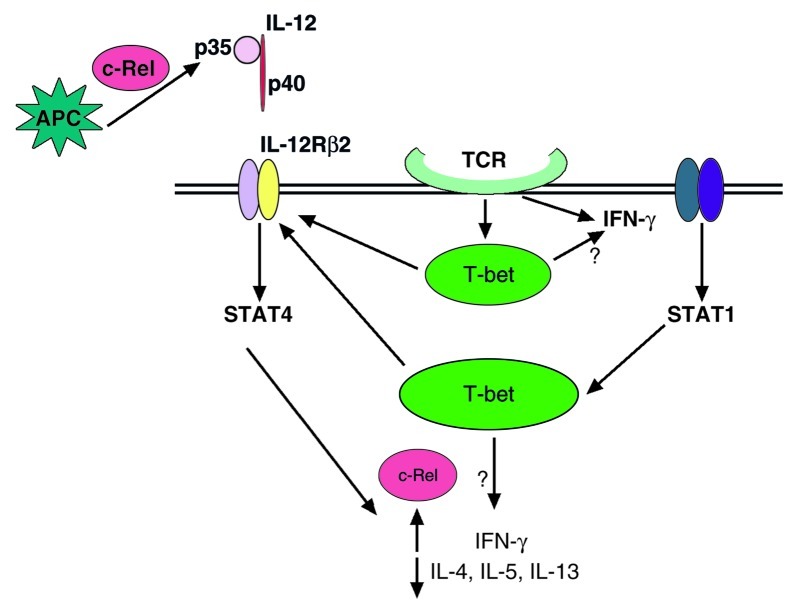
The differentiation of T helper cells into distinct functional subtypes is critical for mediating effective adaptive immune responses. The molecular mechanism by which a Th precursor cell (Thp) adopts a Th1 or Th2 phenotype involves a complex interplay between the induction of lineage-specific transcription factors, such as T-bet, Gata3, and c-Maf, and the stimulatory effects of the cytokine milieu, which are largely provided by antigen-presenting cells (APCs) (1). In the case of Th1 differentiation, antigen stimulation of a naive Thp causes simultaneous low-level expression of both the Th1-specific transcription factor T-bet and the Th2-specific factor Gata3, and coproduction of small amounts of IFN-γ and IL-4 (2, 3).
Subsequent differentiation of Thp into Th1 cell is thought to occur via two other events, as shown in Figure 1. The first is upregulation of IL-12Rβ2 expression by T-bet, which amplifies the effects of IL-12 and strengthens signaling through STAT4 (3, 4). The second is activation of STAT1 via IFN-γ receptor engagement, which further upregulates T-bet expression in a positive feedback loop (4, 5). The net effects of T-bet, STAT4, and STAT1 activation then drive high-level production of IFN-γ by Th1 cells, which is necessary for antiviral immunity and other Th1-mediated immune functions.
Figure 1.
Signaling and transcriptional pathways of Th1 differentiation and the potential role of c-Rel. Induction of T-bet and IFN-γ by T cell receptor engagement is followed by activation of the STAT4 and STAT1 pathways, which lead to still greater expression of IFN-γ. Points at which c-Rel could participate in this pathway are indicated.
In addition to the effects of lineage-specific transcription factors, a number of more widely expressed factors have been shown to affect Th cell differentiation. In this issue of the JCI, Hilliard et al. report the effects of c-Rel deficiency on Th1-dependent immunity both in vitro and in vivo (6). c-Rel is a member of the family of NF-κB/Rel transcription factors, which includes four other mammalian members: RelA (p65), RelB, NF-κB1 (p50/p105), and NF-κB2 (p52/p100) (7). These five factors share a highly conserved Rel homology domain at their N-termini and play important roles in the development, activation, and homeostasis of the immune system.
Studies of the NF-κB family members by targeted deletion of one or more family members in mice have so far shown differential effects on Th immune responses. Universal inhibition of all family members in transgenic mice overexpressing a protease-resistant IκB results in an inability to mount a Th1 immune response (8). In contrast, mice deficient in the single factor NF-κB1 fail to optimally induce Gata3 and are resistant to allergic asthma, a Th2 cell–mediated disease (9). Our understanding of the role of NF-κB family members in Th differentiation is further complicated by their role in cytokine production by APCs. For instance, NF-κB2–deficient mice have clear defects in APC function and are more susceptible to Leishmania infection in vivo, although the intrinsic responses of NF-κB2–deficient Th1 cells appear normal (10). A sophisticated understanding of the role of NF-κB/Rel factors in Th differentiation therefore requires careful dissection of the function of each family member in different cell types by conditional knockout, mixed chimera, and other approaches.
