Abstract
The N-terminal peptide Ac1-11 of myelin basic protein induces experimental autoimmune encephalomyelitis in H-2u and (H-2u × H-2s) mice but does not in H-2s mice. Ac1-11 binds weakly to the class II major histocompatibility complex (MHC) molecule I-Au but not at all to I-As. We have studied the interaction of Ac1-11 and I-Au as a model system for therapeutic intervention in the autoimmune response seen in experimental autoimmune encephalomyelitis. Two polymorphic residues that differ between I-Au and I-As, Y26β and T28β, and one conserved residue, E74β, confer specific binding of Ac1-11 to I-Au. A fourth residue, R70β in I-Au, affects both peptide binding and T cell recognition. These results are consistent with a model that places arginine at position five of Ac1-11 in pockets 4 and 7 of the MHC groove, which is formed in part by residues 26, 28, 70, and 74 of Aβu and places lysine at position four of Ac1-11, previously shown to be a major MHC contact, in hydrophobic pocket 6. The data indicate that the primary region of I-Au that confers specific binding of Ac1-11 lies in the center of the peptide binding groove rather than in the region that contacts the N terminus of the peptide, as has been shown for HLA DR and the homologous I-E molecules.
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune disease mediated by CD4+ T cells in rodents that resembles the human demyelinating disease multiple sclerosis (1). EAE can be induced by immunization with components of the myelin sheath, such as proteolipid protein or myelin basic protein (MBP) or with peptides derived from proteolipid protein or MBP (1, 2). Susceptibility to EAE is correlated with the ability of particular peptides to bind to class II major histocompatibility complex (MHC) molecules in certain strains of mice. The N-terminal peptide Ac1-11 of MBP, which binds weakly to I-Au, can induce EAE in mice that have the H-2u MHC haplotype, such as PL/J, but cannot do so in mice with the H-2s haplotype, such as SJL/J, because Ac1-11 does not bind to I-As (3–6). SJL/J mice, however, develop EAE when injected with the MBP peptide 89–101, which binds to I-As but not to I-Au (1).
Several different immunotherapies have been used successfully in treating or preventing EAE (7). Peptides, either the encephalitogenic epitopes themselves, analogs derived thereof, or unrelated peptides, have been used to prevent or treat EAE (3, 4, 8–11). One example is Ac1-11[4A], an analog in which the lysine at position 4 (Lys P4) is replaced with alanine. Position 4 is a MHC contact, as Ac1-11[4A] binds with 50× higher affinity to I-Au than does Ac1-11 and stimulates Ac1-11-specific T cells in vitro with greater efficiency (3, 6). Ac1-11[4Y], an analog in which tyrosine replaces Lys P4, binds to I-Au with 1,500-fold higher affinity than does Ac1-11 and stimulates Ac1-11-specific T cells in vitro more efficiently than does Ac1-11[4A] (6, 9). Other studies have shown that arginine at position 5 also contacts the MHC (12, 13) whereas glutamine at position 3 and proline at position 6 contact the T cell antigen receptor (TCR) (3). Of interest, Ac1-11[4A], when coimmunized with Ac1-11, will inhibit the development of EAE (3, 8) and does not by itself induce a proliferative response in vivo in (PL/J × SJL/J)F1 mice. The mechanism by which Ac1-11[4A] inhibits EAE is not understood but probably is other than MHC blockade or induction of anergy because it does not inhibit a proliferative response in vivo when coimmunized with Ac1-11, nor does it induce a suppressive response (8).
Understanding the molecular nature of the differences in binding between Ac1-11, Ac1-11[4A], and Ac1-11[4Y] may provide clues to the unusual biological properties of Ac1-11[4A] and Ac1-11[4Y]. Structural data exist for several human HLA DR molecules (14–17), the homologous murine I-Ek (18), and most recently, murine I-Ak and I-Ad (19, 20). No structural data, however, on human HLA DQ or the homologous murine I-Au and I-As molecules have been published. Therefore, we have made site-specific mutants of I-Au and I-As and have tested the ability of these mutants to bind peptide and stimulate Ac1-11-specific T cells as a means to dissect the molecular nature of the Ac1-11/I-Au complex. Hypervariable regions (HVRs) in I-As that differ between I-Au and I-As were mutated to those of I-Au, and single conserved residues thought to be important for peptide binding were mutated nonconservatively by using the class II MHC structures currently available as guides. The results indicate that three key residues on the β chain, Y26β, T28β, and E74β, in pockets 4 and 7 are critical for binding Ac1-11 efficiently in the center of the peptide binding groove. Arg P5 in Ac1-11 is predicted to lie within these pockets, potentially forming a salt bridge with E74β. This model would place LysP4 in Ac1-11 in a hydrophobic pocket, which would be more accommodating to a hydrophobic and/or aromatic residue, explaining why the binding affinities of Ac1-11[4A] and Ac1-11[4Y] for I-Au over that of Ac1-11 is increased.
MATERIALS AND METHODS
Peptides.
