Abstract
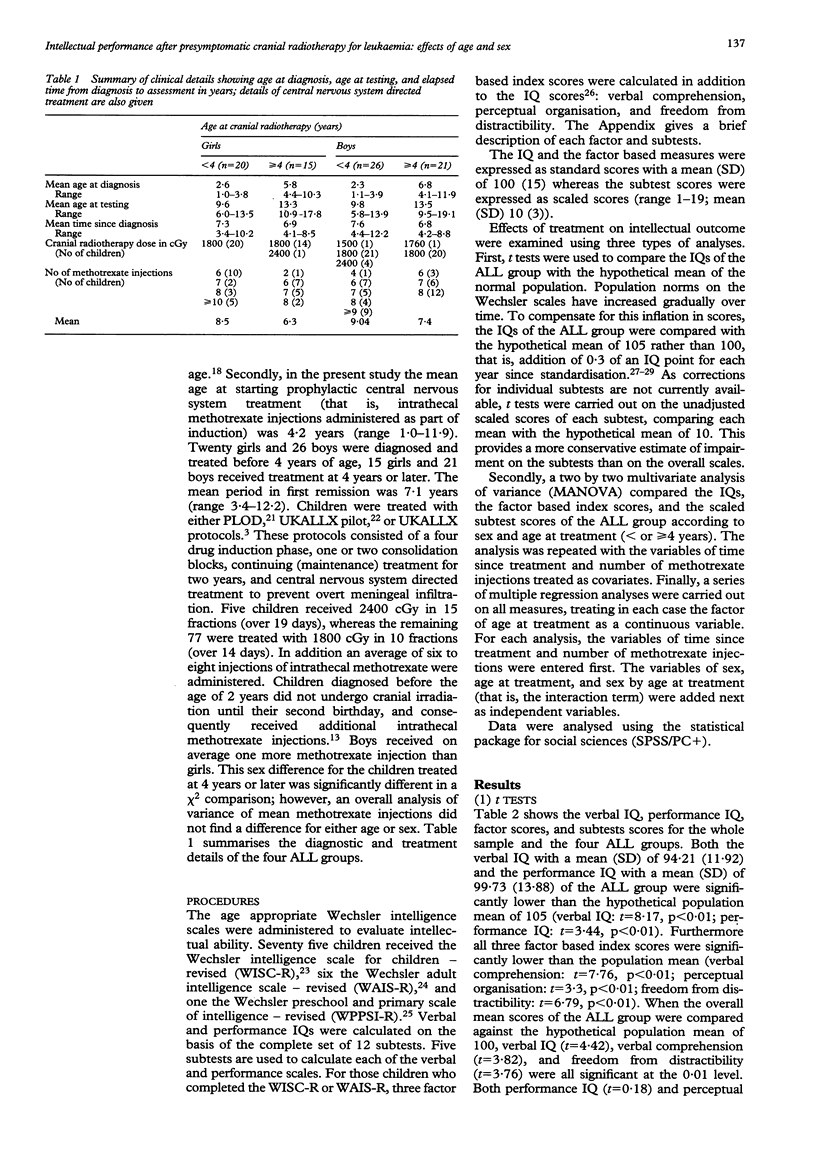
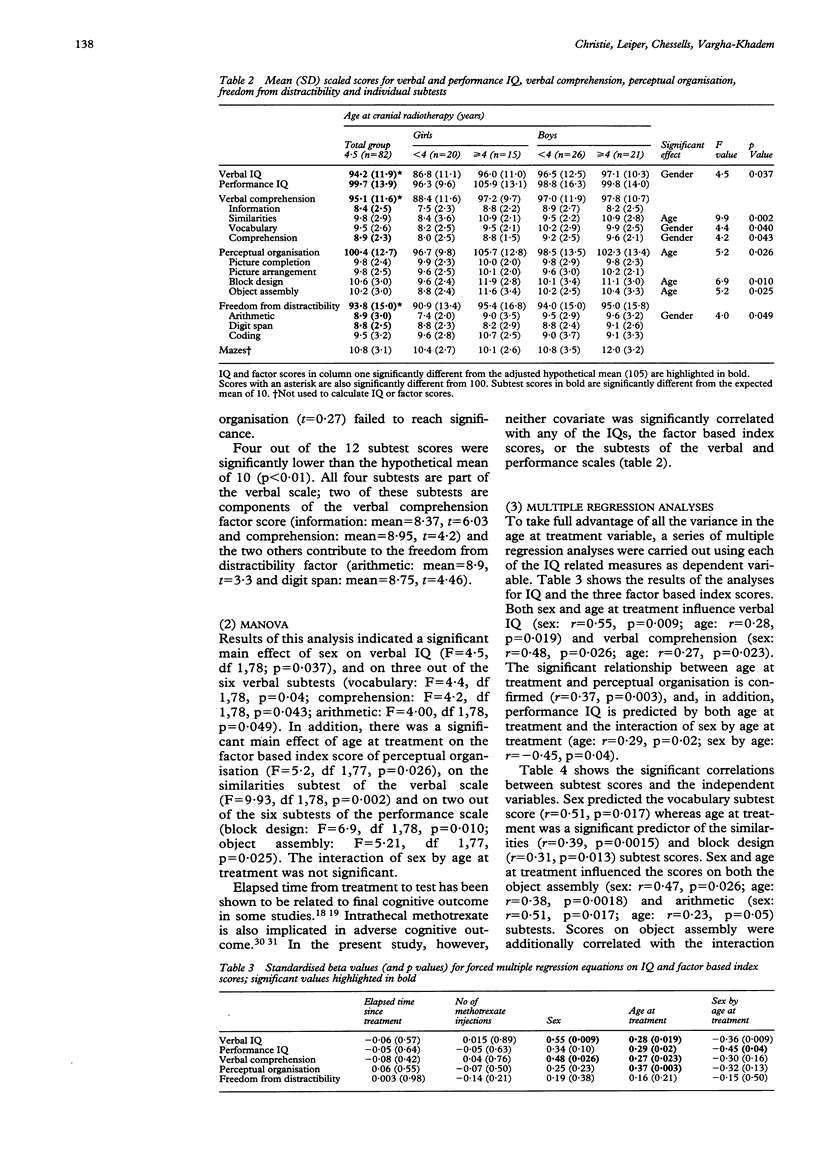
Cognitive outcome, as measured by verbal and performance IQs, was compared in 35 girls and 47 boys who were in first remission for acute lymphoblastic leukaemia. All children had received presymptomatic cranial radiotherapy and intrathecal methotrexate. The mean age at diagnosis was 4.2 years and the mean elapsed time from initial diagnosis to intellectual assessment was 7.1 years. Results showed that children irradiated before the age of 4 years were impaired in certain aspects of non-verbal ability, as well as in measures of short term memory and attention, calculated by factor scores derived from selected subtests of the IQ test. Subtests requiring verbal and non-verbal reasoning showed the greatest impairment after early diagnosis and treatment. In addition girls were selectively impaired in verbal IQ and other aspects of verbal ability, with the degree of impairment exacerbated by early treatment. No relationship was found between degree of impairment and either time since treatment or number of methotrexate injections. It is concluded that early age at irradiation increases the risk of impaired intellectual outcome, particularly in girls.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aur R. J., Simone J., Hustu H. O., Walters T., Borella L., Pratt C., Pinkel D. Central nervous system therapy and combination chemotherapy of childhood lymphocytic leukemia. Blood. 1971 Mar;37(3):272–281. [PubMed] [Google Scholar]
- Brouwers P., Poplack D. Memory and learning sequelae in long-term survivors of acute lymphoblastic leukemia: association with attention deficits. Am J Pediatr Hematol Oncol. 1990 Summer;12(2):174–181. doi: 10.1097/00043426-199022000-00009. [DOI] [PubMed] [Google Scholar]
- Brouwers P., Riccardi R., Poplack D., Fedio P. Attentional deficits in long-term survivors of childhood acute lymphoblastic leukemia (ALL). J Clin Neuropsychol. 1984 Aug;6(3):325–336. doi: 10.1080/01688638408401222. [DOI] [PubMed] [Google Scholar]
- Brown R. T., Madan-Swain A., Pais R., Lambert R. G., Sexson S., Ragab A. Chemotherapy for acute lymphocytic leukemia: cognitive and academic sequelae. J Pediatr. 1992 Dec;121(6):885–889. doi: 10.1016/s0022-3476(05)80333-6. [DOI] [PubMed] [Google Scholar]
