary sterols.
The relationship between ABCG5/ABCG8 activity and sterol transport rates has not been defined. It is also not known whether increased expression of ABCG5 and ABCG8 is sufficient to promote cholesterol secretion, or whether other steps in the transport process are rate-limiting. Furthermore, the primary transport substrate of ABCG5/ABCG8 has not been identified, and the effects of increased expression of these proteins on other major biliary lipids (phospholipids and bile acids) are not known. To address these issues, we developed transgenic mice that contain a large fragment of genomic DNA that includes the human ABCG5 and ABCG8 genes under the control of their own regulatory sequences. We chose this approach since mutations in either gene cause an identical phenotype in humans, presumably because ABCG5 and ABCG8 function as a heterodimer (4). In this study, we show that increased expression of ABCG5 and ABCG8 in liver and small intestine dramatically increases biliary cholesterol secretion, reduces cholesterol absorption, and increases hepatic cholesterol synthesis.
Methods
Generation of transgenic mice expressing human ABCG5 and ABCG8.
A P1 clone (35B6) with a 90-kb insert containing both human ABCG5 and ABCG8 genes was purchased from Incyte Genomics Inc. (Palo Alto, California, USA). The clone was linearized with NotI (Promega Corp., Madison, Wisconsin, USA), size-fractionated on a 1% SeaPlaque agarose gel (BioWhittaker Molecular Applications, Rockland, Maine, USA) by pulse-field gel electrophoresis (Bio-Rad CHEF-DR III System; BioRad Laboratories Inc., Hercules, California, USA), and purified using GELase Agarose Gel-Digesting Preparation Kit (EPICENTRE, Madison, Wisconsin, USA). The purified DNA fragments were dissolved in microinjection buffer (10 mM Tris-HCl [pH 7.5], 0.1 mM EDTA, 100 mM NaCl) at a concentration of about 0.75–1.5 ng/μl and microinjected into fertilized mouse eggs (from C57BL/6J × SJL F2 mice, stock no. 100012; The Jackson Laboratory, Bar Harbor, Maine, USA) to generate human ABCG5 and ABCG8 transgenic mice (13). Founder mice were screened for transgene integration by PCR amplification using oligonucleotides that were specific for the human transgene (5′-CTCCATGTTTCCCAGCACAGCCCTTCTCC-3′ and 5′-CTGCAGCTCTCTTAGACCCAGCTGCTGC-3′), and the results were confirmed by Southern blotting. A total of 23 of the 57 offspring contained at least one copy of the transgene. Founder animals were crossed with C57BL/6J × SJL F1 mice, and genomic DNA was extracted from the tails of the offspring. We estimated the copy number of the transgene by performing Southern blot analysis and comparing band intensities to those of an equal amount of human genomic DNA. Three lines of human ABCG5 and ABCG8 transgenic mice containing different copy numbers of the transgene were established.
Mice were housed in plastic cages in a temperature-controlled room (22°C) with 12-hour-light/12-hour-dark cycling (daylight cycle from 6:00 am to 6:00 pm). Animals were fed ad libitum a cereal-based rodent diet (Diet 7001; Harlan Teklad, Madison, Wisconsin, USA) containing 0.2 mg of cholesterol and 40 mg of total lipid per gram of diet. For cholesterol feeding studies, cholesterol was added to a powdered form of the same cereal-based diet. All animal procedures were performed with the approval of the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center.
Northern blot analysis.
Total RNA was extracted from tissues using the RNA Stat-60 kit (Tel-Test Inc., Friendswood, Texas, USA). Northern blot analysis was performed exactly as previously described (14) using [α-32P]-labeled cDNA probes (Megaprime DNA Labeling System; Amersham Biosciences Corp., Piscataway, New Jersey, USA) for ABCG5, ABCG8, mouse Abcg5, mouse Abcg8, and cyclophilin (4) in Rapid-hyb buffer (Amersham Biosciences Corp.).
Plasma and hepatic lipid analysis.
Plasma and hepatic levels of cholesterol and triglyceride were measured by the methods described previously (15) using the Cholesterol kit (catalog no. 1127771; Roche Molecular Biochemicals, Mannheim, Germany) and Infinity Triglycerides Reagent (catalog no. 343-500P; Sigma, St. Louis, Missouri, USA). Plasma lipoprotein size-fractionation was performed using fast protein liquid chromatography as described (16). Plasma noncholesterol sterols were measured using gas chromatography (8).
Biliary lipid composition.
Bile was removed from the gallbladders of anesthetized mice using a 30.5-gauge needle. The concentrations of cholesterol, phospholipids, and bile acids were measured as described previously (17).
Quantitative real-time PCR.
Real-time PCR was performed on pooled total RNA samples from five mice in each group as described previously (18).
Cholesterol absorption.
Intestinal cholesterol absorption was determined by a fecal dual-isotope ratio method (19). Briefly, individually housed mice were dosed intragastrically with a mixture of 2 μCi [5,6-3H] sitostanol (American Radiolabeled Chemicals Inc., St. Louis, Missouri, USA) and 1 μCi [4-14C] cholesterol (New England Nuclear Corp., Boston, Massachusetts, USA), and then returned to fresh cages. Feces were collected for 3 days, 1 g of feces from each animal was extracted with chloroform/methanol (vol/vol = 2/1), and the ratio of 14C to 3H in each sample was calculated. The percent cholesterol absorption was then determined as described (20).
Fecal neutral and acidic sterol excretion.
