Abstract
Lymphocytes in direct contact with embryonic extravillous trophoblasts constitute more than 40% of decidual cells and appear to play major roles in implantation and early gestation. A unique subset of NK cells, making up 70–80% of decidual lymphocytes, express high levels of CD56 but lack CD16. We have recently demonstrated a novel class I MHC–independent inhibitory mechanism of NK cell cytotoxicity that is mediated by CEACAM1 homotypic interactions. This mechanism is used by some melanoma cells to avoid attack, mainly by CD16– NK cells. We now demonstrate that CEACAM1 is expressed on primary extravillous trophoblasts and is upregulated on the vast majority of IL-2–activated decidual lymphocytes, including NK, T, and NKT cells. Importantly, we present evidence that CEACAM1 interactions inhibit the lysis, proliferation, and cytokine secretion of activated decidual NK, T, and NKT cells, respectively. In vivo analysis of decidual lymphocytes isolated from cytomegalovirus-infected (CMV-infected) pregnant women revealed a dramatic increase in the expression of CEACAM1. Finally, we suggest that a novel ligand for this adhesion molecule is present on the surface of CMV-infected fibroblasts. These combined results demonstrate a major role for the CEACAM1 protein in controlling local decidual immune responses.
Introduction
During embryonic implantation, the extravillous trophoblast (EVT) cells invade the uterine endometrium. At this site, a direct-contact interface forms between maternal and embryonic cells, which locally modifies the properties of the uterine mucosa. Embryonal-maternal interface together with specialized ECM constitutes the decidua basalis. Remarkably, more than 40% of decidual cells are immune cells (1). This suggests that the maternal immune system is involved in the modulation of maternal-embryonal interactions. The decidual lymphocyte composition differs significantly from that of peripheral blood lymphocytes. More than 70% of decidual lymphocytes are CD56bright CD16– (FcRγIII) NK cells, while T cells constitute only 10% (2). In contrast, only 10% of the peripheral blood lymphocytes are NK cells that are characterized by a moderate expression level of the CD56 protein and the expression of the CD16 receptor (2). It is currently believed that decidual lymphocytes are important for control of normal trophoblastic growth, differentiation, and invasion (3). However, their role in combating pathogens in the context of pregnancy is only poorly understood.
The gentle balance between immune tolerance and immune activation that might lead to the rejection of the embryo by the decidual lymphocytes is maintained via several mechanisms, involving both decidual lymphocytes and EVTs. EVT invasion might be controlled by the modulation of the local cytokine profile (4), and therefore the cytokine release of decidual lymphocytes must be tightly regulated. The killing activity of both NK cells and CTLs, belonging to the innate and adaptive branches of the immune system, respectively, is regulated by the class I MHC proteins. While the recognition of the class I MHC proteins by the T cell receptors (TCRs) of CTLs activates T cell–mediated killing, the interactions between NK cells and the same proteins suppress NK cell cytotoxicity. It was reported that EVTs express an unusual combination of two nonclassical class I MHC proteins, the HLA-E (5) and HLA-G (6), along with the classical HLA-C protein (7), but that they do not express the HLA-A and HLA-B proteins (7). As most of the CTLs are directed against HLA-A and -B proteins, this unique pattern of expression of class I MHC proteins probably prevents rejection of the semiallogeneic fetus by CTLs. The HIV virus uses a similar mechanism of specific downregulation of HLA-A and -B proteins, mediated by the Nef protein, to avoid attack by CTL (8).
NK cells compose the vast majority of decidual lymphocytes that are in contact with EVTs. The fetus is protected from rejection by maternal NK cells for several reasons. First, decidual NK cell inhibition appears skewed toward HLA-C recognition, compared with peripheral blood NK cells. Fifty to eighty percent of decidual NK cells are inhibited by HLA-C, compared with only 5–20% of the peripheral blood NK cells (9). Second, virtually all decidual NK cells express the HLA-E–binding inhibitory receptor complex CD94/NKG2A five times more than do peripheral blood NK cells (9). Furthermore, the HLA-E protein, which is expressed on cell surface upon binding of peptides derived from the leader sequence of various class I MHC proteins, binds, with the greatest affinity, the leader peptides of HLA-G and HLA-C proteins (10), which are both expressed on the EVT cells (7). Third, all decidual NK cells express the inhibitory LIR1 (ILT2) or KIR2DL4 receptors (11), both of which are able to interact with the HLA-G proteins (11). Fourth, decidual NK cells have decreased killing activity against class I MHC–negative target cells (12). This wide spectrum of mechanisms aimed at controlling the cytolytic function of decidual NK cells further demonstrates the importance of these cells in the rejection of allogeneic transplants. It also implies that other mechanisms with the ability to control the function of decidual lymphocytes might exist.
The CEACAM1 protein, a member of the CEACAM family, is expressed on a broad spectrum of cells (13). It belongs to the Ig superfamily and interacts in both a homotypic manner and a heterotypic manner with other variants of the CEACAM family, including the CEACAM6 and CEACAM5 proteins (14). Recently, we showed that the CEACAM1 homotypic interactions between NK cells and various target cells inhibit NK cytotoxicity (15). This novel class I MHC–independent mechanism is used mainly by CD16– NK cells and might play an important role in the development of various pathologies, such as melanoma (15).
In this study we show that CEACAM1 is expressed by EVTs as well as by the majority of IL-2–activated decidual lymphocyte subsets. The engagement of the CEACAM1 protein leads to the inhibition of NK killing, T cell proliferation, and IFN-γ secretion by NKT cells. The in vivo upregulation of the CEACAM1 protein on the majority of decidual lymphocytes might have an important role in controlling local immune response. This was demonstrated by the analysis of decidual lymphocyte subsets obtained from decidua of cytomegalovirus-infected (CMV-infected) women, which revealed a dramatic upregulation of surface CEACAM1 expression. In addition, we provide evidence that CEACAM1 binds and functionally interacts with an unidentified molecule present on human primary fibroblasts infected with the laboratory AD169 CMV strain or with a clinical CMV strain isolated from infected decidua. These combined results demonstrate a major role for the CEACAM1 protein in controlling local decidual immune responses.
Methods
Cells, transfections, virus propagation, and antiviral agent.
The cell lines used in this work were the class I MHC–negative Epstein-Barr virus–transformed B cell line 721.221 (.221), .221 cells transfected with the CEACAM1 cDNA (15), and the murine thymoma BW cell line, which lacks expression of α and β chains of the TCR. Stable transfection of .221 cells expressing CEACAM6 and CEACAM5 was performed by electroporation (0.23 kV, Cap [μF] 250 μF). The cDNA for CEACAM6 was amplified by RT-PCR and cloned into pcDNA3 expression vector, and the CEACAM5 cDNA was a kind gift from W. Zimmermann, Ludwig-Maximilians-University, Muenchen, Germany) Human foreskin fibroblasts (HFFs) were used for propagation and infection of human CMV strain AD169 (American Type Culture Collection, Manassas, Virginia, USA), as previously described (16). After a 1-hour period of virus adsorption to cells, 300 μg/ml of the CMV DNA polymerase inhibitor phosphonoformate (PFA; Sigma-Aldrich, St. Louis, Missouri, USA) was added for inhibition of virus replication.