Peptides were synthesized on an Applied Biosystems 431A peptide synthesizer by using standard fluorenylmethoxycarbonyl chemistry. Peptides were purified by HPLC to >90% pure, if necessary. Amino acid compositions were confirmed by amino acid analysis and mass spectroscopy at the Mass Spectroscopy Facility at the University of California, San Francisco. Ova 322–339 and Ac1-14[4A,14G] were biotinylated on either the amino terminus (Ova 322–339) or the lysine at position 13 (Ac1-14[4A,14G]) by using N-hydroxy-succinimide biotin from Pierce as described (4). Peptide sequences are as follows: Ova 322–339, EISQAVHAAHAEINEAGR; Ac1-11, Ac-ASQKRPSQRHG; Ac1-11[4A], Ac-ASQARPSQRHG; and Ac1-14[4A,14G], Ac-ASQARPSQRHGSKG.
Site-Directed Mutagenesis.
A plasmid containing a genomic clone, pI-Aβk-1 (21), was manipulated to create a vector into which the β1 domain of Aβs could be cloned easily. The exon encoding the β1 domain of Aβk was replaced with a multiple cloning site. Because the first two amino acids of the β1 domain are encoded in the leader exon and Aβk differs from Aβs,u,g7 at amino acid position two, the promoter and leader exon of this vector was replaced with a fragment containing the promoter and leader exon from a genomic clone of Aβg7. The β1 domain of Aβs was amplified by PCR. The resulting β1 domain was cloned into pBluescript-KS+ (Stratagene) and was sequenced. This construct was used for site-directed mutagenesis of the β chain by using the method of Kunkel et al. (22). Mutant β1 domains were excised from pBluescript-KS+ and were subcloned into the multiple cloning site in the genomic clone. The Aαu cDNA was cloned into an expression vector containing the neomycin resistance gene.
Transfectants.
Wild-type and mutant constructs were linearized and transfected as described into M12.C3, a B cell lymphoma that lacks class II surface expression (23). Cells that had taken up the constructs were selected with Geneticin (G418) (GIBCO). Because M12.C3 expresses an endogenous Aαd chain, Aβu or mutant Aβ molecules may pair with the endogenous α chain if pairing with Aαu is not favorable. Therefore, transfectants were screened with both fluorescein isothiocyanate-conjugated 10–3.6, an antibody that recognizes the β chain (24), and biotinylated 4D5, an antibody that recognizes Aαu,s but not Aαd (25). Lines expressing low levels of the αβ heterodimer were subjected to one to three rounds of cell sorting in which the brightest 5% cells were selected to obtain transfectants that express MHC levels within 2-fold of that of the wild-type transfectant.
Cell Surface Binding Assay.
Transfectants were assayed for peptide binding as described (4). In brief, 105–5 × 105 cells were incubated with 100 μM biotinylated Ac1-14[4A,14G] or 50 μM biotinylated Ova 322–339 for 2 hours in serum-free medium at 37° in a 5% CO2 atmosphere. Cells then were washed and incubated on ice with avidin (1 μg/ml) for 30 minutes. Samples were washed, were incubated for 30 minutes on ice with biotinylated anti-avidin antibody (1 μg/ml), were washed, then were incubated 30 minutes on ice with streptavidin conjugated to Texas Red (1 μg/ml), and were washed. The median fluorescence of 5 × 103–104 live cells was determined by flow cytometry. The expression levels of MHC molecules were determined by staining the cell lines with antibodies 10–3.6 and 4D5 in a separate sample or by staining concomitantly with a fluorescein isothiocyanate-conjugated form of 4D5 in the same sample.
T Cell Activation Assay.
The T cell hybridoma 1934.4 was created from the Ac1-11-specific, I-Au-restricted T cell clone PJR-25 (3). Tranfectants (5 × 104) and T cells (5 × 104) were incubated in 96-well plates with varying concentrations of peptide for 21 hours. Interleukin 2 production was measured by incubating the supernatants with HT-2 cells, an interleukin 2-dependent cell line, and measuring the uptake of 3H-thymidine in cpm (26).
RESULTS
Generation of Transfectants.
About 25–100× more Ac1-11 than Ac1-11[4A] is required to achieve equivalent stimulation of the T cell hybridoma 1934.4. Several I-Au transfectants that expressed similar levels of I-Au were made. Depending on which transfectant was used as antigen presenting cells, up to 5-fold different amounts of Ac1-11 were required to achieve maximal T cell stimulation (data not shown). Thus, differences in presentation of Ac1-11 and Ac1-11[4A] between cells expressing mutant MHC molecules that were 5-fold or less were not considered significant.
E74β in I-Au Potentially Interacts with Arg P5 of Ac1-11.
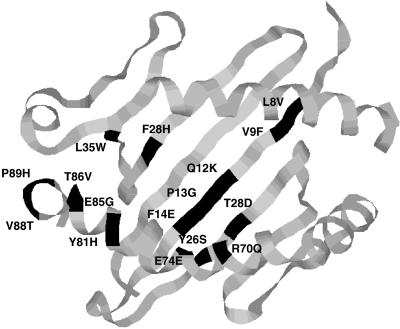
Previous studies indicated that Arg P5 in Ac1-11 may be interacting with a negative charge in the MHC, as alanine at position five reduced binding significantly (6, 12, 13). Glutamic acid at position 74 in the β chain (E74β) was chosen as a likely candidate because the homologous residue in certain DR molecules, R74β, contacts negatively charged residues in peptides (15). The position of residue E74β is shown on the DR1 structure (Fig. 1). β chains in which E74β was mutated were transfected along with Aαu into M12.C3 and were screened for expression by flow cytometry.
