Abstract
A combination of pharmacological and genetic approaches was used to determine the role of type 4 cAMP-specific cyclic nucleotide phosphodiesterase 4 (PDE4) in reversing α2-adrenoceptor–mediated anesthesia, a behavioral correlate of emesis in non-vomiting species. Among the family-specific PDE inhibitors, PDE4 inhibitors reduced the duration of xylazine/ketamine–induced anesthesia in mice, with no effect on pentobarbital-induced anesthesia. The rank order of the PDE4 inhibitors tested was 6-(4-pyridylmethyl)-8-(3-nitrophenyl)quinoline (PMNPQ) > (R)-rolipram > (S)-rolipram >> (R)-N-{4-[1-(3-cyclopentyloxy-4-methoxyphenyl)-2-(4-pyridyl)ethyl]phenyl}N′-ethylurea (CT-2450). The specific roles of PDE4B and PDE4D in this model were studied using mice deficient in either subtype. PDE4D-deficient mice, but not PDE4B-deficient mice, had a shorter sleeping time than their wild-type littermates under xylazine/ketamine–induced anesthesia, but not under that induced with pentobarbital. Concomitantly, rolipram-sensitive PDE activity in the brain stem was decreased only in PDE4D-deficient mice compared with their wild-type littermates. While PMNPQ significantly reduced the xylazine/ketamine–induced anesthesia period in wild-type mice and in PDE4B-null mice, it had no effect in PDE4D-deficient mice. These findings strongly support the hypothesis that inhibition of PDE4D is pivotal to the anesthesia-reversing effect of PMNPQ and is likely responsible for emesis induced by PDE4 inhibitors.
Introduction
Cyclic nucleotides cAMP and cGMP are degraded by at least 11 families of phosphodiesterases (PDEs 1–11) classified according to their gene sequence, substrate specificity, biochemical regulation, and sensitivity to inhibitors (1, 2). The cAMP-specific PDE4 has attracted considerable attention for the treatment of airway inflammatory diseases, since its inhibition results in attenuated inflammatory responses (1, 3, 4). However, the therapeutic potential of PDE4 inhibitors has been limited by the side effects of nausea and emesis, observed both in humans and in various animal species following the administration of structurally diverse compounds (5–9). A major challenge in the development of new generations of PDE4 inhibitors is the improvement of the therapeutic index of this class of compounds.
PDE4 enzymes use a common binuclear ion center as the core catalytic machinery (10). The reversible binding of the cation cofactors (e.g., Mg2+) results in the presence of two coexisting conformers that bind inhibitors differently: the holoenzyme (enzyme bound with Mg2+) and the apoenzyme (free enzyme) (11, 12). In the past, it was observed that the potency of some inhibitors (e.g., rolipram) on PDE activity deviated from their affinity at the high-affinity rolipram binding site (HARBS); this led to the proposal that inhibitors with a reduced potency on the HARBS may have an improved therapeutic index over that of first-generation compounds (13–15). It has now been clarified that the HARBS corresponds to the holoenzyme conformer responsible for PDE4 catalysis (11, 12).
The PDE4 family is composed of four subtypes (PDE4A–D) and multiple splice variants (16). If it were possible to identify the subtype(s) responsible for the beneficial and the side effects associated with PDE4 inhibition, then subtype-selective inhibitors devoid of the tendency to induce nausea and vomiting could be developed. The mechanism of the emetic response associated with PDE4 inhibitors is thought to be a consequence of the inhibition of PDE4 in nontarget tissues (9, 13). It is believed that PDE4 inhibitors produce a pharmacological response analogous to that of a presynaptic α2-adrenoceptor inhibition by elevating intracellular levels of cAMP in noradrenergic neurons. Therefore, by removing an inhibitory mechanism, PDE4 inhibitors are thought to modulate the release of mediators (5-HT, substance P, noradrenaline) involved in the onset of the emetic reflex (17). The ability of PDE4 inhibitors to reverse α2-adrenoceptor agonist–mediated anesthesia confirms the postulate that these inhibitors have effects similar to those of α2-adrenoceptor antagonists (17–19). In the presence of a PDE4 inhibitor, the percentage of animals exhibiting loss of the righting reflex was reduced, and the duration of anesthesia was shortened (17–19). It was further demonstrated that assessing the anesthesia-reversing effect of PDE4 inhibitors is a novel way of evaluating the emetic potential of this class of compounds in species that do not have a vomiting reflex (e.g., rodents) (19). Observations made in rats indicate that this model is functionally dependent on PDE4, specific for α2-adrenoceptor agonist–mediated anesthesia, and related to emesis induced by PDE4 inhibitors (19). In the present study, a combination of pharmacological and genetic approaches was used to clarify the roles of PDE4 subtypes in reversing α2-adrenoceptor–mediated anesthesia, a behavioral correlate of PDE4 inhibitor–induced emesis in non-vomiting species. The relative role of PDE4 subtypes was studied through the use of this model and of mice deficient in PDE4B and PDE4D.
Methods
Duration of anesthesia.