Primary CMV infection, definition of congenital CMV infection, and termination of pregnancy.
Primary CMV infection during pregnancy was diagnosed by documentation of maternal seroconversion, with appearance of CMV antibodies during pregnancy in women known to be CMV-seronegative before gestation. Diagnosis of CMV fetal infection was based on viral isolation (by shell viral culture and conventional culture) from amniotic fluid obtained at the 22nd week of gestation, along with PCR detection of viral DNA in the amniotic fluid. Congenital disease could be predicted by the presence of characteristic ultrasonographic findings including cerebral calcifications and microcephaly. Decision to terminate pregnancy was based on documentation of fetal infection and disease. Deciduae from first-trimester elective terminations were obtained by scraping.
Antibodies.
The mAb’s used in this work were FITC-conjugated Kat4c mAb directed against CEACAM1, -5, and -6 (DAKO, Glostrup, Denmark), phycoerythrin-conjugated anti-CD56 mAb (BDPharmingen, San Diego, California, USA), CyChrome-conjugated anti-CD3 mAb (BDPharmingen), and biotinylated anti-CD16 mAb (Serotec, Oxford, United Kingdom), followed by Cy5-streptavidin (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). The anti-CD4 mAb (DAKO), anti-Vβ3 (BD Pharmingen), anti-Vβ17 (BDPharmingen), and the anti-CEACAM1 5F4 (17) mAb were also used. For blocking assays, rabbit polyclonal anti-CEACAM1, -5, and -6 (DAKO) antibodies and the control rabbit polyclonal antibodies against purified ubiquitin were used. The following anti–IFN-γ mAb’s were purchased from BDPharmingen: mAb B27, used for measuring intracellular IFN-γ production; and biotinylated mAb 4S.B3 (detection) and purified mAb HIB42 (capture), both used in the ELISA assays. The production of mouse IL-2 from BW/CEACAM1ζ-transfected cells was detected by ELISA using purified anti–mouse IL-2 mAb JES6-1A12 (capture) and biotinylated anti–mouse IL-2 mAb JES6-5H4 (detection) (both from BD Pharmingen). ELISA assays were performed according the manufacturer’s instructions (BD Pharmingen).
Isolation of decidual lymphocytes.
The Hadassah Medical Organization Institutional Board approved obtaining deciduae from elective pregnancy-termination procedures, from induced labors, and from caesarian sections, in keeping with the principles of the Helsinki Declaration. The tissue was trimmed into 1-mm pieces and enzymatically digested for 20 minutes, using vigorous shaking, with 1.5 mg type I DNase and 24 mg type IV collagenase present in 15 ml of RPMI-1640 medium. This procedure was repeated three times. After an additional 5 minutes’ incubation at room temperature without shaking, the supernatants were collected and incubated overnight in a tissue culture dish. Nonadherent cells were collected and loaded on Ficoll density gradient to purify the lymphocyte population. Cells were further analyzed by flow cytometry. NK and NKT cells were purified using anti-CD56 mAb followed by incubation with microbeads of conjugated goat anti-mouse IgG antibodies (Miltenyi Biotec Inc., Auburn, California, USA). Separation was performed with the AutoMACS instrument (Miltenyi Biotec Inc.). Positive (NK and NKT cells) and negative (T cells) fractions were collected and cloned (one cell per well) in the presence of IL-2.
Quadruple staining.
For quadruple staining, the following fluorochrome-conjugated mAb’s were used: FITC-conjugated anti-CEACAM Kat4c mAb (DAKO), phycoerythrin-conjugated anti-CD56 mAb (BDPharmingen), and CyChrome-conjugated anti-CD3 mAb (BD Pharmingen). As the fourth color, biotinylated anti-CD16 mAb (Serotec) was used, followed by Cy5-streptavidin (Jackson ImmunoResearch Laboratories Inc.) as a second reagent. To block nonspecific binding, cells were first incubated for 1 hour on ice with 25% human serum, and then incubated with the various antibodies.
Cytotoxicity assays.
The cytotoxic activity of NK cells against the various targets was assayed in 5-hour 35S-release assays, as described previously (15, 18). Briefly, cells were labeled overnight with 35S-methionine and washed, and 5 × 103 labeled target cells were incubated at various effector-to-target ratios. The killing rate was calculated as percent 35S-methionine release = (cpm sample – cpm spontaneous release)/(cpm total – cpm spontaneous release) × 100. Total 35S-methionine release was measured after incubation of the cells with 0.1 M NaOH. In all presented cytotoxic assays, the spontaneous release was less than 25% of maximal release. In experiments where mAb’s were included, the final mAb concentration was 10 μg/ml, or 40 μl/ml in those cases where rabbit polyclonal antibodies were used.
Staphylococcal enterotoxin B–induced T cell proliferation.
These assays were performed as previously described (18). Briefly, target .221 and .221/CEACAM1 cells were irradiated (60 Gy). Thereafter, 50,000 T cells, 25,000 target cells, and various concentrations of superantigen were mixed in a total volume of 200 μl of RPMI–10% FCS in each well of a 96-well plate. After incubation at 37°C and 5% CO2 for 2 days, 1 μCi of 3H-thymidine was added to each well and the cells were further incubated at 37°C overnight. The cells were then harvested and counted on a liquid scintillation counter (1450 MicroBeta PLUS; Wallac, Turku, Finland). In analysis of the cpm from each well, the background cpm from wells in which identical reagents and target cells were placed in the absence of any T cells was subtracted.
Generation of Ig fusion proteins.
The extracellular portion of the CEACAM1 protein was amplified by PCR using the following primers: 5′-CCCAAGCTTGGGGCCGCCACCATGGGGCACCTCTCAGCC (including HindIII restriction site) and 3′-GCGGATCCCCAGGTGAGAGGC (including BamHI restriction site). A silent mutation, adenine 885 guanidine (no change in glycine 281), was performed by site-directed mutagenesis to cancel the BamHI site in the amplified sequence. The generation, production, and staining procedures of the Ig fusion proteins were previously described (19, 20). Briefly, the PCR-generated fragments were cloned into a mammalian expression vector containing the Fc portion of human IgG1 (a kind gift from B. Seed, Massachusetts General Hospital, Department of Molecular Biology, Boston, Massachusetts, USA). Sequencing of the constructs revealed that all cDNAs were in frame with the human Fc genomic DNA and were identical to the reported sequences. COS-7 cells were transiently transfected with the plasmids containing cDNAs using FuGENE6 reagent (Roche Molecular Biochemicals, Indianapolis, Indiana, USA)according to the manufacturer’s instructions, and supernatants were collected and purified on a protein G column. SDS-PAGE analysis revealed that all Ig fusion proteins were approximately 95% pure and of the proper molecular mass. To assay for the CEACAM binding, various cells were incubated with 50 μg/ml of fusion protein for 2 hours on ice. The cells were washed and incubated with Fc fragment–specific (minimal cross-reaction to bovine, horse, and mouse serum proteins), phycoerythrin-conjugated affinity-purified F(ab′)2 fragment of goat anti-human IgG (Jackson ImmunoResearch Laboratories Inc.). Incubation was performed for 1 hour and analyzed by flow cytometry with a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, California, USA).