Figure 1.
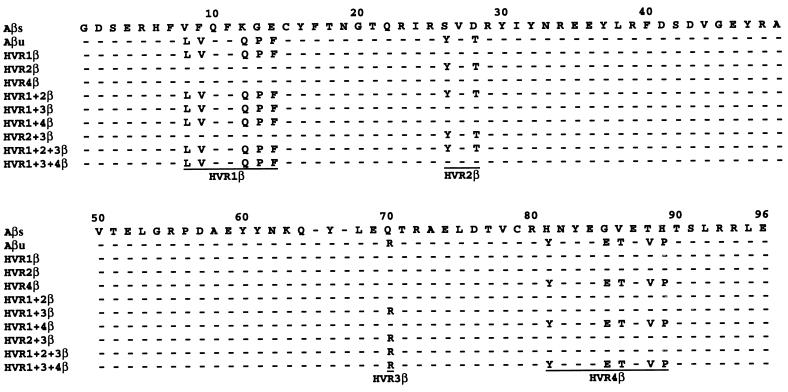
The positions of residues mutated are indicated on the DR1 structure. The amino acid in Aβs is noted on the right of the residue number, and the amino acid in Aβu is noted on the left of the residue number. Residues E74β, L35α, F28α, and T86β were mutated singly. Polymorphic residues that differ between Aβs and Aβu were grouped into HVRs and were mutated on the Aβs backbone to those of Aβu, either as a single HVR or as combinations of HVRs. HVR1, residues 8β, 9β, 12β, 13β, and 14β; HVR2, residues 26β and 28β; HVR3, residue 70β; and HVR4, residues 81β, 85β, 86β, 88β, and 89β.
Alanine and Glutamine at E74β Abrogate Presentation of Ac1-11.
To test the hypothesis that Arg P5 in Ac1-11 contacts a negative residue in I-Au, two mutations at E74β were introduced, alanine (E74A) and glutamine (E74Q). Both E74A and E74Q were unable to present Ac1-11 and were able to present Ac1-11[4A] only at very high concentrations (Table 1).
Table 1.
T cell response to the wild-type I-Au or mutants E74A and E74Q presenting Ac1-11 or Ac1-11[4A] (4A) and the binding signal of Ac1-14[4A, 14G] (4A) or Ova 322–339 (OVA) for each
| Cell line | T cell response*
|
Binding signal†
|
||
|---|---|---|---|---|
| Ac1-11 | 4A | 4A | Ova | |
| u:u | ++ | +++++ | 0.16 ± 0.06 | 0.22 ± 0.11 |
| E74A | − | ± | 0.01 ± 0.01 | 0.17 ± 0.05 |
| E74Q | − | ± | 0.002 ± 0.001 | 0.11 ± 0.04 |
The number of plus symbols indicates the dose of Ac1-11 or Ac1-11[4A] required for maximal T cell response as described in Materials and Methods. The number of plus symbols indicating maximal dose response is as follows: −, no response at any dose; ±, 100–400 μM; +, 25–100 μM; ++, 6–25 μM; +++, 1.5–6 μM; ++++, 0.4–1.5 μM; and +++++, 0.1–0.4 μM.
The binding signals for Ac1-11[4A,14G] and Ova 322–339 were determined by normalizing the binding signal for class II MHC expression. The binding signal generated by the biotinylated peptides was divided by the class II expression signal derived from staining cells with 4D5 in a separate sample. Values for negative controls ranged from 0 to 0.02. The data are from at least four separate experiments.
These two mutants also were assayed for their ability to bind biotinylated Ac1-14[4A,14G] and Ova 322–339, a peptide derived from ovalbumin that binds to I-Au with high affinity (6) (Table 1). Ac1-11 binding to I-Au cannot be detected by using the binding assay described here because it has a very low affinity for I-Au, so a high affinity analog was chosen, Ac1-14[4A,14G]. This peptide consists of the first 14 residues of rat myelin basic protein in which the lysine at position four has been substituted with an alanine, and the tyrosine at position 14 has been substituted with a glycine. The lysine at position 13 provided a site for biotinylation. This peptide binds to I-Au with ≈40× higher affinity than does Ac1-11, and it stimulates T cells in an enhanced fashion, similar to Ac1-11[4A] (data not shown). Neither E74A nor E74Q showed any detectable signal of binding Ac1-14[4A,14G]. In contrast, both mutants were able to bind Ova 322–339, indicating that the lack of Ac1-14[4A,14G] binding on E74A and E74Q is caused by an inability to bind this peptide rather than a loss of structural integrity.
The Roles of Other Residues in I-Au in Peptide Binding.