Experiments were performed on C57BL/6 mice (male, 15–28 g) obtained from Taconic (Germantown, New York, USA), on 129SvJ mice (male, 23–28 g) from The Jackson Laboratory (Bar Harbor, Maine, USA), or on PDE4B- and PDE4D-deficient mice and their littermates (male, 14–48 g) generated at Stanford University on the mixed background C57BL/6 × 129/Ola (20, 21). They were housed in a temperature- and humidity-controlled environment, in groups of four or five, with food and water available ad libitum. A period of 5 to 6 days of acclimatization was allowed prior to experimentation. Experimental procedures were approved by the Animal Care Committee at Merck Frosst Centre for Therapeutic Research, in accordance with the guidelines of the Canadian Council on Animal Care.
Experiments were conducted following procedures previously described (17, 19). Briefly, mice were anesthetized with the combination of xylazine (10 mg/kg) and ketamine (80 mg/kg) or with sodium pentobarbital (50 mg/kg) administered in a single intraperitoneal injection. Fifteen minutes later, the animals were injected subcutaneously with various concentrations of test compound (0.001–30 mg/kg) or vehicle and were subsequently placed in dorsal recumbency. All test compounds were freshly dissolved in 60% (vol/vol) polyethylene glycol (PEG; molecular weight 200) in saline each day and administered in a dosing volume of 10 μl/g of body weight. The return of the righting reflex (i.e., when the animal no longer remained on its back and turned itself spontaneously to prone position) was used as an endpoint to measure the duration of anesthesia. Under sodium pentobarbital–induced anesthesia, the experiments were terminated at 120 minutes after test compound injection. For animals that had not restored their righting reflex by that time, we recorded the maximal amount of time allowed.
Plasma and brain tissue.
At the end of the experiment, samples of plasma and brain tissue were collected for quantification of test compounds. One hour after dosing, sampling was performed from the group treated with the highest dose of the test compound in question. The animals were euthanized with a CO2 chamber. The blood was collected by cardiac puncture in heparinized microtubes (Becton Dickinson and Co., Franklin Lakes, New Jersey, USA) and centrifuged for 5 minutes (5,585 g; VWR scientific microcentrifuge, model V; VWR Canlab, Mississauga, Ontario, Canada), and the plasma was stored at –80°C. The animals were perfused with 15 ml of saline via the left ventricle, and the brains were removed and stored at –80°C until analyzed. The samples were analyzed either by HPLC (Alliance Waters 960; Waters Corp., Milford, Massachusetts, USA) or by liquid chromatography coupled to mass spectrometry (API 100 and API 2000; MDS Sciex, Concord, Ontario, Canada).
PDE4 assays.
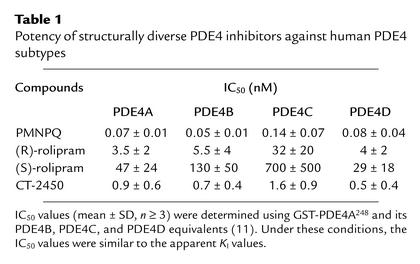
Four representative human enzymes, PDE4A248 (HSPDE4A4B248-886) and its PDE4B, PDE4C, and PDE4D equivalents, with the divergent N-termini of the splice variants truncated at the conserved Gln-Thr dipeptide upstream of the catalytic domain for each PDE4 subtype, were expressed as GST fusion proteins and purified to homogeneity from Sf9 cells according to the published procedure (11). IC50 values (mean ± SD, n ≥ 3) of inhibitors were determined from an 11-point dose-response curve performed in duplicate at 0.1 μM 3H-cAMP in 20 mM HEPES (pH 7.5), 10 mM MgCl2, 1 mM EDTA, and 100 mM KCl at 30°C (12).
Brain stems were dissected from PDE4B- and PDE4D-deficient mice and their wild-type littermates and were homogenized in hypotonic buffer containing protease inhibitors (22). Aliquots of homogenates were assayed for PDE activity using 1 μM cAMP as substrate. PDE activity was assessed in the absence (total activity) or presence of 10 μM rolipram (rolipram-insensitive activity). The rolipram-sensitive activity (PDE4 activity) was obtained by subtracting the rolipram-insensitive activity from the total PDE activity. Values were corrected for the amount of extract protein added to the assay.
Western blot analysis.
Brain stems were dissected from adult PDE4B- and PDE4D-deficient mice and their wild-type littermates and immediately homogenized (30 strokes in a Dounce homogenizer) in a buffer containing 50 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5% glycerol, 10 mM NaF, 1 mM EDTA, 0.2 mM EGTA, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, a protease inhibitor mixture (Roche Applied Science, Indianapolis, Indiana, USA), 1 mM Pefabloc SC (Roche Diagnostics), 1% NP-40, and 10 mM β-mercaptoethanol. After centrifugation at 16,000 g for 20 minutes, the supernatant was immunoprecipitated with a PDE4D-specific mAb, M3S1, or a PDE4B-specific polyclonal antibody, K118. The immunoprecipitated PDE4D and PDE4B proteins were further detected by Western blot analysis using a PDE4D-specific mAb, 61D10E (a gift from ICOS Corp., Bothell, Washington, USA), or a PDE4B-specific polyclonal antibody, K118, respectively. The immunoprecipitation and Western blotting procedures were carried out as previously described (23).
Data presentation and statistical evaluation.