Generation of BW cells expressing the chimeric CEACAM1ζ protein and the production of IL-2.
The extracellular portion of the human CEACAM1 protein was amplified by PCR using the following primers: 5′-CCCAAGCTTGGGGCCGCCACCATGGGGCACCTCTCAGCC (including HindIII restriction site) and 3′-GTAGCAGAGAGGTGAGAGGCCATTTTCTTG (including first nine nucleotides of mouse ζ chain transmembrane portion). The mouse ζ chain was amplified by PCR using the following primers: 5′-CTCTCACCTCTCTGCTACTTGCTAGATGGA (including last nine nucleotides of human CEACAM1 extracellular portion) and 3′-GGAATTCCTTAGCGAGGGGCCAGGGTCTG (including EcoRI restriction site). The two amplified fragments were mixed, and PCR was performed with the 5′ HindIII primer and the 3′ EcoRI primer for the generation of the CEACAM1ζ construct. The CEACAM1ζ construct was cloned into pcDNA3 expression vector (Invitrogen Corp., Carlsbad, California, USA) and stably transfected into BW cells. For measurement of IL-2 production resulting from the homotypic CEACAM1 interactions, 50,000 BW or BW-transfected cells were incubated in RPMI–10% FCS medium for 48 hours at 37°C and 5% CO2. Supernatants were collected and the presence of IL-2 was monitored by using anti–IL-2 mAb and standard ELISA assays (BDPharmingen). For measurement of IL-2 production resulting from the CEACAM1 interactions of different cell types, 50,000 BW or BW-transfected cells were incubated in RPMI–10% FCS with irradiated .221 or with .221/CEACAM1 cells for 24 hours or with CMV-infected HFF cells for 48 hours at 37°C and 5% CO2. The presence of mouse IL-2 in cell supernatants was measured as above.
Cross-linking of NKT cells.
NKT cells (105 per well) were incubated with or without 0.5 μg of Kat4c mAb on ice for 1.5 hours in 96 round bottom microplates (Nalge Nunc, Rochester, New York, USA). Treated NKT cells, present in 200 μl of IL-2–containing medium, were then cultured in 96 flat bottom microplates (Nalge Nunc) precoated with 1 μg/well of sheep anti-mouse IgG antibodies (ICN Biomedicals Inc., Costa Mesa, California, USA) for 24 hours at 37°C. Cells were then analyzed by FACS.
Permeabilization and intracellular IFN-γ staining.
The permeabilization and intracellular IFN-γ staining were performed using the Cytofix/Cytoperm Plus (with GolgiStop) kit (BD Pharmingen) according to the manufacturer’s instruction.
Results
CEACAM1 is expressed on different decidual lymphocytes after activation.
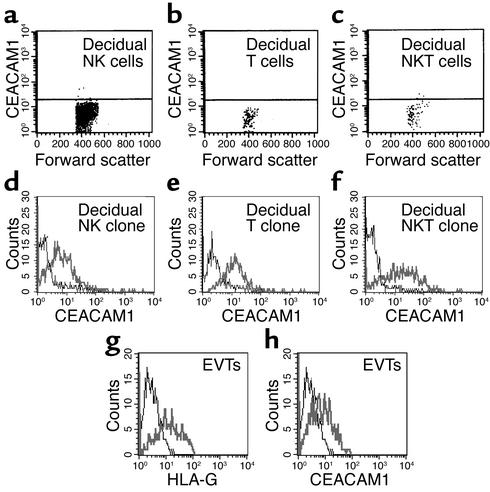
To test the possible role of CEACAM1 in controlling decidual lymphocyte functions, we isolated decidual lymphocytes from first-trimester elective pregnancy terminations as described in Methods. Obtained tissues were identified as decidua by histologic analysis. Lymphocytes were isolated from nine different deciduae and quadruple-stained using flow cytometry for the expression of CD3, CD16, CD56, and CEACAM. In agreement with previous observations (2), the total decidual lymphocyte population contained mainly CD16– NK cells (70–80%, characterized by CD3– CD56bright; data not shown), but T (characterized by CD3+ CD56–) and NKT (characterized by CD3+ CD56+) cells were also identified (5.3% and 3.2%, respectively; data not shown). Little or no staining for the CEACAM1 protein was observed among all decidual lymphocyte populations tested (Figure 1, a–c).
Figure 1.
CEACAM1 staining of decidual lymphocytes. Decidual lymphocytes were isolated and quadruple-stained as described in Methods. (a–c) CEACAM1 staining on nonactivated decidual NK cells (a), T cells (b), and NKT cells (c). One representative experiment is shown out of three performed. Decidual lymphocytes were cultured in the presence of IL-2 as described (20) and then screened for CEACAM1 expression with the 5F4 mAb. (d–f) CEACAM1 staining for activated decidual NK clone (d), T clone (e), and NKT clone (f). Similar results were obtained when other lymphocyte clones were used. (g and h) Staining of EVTs for HLA-G and CEACAM1, respectively. Bold lines represent mAb staining and thin lines show background staining.
Various lymphocytes were cloned (see Methods) and cultured for 3 weeks in the presence of IL-2 (50 U/ml). Remarkably, staining with the 5F4 anti-CEACAM1 mAb (see Figure 2) revealed a dramatic increase in the CEACAM1 protein expression on the surface of the vast majority of NK, T, and NKT cell clones tested (85%, 86%, and 95%, respectively; surface expression of CEACAM1 on representative clones is shown in Figure 1, d–f, and data not shown). This is in marked contrast to NK cells derived from peripheral blood, in which surface CEACAM1 expression could be detected on only 2–3% of IL-2–activated CD16+ NK clones and on about 45% of the IL-2–activated CD16– clones (15, 21). Notably, the expression levels of the CEACAM1 on the surface of all tested clones were more than threefold above background (data not shown). This level of expression was reported to be sufficient for effective inhibition of NK cytotoxicity (15).
Figure 2.
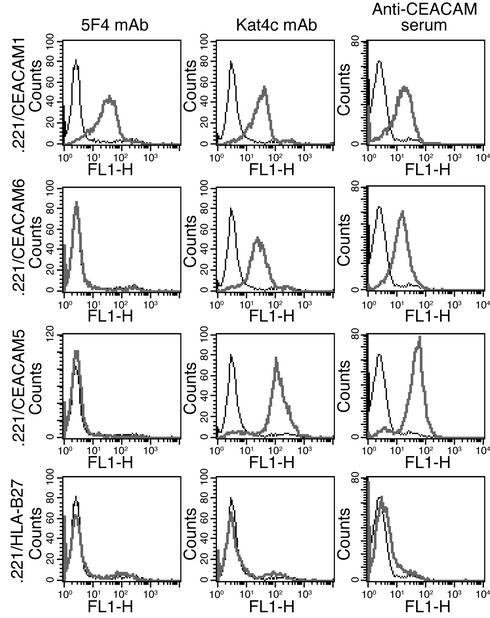
Staining of .221 cells expressing various members of the CEACAM family using specific anti-CEACAM antibodies. We generated .221 transfectants as described in Methods. Each row shows the staining performed on a particular transfectant (indicated at left), and each column shows the staining with a particular antibody (indicated at top). Bold lines represent antibody staining and thin lines show background staining on .221 cells. One representative experiment is shown out of three performed.