- Chessells J. M., Bailey C. C., Richards S. MRC UKALL X. The UK protocol for childhood ALL: 1985-1990. The Medical Research Council Working Party on Childhood Leukaemia. Leukemia. 1992;6 (Suppl 2):157–161. [PubMed] [Google Scholar]
- Chessells J. M., Leiper A. D., Tiedemann K., Hardisty R. M., Richards S. Oral methotrexate is as effective as intramuscular in maintenance therapy of acute lymphoblastic leukaemia. Arch Dis Child. 1987 Feb;62(2):172–176. doi: 10.1136/adc.62.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D., Battin M., Leiper A. D., Chessells J., Vargha-Khadem F., Neville B. G. Neuropsychological and neurological outcome after relapse of lymphoblastic leukaemia. Arch Dis Child. 1994 Apr;70(4):275–280. doi: 10.1136/adc.70.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland D. R., Dowell R. E., Jr, Fletcher J. M., Bordeaux J. D., Sullivan M. P., Jaffe N., Frankel L. S., Ried H. L., Cangir A. Neuropsychological effects of childhood cancer treatment. J Child Neurol. 1988 Jan;3(1):53–62. doi: 10.1177/088307388800300113. [DOI] [PubMed] [Google Scholar]
- Cousens P., Waters B., Said J., Stevens M. Cognitive effects of cranial irradiation in leukaemia: a survey and meta-analysis. J Child Psychol Psychiatry. 1988 Nov;29(6):839–852. doi: 10.1111/j.1469-7610.1988.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Dowell R. E., Jr, Copeland D. R., Francis D. J., Fletcher J. M., Stovall M. Absence of synergistic effects of CNS treatments on neuropsychologic test performance among children. J Clin Oncol. 1991 Jun;9(6):1029–1036. doi: 10.1200/JCO.1991.9.6.1029. [DOI] [PubMed] [Google Scholar]
- Fletcher J. M., Copeland D. R. Neurobehavioral effects of central nervous system prophylactic treatment of cancer in children. J Clin Exp Neuropsychol. 1988 Aug;10(4):495–537. doi: 10.1080/01688638808408255. [DOI] [PubMed] [Google Scholar]
- Fuggle P. W., Tokar S., Grant D. B., Smith I. Rising IQ scores in British children: recent evidence. J Child Psychol Psychiatry. 1992 Oct;33(7):1241–1247. doi: 10.1111/j.1469-7610.1992.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Gangji D., Reaman G. H., Cohen S. R., Bleyer W. A., Poplack D. G. Leukoencephalopathy and elevated levels of myelin basic protein in the cerebrospinal fluid of patients with acute lymphoblastic leukemia. N Engl J Med. 1980 Jul 3;303(1):19–21. doi: 10.1056/NEJM198007033030106. [DOI] [PubMed] [Google Scholar]