The duration of anesthesia, following a given treatment, is expressed in time (minutes). Comparisons between vehicle- and test compound–treated mice were performed in separate animals, and each animal was used to generate only one data point at any given concentration of drug treatment. All data in the text and figures are expressed as the mean ± SEM, and n represents the number of animals tested for each concentration of test compound (n = 5 unless indicated otherwise). Statistically significant differences among groups were determined using ANOVA with multiple comparisons (Bonferroni test). Differences were considered to be statistically significant for P values less than 0.05.
Drugs.
The PDE4 inhibitors (R)-rolipram, (S)-rolipram (15), and 6-(4–pyridylmethyl">)-8-(3–nitrophenyl)quinoline (PMNPQ) (24) were synthesized at Merck Research Laboratories (Montreal, Quebec, Canada). (R)-N-{4-[1-(3-cyclopentyloxy-4-methoxyphenyl)-2-(4–Bpyridyl)ethyl]phenyl}N′-ethylurea (CT-2450) (25) was synthesized at Celltech Pharmaceuticals Ltd. (Slough, United Kingdom). Their potency against the PDE4 subtypes is presented in Table 1. The biochemical characterization of PMNPQ was previously published under the incorrect name of RS14203 following a mistake in the identification of the compound (11, 26). The following drugs and test compounds were also used: erythro-9-(2–hydroxy–3–nonyl)adenine hydrochloride (EHNA; BIOMOL Research Laboratories Inc., Plymouth Meeting, Pennsylvania, USA), milrinone and dipyridamole (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada), xylazine (Rompun; Bayer, Etobicoke, Ontario, Canada), ketamine (Ketaset; Ayerst, Montreal, Quebec, Canada), polyethylene glycol (PEG; molecular weight 200; Sigma, Milwaukee, Wisconsin, USA), vinpocetine and dipyridamole (Tocris Cookson Inc., Ballwin, Missouri, USA), sodium pentobarbital (Somnotol; MTC Pharmaceuticals, Cambridge, Ontario, Canada), and MK-912 (synthesized at Merck Research Laboratories, Rahway, New Jersey, USA).
Table 1.
Potency of structurally diverse PDE4 inhibitors against human PDE4 subtypes
Results
C57BL/6 mice
Duration of anesthesia.
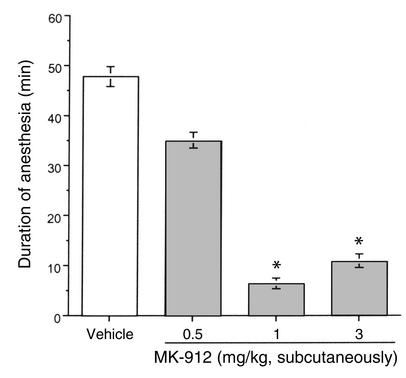
In C57BL/6 mice, the combined administration of xylazine (10 mg/kg) and ketamine (80 mg/kg) induced loss of righting reflex within 15 minutes following injection. The duration of anesthesia measured was approximately 50 minutes. A difference in the anesthesia period could be seen when using a different strain of mice (129SvJ strain: 35 ± 2 min; n = 8). In C57BL/6 mice, the duration of anesthesia induced by the xylazine/ketamine combination was significantly reduced by the administration of MK-912, a potent and brain-penetrant α2-antagonist (27) (Figure 1).
Figure 1.
Effect of MK-912 on the duration of anesthesia induced by the combination of xylazine (10 mg/kg) and ketamine (80 mg/kg) in C57BL/6 mice. Fifteen minutes after the induction of anesthesia, mice were injected with increasing doses of MK-912 (n = 5 per dose) or vehicle (PEG 60%; n = 34). The duration of anesthesia was assessed by the return of the righting reflex. Results are expressed as mean ± SEM. *Significantly different from vehicle group at P < 0.05.
PDE inhibitors.
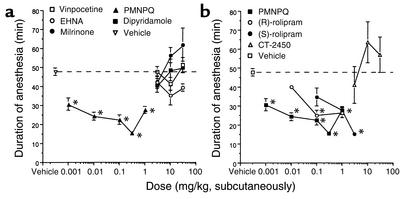
The roles of various PDEs in the hypnotic effect of the α2-adrenoceptor–mediated anesthetic regimen were studied using selective and mixed-type PDE inhibitors. Vinpocetine (PDE1 inhibitor), EHNA (PDE2 inhibitor), milrinone (PDE3 inhibitor), and dipyridamole (PDE5/6/9/10/11 inhibitor) (28) had no significant effect on the duration of xylazine/ketamine–induced anesthesia at the doses tested (3–30 mg/kg, subcutaneously) (Figure 2a). In contrast, PMNPQ (PDE4 inhibitor; 0.001–1 mg/kg, subcutaneously) reduced the duration of anesthesia in a dose-dependent manner (Figure 2). PMNPQ was the most potent PDE4 inhibitor tested in this model, followed in potency by (R)-rolipram and (S)-rolipram (the less active enantiomer) (Figure 2b). CT-2450 had no effect at the doses tested (3–30 mg/kg) following a subcutaneous administration (Figure 2b).
Figure 2.