As the CEACAM1 protein interacts homotypically with other CEACAM1 proteins (15, 22, 23) (see Figures 6 and 7) and decidual lymphocytes are in direct contact with embryonic EVT cells in vivo (3), it was important to test whether EVT cells express the CEACAM1 protein. EVT cells were obtained from the same elective pregnancy terminations from which decidual lymphocytes were isolated and were tested for the expression of HLA-G and CEACAM1. As the expression of HLA-G is restricted to EVT cells only (6), isolated cells were identified as EVT cells by using specific staining with the anti–HLA-G specific mAb MEM-G/13B. The mAb MEM-G/13B specifically stains the class I MHC–negative .221 cells transfected with HLA-G; it did not stain .221 cells transfected with other class I MHC cDNA (data not shown). FACS staining analysis of isolated EVT cells showed that these cells express the HLA-G (Figure 1g) and the CEACAM1 (Figure 1h) proteins. These findings suggest that CEACAM1 might mediate direct interactions between activated decidual lymphocytes and EVTs and thus might display a novel control mechanism protecting the embryo from sustaining damage.
Figure 6.
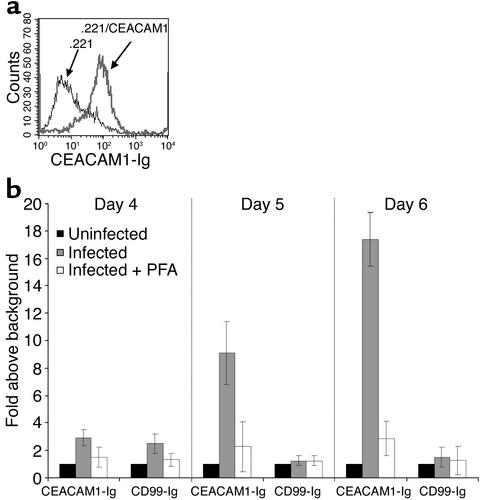
CEACAM1-Ig specifically binds to CMV-infected fibroblasts. (a) Binding of CEACAM1-Ig to .221/CEACAM1 cells (bold line) but not to parental .221 (thin line). The figure shows a representative experiment out of three performed. (b) Day-by-day staining of uninfected and CMV-infected HFF cells in the presence or absence of 300 μg/ml of the antiviral agent PFA. Cells were stained with CEACAM1-Ig and with the control CD99-Ig fusion protein as described in Methods. Data are presented as fold increase above the staining of uninfected cells. The average of two independent experiments is shown.
Figure 7.
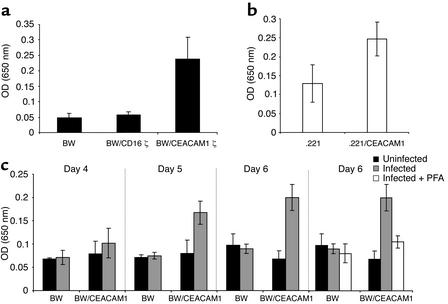
The functional interactions between BW/CEACAM1ζ and CMV-infected HFFs elicit IL-2 secretion. (a) Spontaneous IL-2 secretion by BW and various BW transfectants after 48 hours of incubation. The average of 20 independent experiments is shown. (b) IL-2 secretion by BW/CEACAM1ζ cells coincubated for 24 hours with irradiated .221 or with .221/CEACAM1 cells. The average of six independent experiments is shown. (c) IL-2 secretion after coincubation of BW or BW/CEACAM1ζ cells with uninfected or CMV-infected HFF cells for 48 hours. No IL-2 secretion above background levels was observed when PFA was included in the assay (only day 6 is shown). Experiments were performed concomitantly with the flow cytometry binding assays of CEACAM1-Ig shown in Figure 5. The average of two independent experiments is shown.
CEACAM1 interactions inhibit decidual NK cytotoxicity.
We have previously demonstrated that the CEACAM1-mediated inhibition of NK cells can be blocked by using rabbit polyclonal anti-CEACAM antibodies and not by the mAb 5F4 or the mAb Kat4c (15). It was previously reported that the CEACAM1 protein interacts with other CEACAM proteins, such as CEACAM5 and CEACAM6, and that the binding site of CEACAM1 was located at the N-terminal Ig-V–type domain of the CEACAM1 protein (23). The N-terminal Ig-V–type domain of the CEACAM family reveals 70–90% sequence similarity among the different variants. It was therefore important to determine the specificity of all anti-CEACAM1 antibodies used in this work. We transfected .221 cells with CEACAM1 (15), CEACAM6, and CEACAM5 and stained them for surface expression using the various anti-CEACAM antibodies.
Figure 2 shows that all anti-CEACAM antibodies specifically recognized members of the CEACAM family. This is because no staining was observed on either nontransfected .221 cells or the control HLA-B27–transfected .221 cells. The 5F4 mAb recognized the CEACAM1 protein only, whereas the Kat4c mAb and the rabbit polyclonal antibodies directed against CEACAM recognized CEACAM1, CEACAM6, and CEACAM5 proteins (Figure 2).
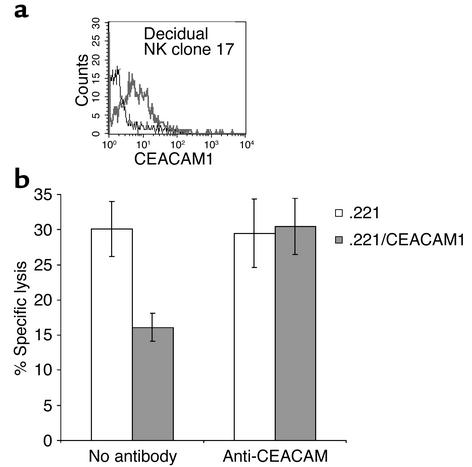
To investigate whether the CEACAM1 protein is functional, IL-2–activated decidual NK clones, expressing the CEACAM1 protein (a representative clone is shown in Figure 3a), were tested in killing assays against .221 cells and .221 cells transfected with CEACAM1 (.221/CEACAM1). The generation of these transfectants was described previously (15). The CEACAM1+ NK clones effectively killed .221 cells, whereas inhibition of lysis was observed when .221/CEACAM1 cells were used (Figure 3b). The inhibition of NK killing by .221/CEACAM1 cells was the result of the CEACAM1 homotypic interactions, as lysis of .221/CEACAM1 cells was restored when rabbit anti-human CEACAM antibodies were included in the assay. The addition of a control rabbit serum derived from ubiquitin-immunized rabbit had no effect (data not shown). No difference in the lysis of .221 or .221/CEACAM1 cells was observed when CEACAM1– NK clones were used (data not shown). In agreement with a previous report (12), most decidual NK clones displayed only limited cytotoxicity against the .221 target cells (10–20% lysis; data not shown). Importantly, the killing of .221/CEACAM1 cells by “low killer” decidual NK clones was also decreased because of the homotypic CEACAM1 interactions, and the addition of anti-CEACAM polyclonal antibodies restored lysis (data not shown).