- Giralt J., Ortega J. J., Olive T., Verges R., Forio I., Salvador L. Long-term neuropsychologic sequelae of childhood leukemia: comparison of two CNS prophylactic regimens. Int J Radiat Oncol Biol Phys. 1992;24(1):49–53. doi: 10.1016/0360-3016(92)91020-n. [DOI] [PubMed] [Google Scholar]
- Kinney H. C., Brody B. A., Kloman A. S., Gilles F. H. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988 May;47(3):217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Leiper A. D., Chessells J. Acute lymphoblastic leukaemia under 2 years. Arch Dis Child. 1986 Oct;61(10):1007–1012. doi: 10.1136/adc.61.10.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiper A. D., Stanhope R., Kitching P., Chessells J. M. Precocious and premature puberty associated with treatment of acute lymphoblastic leukaemia. Arch Dis Child. 1987 Nov;62(11):1107–1112. doi: 10.1136/adc.62.11.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H. A., Nannis E. D., Poplack D. G. The effects of prophylactic treatment of the central nervous system on the intellectual functioning of children with acute lymphocytic leukemia. Am J Med. 1981 Jul;71(1):47–52. doi: 10.1016/0002-9343(81)90257-6. [DOI] [PubMed] [Google Scholar]
- Mulhern R. K., Kovnar E., Langston J., Carter M., Fairclough D., Leigh L., Kun L. E. Long-term survivors of leukemia treated in infancy: factors associated with neuropsychologic status. J Clin Oncol. 1992 Jul;10(7):1095–1102. doi: 10.1200/JCO.1992.10.7.1095. [DOI] [PubMed] [Google Scholar]
- Ochs J., Mulhern R. K. Late effects of antileukemic treatment. Pediatr Clin North Am. 1988 Aug;35(4):815–833. doi: 10.1016/s0031-3955(16)36511-7. [DOI] [PubMed] [Google Scholar]
- Peylan-Ramu N., Poplack D. G., Pizzo P. A., Adornato B. T., Di Chiro G. Abnormal CT scans of the brain in asymptomatic children with acute lymphocytic leukemia after prophylactic treatment of the central nervous system with radiation and intrathecal chemotherapy. N Engl J Med. 1978 Apr 13;298(15):815–818. doi: 10.1056/NEJM197804132981504. [DOI] [PubMed] [Google Scholar]