Effect of PDE inhibitors on the duration of anesthesia induced by the combination of xylazine (10 mg/kg) and ketamine (80 mg/kg) in C57BL/6 mice. Fifteen minutes after the induction of anesthesia, mice were injected with (a) increasing doses of vinpocetine (PDE1 inhibitor; n = 5 per dose), EHNA (PDE2 inhibitor; n = 4–8 per dose), milrinone (PDE3 inhibitor; n = 2–6 per dose), PMNPQ (PDE4 inhibitor; n = 5 per dose), dipyridamole (PDE5/6/9/10/11 inhibitor; n = 4–5 per dose) or vehicle (PEG 60%; n = 34); or (b) increasing doses of PMNPQ, (R)-rolipram, (S)-rolipram, CT-2450 (n = 5 per dose tested), or vehicle (PEG 60%; n = 34). The duration of anesthesia was assessed by the return of the righting reflex. Results are expressed as mean ± SEM. *Significantly different from vehicle group at P < 0.05.
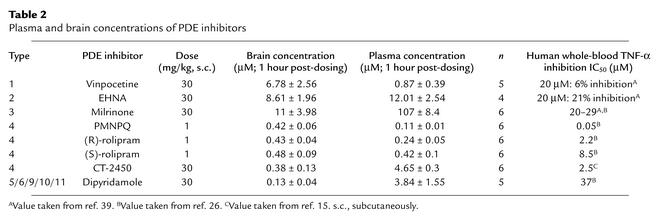
To rule out the possibility of a decreased absorption or distribution to the brain of the inactive PDE inhibitors tested, samples of plasma and brain tissue were collected at the end of the experiment from treated animals. Analysis of these samples revealed significant amounts of the PDE inhibitors both in plasma and in brain tissue (Table 2).
Table 2.
Plasma and brain concentrations of PDE inhibitors
Sodium pentobarbital.
The ability of the PDE4 inhibitor PMNPQ to reduce the duration of anesthesia was tested against an anesthetic regimen that was not α2-adrenoceptor–mediated (sodium pentobarbital, 50 mg/kg, intraperitoneally) (29). Under these experimental conditions, vehicle-treated C57BL/6 mice slept for a mean period of 98 ± 5 minutes (n = 4). The duration of anesthesia was affected neither by 3 mg/kg subcutaneous MK-912 (96 ± 6 minutes; n = 6) nor by 0.1–1 mg/kg subcutaneous PMNPQ (103 ± 2 minutes at highest dose; n = 5).
Characterization of the PDE4D-deficient and PDE4B-deficient mouse phenotypes
PDE protein expression and activity.
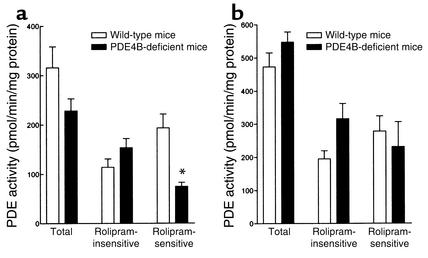
The hypnotic action of α2-adrenoceptor agonists is mediated at the brain stem level (30). Western blot analysis demonstrated that immunoreactive PDE4D and PDE4B could not be detected in brain stems of PDE4D-deficient and PDE4B-deficient mice, respectively (Figure 3). cAMP hydrolysis was measured in brain stem extracts from PDE4D- and PDE4B-deficient mice and their respective wild-type littermates in the presence or absence of 10 μM of rolipram. Total PDE activity was decreased in the PDE4D-deficient mice compared with their wild-type littermates. This attenuated PDE activity was solely due to a decrease in rolipram-sensitive PDE activity (PDE4 activity) (Figure 4a). In contrast, no significant changes in the total or in the rolipram-sensitive PDE activity were observed in the PDE4B-deficient mice compared with their wild-type littermates (Figure 4b).
Figure 3.
Western blot analysis from the brain stem of PDE4D- and PDE4B-deficient mice and their wild-type littermates. Left: Expression of PDE4D proteins in the brain stem of PDE4D-deficient mice (–/–) and their wild-type littermates (+/+). A mixture of recombinant PDE4D3 and PDE4D5 (Rec) was loaded for control. Right: Expression of PDE4B proteins in the brain stem of PDE4B-deficient mice (–/–) and their wild-type littermates (+/+).
Figure 4.
PDE activity in the brain stem of PDE4D-deficient (a) and PDE4B-deficient (b) mice and their wild-type littermates. PDE activity was determined using 1 μM cAMP as substrate in the absence (total activity) or presence (rolipram-insensitive activity) of 10 μM rolipram. The rolipram-sensitive activity (PDE4 activity) was obtained by subtracting the rolipram-insensitive activity from the total PDE activity. Values were corrected for the amount of protein added to the assay. Results are expressed as mean ± SEM (n = 3–9). *Significantly different at P < 0.05.
Duration of anesthesia.