Figure 3.
CEACAM1-mediated inhibition of decidual NK cytotoxicity. Decidual NK clones were stained for CEACAM1 expression. (a) CEACAM1 staining of decidual NK clone 17 using the anti-CEACAM1 mAb 5F4 (bold line). The thin line shows the control staining. (b) Killing and inhibition of NK clone 17 by .221 cells and by .221 cells transfected with CEACAM1 (.221/CEACAM1). Blocking experiments were performed using 40 μl/ml of anti-CEACAM antibodies. Average of three independent experiments is shown. Similar results were obtained when other CEACAM1+ NK clones were used.
CEACAM1 interactions inhibit staphylococcal enterotoxin B–induced decidual T cell proliferation.
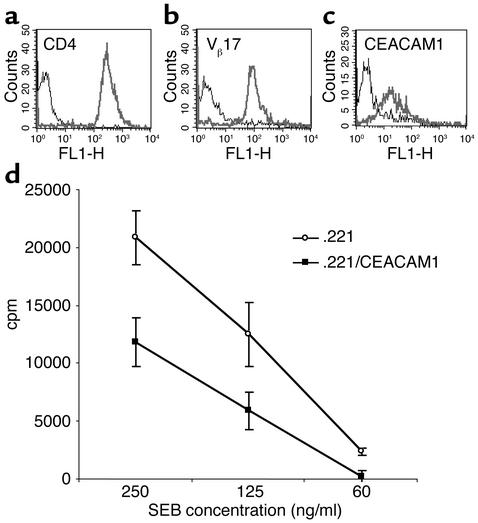
As the expression of CEACAM1 protein was also demonstrated on the vast majority of T lymphocytes activated by IL-2, we also tested the effect of CEACAM1 interactions on T cell proliferation. Superantigens can induce T cell proliferation by binding to class II MHC proteins and specific TCR Vβ chains. The staphylococcal enterotoxin B (SEB) superantigen interacts with various TCR Vβ chains, including Vβ3 and Vβ17. Decidual T cell clones were obtained as described in Methods and screened by flow cytometry for the expression of CD4, Vβ3, and Vβ17 by using specific mAb’s. A representative T cell clone, no. 1, stained brightly for both CD4 and Vβ17 (Figure 4, a and b), and moderately for CEACAM1 (Figure 4c). The SEB-induced proliferation of this T cell clone was assayed as described in Methods. A dramatic increase in the T cell proliferation was observed when cells were incubated with .221 cells in the presence of 250 ng/ml of SEB (50-fold above the background proliferation without SEB; data not shown). Efficient inhibition of the T cell proliferation (around 50%) was observed in all SEB concentrations tested when cloned T cells were incubated with .221/CEACAM1 cells (Figure 4d). The expression levels of the class II MHC proteins were similar on both .221 and .221/CEACAM1 cells (data not shown).
Figure 4.
CEACAM1-mediated interactions inhibit SEB-induced T cell proliferation. Decidual T cell clones were tested for expression of CD4 (a), Vβ17 (b), and CEACAM1 (c) by flow cytometry. Bold lines indicate mAb staining and thin lines indicate control staining. (d) Fifty thousand cells of the presented T cell clone were incubated for 2 days with 25,000 irradiated .221 cells or with .221 cells transfected with CEACAM1 (.221/CEACAM1), in the presence of decreasing SEB concentrations as indicated in the figure. Proliferation was measured with 3H-thymidine incorporation. The figure represents the average of ten independent experiments. Similar results were obtained when other T cell clones were used.
CEACAM1 interactions inhibit secretion of cytokines from decidual NKT cells.
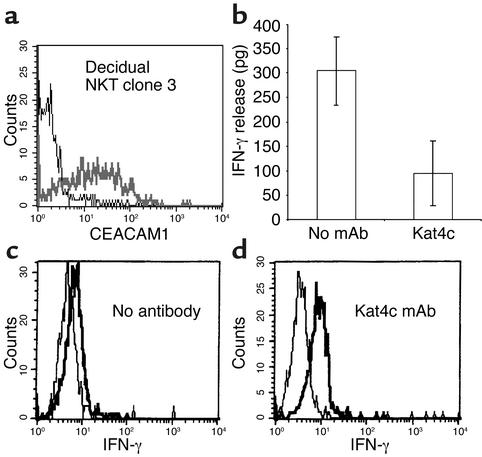
Cytokines might play an important role in fetus development (24). NKT cells that are present among the decidual lymphocyte population (see Figure 1) are able to produce large amount of cytokines. The functional effect of CEACAM1 interactions on cytokine secretion of decidual NKT cells has never been investigated. Decidual NKT clones were cultured as described in Methods and screened for CEACAM1 expression by flow cytometry, using the anti-CEACAM1 5F4 mAb (a representative NKT cell clone, no. 3, is shown in Figure 5a). NKT clone 3 spontaneously secreted IFN-γ into the media, as measured by ELISA (Figure 5b). Other cytokines such as IL-4, IL-5, IL-13, TNF, and macrophage inflammatory protein-1α could not be detected in culture supernatant of this clone (data not shown). Cross-linking of CEACAM1 for 24 hours with the Kat4c mAb dramatically decreased the amount of IFN-γ detected in the medium of this NKT cell clone (Figure 5b). In order to determine whether the inhibitory effect observed after cross-linking of CEACAM1 on NKT cells is the result of decreased secretion or decreased production of IFN-γ, we stained for the presence of intracellular IFN-γ, before and after cross-linking of CEACAM1, as described in Methods. Untreated NKT cells showed little staining for intracellular IFN-γ (median fluorescence intensity twofold above background; Figure 5c). After cross-linking with the Kat4c mAb, the staining for intracellular IFN-γ increased significantly (median fluorescence intensity 4.5-fold above background; Figure 5d). These findings therefore suggest that CEACAM1 engagement on NKT cells suppresses the cytokine secretion machinery and not de novo synthesis.
Figure 5.
CEACAM1-mediated inhibition of IFN-γ secretion from NKT cells. (a) CEACAM1 expression on isolated activated NKT clone. The bold line shows the staining with 5F4 mAb, and the thin line shows the control staining. (b) The amount of IFN-γ in culture supernatant of mAb-treated and untreated NKT clone cells measured by ELISA. The average of two independent experiments is shown. Cross-linking of surface CEACAM1 was performed without (c) or with (d) the Kat4c mAb, and intracellular staining for IFN-γ was performed. One representative experiment is shown out of two performed. Similar results were obtained when other NKT cell clones were used.