- Pinkerton C. R., Bowman A., Holtzel H., Chessells J. M. Intensive consolidation chemotherapy for acute lymphoblastic leukaemia (UKALL X pilot study). Arch Dis Child. 1987 Jan;62(1):12–18. doi: 10.1136/adc.62.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochedly C. Prophylactic CNS therapy in childhood acute leukemia: review of methods used. Am J Pediatr Hematol Oncol. 1979 Summer;1(2):119–126. [PubMed] [Google Scholar]
- Price R. A., Jamieson P. A. The central nervous system in childhood leukemia. II. Subacute leukoencephalopathy. Cancer. 1975 Feb;35(2):306–318. doi: 10.1002/1097-0142(197502)35:2<306::aid-cncr2820350203>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Robison L. L., Nesbit M. E., Jr, Sather H. N., Meadows A. T., Ortega J. A., Hammond G. D. Factors associated with IQ scores in long-term survivors of childhood acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1984 Summer;6(2):115–121. doi: 10.1097/00043426-198406020-00001. [DOI] [PubMed] [Google Scholar]
- Rubenstein C. L., Varni J. W., Katz E. R. Cognitive functioning in long-term survivors of childhood leukemia: a prospective analysis. J Dev Behav Pediatr. 1990 Dec;11(6):301–305. [PubMed] [Google Scholar]
- Shalet S. M. Irradiation-induced growth failure. Clin Endocrinol Metab. 1986 Aug;15(3):591–606. doi: 10.1016/s0300-595x(86)80011-1. [DOI] [PubMed] [Google Scholar]
- Uruena M., Stanhope R., Chessells J. M., Leiper A. D. Impaired pubertal growth in acute lymphoblastic leukaemia. Arch Dis Child. 1991 Dec;66(12):1403–1407. doi: 10.1136/adc.66.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber D. P., Tarbell N. J., Kahn C. M., Gelber R. D., Sallan S. E. The relationship of sex and treatment modality to neuropsychologic outcome in childhood acute lymphoblastic leukemia. J Clin Oncol. 1992 May;10(5):810–817. doi: 10.1200/JCO.1992.10.5.810. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Nitschke R., Bowman M. E., Chaffin M. J., Sexauer C. L., Prince J. R. Transient white matter changes on MR images in children undergoing chemotherapy for acute lymphocytic leukemia: correlation with neuropsychologic deficiencies. Radiology. 1991 Jul;180(1):205–209. doi: 10.1148/radiology.180.1.2052695. [DOI] [PubMed] [Google Scholar]