The structure of DR1 revealed a deep hydrophobic pocket (pocket 1) made up in part by residues F24α, I31α, and G86β (14, 15). The predicted sites of the homologous residues in I-Au, F28α, L35α, and T86β are shown in Fig. 1. To test whether pocket 1 contacts Ac1-11 in I-Au, these three residues were changed singly to a nonconservative glutamic acid or a semiconservative serine or glycine. Mutations F28E, F28S, T86E, and T86G had a beneficial effect on binding Ac1-14[4A,14G] (Table 2). There was, despite the improvement in binding efficiency, no difference in the abilities of transfectants F28E, F28S, T86E, or T86G and that of a transfectant expressing the wild-type I-Au molecule to present Ac1-11 or Ac1-11[4A] to 1934.4. One mutation at L35α, L35E, did not affect the binding signal but did reduce the ability to present peptide. The mutation L35S reduced binding by 3× and concomitantly reduced the ability to present Ac1-11[4A] by 50×. In contrast, most of these mutations had a negative (F28E, F28S, L35S, T86E) or neutral (L35E and T86G) effect on the binding of Ova 322–339 peptide.
Table 2.
T cell response to the wild-type I-Au (u:u) or mutants at F28α, L35α, or T86β presenting Ac1-11 or Ac1-1-11[4A] (4A) and the binding signal of Ac1-14[4A,14G] (4A) or Ova 322–399 (OVA) for each
| Cell line | T cell response*
|
Binding signal†
|
||
|---|---|---|---|---|
| Ac1-11 | 4A | 4A | OVA | |
| u:u | ++ | +++++ | 29.6 ± 9.5 | 50.6 ± 12.7 |
| F28E | ++ | +++++ | 89.0 ± 54.5 | 19.8 ± 4.6 |
| F28S | ++ | +++++ | 76.2 ± 27.1 | 22.6 ± 2.9 |
| L35E | + | ++ | 23.1 ± 8.3 | 57.0 ± 12.2 |
| L35S | − | ± | 10.8 ± 6.4 | 28.1 ± 7.9 |
| T86E | ++ | +++++ | 89.7 ± 5.8 | 9.3 ± 1.6 |
| T86G | ++ | +++++ | 53.2 ± 5.9 | 81.0 ± 14.5 |
The number of plus symbols indicating maximal dose response is as described in Table 1.
The ability of the relevant MHC molecules to bind Ac1-11[4A,14G] or OVA 323–339 is expressed as the median fluorescence of the signal derived from the biotinylated peptide on cells gated for the same MHC expression as determined by concomitantly staining with fluorescein isothiocyanate-conjugated 4D5 antibody. Values for negative controls ranged from 2.0 to 4.0. The data are the average of three separate experiments.
Creation of Transfectants Expressing β Chain HVR Mutants.
Aβs and Aβu differ by 13 amino acids, which were grouped into four HVRs. Fig. 1 shows where on the DR1 structure each of these residues are predicted to lie in I-Au. The roles of the β chain residues were examined by changing each of the hypervariable regions of Aβs to those of Aβu, either singly or in combination. The sequences of the β1 domains of each of the mutants and those of Aβu and Aβs are shown in Fig. 2. DNA encoding each of the β chain mutants and Aαu was cotransfected into M12.C3, and transfectants were screened for expression by flow cytometry.
Figure 2.
Sequences of the β1 domains of I-Au and I-As. Nine of the HVR mutant class II molecules are shown. The residues included in each HVR are underlined in the last mutant listed for the β chains.
Aβs Chain HVR Mutants that Contain HVR2β of Aβu Bind Ac1-11.
Table 3 shows the binding signals of each mutant β chain expressing transfectant. Because transfectants varied in their MHC expression, the fluorescence signal generated by peptide binding was normalized by dividing it by the signal generated when the cells were stained with an antibody, 4D5, which recognizes the α chain. Heterodimers AαuAβs and AαsAβu and the M12.C3 cell line did not have binding signals significantly above background (data not shown), indicating that both α and β chains are needed for efficient peptide binding. Only those mutants that contain HVR2β consistently bind peptide with levels equal to or greater than that of the I-Au transfectant (u:u). Of these, HVR1+2β and HVR2β consistently bind peptide at much higher levels than that of the wild type whereas HVR1+2+3β and HVR2+3β bind peptide at levels equal to or slightly less than that of the wild type. These results indicate that only HVR2β, residues Y26β and T28β, is sufficient for binding Ac1-14[4A,14G] whereas HVR3β, residue R70β, inhibits such binding. Mutation of residue 26 alone was sufficient to bind Ac1-14[4A,14G] at a level similar to that of I-Au whereas mutation of residue 28 alone did not bind peptide (data not shown), indicating that both residues are needed for maximally efficient binding.
Table 3.
The ability of HVR mutants to bind Ac1-14[4A,14G]
| Cell line | Binding signal*
|
|
|---|---|---|
| Ac1-14[4A,14G] | Relative to I-Au† | |
| u:u | 0.156 | 1.0 |
| HVR1β | 0.037 | 0.24 |
| HVR2β | 0.571 | 3.66 |
| HVR4β | 0.057 | 0.37 |
| HVR1+2β | 0.309 | 1.98 |
| HVR1+3β | 0.063 | 0.40 |
| HVR1+4β | 0.051 | 0.33 |
| HVR2+3β | 0.135 | 0.87 |
| HVR1+2+3β | 0.169 | 1.08 |
| HVR1+3+4β | 0.062 | 0.40 |
| s:s | 0.022 | 0.14 |
The binding signals for Ac1-11[4A,14G] and OVA 323–339 were determined as described in Table 1. Values similar to or greater than that of wild-type binding are underlined. The data are from at least three separate experiments.