In vivo upregulation of CEACAM1 on decidual lymphocytes.
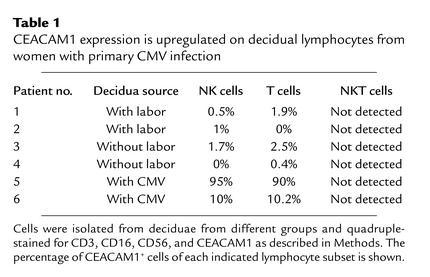
The above observations suggest a major role for the CEACAM1 protein in the regulation of decidual lymphocyte functions after IL-2 activation. In vivo activation of decidual lymphocytes might occur as a result of viral infection. CMV is the leading cause of congenital viral infections in Western countries (25, 26). We therefore tested whether CEACAM1 expression could be detected on the surface of lymphocytes obtained from deciduae of women who had primary CMV infection during gestation with documented intrauterine manifestations. Second and third-trimester pregnancy terminations of women diagnosed with primary CMV infection necessitate the administration of labor-promoting agents that might have some immunological effects. To control the experiment, we analyzed the expression of CEACAM1 protein on the surface of lymphocytes obtained either from third-trimester caesarian sections with labor (Table 1) or from the deciduae taken from caesarian sections without labor (Table 1). Decidual lymphocytes were obtained and stained for the presence of CEACAM1 on NK, NKT, and T cells as above. Only very limited numbers of NKT cells were isolated, and therefore the expression of CEACAM1 on NKT cells could not be determined. Remarkably, a significant elevation of CEACAM1 expression was observed in NK and T cells obtained from deciduae of CMV-infected women, whereas little or no expression of CEACAM1 was observed in the two control groups (Table 1). CEACAM1 expression can vary significantly between different CMV-infected deciduae. In one patient, 90% and 95% of the NK and T cells, respectively, expressed the CEACAM1 protein, whereas in the second patient the expression of the CEACAM1 protein was limited to 10% and 10.2% of NK and T cells, respectively. However, the expression of the CEACAM1 protein, even in the second patient, was still very significant compared with that of the control groups, and it was similar to the expression level of other class I MHC inhibitory receptors, which vary between 5% and 20% (9). There are several possible reasons for the differences in the level of CEACAM1 expression, such as subjective local immune response, course of CMV infection, and the time of pregnancy termination after the initiation of infection. The mild expression of the CEACAM1 protein on trophoblasts obtained from normal decidua (Figure 1h) was still maintained on trophoblasts obtained from infected decidua (data not shown).
Table 1.
CEACAM1 expression is upregulated on decidual lymphocytes from women with primary CMV infection
CMV-infected fibroblasts express a novel ligand for CEACAM1.
The results presented above demonstrate that CEACAM1 expression is upregulated in vivo in lymphocytes obtained from CMV-infected deciduae. Expression of CEACAM1 was observed on EVT cells obtained from either normal or CMV-infected deciduae (Figure 1 and data not shown). Thus, CEACAM1 homotypic interactions might be expected to occur in vivo, leading to lymphocyte inhibition.
Only two cases of CMV-infected deciduae are presented here, as studies in vivo are limited for several reasons. In addition to the fact that primary CMV infection during pregnancy is quite rare (26), the detection and diagnosis are quite difficult. Furthermore, deciduae from CMV-infected women were used only if they spontaneously detached, to avoid unnecessary additional procedures. It is clear, however, that in both presented cases, a significant CEACAM1 upregulation was observed. To further establish the effect of CMV infection with regard to CEACAM1 inhibition and to test whether the CMV uses the CEACAM1 inhibitory mechanism to avoid attack by the immune system, we used the CMV-infected HFFs. HFF cells were infected with CMV strain AD169 with moi 2–3. Importantly, no staining of either infected or uninfected HFF cells with anti-CEACAM1, -5, and -6 Kat4c mAb was observed at any time point before or after the infection. Infected cells were harvested at different time points at 24-hour intervals after the infection and stained for the presence of CEACAM1 ligand using CEACAM1-Ig fusion protein, as described in Methods.
The CEACAM1-Ig fusion protein specifically stained the .221/CEACAM1 cells and did not stain the .221 cells (Figure 6a), indicating that CEACAM1 homotypic interactions are strong enough to be detected by this method. No staining of CEACAM1-Ig was observed in the first 4 days after the infection (Figure 6b and data not shown). Importantly, CEACAM1-Ig staining was observed starting on day 5 and reaching maximum on day 6 after the infection. All infected cells were positively stained with anti-pp65 mAb (data not shown). The CEACAM1-Ig binding observed was only to the HFF-infected cells, not to the uninfected cells (data not shown). No changes in the level of the control CD99-Ig fusion protein staining were observed at any time point (Figure 6b). As CEACAM1 can interact only with the CEACAM1, -5, and -6 variants (14), and as it was also reported that CEACAM variants cannot be detected on the surface of human fibroblasts (27), these results strongly suggest the existence of a novel ligand for CEACAM1 on the surface of CMV-infected HFF cells. This novel ligand appears late after the infection. To further test this hypothesis, we performed similar experiments in the presence of the antiviral agent PFA, which is known to block viral DNA synthesis and early-late-phase transition. Progeny virus titers in culture supernatants were determined on day 4 after infection by a standard plaque titration assay on HFFs. In the absence of PFA, virus titer was 3 × 106 plaque-forming units/ml, whereas in the presence of PFA no virus could be detected. In agreement with the above observations demonstrating the appearance of CEACAM1 ligand on the surface of CMV-infected HFFs, the addition of PFA completely abolished the binding of CEACAM1-Ig to the infected HFF cells (Figure 6b).
We next tested whether the CEACAM1 interactions with the CMV-infected HFFs are functional. Mouse BW cells were stably transfected with a chimeric molecule composed of the extracellular portion of CEACAM1 fused to mouse ζ chain(as described in Methods). Engagement of CEACAM1 leads to the secretion of mouse IL-2, mediated by the ζ chain. The IL-2 amounts in the cell supernatants can be measured by ELISA. Secretion of IL-2 could be detected in the culture supernatants of the BW cells transfected with CEACAM1ζ, but not in the culture supernatants of the BW cells or BW cells transfected with CD16ζ (Figure 7a). Moreover, IL-2 secretion was also detected in the supernatants of BW/CEACAM1ζ cells when cells were incubated with .221/CEACAM1 cells, but not with they were incubated with .221 cells (Figure 7b). Thus, homotypic CEACAM1 interactions are strong enough to induce IL-2 secretion in this system. In agreement with the CEACAM1-Ig staining data, efficient secretion of IL-2 was observed (on days 5 and 6 after the infection) in the supernatants of BW/CEACAM1ζ cells cultured with infected HFFs. This IL-2 secretion was blocked by the addition of PFA (Figure 7c). No IL-2 secretion was observed in the culture supernatants of BW/CD16ζ cells incubated with uninfected or infected HFF cells (data not shown).