Feces were collected from individually housed mice over a 3-day period and were dried, weighed, and ground to a powder. The total bile acid content in 0.5 g of the ground feces was determined using a 3α-hydroxysteroid dehydrogenase assay (15, 21). A second 0.5 g aliquot of feces was subjected to alkaline hydrolysis at 100–120°C for 8 hours and dried prior to the addition of 10 ml of water and 10 ml of ethanol. Samples were then extracted in 15 ml of petroleum ether to which 1.0 mg of 5-cholestene (Sigma) had been added as an internal standard. The amount of neutral sterols (cholesterol, coprostanol, and cholestanone) in the extracts was quantitated by gas chromatography (19).
Bile acid pool size.
The bile acid pool size was determined as described previously (15). The gallbladder, liver, and small intestine with its content were collected and extracted with ethanol in the presence of 14C-taurocholic acid (TCA), which was used as an internal recovery control. The bile acids were further purified using a Bond Elut C18 column (part no. 12102052; Varian Inc., Harbor City, California, USA). Individual bile acids including TCA, tauro-β-muricholic acid, tauroursocholic acid, taurohyodeoxycholic acid, taurochenodeoxycholic acid, taurodeoxycholic acid, taurolithocholic acid, and glycocholic acid were quantified using HPLC by comparisons with standards of known concentration. The bile acid pool size was calculated as the sum of all the bile acids.
In vivo cholesterol synthesis.
The rates of in vivo cholesterol synthesis in liver and intestine were measured as described (15). Mice were given an intraperitoneal injection of 40 mCi of [3H] water (New England Nuclear Corp.), anesthetized, and exsanguinated after 1 hour. Aliquots of liver, and of small intestine (separated into three equal parts), were saponified, and the radiolabeled digitonin-precipitable sterols were extracted and measured.
Statistical analysis.
All the data are reported as the mean ± SEM. The differences between the mean values were tested for statistical significance by the two-tailed Student’s t test.
Results
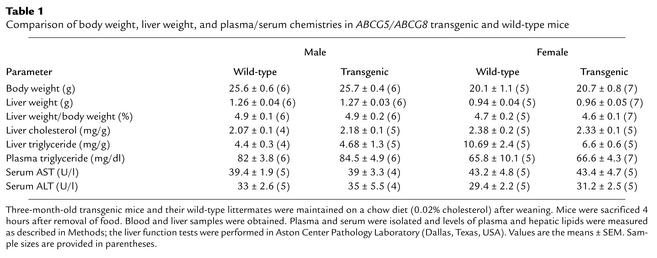
Three independent lines of transgenic mice were established with 4 to 14 copies of a linearized P1 plasmid containing a 90-kb insert. The insert contained the whole human ABCG5 and ABCG8 genomic sequences, about 30 kb of flanking sequence at the 3′ end of ABCG5, and 6 kb of flanking sequence at the 3′ end of ABCG8. Exons 6–13 of a gene of unknown function (CG1-60; GenBank accession no. AF151818) were included in the insert at the 3′ end of ABCG5. The mouse line (14–2) with the highest copy number of the transgene (∼14) was used for the studies described in this paper. Selected studies were also performed with another transgenic line (6–1) containing approximately ten copies of the transgene to confirm observations made in the first line. No significant differences in litter size, body weight, liver weight, or liver enzymes were detected between transgenic and wild-type mice (Table 1).
Table 1.
Comparison of body weight, liver weight, and plasma/serum chemistries in ABCG5/ABCG8 transgenic and wild-type mice
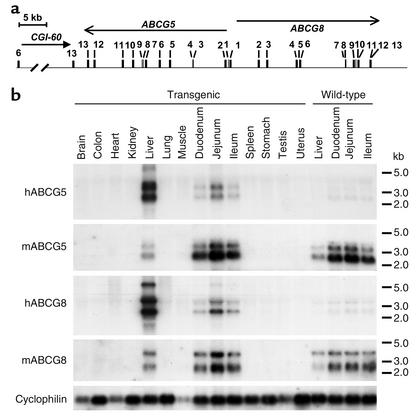
The human transgenes were expressed in the liver and in the small intestine (Figure 1b), which is similar to the expression patterns of the endogenous genes. Two major mRNAs for human ABCG5 (2.4 and 3.4 kb) and human ABCG8 (2.6 and 3.8 kb) that differ in their 3′-untranslated region were detected (22). A third human transcript of higher molecular weight was detected in the liver and the jejunum of the transgenic animals (5.9 and 7.4 kb, respectively, for human ABCG5 and ABCG8); these larger mRNA species correlated directly with the amounts of the other two mRNA species. The expression of human ABCG5 and ABCG8 in the liver and intestine of the transgenic animals was not associated with major changes in the expression levels of Abcg5 and Abcg8; however, the relative levels of expression of human ABCG5 and ABCG8 and the endogenous murine genes differed in the liver and small intestine. The human transgenes were expressed at higher levels in the liver than in the small intestine, resembling the pattern seen in human tissues (4). In contrast, the mouse genes were expressed at a higher level in the intestine than in the liver. These results suggest that cis-acting elements present in the human transgene confer increased expression in the liver. An identical tissue-specific pattern and relative level of expression was seen in a different line of transgenic mice (6–1) (data not shown). RT-PCR was performed to determine whether human ABCG5 and ABCG8 were expressed in other tissues at levels that were too low to detect by mRNA blotting. Trace amounts of human ABCG5 and ABCG8 mRNA were detected in the ovaries of the transgenic mice. Neither the human transgenes nor the endogenous mouse genes were expressed in peritoneal macrophages of the transgenic animals (data not shown).