The binding signal for each mutant was normalized to that of I-Au by dividing the binding signal by that of I-Au.
T Cell Responses to the β Chain HVR Mutants.
We tested each of the nine HVR β chain shuffle mutants for their ability to present Ac1-11 and Ac1-11[4A] to an Ac1-11-specific, I-Au restricted T cell hybridoma, 1934.4. The responses are summarized in Table 4. Cells expressing AαsAβs, AαsAβu, or AαuAβs did not stimulate 1934.4 when presenting either Ac1-11 or Ac1-11[4A] (data not shown).
Table 4.
Summary of the responses to the β chain mutants presenting Ac1-11 and Ac1-11[4A] to the T cell hybridoma 1934.4
| Peptide
|
||
|---|---|---|
| Ac1-11 | Ac1-11[4A] | |
| u:u | ++ | +++++ |
| HVR1β | − | − |
| HVR1+4β | − | − |
| HVR1+3β | − | − |
| HVR1+2β | − | − |
| HVR1+2+3β | ± | ++ |
| HVR1+3+4β | − | + |
| HVR2+3β | ± | ++++ |
| HVR2β | − | − |
| HVR4β | − | − |
The maximal response is as described in Table 1.
Although mutants HVR2β and HVR1+2β bind peptide efficiently, neither was recognized by 1934.4. In contrast, 1934.4 recognized HVR2+3β and HVR1+2+3β presenting Ac1-11 and Ac1-11[4A]. These observations suggest that 1934.4 required only HVR3β or R70β to recognize the peptide/MHC complex. Although HVR1+2+3β requires ≈20× more Ac1-11 and Ac1-11[4A] to stimulate 1934.4 than does the wild-type transfectant, the binding signal of Ac1-14[4A,14G] on HVR1+2+3β is similar to that on I-Au, suggesting that these differences in ability to present Ac1-11 and Ac1-11[4A] may be caused by factors other than peptide affinity. Mutant HVR1+3+4β, which showed no measurable binding signal for Ac1-14[4A,14G], was able to present Ac1-11[4A] at high doses to 1934.4, indicating that this mutant is able to bind peptide, but at a level that the binding assay cannot detect.
DISCUSSION
Studies with Ac1-11 analogs indicate that the N-terminal acetyl group and residues Lys P4 and Arg P5 in Ac1-11 contact the MHC whereas residues Gln P3 and Pro P6 contact the TCR (3, 6, 12, 13). The present study extends these findings by defining residues in I-Au that are critical for conferring specific binding of Ac1-11 to I-Au. Through site-specific mutagenesis of residues in I-Au, three residues in the β chain have been identified as key peptide binding contacts: Y26β, T28β, and E74β. A fourth residue, R70β, affects both peptide binding and T cell recognition of the Ac1-11/I-Au complex by an Ac1-11-specific T cell hybridoma. All four residues lie within close proximity of each other in the center of the MHC and compose, in part, pockets 4 and 7 (as defined by the crystal structure of HLA DR1) (14, 15).
Position E74β is a conserved, negatively charged residue in murine β chains and thus in I-Au was a likely candidate for contacting the positively charged arginine at position 5 (P5) in Ac1-11. Substitutions of neutrally charged residues, alanine and glutamine, were expressed at E74β. Neither of these mutants could bind Ac1-14[4A,14G] detectably, indicating that E74β is critical for peptide binding. In contrast, mutations at E74β had little effect on Ova 322–339 peptide binding, indicating that (1) the structural integrity of I-Au was not compromised by the alanine or glutamine substitutions at 74β and (2) Ova 322–339 binding to I-Au does not depend on interactions between the peptide and residue E74β. Concordantly, T cell recognition of Ac1-11 presented by E74A or E74Q mutant was abolished whereas recognition of Ac1-11[4A] was reduced by >1000×.
Only 2 of the 13 residues that differ between the β chains of I-Au and I-As, Y26β and T28β, are needed to confer specific binding of Ac1-11 to I-Au. Residues Y26β and T28β are predicted to border pocket 4 on the left and pocket 7 on the right in the MHC binding groove (Fig. 3) (14–20). Based on computer modeling, Y26β and T28β in I-Au create a different-sized and more hydrophobic pocket than do S26β and D28β in I-As (data not shown). Residues homologous to 28β and 74β in I-E and DR molecules have been shown previously to confer specific binding of other peptides by site-specific mutagenesis (27, 28) and structural data (14–20). In addition, residue 26β in I-Ak, which is closely related to I-Au, is important for binding Ac1-11 (K. Tate and P. Jones, personal communication).