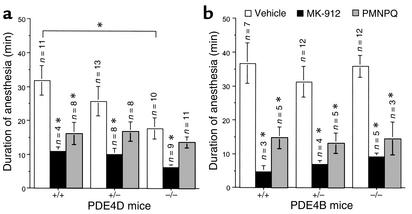
The contribution of PDE4B and PDE4D to the anesthesia-reversing effect of PDE4 inhibitors was evaluated using mice deficient in either subtype and their littermates. Loss of righting reflex was observed in all mice during the first 15 minutes following administration of the xylazine (10 mg/kg)/ketamine (80 mg/kg) combination, independent of the genotype. Under vehicle conditions, anesthesia lasted an average of 37 ± 6 minutes (n = 7) in PDE4B wild-type mice and 32 ± 4 minutes in the PDE4D wild-type ones. Although the PDE4B-deficient mice exhibited a duration of anesthesia similar to that of their wild-type and heterozygote littermates (Figure 5b), the PDE4D-deficient mice had shorter duration of anesthesia under vehicle treatment compared with their wild-type littermates (Figure 5a). However, under sodium pentobarbital–induced anesthesia (50 mg/kg, intraperitoneally), the PDE4D-deficient mice had a sleeping time (71 ± 11 minutes; n = 6) similar to that of their heterozygote (70 ± 13 minutes; n = 6) or wild-type littermates (71 ± 16 minutes; n = 6).
Figure 5.
Effect of MK-912 and PMNPQ on the duration of anesthesia induced by the combination of xylazine (10 mg/kg) and ketamine (80 mg/kg) in PDE4D-deficient (a) and PDE4B-deficient (b) mice (–/–) and their littermates (wild-type, +/+; heterozygote, +/–). Fifteen minutes after the induction of anesthesia, mice were injected with MK-912 (1 mg/kg, subcutaneously), PMNPQ (0.3 mg/kg, subcutaneously), or vehicle (PEG 60%). The duration of anesthesia was assessed by the return of the righting reflex. Results are expressed as mean ± SEM. *Significantly different at P < 0.05.
α2-Antagonist.
Compared with vehicle treatment, MK-912 (1 mg/kg, subcutaneously; positive control) significantly reduced the duration of anesthesia of all mice independent of their genotype. In PDE4D- and PDE4B-deficient mice the reduction in the anesthesia period was similar in magnitude to that observed in their wild-type and heterozygote littermates (Figure 5).
PDE4 inhibitor.
The contribution of PDE4 subtypes other than PDE4B and PDE4D was evaluated in this model by treatment of PDE4B- and PDE4D-deficient mice with the PDE4 inhibitor PMNPQ (0.3 mg/kg, subcutaneously). Treatment with PMNPQ significantly reduced the duration of anesthesia induced by the combination of xylazine and ketamine in the PDE4B-deficient mice (60% ± 14%) as well as in the wild-type (60% ± 9%) and heterozygote mice (58% ± 9%) compared with their respective vehicle-treated littermates (Figure 5b).
In the PDE4D mice, treatment with PMNPQ also significantly reduced the duration of anesthesia induced by the combination of xylazine and ketamine in the wild-type mice compared with their vehicle-treated littermates (49% ± 10%) (Figure 5a). In contrast, the duration of anesthesia was not significantly modified in the PDE4D-deficient mice treated with PMNPQ compared with their respective vehicle-treated littermates (reduction of 27% ± 7%). An intermediate effect was observed in the group of PDE4D-heterozygote mice treated with PMNPQ (mean reduction of 36% ± 10%) (Figure 5a).
Discussion
The administration of many PDE4 inhibitors has been associated with the side effect of nausea and vomiting. These debilitating adverse events are a significant issue in the therapeutic use of PDE4 inhibitors. Consequently the improvement of the therapeutic window of new generations of PDE4 inhibitors has been a major challenge. It has been previously suggested that the activity on the HARBS of PDE4 correlates with the side effects of emesis and that, hence, PDE4 inhibitors exhibiting a reduced activity on this conformer should have attenuated side effects (13, 15, 31). However, it has now been clarified that the HARBS coincides with the holoenzyme responsible for PDE4 catalysis (11, 12).
An alternative approach to improving the therapeutic index of new generations of PDE4 inhibitors would be to design PDE4 subtype–selective inhibitors, if it could be shown that nausea and vomiting are dependent on a specific PDE4 subtype. The recent demonstration that the emetic potential of PDE4 inhibitors can be assessed in non-vomiting species (e.g., rodents) has opened the possibility of using a genetic approach to evaluate the role of specific PDE4 subtypes in knockout mice (17, 19).
It has been shown that the ability of PDE4 inhibitors to reduce the duration of anesthesia induced by an α2-adrenoceptor agonist–mediated anesthetic regimen (xylazine/ketamine) can be used as a behavioral correlate of emesis in non-vomiting species (e.g., rodents) (17, 19). Our present findings in normal mice are in agreement with this notion. As previously observed in rats (19), PDE4 plays a pivotal role in anesthesia induced by α2-adrenoceptor activation in mice. The administration of PDE4 inhibitors significantly and specifically reduced the duration of anesthesia induced by the xylazine/ketamine combination. Furthermore, the potency of the various PDE4 inhibitors in reducing the anesthesia period in mice was reflective of their respective propensity to induce emesis in ferrets (9). The tendency of PDE4 inhibitors to reduce the anesthesia period was correlated with the proportion of compound present in the brain relative to plasma [PMNPQ, 391% ± 55%; (R)-rolipram, 291% ± 37%; (S)-rolipram, 114% ± 20%; CT-2450, 9% ± 3%]. However, it is important to note that the concentrations of the various PDE4 inhibitors in the brain were studied at the end of the experiment (1 hour after dosing) rather than at wake-up time. With the readily brain-permeable PDE4 inhibitors (PMNPQ, (R)-rolipram, and (S)-rolipram), the ability to shorten the duration of anesthesia correlated with the potency of inhibition of PDE4 enzyme and inhibition of TNF-α release from LPS-stimulated whole blood, a cellular marker of nonselective PDE4 inhibition (32). CT-2450, which was more potent than (R)- or (S)-rolipram in the PDE4 enzyme assay, was less active at reducing the anesthesia period. This is probably related to its weak capacity for brain penetration or to its pharmacokinetics. Nevertheless, since the absolute brain concentration of CT-2450 was similar to that of the other PDE4 inhibitors studied, it is possible that CT-2450 further fractionates within specific areas of the brain because of its limited partitioning between plasma and brain.
