Abstract
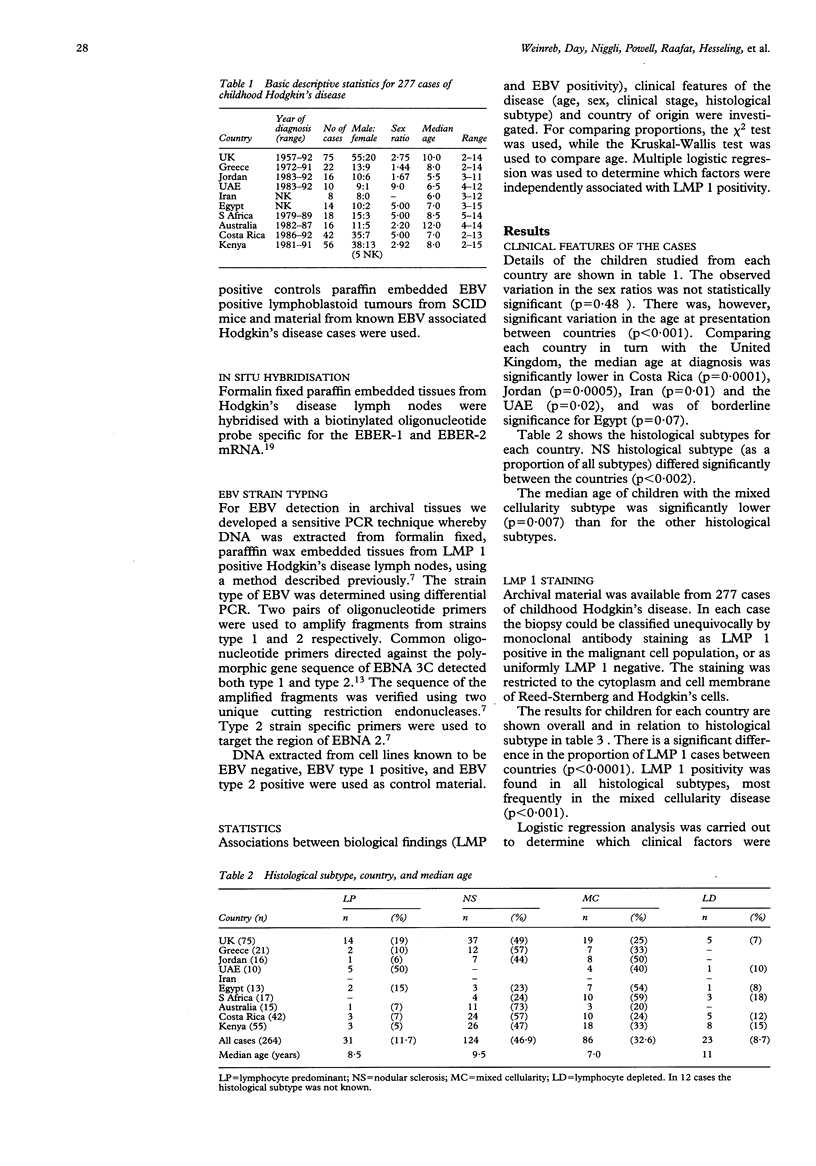
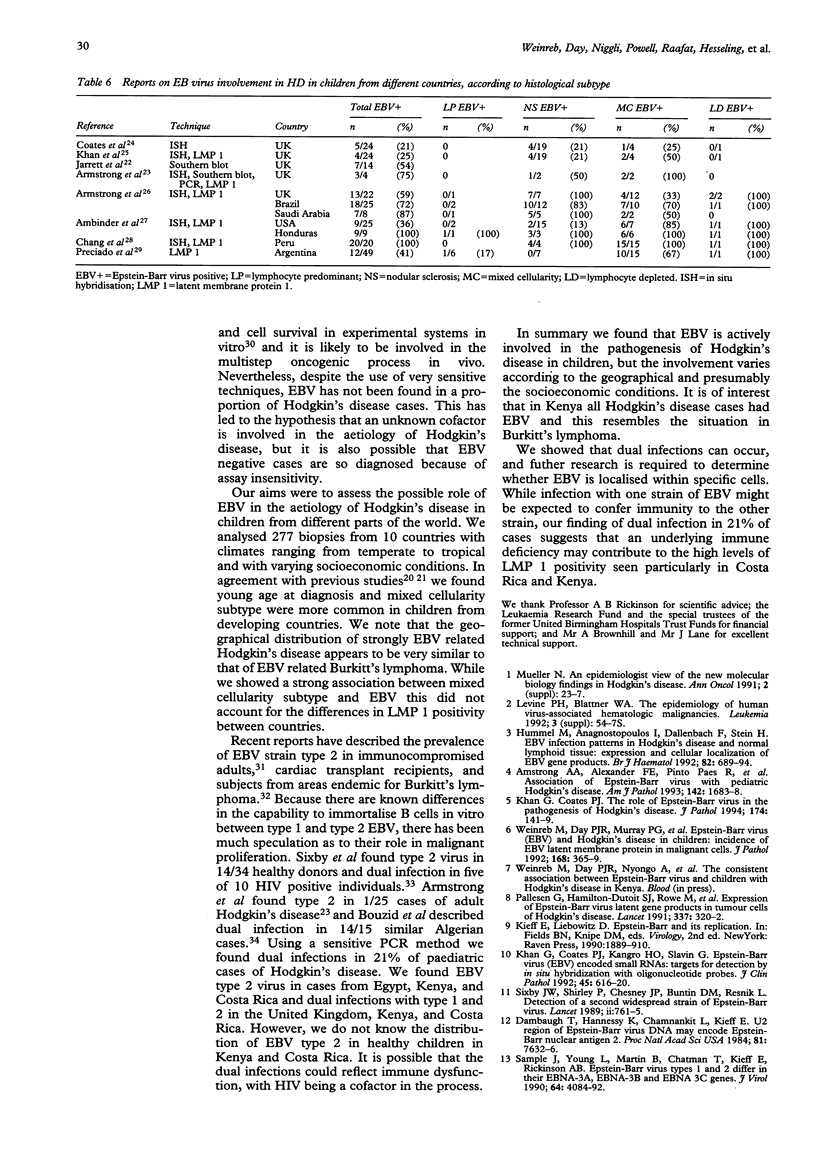
Recent studies have suggested that Epstein-Barr virus (EBV) may play a role in the aetiology of Hodgkin's disease. To determine the role of EBV in childhood Hodgkin's disease in different geographical areas, immunohistochemical staining and in situ hybridisation were used to analyse latent membrane protein 1 (LMP 1) and small nuclear non-transcribed RNAs (EBER-1) respectively. Testing for EBV within the Reed-Sternberg and Hodgkin's cells was carried out in childhood Hodgkin's disease from 10 different countries. The proportion of LMP 1 positive cases varied significantly, being 50% of cases from the United Kingdom (38/75), South Africa (9/18), Egypt (7/14), and Jordan (8/16), 60% from the United Arab Emirates (6/10), 70% from Australia (11/16), 81% from Costa Rica (34/42), 88% from Iran (7/8), 90% from Greece (20/22), and 100% of the 56 cases from Kenya. A sensitive polymerase chain reaction based EBV strain typing technique was established using archival tissues. EBV strain type 1 was shown to be predominant in childhood Hodgkin's disease from the United Kingdom, South Africa, Australia, and Greece. Type 2 was predominant in Egypt. EBV strain types 1 and 2 were both detected in some cases of childhood Hodgkin's disease in the United Kingdom, Costa Rica, and Kenya. The high incidence of EBV and the presence especially in developing countries of dual infection with both strain types 1 and 2 may reflect socioeconomic conditions leading to malnutrition induced immunological impairment. The possibility of HIV infection also needs to be explored.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambinder R. F., Browning P. J., Lorenzana I., Leventhal B. G., Cosenza H., Mann R. B., MacMahon E. M., Medina R., Cardona V., Grufferman S. Epstein-Barr virus and childhood Hodgkin's disease in Honduras and the United States. Blood. 1993 Jan 15;81(2):462–467. [PubMed] [Google Scholar]
- Armstrong A. A., Alexander F. E., Paes R. P., Morad N. A., Gallagher A., Krajewski A. S., Jones D. B., Angus B., Adams J., Cartwright R. A. Association of Epstein-Barr virus with pediatric Hodgkin's disease. Am J Pathol. 1993 Jun;142(6):1683–1688. [PMC free article] [PubMed] [Google Scholar]
- Armstrong A. A., Alexander F. E., Paes R. P., Morad N. A., Gallagher A., Krajewski A. S., Jones D. B., Angus B., Adams J., Cartwright R. A. Association of Epstein-Barr virus with pediatric Hodgkin's disease. Am J Pathol. 1993 Jun;142(6):1683–1688. [PMC free article] [PubMed] [Google Scholar]