Figure 1.
(a) The structure of the human ABCG5/ABCG8 transgene. A P1 clone (35B6; Incyte Genomics Inc.) containing a 90-kb insert was linearized with NotI, purified, and injected into fertilized embryos as described in Methods. The insert contains the entire coding regions of human ABCG5 and ABCG8, exons 6–13 of the putative gene CGI-60 at the 3′ end of ABCG5, and 6 kb of flanking sequence at the 3′ end of ABCG8. (b) Tissue distribution of human ABCG5 and ABCG8 mRNA in the transgenic mice. Total RNA was extracted from tissues of wild-type and transgenic mice consuming a chow diet. A total of 15 μg of RNA was fractionated on a 1% formaldehyde gel, transferred to a nylon membrane, and hybridized with 1 × 106 cpm/ml of [α-32P]-labeled cDNA probes for human and mouse ABCG5 and ABCG8 at 65°C in Rapid-hyb Buffer (Amersham Biosciences Corp.). After stringent washing, the membrane was exposed to Kodak X-OMAT Blue XB-1 films (Eastman Kodak Co., Rochester, New York, USA) for 12–36 h at –80°C. This experiment was repeated, and the results were similar.
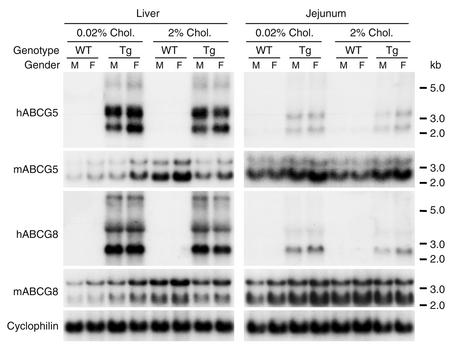
The hepatic mRNA levels of mouse Abcg5 and Abcg8 were 1.5-fold higher in the female wild-type mice than in the male wild-type mice. No sex-specific differences in the expression levels of the endogenous genes were detected in the intestine (Figure 2). Human ABCG5 was expressed in the livers at a 1.5-fold higher level in the female transgenic mice than in the male transgenic mice, whereas no sex-specific significant differences in the expression of human ABCG8 were seen. In mice (6–1) with a lower copy number of the transgene (∼10), expression of both ABCG5 and ABCG8 was more than twofold higher in the liver of the female transgenic mice compared with the male transgenic mice (data not shown). In wild-type animals, the high-cholesterol diet increased expression levels of the endogenous mouse genes twofold in the liver and 1.5-fold in the intestine. In transgenic animals, no significant changes were seen in the levels of endogenous mRNA or the transgene mRNAs after 3 weeks on the high-cholesterol diet.
Figure 2.
Northern blot analysis of ABCG5 and ABCG8 in male and female transgenic mice. Total RNA was isolated from the liver and jejunum of mice on a chow or 2% cholesterol diet. Equal amounts of total RNA from each mouse in each group were pooled and subjected to Northern blotting as described for Figure 1b. This experiment was repeated, and the results were similar. WT, wild-type transgene.
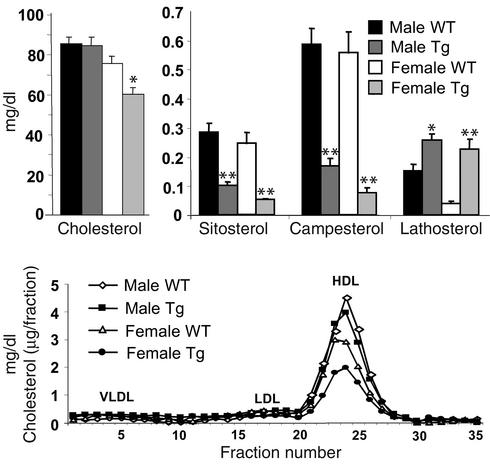
The mean fasting plasma cholesterol levels were not significantly different in the male transgenic and control mice (Figure 3, left top) but were significantly lower in the female transgenic mice (60.2 vs. 75.7 mg/dl). Pooled fasting plasma samples from chow-fed transgenic and wild-type mice were subjected to fast protein liquid chromatography to determine the distribution of lipoprotein cholesterol (16). No significant difference in the fractionation profile was seen in the two groups of male mice (Figure 3, bottom). Although female transgenic mice had reduced plasma levels of HDL-cholesterol, which accounts for the reduction in plasma cholesterol in these animals, transgene expression did not influence the size distribution of lipoprotein particles.
Figure 3.
Levels of plasma sterols and lipoprotein profiles of ABCG5 and ABCG8 transgenic mice. Male and female 12-week-old transgenic mice and their littermate controls (n = 4–7 in each group) were maintained on a chow diet. After a 4-hour fast, the animals were sacrificed and the venous blood collected. The plasma was isolated by centrifugation, the plasma levels of cholesterol were measured as described in Methods, and the noncholesterol sterol levels of plasma were assayed by gas chromatography as described (8). Pooled plasma from each group of mice was subject to fast protein liquid chromatography fractionation, and the cholesterol content in each fraction was assayed as described in Methods. WT, wild-type; Tg, transgenic. *P < 0.05, **P < 0.01.