The contribution of the different subtypes of PDE4 to the anesthesia-reversing effect of PDE4 inhibitors was evaluated using mice that were deficient in PDE4D and PDE4B. Loss of the righting reflex following injection with the α2-adrenoceptor agonist–mediated anesthetic regimen occurred in all mice, independent of their genotype. Moreover, following treatment with MK-912, a significant genotype-independent reduction in the duration of the anesthesia was observed, suggesting the presence of a functional cell surface α2-adrenoceptor in all groups of mice.
While PDE4B-deficient mice injected with xylazine/ketamine had a duration of anesthesia similar to that of their wild-type or heterozygote littermates, PDE4D-deficient mice had a shorter sleeping time compared with their wild-type littermates. However, no genotype-related differences were seen in PDE4D-deficient mice under sodium pentobarbital–induced anesthesia. The anesthetic effect of sodium pentobarbital is mediated not by the α2-adrenoceptor, but rather through an enhancement of the γ-aminobutyric acid–mediated (GABA-mediated) inhibition of synaptic transmission (29). Taken together, these results indicate that PDE4D, but not PDE4B, has a modulating effect on the activity of the α2A-adrenoceptor, the receptor subtype responsible for the hypnotic effect of α2-adrenoceptor agonists (19, 33).
The hypnotic action of α2-adrenoceptor agonists is believed to be mediated at the locus coeruleus (30). Both ascending and descending noradrenergic fibers originate from that brain stem nucleus to innervate the CNS (34). Consistently, PDE4D-deficient mice showed a decrease in rolipram-sensitive PDE activity in the brain stem, compared with their wild-type littermates. Although immunoreactive PDE4B was detected in the hindbrain, the contribution of this subtype to the overall PDE activity must be minimal since no changes were observed in PDE4B-deficient mice. The α2-adrenoceptor is negatively coupled to adenylyl cyclase, and the inhibition of the activity of adenylyl cyclase is believed to play a critical role in the hypnotic effect of α2-adrenoceptor agonists (18). Hence, by elevating the intracellular levels of cAMP, the inhibition of PDE4D may trigger a series of downstream events offsetting the anesthesia induced by α2-adrenoceptor agonists.
We studied the possible role of PDE4 subtypes other than PDE4D or PDE4B in this model by evaluating the effect of PMNPQ (pan-specific PDE4 inhibitor) on the duration of anesthesia induced by xylazine/ketamine in PDE4D- and PDE4B-deficient mice and their littermates. In contrast to what was observed with PDE4B-deficient mice and with PDE4D wild-type mice, the pharmacological inhibition of the remaining PDE4 subtypes in the PDE4D-deficient mice with PMNPQ had no effect on the duration of anesthesia compared with that of the vehicle-treated PDE4D-deficient mice. Thus, these observations demonstrate that inhibition of PDE4D is playing a key role in the reduction of the duration of anesthesia seen with the PDE4 inhibitor PMNPQ. In agreement with this view, it is interesting that the pharmacological inhibition of all subtypes of PDE4 with the pan-specific inhibitor PMNPQ in the wild-type PDE4D mice produced a reduction in the duration of anesthesia similar in magnitude to that produced by the single genetic inactivation of PDE4D (Figure 5).
The fact that MK-912 was effective at reversing the duration of anesthesia in PDE4D-deficient mice while PMNPQ was not suggests that these two agents produce their pharmacological actions by different mechanisms. MK-912 is an α2-adrenoceptor antagonist (27) and is thought to reverse the anesthetic effect of xylazine/ketamine by displacing the agonist at the cell surface receptor level. In contrast, the PDE4 inhibitor PMNPQ is believed to produce its effect via the intracellular inhibition of PDE4D, as discussed above. Since this particular subtype is inactivated in PDE4D-deficient mice, no further decrease in the duration of anesthesia could be achieved with PMNPQ.
The present findings support a critical role of PDE4D in α2A-adrenoceptor signaling. In situ hybridization of the rat brain using 35S-RNA probes revealed that only α2A mRNA was detected in the locus coeruleus, giving the most intense signal of the entire brain (35). Moreover, the expression of α2A mRNA in noradrenergic neurons strongly suggests that this subtype of the α2-adrenoceptor functions as a presynaptic autoreceptor (35). Thus, the genetic inactivation of PDE4D may produce an elevated level of intracellular cAMP in presynaptic sympathetic neurons, opposing the inhibitory mechanism associated with the activation of the α2A-adrenoceptor. Consequently, it may imply that PDE4D-deficient mice have an exaggerated sympathetic nerve activity compared with wild-type mice under stress conditions. This would be consistent with the observation that PDE4D-deficient mice had a reduced sleeping time under xylazine/ketamine–induced anesthesia.