To further substantiate the above results, we cultured the clinical CMV strain isolated from the infected decidua (patient 6; Table 1) with infected HFF cells. The propagation of the virus was much slower than that of the laboratory AD169 strain (data not shown). Consistent microscopic monitoring of infected HFF cells revealed that even after prolonged propagation time, only partial infection could be achieved. One month after initiation of infection, infected HFF cells were analyzed for recognition by CEACAM1. HFF cells were stained with Ig-fused proteins, including CEACAM1-Ig and the control CD99-Ig. No staining of uninfected HFF cells was observed (data not shown). Specific staining of the infected HFF cells could be observed with the CEACAM1-Ig but not with the CD99-Ig (20% and 2% staining, respectively; Figure 8a). No staining was observed when anti-CEACAM antibodies were used (data not shown), indicating that CEACAM1-Ig recognizes a novel CMV-induced ligand on infected HFFs. We also tested whether this recognition is capable of eliciting IL-2 secretion from BW/CEACAM1ζ cells. IL-2 levels were measured in the supernatants of BW or BW/CEACAM1ζ cells cocultured with HFF cells infected with the clinical CMV strain isolated from patient 6. In agreement with the CEACAM1-Ig staining, increased IL-2 secretion could be detected only in the supernatants of the BW/CEACAM1ζ cells coincubated with infected HFF cells (Figure 8b). The moderate elevation of IL-2 secretion and the partial staining of CEACAM1-Ig (Figure 8, a and b) are correlated with the low infection levels of this clinical CMV strain observed in vitro, and with the moderate percentages of CEACAM1+ lymphocytes isolated from infected decidua no. 2 (patient 6; Table 1). Similar results were obtained with another clinical CMV strain, isolated from a neonate’s urine (data not shown).
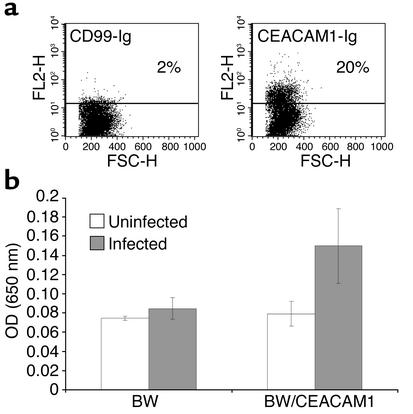
Figure 8.
CMV isolated from infected decidua induces a ligand for the CEACAM1 on infected HFF cells. (a) Staining of HFF cells infected with clinical CMV strain with CD99-Ig or with CEACAM1-Ig. No staining was observed when proteins were omitted, indicated by the horizontal line. FSC, forward scatter. (b) IL-2 secretion from BW or BW/CEACAM1ζ cells coincubated with HFF-infected cells for 48 hours. The average of two experiments is shown.
These combined results show a major role for the CEACAM1 protein in the inhibition of decidual lymphocyte functions after activation and suggest that the CMV virus might have developed a unique mechanism, mediated via the CEACAM1 protein interactions with an unknown ligand, to avoid decidual immune cell attack.
Discussion
The presence of a semiallogeneic fetus in the maternal uterus presents a major problem to the immune system. This is emphasized by observations demonstrating that more than 40% of decidual cells are immune cells (1). One of the ways in which the immune system deals with the problem is the unique distribution of decidual lymphocytes. More than 70% of decidual lymphocytes are CD16– NK cells, in contrast to only about 1% CD16– NK cells in peripheral blood lymphocytes, whereas T cells constitute only 10% of decidual cells. The fact that NK cells are so highly represented in the decidua suggests that they might play an important role in controlling trophoblast invasion and preventing rejection (3). T cells are probably strictly controlled, as only a minor fraction of decidual lymphocytes are T cells and thus cytokine secretion or T cell proliferation is expected to be minimal under normal conditions.
The lytic function of the decidual NK cells, the major subset of decidual lymphocyte population, must be tightly controlled. This is achieved by several mechanisms, including low lytic ability (12), unique receptor repertoire (9, 11), and the presence of ligands such as HLA-G, -C, and -E, on EVT cells, interacting with inhibitory receptors on NK cells (5–7). However, if local bacterial (28) or viral (29) infection occurs, it may lead to decidual lymphocyte activation. This might result in changes in the composition, cytotoxicity, and cytokine profile (30) of decidual lymphocytes that might eventually damage fetal tissue (31). A gentle immunological balance thus has to be maintained in the decidua, where immunological activity operates to properly eliminate a pathogen without damaging the fetus. Therefore, there must be mechanisms to control the different functions of activated decidual lymphocytes. The results presented here demonstrate a novel inhibitory mechanism of activated decidual lymphocytes that is mediated by the CEACAM1 protein interactions. Here, we show that the CEACAM1 protein, expressed on virtually all IL-2–activated decidual lymphocytes, can inhibit the killing, proliferation, and cytokine secretion of IL-2–activated NK, CD4+ T, and NKT cells, respectively. The expression of CEACAM1 on the surface of activated decidual lymphocytes was observed using the 5F4 mAb (Figure 1). This mAb specifically recognizes CEACAM1 but not CEACAM5 or CEACAM6 (Figure 2). In addition, RT-PCR analysis performed on activated decidual lymphocytes showed expression of CEACAM1 only (data not shown). It is still possible that other members of the CEACAM family are expressed on the surface of activated decidual lymphocytes. However, it is very unlikely that members other than CEACAM1 are involved in decidual lymphocyte inhibition, as the inhibitory signal is transmitted via the immunoreceptor tyrosine-based inhibitory motif sequences (15), which are only found in the CEACAM1 protein. The CEACAM1 interactions might, therefore, play a pivotal role in cases of lymphocyte activation. Normally, EVT cells constitutively express the CEACAM1 protein on their surface. Once activated, the vast majority of the decidual lymphocytes also express the CEACAM1 protein. We therefore hypothesize that the CEACAM1 interactions serve as a general inhibitory mechanism of various populations of activated decidual lymphocytes. The inhibition of lymphocyte function might occur via homotypic interactions with other CEACAM1 molecules present on EVT cells or on neighboring lymphocytes.
Importantly, we show in vivo that lymphocytes isolated from the deciduae of CMV-infected patients express the CEACAM1 protein in increased levels. This upregulation probably serves as a self-defense mechanism of the maternal immune system to avoid fetal damage. The mechanism of the observed CEACAM1 upregulation on the surface of lymphocytes derived from CMV-infected deciduae is yet unknown.
We have recently demonstrated that efficient CEACAM1-mediated inhibition of NK cell cytotoxicity is dependent on the expression level of CEACAM1 protein on both NK and target cells (15). Here we suggest that an unidentified ligand present on the surface of CMV-infected HFFs is capable of interacting with the CEACAM1 protein. The CEACAM1 protein is expressed on the surface of trophoblast cells derived from CMV-infected decidua (data not shown), and it was also previously suggested that transmission of CMV to the fetus occurs via the EVT cells (29). The combined inhibitory effect of both CEACAM1 and the putative unknown CMV-induced ligand might therefore be advantageous to the virus in vivo, enabling efficient inhibition. In addition, the increased CEACAM1 expression on the decidual lymphocytes might further diminish the local immune response, enabling the virus to avoid clearance.