Plasma levels of other neutral sterols were measured by gas chromatography (Figure 3, right top). The mean plasma levels of sitosterol in fasted mice were reduced by 64% and 79% in the male and female transgenic animals, respectively. Marked reductions in another major plant sterol, campesterol, were also detected. In contrast to the plant sterols, the levels of lathosterol, a biosynthetic precursor to cholesterol, were significantly increased in the transgenic animals, suggesting that cholesterol synthesis was elevated (8).
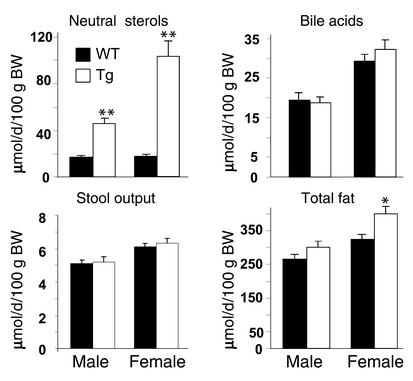
To determine the effect of ABCG5 and ABCG8 transgene expression on whole-body cholesterol turnover, the daily output of neutral sterols in the feces was measured in transgenic mice and their littermate controls. The amount of neutral sterol in the feces (μmol/d/100 g body weight) was threefold higher in transgenic male mice, and sixfold higher in the transgenic female mice. No significant differences in fecal bile acid excretion were found. The daily stool output (g/100 g body weight) did not differ between transgenic and control mice. Total fecal fat was significantly higher in female transgenic mice but not in male transgenics (Figure 4).
Figure 4.
The fecal lipid content and composition of ABCG5 and ABCG8 transgenic mice. Feces were collected from 9- to 10-week-old transgenic mice and their littermate controls (n = 10 in each group) for 3 days while they consumed a chow diet. The daily total fecal weight, neutral sterols, bile acids, and total fat were measured and expressed per 100 g body weight (BW). *P < 0.01, **P < 0.001.
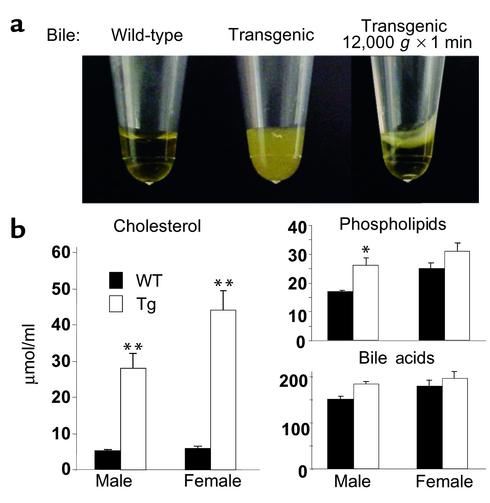
The increased neutral sterol excretion in the transgenic mice may be caused by an increased biliary cholesterol secretion and/or by decreased cholesterol absorption. To determine which of these processes was affected, the concentrations of cholesterol, phospholipids, and bile acids were first measured in bile from transgenic and wild-type animals (four to five in each group). The genotypes of the animals could be accurately assigned by visual inspection of the bile; the bile from transgenic animals was opaque, whereas that of the control animals was clear (Figure 5a). The bile was subjected to centrifugation (12,000 g for 1 minute), which resulted in a phase separation with the opaque material floating to the top of the tube over a clear yellow infranatant (Figure 5a, right). Pooled bile samples (n = 5) were delipidated (23) and assayed for protein. The concentration of protein in the two bile samples differed slightly (6.1 vs. 7.3 mg/ml). Microscopic examination of the bile revealed large amounts of amorphous material in the transgenic animals that was not present in the bile from littermate wild-type controls. The bile from the transgenic animals was inspected using a microscope with a polarized filter, and no crystals were observed (data not shown). The most striking difference between the bile of transgenic and that of wild-type mice was the concentration of cholesterol, which was more than fivefold and more than sevenfold higher in the male and female transgenic mice, respectively. There was a modest but significant (P < 0.05) increase in phospholipid concentration of the bile from the male transgenic animals, but no significant differences in bile acid concentration between transgenic and wild-type animals (Figure 5b).
Figure 5.
A comparison of the appearance of the bile (a) and the biliary lipid concentrations and composition (b) in the ABCG5/ABCG8 transgenic mice and littermate controls. The gallbladder bile was collected from 12-week-old wild-type (n = 4 in each group) and transgenic (n = 5 in each group) mice. The mice were fed ad libitum a chow diet and sacrificed nonfasted in the mid–light cycle. The biliary cholesterol, bile acids, and phospholipids were measured as described in Methods. This experiment was repeated in another line of transgenic mice (6–1), and the results were similar. * P < 0.05, **P < 0.01.
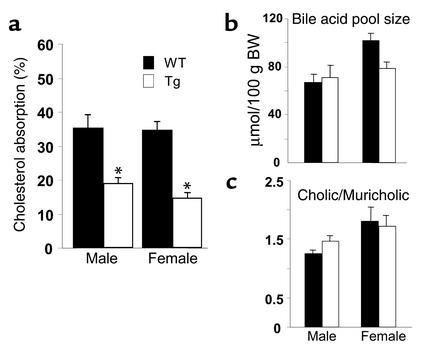
The fractional absorption of cholesterol was then measured by the fecal dual-isotope ratio method in the transgenic and wild-type mice. The intestinal absorption of cholesterol was reduced about 50% in both male and female transgenic animals compared with their wild-type counterparts (Figure 6a). The decrease in fractional absorption of cholesterol could not be attributed to a change in the amount or composition of bile acids, as bile acid pool size and the ratios of cholic acid and muricholic acid were not significantly different in transgenic and wild-type mice (Figure 6, b and c).