In conclusion, on the basis of the present functional study, we propose that PDE4D is the subtype responsible for the emetic side effect associated with the administration of pan-specific PDE4 inhibitors. The nature of the PDE4D splice variant involved remains to be determined, along with its phosphorylation status, since phosphorylation has been shown to influence the potency of certain PDE4 inhibitors in increasing cAMP concentrations (36–38). Furthermore, the possible contribution(s) of PDE4A and/or PDE4C remains to be investigated.
Acknowledgments
The authors wish to thank Micheal Gresser and George Robertson for supporting this collaborative work, which was funded by Merck Frosst Canada & Co. and by National Institute of Child Health and Human Development grant HD-20788 (to M. Conti).
Footnotes
Conflict of interest: A. Robichaud, N. Lachance, D. MacDonald, F. Laliberté, S. Liu, Z. Huang, and C.-C. Chan are currently employees of Merck and Co. and own Merck stock and/or stock options.
Nonstandard abbreviations used: phosphodiesterase (PDE); high-affinity rolipram binding site (HARBS); 6-(4-pyridylmethyl)-8-(3-nitrophenyl)quinoline (PMNPQ); (R)-N-{4-[1-(3-cyclopentyloxy-4-methoxyphenyl)-2-(4-pyridyl)ethyl]phenyl}N′-ethylurea (CT-2450); polyethylene glycol (PEG); erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA).
References
- 1.Giembycz MA. Cilomilast: a second generation phosphodiesterase 4 inhibitor for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2001;10:1361–1379. doi: 10.1517/13543784.10.7.1361. [DOI] [PubMed] [Google Scholar]
- 2.Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001;108:671–680. doi: 10.1067/mai.2001.119555. [DOI] [PubMed] [Google Scholar]
- 3.Timmer W, et al. The new phosphodiesterase 4 inhibitor roflumilast is efficacious in exercise-induced asthma and leads to suppression of LPS-stimulated TNF-α ex vivo. J Clin Pharmacol. 2002;42:297–303. doi: 10.1177/00912700222011328. [DOI] [PubMed] [Google Scholar]
- 4.Torphy TJ, et al. Ariflo™ (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinic. Pulm Pharmacol Ther. 1999;12:131–135. doi: 10.1006/pupt.1999.0181. [DOI] [PubMed] [Google Scholar]
- 5.Horowski R, Sastre-y-Hernandez M. Clinical effects of neurotropic selective cAMP phosphodiesterase inhibitor rolipram in depressed patients: global evaluation of the preliminary reports. Current Therapeutic Research. 1985;38:23–29. [Google Scholar]
- 6.Humpel M, Kühne G, Lehmann M, Poggel A. Pharmacokinetically governed design of animal toxicity studies of a new antidepressant drug. Arch Toxicol. 1986;9(Suppl.):251. (Abstr.) [Google Scholar]
- 7.Heaslip RJ, Evans DY. Emetic, central nervous system and pulmonary activities of rolipram in the dog. Eur J Pharmacol. 1995;286:281–290. doi: 10.1016/0014-2999(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 8.Silvestre J, Graul A, Castañer J. SB-207499. Antiasthmatic/antiinflamatory, phosphodiesterase IV inhibitor. Drugs of the Future. 1998;23:607–615. [Google Scholar]
- 9.Robichaud A, Tattersall FD, Choudhury I, Rodger IW. Emesis induced by inhibitors of type IV cyclic nucleotide phosphodiesterase (PDE IV) in the ferret. Neuropharmacology. 1999;38:289–297. doi: 10.1016/s0028-3908(98)00190-7. [DOI] [PubMed] [Google Scholar]
- 10.Xu RX, et al. Atomic structure of PDE4: insights into phosphodiesterase mechanism and specificity. Science. 2000;288:1822–1825. doi: 10.1126/science.288.5472.1822. [DOI] [PubMed] [Google Scholar]
- 11.Laliberté F, et al. Conformational difference between PDE4 apoenzyme and holoenzyme. Biochemistry. 2000;39:6449–6458. doi: 10.1021/bi992432w. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, et al. Dissecting the cofactor-dependent and independent bindings of PDE4 inhibitors. Biochemistry. 2001;40:10179–10186. doi: 10.1021/bi010096p. [DOI] [PubMed] [Google Scholar]
- 13.Barnette MS. Phosphodiesterase 4 (PDE4) inhibitors in asthma and chronic obstructive pulmonary disease (COPD) Prog Drug Res. 1999;53:193–229. doi: 10.1007/978-3-0348-8735-9_5. [DOI] [PubMed] [Google Scholar]
- 14.Barnette MS, Underwood DC. New phosphodiesterase inhibitors as therapeutics for the treatment of chronic lung disease. Curr Opin Pulm Med. 2000;6:164–169. doi: 10.1097/00063198-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Christensen SB, et al. 1,4-Cyclohexanecarboxylates: potent and selective inhibitors of phosphodiesterase 4 for the treatment of asthma. J Med Chem. 1998;41:821–835. doi: 10.1021/jm970090r. [DOI] [PubMed] [Google Scholar]
- 16.Conti M, Jin S-LC. The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 2000;63:1–38. doi: 10.1016/s0079-6603(08)60718-7. [DOI] [PubMed] [Google Scholar]