- Bouzid M., Belkaid M. I., Colonna P., Bouguermouh A. M., Ooka T. Co-existence of the A and B types of Epstein-Barr virus DNA in lymph node biopsies from Algerian patients with Hodgkin's disease and non-Hodgkin's lymphoma. Leukemia. 1993 Sep;7(9):1451–1455. [PubMed] [Google Scholar]
- Boyle M. J., Vasak E., Tschuchnigg M., Turner J. J., Sculley T., Penny R., Cooper D. A., Tindall B., Sewell W. A. Subtypes of Epstein-Barr virus (EBV) in Hodgkin's disease: association between B-type EBV and immunocompromise. Blood. 1993 Jan 15;81(2):468–474. [PubMed] [Google Scholar]
- Chang K. L., Albújar P. F., Chen Y. Y., Johnson R. M., Weiss L. M. High prevalence of Epstein-Barr virus in the Reed-Sternberg cells of Hodgkin's disease occurring in Peru. Blood. 1993 Jan 15;81(2):496–501. [PubMed] [Google Scholar]
- Chang K. L., Chen Y. Y., Shibata D., Weiss L. M. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol. 1992 Dec;1(4):246–255. [PubMed] [Google Scholar]
- Coates P. J., d'Ardenne A. J., Slavin G., Kingston J. E., Malpas J. S. Detection of Epstein-Barr virus in Reed-Sternberg cells of Hodgkin's disease arising in children. Med Pediatr Oncol. 1993;21(1):19–23. doi: 10.1002/mpo.2950210105. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidts W. L., Bhatia K., Johnson J. F., Akar N., Gutiérrez M. I., Shibata D., Carolan M., Levine A., Magrath I. T. Epstein-Barr virus genotypes in AIDS-associated lymphomas are similar to those in endemic Burkitt's lymphomas. Leukemia. 1992 Sep;6(9):875–878. [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hummel M., Anagnostopoulos I., Dallenbach F., Korbjuhn P., Dimmler C., Stein H. EBV infection patterns in Hodgkin's disease and normal lymphoid tissue: expression and cellular localization of EBV gene products. Br J Haematol. 1992 Dec;82(4):689–694. doi: 10.1111/j.1365-2141.1992.tb06945.x. [DOI] [PubMed] [Google Scholar]
- Khan G., Coates P. J., Kangro H. O., Slavin G. Epstein Barr virus (EBV) encoded small RNAs: targets for detection by in situ hybridisation with oligonucleotide probes. J Clin Pathol. 1992 Jul;45(7):616–620. doi: 10.1136/jcp.45.7.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan G., Coates P. J. The role of Epstein-Barr virus in the pathogenesis of Hodgkin's disease. J Pathol. 1994 Nov;174(3):141–149. doi: 10.1002/path.1711740302. [DOI] [PubMed] [Google Scholar]