Figure 6.
(a) The fractional absorption of dietary cholesterol in the ABCG5/ABCG8 transgenic mice. A total of six male and six female 9- to 10-week-old transgenic mice and their littermate controls were gavaged with [14C] cholesterol and [3H] sitostanol. Stool was collected for 3 days, and the ratio of the two isotopes in feces was measured to determine the fractional absorption of cholesterol, as described in Methods. (b) The bile acid pool size in the ABCG5/ABCG8 transgenic and wild-type mice. The content of total bile acids from the small intestine, gallbladder, and liver of chow-fed mice (12–13 weeks old) of the indicated genotype (n = 6 in each group) was determined by extracting the bile acids in ethanol. The bile acids were analyzed using HPLC as described in Methods. (c) The ratios of cholic to muricholic acids in the total bile acid pool. *P < 0.01.
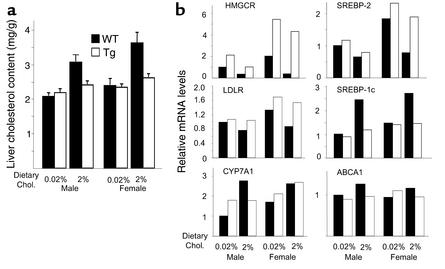
Susceptibility to diet-induced hepatic cholesterol accumulation was determined next in transgenic and wild-type mice fed a high-cholesterol (2%) diet for 3 weeks. The mean levels of hepatic cholesterol are shown in Figure 7a. On a chow diet, the cholesterol content was similar in the transgenic and wild-type animals. As expected, ingestion of a high-cholesterol diet was associated with an increase in hepatic cholesterol in both the male and the female wild-type animals. In contrast, the levels of cholesterol in the liver did not change significantly in the transgenic mice fed a high-cholesterol diet.
Figure 7.
(a) The hepatic cholesterol levels in the ABCG5/ABCG8 transgenic and wild-type mice. Individually housed male and female 12-week-old mice of the indicated genotypes were fed a powdered chow diet (0.02% cholesterol) or the same diet containing 2% cholesterol for 21 days. The mice were sacrificed without fasting in the mid–light cycle. The livers were obtained and rapidly frozen in liquid nitrogen. Lipids were extracted and the hepatic cholesterol content was measured as described in Methods. The values shown represent the means of individual measurements in each experimental group (n = 5). (b) Quantitative real-time PCR from liver RNA of ABCG5/ABCG8 transgenic and wild-type mice. Total RNA from the liver of mice in each group (n = 5) was isolated and pooled for real-time PCR using oligonucleotides as described (18, 39). Cyclophilin was used as an internal control for these studies, and the values represent the amount relative to the amount in the chow-fed wild-type males, which was arbitrarily standardized to 1. This experiment was repeated in the 6-1 transgenic mouse line, and the results were similar.
Quantitative real-time PCR was used to measure the mRNA levels of key genes of cholesterol metabolism in the livers of chow- and cholesterol-fed mice (Figure 7b). The data are expressed relative to the level of the transcript in the wild-type male animals on a chow diet. High-level expression of human ABCG5 and ABCG8 appeared to selectively increase mRNA levels of genes participating in cholesterol synthesis (HMG-CoA reductase; HMGCR) without major changes in the levels of mRNAs encoding proteins involved in transcriptional regulation of cholesterol (sterol regulatory element–binding proteins-1c and -2; SREBP-1c and SREBP-2), bile acid synthesis (cholesterol 7α-hydroxylase; CYP7A1), hepatic cholesterol uptake (LDL receptor; LDLR), and cholesterol trafficking (ABC transporter A1; ABCA1). We also checked the hepatic mRNA levels of scavenger receptor class B type I (SR-BI), fatty acid synthase, sterol 12α-hydroxylase, short heterodimer partner, farnesoid X receptor, and Na+-taurocholate–cotransporting polypeptide, and no significant changes in these mRNAs were seen between transgenic and wild-type chow-fed animals (data not shown).
As expected, ingestion of a high-cholesterol diet was associated with reduced expression of HMGCR and increased mRNA levels of CYP7A1. The levels of SREBP-1c mRNA, which is an LXR target (24), were also increased. In general, expression of the transgene attenuated the effect of cholesterol feeding on suppression of cholesterol-regulated genes.
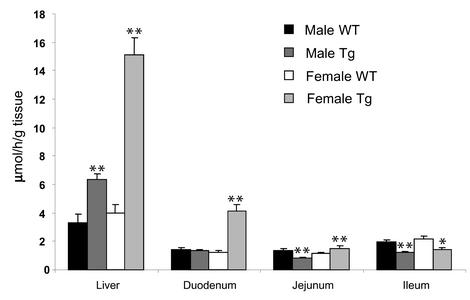
To examine the effect of robust expression of ABCG5 and ABCG8 on cholesterol synthesis in the liver and intestine, we measured in vivo cholesterol synthesis using tritiated water (22). Cholesterol synthesis in the liver was increased in both the male (twofold) and the female (about fourfold) transgenic mice (Figure 8), which is consistent with the larger increase in HMGCR mRNA levels in the female transgenic animals. Cholesterol synthesis was increased threefold in the duodenum of female transgenic mice, decreased in the jejunum and ileum of male transgenic mice, and decreased in the ileum of female transgenic mice. No significant differences in fatty acid synthesis were found between the transgenic and wild-type mice (data not shown).