In the current study by Hilliard et al., c-Rel deficiency led to a defect in Th1 immune responses both in vitro and in vivo. c-Rel–deficient mice proved resistant to experimental autoimmune encephalomyelitis (EAE), a Th1-mediated autoimmune disease. By studying mixed chimeric mice, the authors found that the resistance to EAE was mainly conferred by bone marrow–derived cells and was thus independent of NF-κB function in the brain. Splenocytes from immunized c-Rel–deficient mice produced very low levels of IFN-γ upon rechallenge with antigen but secreted higher than normal levels of IL-4. Further analysis revealed that c-Rel–deficient APCs express very low levels of Th1-promoting cytokines, including IL-12p35 (consistent with previous work; see ref. 11) and IL-23p19. This APC defect alone could easily explain the resistance to EAE. Somewhat surprisingly, however, the authors also found that c-Rel–deficient Th cells are intrinsically defective in developing into IFN-γ–producing cells and that this defect cannot be rescued by wild-type APCs or exogenous IL-12. Taken together, these results strongly suggest that c-Rel can regulate the differentiation of Th cells by both T cell–dependent and APC-dependent mechanisms.
These findings bring up a number of interesting questions. IL-12, IL-23, and the newly discovered IL-27 are structurally related cytokines all capable of promoting the differentiation of Th1 cells, but their expression kinetics and cell type distribution are quite distinct (12, 13). In particular, very little is known regarding the transcriptional regulation of IL-23p19 and IL-27, and, based on the current study, the role of NF-kB family members in this regulation merits further examination. Also intriguing is the marked defect in IFN-γ production by c-Rel–deficient Th1 cells in the face of normal levels of T-bet and normal activation of STAT4, both of which are clearly important for optimal production of this cytokine (4, 14). As Hilliard et al. note, c-Rel may thus act downstream of T-bet and regulate the expression of IFN-γ either directly or indirectly. Recently, a Th1 cell–specific homeobox protein, Hlx, has been shown to synergize with T-bet in promoting the transcription of IFN-γ (15). Therefore, one attractive explanation for the data presented is that c-Rel might indirectly regulate the expression of IFN-γ by controlling the levels of Hlx. The defect in production of IFN-γ by c-Rel–deficient Th1 cells could also be due to a failure to remodel the IFN-γ locus; such remodeling has been shown to be necessary to stabilize the Th1 phenotype (16, 17). Alternatively, c-Rel could be involved in a pathway parallel to T-bet, or it could even serve as a common effector molecule downstream of both the T-bet and STAT4 pathways. Thus, the precise position of c-Rel in the hierarchy of the Th1 transcriptional cascade leading to the production of IFN-γ has yet to be determined. Continued elucidation of the pathways controlling IFN-γ production will further our ability to manipulate Th1-mediated im-mune responses and should ultimately affect our ability to manage human diseases by modulating patterns of T cell differentiation.
Footnotes
See the related article beginning on page 843.
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: Th precursor cell (Thp); antigen-presenting cell (APC); experimental autoimmune encephalomyelitis (EAE).
References
- 1.Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell. 2002;109(Suppl.):S109–S120. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 2.Grogan JL, et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 3.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 4.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 5.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilliard BA, et al. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–850. doi:10.1172/JCI200215254. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 8.Aronica MA, et al. Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J Immunol. 1999;163:5116–5124. [PubMed] [Google Scholar]
- 9.Das J, et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 10.Speirs K, Caamano J, Goldschmidt MH, Hunter CA, Scott P. NF-kappa B2 is required for optimal CD40-induced IL-12 production but dispensable for Th1 cell differentiation. J Immunol. 2002;168:4406–4413. doi: 10.4049/jimmunol.168.9.4406. [DOI] [PubMed] [Google Scholar]
- 11.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–270. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 12.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 13.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 14.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 15.Mullen AC, et al. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nat Immunol. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 17.Avni O, et al. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]