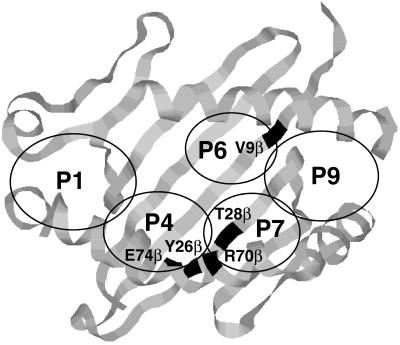
Figure 3.
Residues in pockets 4, 6, and 7 that are predicted to interact with Ac1-11 as outlined on the DR1 structure. E74β is predicted to interact with arginine at position five in Ac1-11. V9β is predicted to interact with lysine, alanine, or tyrosine at position 4 in Ac1-11, Ac1-11[4A], or Ac1-11[4Y], respectively. The regions that compose pockets 1, 4, 6, 7, and 9 have been circled and labeled accordingly.
A fourth residue, R70β, which also forms part of pockets 4 and 7, inhibits peptide binding but is required for T cell recognition by 1934.4, an Ac1-11-specific, I-Au-restricted T cell hybridoma. Inhibition of peptide binding presumably results from electrostatically unfavorable interactions between Arg P5 in Ac1-11 and R70β in I-Au. R70β in I-A and the homologous residue in I-E, DR, and DQ molecules have been shown to contact the TCR and the peptide through both structure/function (29, 30) and structural studies (14–20). Several I-A molecules, including I-Ak, I-As, and I-Au, have deletions at residues 65β and 67β. These changes distinguish these molecules from other I-A molecules as well as from DR and I-E molecules and have been shown to alter the structure of the β chain helix in I-Ak (19). Position R70β, however, remains in the same position in I-Ak as in DR and I-E, and our data that R70β binds both peptide and TCR in the Ac1-11/I-Au complex are consistent with that finding.
Although several residues in the center of I-Au are critical for binding of Ac1-11 to I-Au, pockets on the left side of the binding groove are not, as the single mutations at residues F28α, L35α, and T86β had a neutral or even beneficial impact on MHC binding of Ac1-11 and Ac1-11[4A], with the exception of the L35S mutant. The mutations at HVR4β also had little impact. The appearance of a tyrosine in I-Au at position 81β rather than a histidine found in I-As and other class II MHC molecules may allow the N-terminal region of the groove to stabilize if solvent exposed. The importance of pockets in the center of the groove correlates with peptide binding motifs for I-Au, I-Ad, and I-Ag7, as well as DQ molecules, which indicate that anchor residues appear in the middle of the peptide molecule (6, 31–36).
Although we have not shown a direct contact between Arg P5 in Ac1-11 and Y26β, T28β, and E74β, their interaction is a reasonable hypothesis. Recent data show that, in I-Au and I-Ak, position 9β, which is adjacent to both pockets 6 and 9 of the MHC binding groove, contacts Lys P4 in Ac1-11. Based on these data and those presented here, Lee et al. (37) have proposed that Ac1-11 occupies only the center and right portions of the MHC binding groove, leaving pocket 1 on the left side of the cleft empty. In this model, the hydrophobic CH2 units of Arg P5 lie in pocket 7, and the positively charged amino group extends into pocket 4 to form a salt bridge with E74β. Lys P4 then interacts with V9β in the hydrophobic pocket 6 (Fig. 3). This model would explain why Ac1-11 has a low affinity for I-Au, as stabilizing anchors in pocket 1 and elsewhere in the binding groove are not occupied. Furthermore, the model shows why lysine at position four confers a low affinity for the MHC whereas the hydrophobic residues alanine and tyrosine confer higher affinities (3, 6). Both our data and those of Lee et al. correlate with direct peptide binding data that show that the last four residues of Ac1-11 are not needed for efficient binding when tyrosine is placed at position four (6) and with biological data that show that Ac1-6 is sufficient to induce EAE in susceptible mouse strains (12).
In contrast to the model of Ac1-11 bound to I-Au, structural data of DR1 show that a major contact between DR1 and the HA peptide consists of the P1 residue binding to the deep hydrophobic pocket 1 on the left side of the MHC groove (14, 15), a finding that correlates with binding motifs of peptides that bind to DR (38–41), I-E (42–44), and DQ (34, 45, 46) molecules. In addition, I-Ak preferentially binds peptides with a glutamate or aspartate at the P1 position (47). These data are confirmed by the structure of the HEL peptide bound to I-Ak, which indicates that pocket 1 binds the aspartate at P1 in the HEL peptide (19). High affinity peptide binding to I-Ad, however, does not require large anchor residues at position P1 or P4 (20). The structural data for I-Ak and I-Ad emphasize that peptide binding relies on the conserved network of hydrogen bonds that connect the peptide main chain to conserved residues of the MHC seen in other peptide/class II MHC structures so far studied (14–18). Moreover, Ova 322–339 probably occupies the N-terminal region of the groove, as mutations in I-Au on the left side of the MHC had an adverse effect on Ova 322–339 binding whereas mutations in the center of the MHC had a neutral effect. Thus, the model of Ac1-11 binding to I-Au may be an atypical example of peptide binding to I-Au.