Figure 8.
In vivo cholesterol synthesis rates in the ABCG5/ABCG8 transgenic mice and wild-type controls. Four-month-old transgenic and control mice (n = 6 in each group) maintained on a chow diet were injected intraperitoneally with 40 mCi of [3H] water. The mice were sacrificed 1 hour later, and the tissues were collected and processed as described in Methods. *P < 0.05, **P < 0.01. This experiment was repeated, and the results were similar.
Discussion
The major finding of this paper is that increased expression of ABCG5 and ABCG8 in mice causes profound alterations in cholesterol trafficking. Expression of a human transgene encoding ABCG5 and ABCG8 increased biliary cholesterol concentrations more than fivefold but did not significantly affect the bile acid pool size or composition. The phospholipid concentration of the bile was only modestly increased (10%) in the male mice. These data demonstrate that increased expression of ABCG5 and ABCG8 had a specific effect on neutral sterol transport into bile. The transgenic mice also had a 50% reduction in the fractional absorption of dietary cholesterol. The increase in biliary cholesterol secretion and the decrease in fractional cholesterol absorption in the transgenic animals were associated with a three- to sixfold increase in fecal neutral sterol excretion and a two- to fourfold increase in hepatic cholesterol synthesis. We conclude that ABCG5 and ABCG8 play a crucial role in the enterohepatic trafficking of cholesterol in mammals and that the expression level of these proteins directly affects the rate of biliary sterol excretion.
ABCG5 and ABCG8 are coordinately expressed and regulated (4, 11), which is consistent with their participation in the same transport process. Moreover, the intracellular trafficking of ABCG5 and ABCG8 is dependent on their coexpression in cells. Expression of either half-transporter alone in cultured hepatocytes results in the subunit being confined to the endoplasmic reticulum, whereas coexpression of both proteins in cells allows for transport to the plasma membrane (25). To avoid artifacts associated with unequal levels of expression of ABCG5 and ABCG8, we exploited the close physical apposition of the two genes and used a large (90 kb) genomic fragment containing both genes in their own genomic context to make transgenic mice. The human ABCG5 and ABCG8 transcripts were predominantly expressed in the liver and proximal small intestine, which mirrors the pattern of expression in mice and humans (4). The strong concordance between the tissue distributions of the transgenes and the endogenous genes indicates that the regulatory sequences conferring tissue-specific expression are contained within the P1 transgene. The mouse genes (Abcg5 and Abcg8) were more highly expressed in the intestine than in the liver, whereas the human transgenes were more highly expressed in the liver than in the intestine (Figure 1b). This pattern of tissue expression recapitulates that observed in human tissues (4), and the data suggest that cis-acting elements contribute to the species-specific differences in the relative expression levels of ABCG5 and ABCG8 in the liver and intestine. Systematic comparison of the genomic sequences of the ABCG5 and ABCG8 genes among several species may reveal sequences that contribute to the observed differences in expression.
The mechanism by which cholesterol is secreted into the bile remains poorly characterized at a molecular level. ABCG5 and ABCG8 were implicated in this process when it was discovered that genetic defects in either gene caused sitosterolemia (4, 26), a disorder in which biliary cholesterol secretion is decreased about fivefold and the preferential excretion of plant sterols observed in normal individuals is abolished (2, 27). The finding that increased expression of ABCG5 and ABCG8 in mice is associated with very high levels of biliary cholesterol indicates that changes in the activity of ABCG5 and ABCG8 directly influence the flux of cholesterol into the bile. The marked increase in biliary cholesterol secretion was associated with only a modest increase in phospholipid secretion. This finding is consistent with the observation that sitosterolemic patients have reduced biliary sterol excretion but maintain normal biliary phospholipid levels (28). Expression of the transgenes had no effect on the composition, pool size, biliary secretion, and fecal excretion of bile acids. These data, together with the localization of ABCG5 and ABCG8 to the bile canalicula in cultured hepatocytes (25), indicate that they are directly and specifically involved in the translocation of neutral sterols across the plasma membranes of hepatocytes, and, by extension, of enterocytes. Neutral sterols may therefore constitute the primary transport substrate(s) for the ABCG5/ABCG8 heterodimer.
The biliary excretion of cholesterol into the bile is usually closely coupled to phospholipid excretion. Mice in which the phosphatidylcholine transporter (ABCB4/mdr2) has been inactivated have only trace amounts of cholesterol in bile (29), which suggested that the biliary excretion of cholesterol was mechanistically coupled to the secretion of phospholipids. Follow-up studies revealed that acute infusion of bile acids partially restored biliary cholesterol secretion in mdr2-deficient mice, indicating that excretion of cholesterol and phospholipids may be transiently uncoupled in the absence of mdr2 (30). However, the apparent increase in biliary cholesterol secretion in the bile acid–treated animals may simply represent leaching of cholesterol from the bile canalicular membrane. The results of the present study indicate that biliary cholesterol secretion can be increased substantially with little concomitant increase in phospholipid secretion. Therefore, while it appears that cholesterol cannot be efficiently secreted into bile in the absence of phospholipids, large changes in the rate of cholesterol secretion into bile can be achieved without altering the biliary phospholipid concentrations.