- 17.Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway (erratum 2001, 40:465) Neuropharmacology. 2001;40:262–269. doi: 10.1016/s0028-3908(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 18.Correa-Sales C, Nacif-Coelho C, Reid K, Maze M. Inhibition of adenylate cyclase in the locus coeruleus mediates the hypnotic response to an alpha2agonist in the rat. J Pharmacol Exp Ther. 1992;263:1046–1049. [PubMed] [Google Scholar]
- 19.Robichaud A, et al. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002;135:113–118. doi: 10.1038/sj.bjp.0704457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin S-LC, Richard FJ, Kuo W-P, D’Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci USA. 1999;96:11998–12003. doi: 10.1073/pnas.96.21.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin S-LC, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-α responses. Proc Natl Acad Sci USA. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sette C, Iona S, Conti M. Short-term activation of a rolipram-sensitive, cAMP specific phosphodiesterase by thyroid-stimulating hormone in thyroid FRTL-5 cells is mediated by a cAMP-dependent phosphorylation. J Biol Chem. 1994;269:9245–9252. [PubMed] [Google Scholar]
- 23.Iona S, et al. Characterization of the rolipram-sensitive, cyclic AMP specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol Pharmacol. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm, R.S., Fatheree, P.R., and Chin, R.L., inventors; Syntex (USA) Inc., assignee. October 13, 1994. Quinolines as type IV phosphodiesterase inhibitors. World patent WO9422852.
- 25.Alexander RP, et al. CDP840. A prototype of a novel class of orally active anti-inflammatory phosphodiesterase 4 inhibitors. Bioorg Med Chem Lett. 2002;12:1451–1456. doi: 10.1016/s0960-894x(02)00202-0. [DOI] [PubMed] [Google Scholar]
- 26.Brideau C, Van Staden C, Styhler A, Rodger IW, Chan C-C. The effect of phosphodiesterase type 4 inhibitors on tumor necrosis factor-α and leukotriene B4 in a novel human whole blood assay. Br J Pharmacol. 1999;126:979–988. doi: 10.1038/sj.bjp.0702387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettibone DJ, et al. Pharmacological profile of a new potent and specific α2-adrenoceptor antagonist, L-657,743. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:169–175. doi: 10.1007/BF00165801. [DOI] [PubMed] [Google Scholar]
- 28.Fawcett L, et al. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA. 2000;97:3702–3707. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fish, R.E. 1997. Pharmacology of injectable anesthetics. In Anesthesia and analgesia in laboratory animals. D.F. Kohn, S.K. Wixson, W.J. White, and G.J. Benson, editors. Academic Press. New York, New York, USA. 1–28.
- 30.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha2agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Griswold DE, et al. SB 207499 (Ariflo), a second generation phosphodiesterase 4 inhibitor, reduces tumor necrosis factor α and interleukin-4 production in vivo. J Pharmacol Exp Ther. 1998;287:705–711. [PubMed] [Google Scholar]
- 32.Muise ES, et al. Comparison of inhibition of ovalbumin-induced bronchoconstriction in guinea pigs and in vitro inhibition of tumor necrosis factor-α formation with phosphodiesterase 4 (PDE4) selective inhibitors. Biochem Pharmacol. 2002;63:1527–1535. doi: 10.1016/s0006-2952(02)00903-6. [DOI] [PubMed] [Google Scholar]
- 33.Mizobe T, et al. Antisense technology reveals the α2Aadrenoceptor to be the subtype mediating the hypnotic response to the highly selective agonist, dexmedetomidine, in the locus coeruleus of the rat. J Clin Invest. 1996;98:1076–1080. doi: 10.1172/JCI118887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald E, Scheinin M. Distribution and pharmacology of α2-adrenoceptors in the central nervous system. J Physiol Pharmacol. 1995;46:241–258. [PubMed] [Google Scholar]
- 35.Scheinin M, et al. Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez R, et al. Activation and selective inhibition of a cyclic AMP-specific phosphodiesterase, PDE-4D3. Mol Pharmacol. 1995;48:616–622. [PubMed] [Google Scholar]
- 37.MacKenzie SJ, et al. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in upstream conserved region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laliberté F, et al. In vitro PKA phosphorylation-mediated human PDE4A4 activation. FEBS Lett. 2002;512:205–208. doi: 10.1016/s0014-5793(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 39.Marx D, Tassabehji M, Heer S, Hüttenbrink K-B, Szelenyi I. Modulation of TNF and GM-CSF release from dispersed human nasal polyp cells and human whole blood by inhibitors of different PDE isoenzymes and glucocorticoids. Pulm Pharmacol Ther. 2002;15:7–15. doi: 10.1006/pupt.2001.0315. [DOI] [PubMed] [Google Scholar]