Replacement of Lys P4 with a hydrophobic residue alanine (Ac1-11[4A]) increases the affinity of the peptide for the MHC and preserves recognition of the peptide/MHC complex by Ac1-11-specific T cells in vitro yet renders the peptide nonimmunogenic in vivo (3, 8). Preliminary data indicate that the analog Ac1-11[5K], in which lysine at position 5 preserves the positive charge, can stimulate Ac1-11-specific T cells in vitro and can induce EAE (48) whereas Ac1-11[4A,5K] can bind to I-Au but cannot stimulate Ac1-11-specific T cells in vitro (C.I.P. and H.O.M., unpublished work). Moreover, Ac1-11[5E] antagonizes T cell activation (C.I.P. and H.O.M., unpublished work), suggesting that position 5 contacts the TCR in addition to the MHC. These results suggest that Ac1-11[4A] binds to I-Au in a different conformation than does Ac1-11. This different conformation may allow recognition of the Ac1-11[4A]/I-Au complex by Ac1-11-specific T cells in vitro but not in vivo, preventing an immune response (because of a low affinity of the TCR for the Ac1-11[4A]/I-Au complex).
The structure–function studies presented here and the model put forward by Lee et al. (37) show that MBP Ac1-11 binds to the center and right part of the peptide-binding groove, with a very low binding affinity, primarily because of the necessity of placing LysP4 into the hydrophobic pocket 6. This is an unusual peptide/MHC configuration. Despite these two distinctive characteristics, Ac1-11 is the immunodominant peptide epitope of MBP in I-Au-expressing mice. One possible reason for this immunodominance might be an unusually high affinity of the T cell receptor for the Ac1-11/I-Au complex. Another possibility is that T cells bearing TCR specific for more stable MBP peptide/I-Au complexes, such as MBP121–150/I-Au, are tolerized whereas T cells specific for the unstable Ac1-11/I-Au complex escape tolerance (49).
These structure–function studies provide valuable information concerning the important interactions between Ac1-11 and I-Au. We have shown that the major contact between Ac1-11 and I-Au lies at the center of the peptide binding groove in pockets 4 and 7, and we provide evidence to explain why Ac1-11 has such a low affinity for I-Au. Until structural data on the Ac1-11 and I-Au complex are available, the details of this peptide/MHC complex must rely on structure–function studies such as those presented here.
Acknowledgments
We thank D. Fremont and J. Rothbard for critical discussion of the manuscript and J. Rothbard for help with computer modeling. This work was supported by grants from the National Institutes of Health.
ABBREVIATIONS
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- HVR
hypervariable region
- MHC
major histocompatibility complex
- TCR
T cell antigen receptor
References
- 1.Zamvil S, Steinman L. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 2.Sobel R A, Tuohy V K, Lu Z J, Laursen R A, Lees M B. J Neuropathol Exp Neurol. 1990;49:468–479. doi: 10.1097/00005072-199009000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Wraith D C, Smilek D E, Mitchell D J, Steinman L, McDevitt H O. Cell. 1989;59:247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- 4.Gautam A M, Pearson C I, Sinha A A, Smilek D E, Steinman L, McDevitt H O. J Immunol. 1992;148:3049–3054. [PubMed] [Google Scholar]
- 5.Mason K, Denney D W, Jr, McConnell H M. J Immunol. 1995;154:5216–5227. [PubMed] [Google Scholar]
- 6.Fugger L, Liang J, Gautam A, Rothbard J B, McDevitt H O. Mol Med. 1996;2:181–188. [PMC free article] [PubMed] [Google Scholar]
- 7.Martin R, McFarland H F, McFarlin D E. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 8.Smilek D E, Wraith D C, Hodgkinson S, Dwivedy S, Steinman L, McDevitt H O. Proc Natl Acad Sci USA. 1991;88:9633–9637. doi: 10.1073/pnas.88.21.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzler B, Wraith D C. Int Immunol. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 10.Samson M F, Smilek D E. J Immunol. 1995;155:2737–2746. [PubMed] [Google Scholar]
- 11.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, et al. Nature (London) 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 12.Gautam A M, Pearson C I, Smilek D E, Steinman L, McDevitt H O. J Exp Med. 1992;176:605–609. doi: 10.1084/jem.176.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wraith D C, Bruun B, Fairchild P J. J Immunol. 1992;149:3765–3770. [PubMed] [Google Scholar]