The ABCG5/ABCG8 transgenic mice have reduced fractional absorption of dietary cholesterol. This reduction in fractional cholesterol absorption may be due in part to an increased efflux of cholesterol from enterocytes via the action of ABCG5 and ABCG8. Solubilized sterols enter the enterocytes of the duodenum and the jejunum either by passive diffusion or by a yet-to-be-defined protein-mediated process (31). Cholesterol is esterified by acyl-CoA:cholesterol acyltransferase-2 (ACAT-2) to form cholesterol esters, which are secreted from the basolateral surfaces of the enterocytes as part of chylomicrons. The absence of ABCG5 and ABCG8 in sitosterolemic patients is associated with increased fractional cholesterol absorption (3, 8). Increased expression of these two proteins in the enterocytes likely limits the amount of cholesterol delivered to ACAT-2 for esterification. Alternatively, the reduction in cholesterol absorption in our transgenic mice may be secondary to the increased delivery of cholesterol to the intestinal lumen from the liver. The delivery of increased biliary cholesterol to the intestine may dilute the labeled cholesterol tracer, or compete with dietary cholesterol for micellar solubilization and uptake (32, 33). The development of mice in which ABCG5 and ABCG8 are expressed exclusively in the intestine or in the liver will be required to determine the role of each tissue in the reduction in cholesterol absorption in these mice.
Expression of ABCG5 and ABCG8 in mice had little effect on cholesterol levels in the liver and plasma but caused a marked reduction in plasma levels of plant sterols. The accelerated loss of cholesterol from the body caused by reduced absorption of dietary cholesterol and increased secretion of cholesterol into the bile was balanced by an increase in hepatic cholesterol synthesis. Animals do not have the enzymatic machinery to synthesize plant sterols from cholesterol (1), and thus the plasma levels of sitosterol and campesterol fall more dramatically than those of cholesterol in the transgenic mice.
It is not clear why the metabolic effects of transgene expression were more marked in the female mice. This difference is not attributable to sex-associated differences in the level of expression of the transgene. Although the mRNA levels of human ABCG5 were higher in the livers of the female transgenic mice, the expression levels of human ABCG8 were similar in the male and female transgenic animals. The expression levels of hepatic, but not intestinal, mouse Abcg5 and Abcg8 were slightly higher in female mice than in male mice. Male and female rodents differ significantly in many aspects of cholesterol and bile acid metabolism (34). Female mice generally have a larger bile acid pool size and absorb a higher fraction of dietary cholesterol (34). Overexpression of ABCG5 and ABCG8 did not affect bile acid pool size and was associated with a decreased fractional absorption of dietary cholesterol. Thus, differences in cholesterol metabolism between male and female mice appear not to reflect sex differences in expression of Abcg5 and Abcg8.
Mouse Abcg5 and Abcg8 expression is normally increased by cholesterol feeding through an LXR-mediated process (4, 11). The expression levels of human ABCG5 and ABCG8 transgenes were not increased by cholesterol feeding. The reasons for the lack of increase in the levels of human ABCG5 and ABCG8 mRNAs in the cholesterol-fed mice are not clear. Interestingly, the levels of mRNA for both human ABCG5 and human ABCG8 did increase in the small intestine in another line of transgenic mice that contained fewer copies (about ten) of the transgene (data not shown). The increase in mouse Abcg5 and Abcg8 expression levels in response to a high-cholesterol diet requires the action of the nuclear hormone receptor LXRα (11, 24). The ligand for LXRα is thought to be a hydroxylated cholesterol, which may not accumulate in enterocytes or hepatocytes expressing high levels of human ABCG5 and ABCG8 because of enhanced excretion of cholesterol and other sterols into either the gut lumen or the bile. Alternatively, the expression of human ABCG5 and ABCG8 may reduce the intracellular concentration of the regulatory oxysterols by directly excreting these molecules from cells.
The concentration of cholesterol in bile is the principal determinant of susceptibility to the development of cholesterol gallstones (35). In the human ABCG5/ABCG8 transgenic mice, high levels of biliary cholesterol were associated with amorphous materials but not cholesterol crystals in bile. Mouse strains differ markedly in their susceptibility to form gallstones, and it is possible that expression of the transgenes in a susceptible strain will result in gallstones (36). Humans are more susceptible to cholesterol gallstone formation because the molar ratio of cholesterol to phospholipids and bile acids (the cholesterol saturation index) in bile is near saturation (35, 37). Interindividual variation in ABCG5/ABCG8 activity may influence susceptibility to cholesterol gallstone formation in humans. We have recently identified common DNA sequence polymorphisms in ABCG8 that are associated with variation in the plasma levels of plant sterols, suggesting that these polymorphisms influence ABCG5/ABCG8 activity (38). Sequence variations in ABCG5/ABCG8 that increase biliary cholesterol secretion may confer genetic susceptibility to cholesterol gallstone disease in humans.
Acknowledgments
We thank Robert Guzman, Yinyan Ma, and Tommy Hyatt for excellent technical assistance. We also thank Scott Clark, Scott M. Grundy, and Gloria Vega for measuring the plasma lipids and noncholesterol sterols, Ingrid Gelissen for measuring the biliary phospholipids, and Atilla Kumanovics for pulse-field gel electrophoresis. We wish to thank Stephen D. Turley for very helpful suggestions, Richard Anderson for assistance with microscopy, and David W. Russell for review of the manuscript. K.E. Berge is supported by the Norwegian Research Council and the Thoresen Foundation. H.H. Hobbs is supported by the NIH (HL-20948), the Perot Family Fund, the W.M. Keck Foundation, and the Donald W. Reynolds Clinical Cardiovascular Research Center in Dallas, Texas, USA.