- Khan G., Norton A. J., Slavin G. Epstein-Barr virus in Hodgkin disease. Relation to age and subtype. Cancer. 1993 May 15;71(10):3124–3129. doi: 10.1002/1097-0142(19930515)71:10<3124::aid-cncr2820711038>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Medeiros L. J., Greiner T. C. Hodgkin's disease. Cancer. 1995 Jan 1;75(1 Suppl):357–369. doi: 10.1002/1097-0142(19950101)75:1+<357::aid-cncr2820751318>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mueller N. An epidemiologist's view of the new molecular biology findings in Hodgkin's disease. Ann Oncol. 1991 Feb;2 (Suppl 2):23–28. doi: 10.1007/978-1-4899-7305-4_3. [DOI] [PubMed] [Google Scholar]
- Murray P. G., Young L. S., Rowe M., Crocker J. Immunohistochemical demonstration of the Epstein-Barr virus-encoded latent membrane protein in paraffin sections of Hodgkin's disease. J Pathol. 1992 Jan;166(1):1–5. doi: 10.1002/path.1711660102. [DOI] [PubMed] [Google Scholar]
- Pallesen G., Hamilton-Dutoit S. J., Rowe M., Young L. S. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet. 1991 Feb 9;337(8737):320–322. doi: 10.1016/0140-6736(91)90943-j. [DOI] [PubMed] [Google Scholar]
- Preciado M. V., De Matteo E., Diez B., Grinstein S. Epstein-Barr virus (EBV) latent membrane protein (LMP) in tumor cells of Hodgkin's disease in pediatric patients. Med Pediatr Oncol. 1995 Jan;24(1):1–5. doi: 10.1002/mpo.2950240102. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Young L. S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987 May;61(5):1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Evans H. S., Young L. S., Hennessy K., Kieff E., Rickinson A. B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987 Jun;68(Pt 6):1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixbey J. W., Shirley P., Chesney P. J., Buntin D. M., Resnick L. Detection of a second widespread strain of Epstein-Barr virus. Lancet. 1989 Sep 30;2(8666):761–765. doi: 10.1016/s0140-6736(89)90829-5. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Shirley P., Chesney P. J., Buntin D. M., Resnick L. Detection of a second widespread strain of Epstein-Barr virus. Lancet. 1989 Sep 30;2(8666):761–765. doi: 10.1016/s0140-6736(89)90829-5. [DOI] [PubMed] [Google Scholar]
- Weinreb M., Day P. J., Murray P. G., Raafat F., Crocker J., Parkes S. E., Coad N. A., Jones J. T., Mann J. R. Epstein-Barr virus (EBV) and Hodgkin's disease in children: incidence of EBV latent membrane protein in malignant cells. J Pathol. 1992 Dec;168(4):365–369. doi: 10.1002/path.1711680405. [DOI] [PubMed] [Google Scholar]