- 14.Brown J H, Jardetzky T S, Gorga J C, Stern L J, Urban R G, Strominger J L, Wiley D C. Nature (London) 1993;364:33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 15.Stern L J, Brown J H, Jardetzky T S, Gorga J C, Urban R G, Strominger J L, Wiley D C. Nature (London) 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P, Amaya M, Mellins E, Wiley D C. Nature (London) 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 17.Dessen A, Lawrence C M, Cupo S, Zaller D M, Wiley D C. Immunity. 1997;7:473–481. doi: 10.1016/s1074-7613(00)80369-6. [DOI] [PubMed] [Google Scholar]
- 18.Fremont D H, Hendrickson W A, Marrack P, Kappler J. Science. 1996;272:1001–1004. doi: 10.1126/science.272.5264.1001. [DOI] [PubMed] [Google Scholar]
- 19.Fremont D H, Monnaie D, Nelson C A, Hendrickson W A, Unanue E R. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- 20.Scott C A, Peterson P A, Teyton L, Wilson I A. Immunity. 1998;8:319–329. doi: 10.1016/s1074-7613(00)80537-3. [DOI] [PubMed] [Google Scholar]
- 21.Germain R N, Norcross M A, Margulies D H. Nature (London) 1983;306:190–194. doi: 10.1038/306190a0. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 23.Glimcher L, McKean D, Choi E, Seidman J. J Immunol. 1985;135:3542–3550. [PubMed] [Google Scholar]
- 24.Oi V T, Jones P P, Goding J W, Herzenberg L A, Herzenberg L A. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- 25.Beck B N, Buerstedde J-M, Krco D J, Nilson A E, Chase C G, McKean D J. J Immunol. 1986;136:2953–2961. [PubMed] [Google Scholar]
- 26.Kappler J, Skidmore B, White J, Marrack P. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McElligott D L, Sorger S B, Matis L A, Hedrick S M. J Immunol. 1988;140:4123–4131. [PubMed] [Google Scholar]
- 28.Racioppi L, Ronchese F, Schwartz R H, Germain R N. J Immunol. 1991;147:3718–3727. [PubMed] [Google Scholar]
- 29.Coppin H L, Carmichael P, Lombardi G, L’Faqihi F E, Salter R, Parham P, Lechler R I, de Preval C. Eur J Immunol. 1993;23:343–349. doi: 10.1002/eji.1830230207. [DOI] [PubMed] [Google Scholar]
- 30.Itoh Y, Ogasawara K, Gotohda T, Takami K, Naruse H, Onoe K. Int Immunol. 1992;4:779–787. doi: 10.1093/intimm/4.7.779. [DOI] [PubMed] [Google Scholar]
- 31.Sette A, Southwood S, O’Sullivan D, Gaeta F C, Sidney J, Grey H M. J Immunol. 1992;148:844–851. [PubMed] [Google Scholar]
- 32.Sette A, Sidney J, Albertson M, Miles C, Colon S M, Pedrazzini T, Lamont A G, Grey H M. J Immunol. 1990;145:1809–1813. [PubMed] [Google Scholar]
- 33.Wall M, Southwood S, Sidney J, Oseroff C, del Guercio M F, Lamont A G, Colon S M, Arrhenius T, Gaeta F C, Sette A. Int Immunol. 1992;4:773–777. doi: 10.1093/intimm/4.7.773. [DOI] [PubMed] [Google Scholar]
- 34.Harrison L C, Honeyman M C, Trembleau S, Gregori S, Gallazzi F, Augstein P, Brusic V, Hammer J, Adorini L. J Exp Med. 1997;185:1013–1021. doi: 10.1084/jem.185.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidney J, Oseroff C, del Guercio M F, Southwood S, Krieger J I, Ishioka G Y, Sakaguchi K, Appella E, Sette A. J Immunol. 1994;152:4516–4525. [PubMed] [Google Scholar]
- 36.Chicz R M, Lane W S, Robinson R A, Trucco M, Strominger J L, Gorga J C. Int Immunol. 1994;6:1639–1649. doi: 10.1093/intimm/6.11.1639. [DOI] [PubMed] [Google Scholar]
- 37.Lee C, Liang M N, Tate K M, Rabinowitz J D, Beeson C, Jones P P, McConnell H M. J Exp Med. 1998;187:1505–1516. doi: 10.1084/jem.187.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee H G. Immunogenetics. 1994;39:230–242. doi: 10.1007/BF00188785. [DOI] [PubMed] [Google Scholar]
- 39.Demotz S, Barbey C, Corradin G, Amoroso A, Lanzavecchia A. Eur J Immunol. 1993;23:425–432. doi: 10.1002/eji.1830230219. [DOI] [PubMed] [Google Scholar]
- 40.Newton-Nash D K, Eckels D D. J Immunol. 1993;150:1813–1821. [PubMed] [Google Scholar]
- 41.Busch R, Hill C M, Hayball J D, Lamb J R, Rothbard J B. J Immunol. 1991;147:1292–1298. [PubMed] [Google Scholar]
- 42.Brusic V, Rudy G, Harrison L. Nucleic Acids Res. 1994;22:3663–3665. doi: 10.1093/nar/22.17.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marrack P, Ignatowicz L, Kappler J W, Boymel J, Freed J H. J Exp Med. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reay P A, Kantor R M, Davis M M. J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- 45.Wucherpfennig K W, Strominger J L. J Exp Med. 1995;181:1597–1601. doi: 10.1084/jem.181.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwok W W, Nepom G T, Raymond F C. J Immunol. 1995;155:2468–2476. [PubMed] [Google Scholar]
- 47.Nelson C A, Viner N J, Young S P, Petzold S J, Unanue E R. J Immunol. 1996;157:755–762. [PubMed] [Google Scholar]
- 48.Pearson C I. Ph.D. thesis. Stanford, CA: Stanford Univ.; 1994. [Google Scholar]
- 49.Harrington C J, Paez A, Hunkapiller T, Mannikko V, Brabb T, Ahearn M, Beeson C, Goverman J. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]