Footnotes
See the related Commentary beginning on page 605.
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: ATP-binding cassette (ABC); 14C-taurocholic acid (TCA); HMG-CoA reductase (HMGCR); sterol regulatory element–binding protein (SREBP); LDL receptor (LDLR); ATP-binding cassette transporter A1 (ABCA1); acyl-CoA:cholesterol acyltransferase-2 (ACAT-2).
References
- 1.Schoenheimer R. New contributions in sterol metabolism. Science. 1931;74:579–584. doi: 10.1126/science.74.1928.579. [DOI] [PubMed] [Google Scholar]
- 2.Gould RG, Jones RJ, LeRoy GV, Wissler RW, Taylor CB. Absorbability of beta-sitosterol in humans. Metabolism. 1969;18:652–662. doi: 10.1016/0026-0495(69)90078-x. [DOI] [PubMed] [Google Scholar]
- 3.Salen G, Ahrens EH, Jr, Grundy SM. Metabolism of beta-sitosterol in man. J Clin Invest. 1970;49:952–967. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 5.Lee MH, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg RE, Connor WE, Lin DS, Brewer HB., Jr Abnormal metabolism of shellfish sterols in a patient with sitosterolemia and xanthomatosis. J Clin Invest. 1986;77:1864–1872. doi: 10.1172/JCI112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36:1763–1773. [PubMed] [Google Scholar]
- 9.Bjorkhem, I., Boberg, K., and Leitersdorf, E. 2001. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In The metabolic and molecular bases of inherited disease. Volume II. C. Scriver, A. Beaudet, W. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 2961–2988.
- 10.Goldstein, J., Hobbs, H., and Brown, M. 2001. Familial hypercholesterolemia. In The metabolic and molecular bases of inherited disease. Volume II. C. Scriver, A. Beaudet, W. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 2863–2913.
- 11.Repa JJ, et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 12.Peet DJ, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann SL, Russell DW, Brown MS, Goldstein JL, Hammer RE. Overexpression of low density lipoprotein (LDL) receptor eliminates LDL from plasma in transgenic mice. Science. 1988;239:1277–1281. doi: 10.1126/science.3344433. [DOI] [PubMed] [Google Scholar]
- 14.Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7 alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]
- 16.Sanan DA, et al. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a) Proc Natl Acad Sci USA. 1998;95:4544–4549. doi: 10.1073/pnas.95.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turley SD, Daggy BP, Dietschy JM. Cholesterol-lowering action of psyllium mucilloid in the hamster: sites and possible mechanisms of action. Metabolism. 1991;40:1063–1073. doi: 10.1016/0026-0495(91)90131-f. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, et al. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc Natl Acad Sci USA. 2001;98:13607–13612. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turley SD, Herndon MW, Dietschy JM. Reevaluation and application of the dual-isotope plasma ratio methods for the measurement of intestinal cholesterol absorption in the hamster. J Lipid Res. 1994;35:328–339. [PubMed] [Google Scholar]
- 20.Turley SD, Daggy BP, Dietschy JM. Psyllium augments the cholesterol-lowering action of cholestyramine in hamsters by enhancing sterol loss from the liver. Gastroenterology. 1994;107:444–452. doi: 10.1016/0016-5085(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 21.Turley SD, Dietschy JM. Re-evaluation of the 3α-hydroxy-steroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978;19:924–928. [PubMed] [Google Scholar]
- 22.Lu K, et al. Molecular cloning, genomic organization, genetic variations, and characterization of murine sterolin genes Abcg5 and Abcg8. J Lipid Res. 2002;43:565–578. [PMC free article] [PubMed] [Google Scholar]
- 23.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 24.Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graf GA, et al. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–670. doi:10.1172/JCI200216000. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M-H, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salen G, et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res. 1989;30:1319–1330. [PubMed] [Google Scholar]
- 28.Miettinen TA. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur J Clin Invest. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 29.Smit JJ, et al. Homozygous disruption of the murine Mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 30.Elferink RPJO, et al. Uncoupling of biliary phospholipid and cholesterol secretion in mice with reduced expression of mdr2 P-glycoprotein. J Lipid Res. 2001;37:1065–1075. [PubMed] [Google Scholar]
- 31.Dawson PA, Rudel LL. Intestinal cholesterol absorption. Curr Opin Lipidol. 1999;10:315–320. doi: 10.1097/00041433-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annu Rev Physiol. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- 33.Sehayek E, et al. Biliary cholesterol excretion: a novel mechanism that regulates dietary cholesterol absorption. Proc Natl Acad Sci USA. 1998;95:10194–10199. doi: 10.1073/pnas.95.17.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turley SD, Schwarz M, Spady DK, Dietschy JM. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology. 1998;28:1088–1094. doi: 10.1002/hep.510280425. [DOI] [PubMed] [Google Scholar]
- 35.Bilhartz, L.E., and Horton, J.D. 1998. Gallstone disease and its complications. In Gastrointestinal and liver disease. Volume 1. M. Feldman, B.F. Scharschmidt, and M.H. Sleisenger, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 948–972.
- 36.Wang DQ, Lammert F, Paigen B, Carey MC. Phenotypic characterization of lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice. Pathophysiology of biliary lipid secretion. J Lipid Res. 1999;40:2066–2079. [PubMed] [Google Scholar]
- 37.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945–955. [PubMed] [Google Scholar]
- 38.Berge KE, et al. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res. 2002;43:486–494. [PubMed] [Google Scholar]
- 39.Liang G, et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]