CMV exists in equilibrium with the immune system of the normal host and developed mechanisms to counteract or evade immune surveillance. These mechanisms include, for example, the downregulation of class I MHC proteins to avoid CTL recognition (32), and the concomitant upregulation of the class I homologue UL18 to avoid NK-mediated elimination (33). The CMV also induces the expression of the nonclassical HLA-E protein via the leader peptide derivative of the UL40 protein (34). On the other hand, NK cells are known to play an important role in controlling CMV infection. Patients with defective NK cells commonly suffer from herpesvirus infections (35). It was reported that the class I MHC-related MIC proteins are induced following CMV infection (36). These MIC proteins are recognized by the activating NKG2D-complex receptors, present on NK cells and other immune cells (36). CMV uses the UL16 protein to mask MICB and ULBP recognition by the activating NKG2D (37). The expression of a putative CMV-induced ligand for CEACAM1 protein might be considered as yet another mechanism developed by the virus to avoid recognition primarily by activated decidual lymphocytes.
Acknowledgments
The authors would like to thank Jon Boyson for the detailed protocol and for his help in the decidual lymphocyte isolation, W. Zimmermann for the CEACAM5 cDNA, R.E. Blumberg for the 5F4 mAb, and Vaclav Horesji for providing the anti–HLA-G mAb. We thank Niveen Saleh, Caryn Greenfield, and Shira Natanson-Yaron for excellent assistance. O. Mandelboim is supported by research grants from the Israel Cancer Research Foundation, the joint German-Israeli Research Program, the Israel Science Foundation, and the Charles H. Revson Foundation (no. 153/00), and by the Cancer Research Institute. The virological work was supported by a grant from the Samuel and Dora Straus Foundation.
Footnotes
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: extravillous trophoblast (EVT); T cell receptor (TCR); cytomegalovirus (CMV); human foreskin fibroblast (HFF); phosphonoformate (PFA); staphylococcal enterotoxin B (SEB).
References
- 1.Bulmer JN, Morrison L, Longfellow A, Riston A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 2.Loke, Y.W., and King, A. 1995. Human implantation: cell biology and immunology. Cambridge University Press. Cambridge, United Kingdom. 1–40.
- 3.King A, et al. On the nature and function of human uterine granular lymphocytes. Immunol Today. 1991;12:432–435. doi: 10.1016/0167-5699(91)90014-K. [DOI] [PubMed] [Google Scholar]
- 4.King A, et al. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors: a review. Placenta. 2000;21(Suppl. A):s81–s85. doi: 10.1053/plac.1999.0520. [DOI] [PubMed] [Google Scholar]
- 5.King A, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Rouas-Friess N, Marchal-Bras Goncalie R, Manier C, Dausset J, Carosella DC. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King A, et al. Surface expression of HLA-C antigen by human extravillous trophoblasts. Placenta. 2000;21:376–387. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- 8.Cohen GB, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 9.Verma S, King A, Loke YW. Expression of killer cell inhibitory receptors (KIR) on human uterine NK cells. Eur J Immunol. 1997;27:979–983. doi: 10.1002/eji.1830270426. [DOI] [PubMed] [Google Scholar]
- 10.Braud VM, Jones EY, McMichael AJ. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27:1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 11.Biassoni R, Bottino C, Millo R, Moretta L, Moretta A. Natural killer cell-mediated recognition of human trophoblast. Semin Cancer Biol. 1999;1:13–18. doi: 10.1006/scbi.1998.0108. [DOI] [PubMed] [Google Scholar]
- 12.Ponte M, et al. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc Natl Acad Sci USA. 1999;10:5674–5679. doi: 10.1073/pnas.96.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 14.Watt SM, et al. CD66 identifies the biliary glycoprotein (BGP) adhesion molecule: cloning, expression, and adhesion functions of the BGPc splice variant. Blood. 1994;84:200–205. [PubMed] [Google Scholar]
- 15.Markel G, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–2810. doi: 10.4049/jimmunol.168.6.2803. [DOI] [PubMed] [Google Scholar]
- 16.Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc Natl Acad Sci USA. 2001;98:1895–1900. doi: 10.1073/pnas.98.4.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales VM, et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a) J Immunol. 1999;163:1363–1370. [PubMed] [Google Scholar]
- 18.Mandelboim O, et al. Enhancement of class II-restricted T cell responses by costimulatory NK receptors for class I MHC proteins. Science. 1996;274:2097–2100. doi: 10.1126/science.274.5295.2097. [DOI] [PubMed] [Google Scholar]
- 19.Katz G, Markel G, Mizrahi S, Arnon TI, Mandelboim O. Recognition of HLA-Cw4 but not HLA-Cw6 by the NK cell receptor killer cell Ig-like receptor two-domain short tail number 4. J Immunol. 2001;166:7260–7267. doi: 10.4049/jimmunol.166.12.7260. [DOI] [PubMed] [Google Scholar]
- 20.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 21.Kammerer R, Hahn S, Singer BB, Luo JS, Von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–3674. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664::AID-IMMU3664>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Oikawa S, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Homotypic and heterotypic Ca++-independent cell adhesion activities of biliary glycoprotein, a member of carcinoembryonic antigen family, expressed on CHO cell surface. Biochem Biophys Res Commun. 1992;186:881–887. doi: 10.1016/0006-291x(92)90828-9. [DOI] [PubMed] [Google Scholar]
- 23.Kuroki M, et al. Identification and comparison of residues critical for cell adhesion activities of two neutrophil CD66 antigens, CEACAM6 and CEACAM8. J Leukoc Biol. 2001;70:543–550. [PubMed] [Google Scholar]
- 24.Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47:87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 25.Demmler GJ. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Reviews of infectious diseases. 1991;13:315–329. doi: 10.1093/clinids/13.2.315. [DOI] [PubMed] [Google Scholar]
- 26.Knipe, D.M., and Howley, P.M. 2001. Fields virology. 4th edition. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 2675–2705.
- 27.Barclay, A.N., et al. 1997. The leucocyte antigen factsbook. 2nd edition. Academic Press Inc. San Diego, California, USA. 1–613.
- 28.Parkash V, et al. Immunohistochemical detection of Listeria antigens in the placenta in perinatal listeriosis. Int J Gynecol Pathol. 1998;17:343–350. doi: 10.1097/00004347-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol. 2000;74:6808–6820. doi: 10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unanue ER. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 31.Haddad EK, Duclos AJ, Lapp WS, Baines MG. Early embryo loss is associated with the prior expression of macrophage activation markers in the decidua. J Immunol. 1997;158:4886–4892. [PubMed] [Google Scholar]
- 32.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 33.Cosman D, et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 34.Tomasec P, et al. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287:1031–1033. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 35.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 36.Groh V, et al. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 37.Cosman D, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]