Abstract
Purpose
To characterize the molecular composition of cross-linked actin networks (CLANs) and the regulation of their formation by integrins in normal human trabecular meshwork (TM) cells. CLANs have been observed in steroid-treated and glaucomatous TM cells and have been suggested to contribute to decreased outflow facility by altering the contractility of the TM.
Methods
Immunofluorescence microscopy was used to identify molecular components of CLANs and quantitate CLAN formation in HTM cells plated on coverslips coated with various extracellular matrix (ECM) proteins (fibronectin, types I and IV collagen, and vitronectin), vascular cell adhesion molecule (VCAM)-1, or activating antibodies against β1, β3, or α2β1 integrins. These integrin antibodies were also used as soluble ligands.
Results
CLAN vertices contained the actin-binding proteins α-actinin and filamin and the signaling molecules syndecan-4 and PIP2. CLANs lacked Arp3 and cortactin. CLAN formation was dependent on the ECM substrate and was significantly higher on fibronectin and VCAM-1 compared with vitronectin, types I or IV collagen. Adsorbed β1 integrin antibodies also induced CLANs, whereas adsorbed β3 or α2β1 integrin antibodies did not. Soluble β3 integrin antibodies, however, induced CLANs and actually enhanced CLAN formation in cells spread on fibronectin, VCAM-1, type I or type IV collagen, or β1 integrin antibodies.
Conclusions
CLANs are unique actin-branched networks whose formation can be regulated by β1 and β3 integrin signaling pathways. Thus, integrin-mediated signaling events can modulate the organization of the actin cytoskeleton in TM cells and hence could participate in regulating cytoskeletal events previously demonstrated to be involved in controlling outflow facility.
The actin cytoskeleton plays a fundamental role in regulating a variety of cell processes including morphogenesis, motility, secretion, endocytosis, and cytokinesis.1 Actin-based processes may also play an important role in regulating aqueous humor outflow through the trabecular meshwork (TM).2–4 Agents that disrupt the cytoskeleton, such as cytochalasin, H-7, BDM, latrunculins, ethacrynic acid, vinblastine, and Y-27632,2,4–6 can increase aqueous humor outflow facility.
Recent studies suggest that alterations in the organization of the actin cytoskeleton may also play a role in outflow facility and may be involved in the pathogenesis of steroid-induced glaucoma7–9 and possibly other forms of POAG as well.7–9 Treatment with glucocorticoids such as dexamethasone (DEX) can increase intraocular pressure (IOP) both in vivo and in cultured anterior segments.10–14 Examination of the actin cytoskeleton in DEX-treated anterior segment cultures14 and TM cell cultures8,9,15 show that the actin cytoskeleton undergoes reorganization into highly cross-linked actin networks (CLANs) or actin geodesic domes. Similar structures have been observed in glaucomatous TM cells in the absence of DEX treatment,16 suggesting that CLANs may also be the result of pressure-induced changes in the actin network. It has been suggested by others that DEX-induced CLAN formation in TM cells decreases the overall contractility of the tissue by making the cells more rigid and unable to change shape and “relax” under pressure.14 Alternatively, it could affect other actin-mediated biological processes of the TM needed for normal outflow facility, such as gene expression.17
CLANs are composed of a series of interconnected F-actin bundles that radiate outward from central vertices and resemble a geodesic dome. Besides actin, CLANs are known to be composed of α-actinin, tropomyosin, and filamin.18–20 CLANs are located just beneath the apical cell surface and may be physically attached to the plasma membrane.18,20–22 They are often found within lamellipodia, but they can also be anywhere throughout the cell.9,19,22 CLANs can be found in both spreading19 and nonspreading cells22,23 and were originally thought to be precursors to actin stress fibers19 or reorganized sarcomeres.24 CLANs have also been proposed to be specialized structures that help maintain cellular tensegrity.25 The function of CLANs, however, remains unclear, and little is known about the structural components and signaling pathways that regulate their formation and disassembly.
The formation of other actin-containing structures such as filopodia, lamellipodia, and stress fibers is regulated by signaling pathways involving Rho family GTPases26 and cell surface receptors, such as integrin family members. Integrins are heterodimeric transmembrane glycoproteins composed of an α and β subunit. They bind several extracellular matrix (ECM) proteins via a tripeptide sequence, arg-gly-asp (RGD) in the ECM protein, whereas their cytoplasmic tails interact with a variety of tyrosine kinases, adaptor proteins, and actin-binding proteins. Thus, integrins not only form an important physical link between the extracellular environment and the actin cytoskeleton, but the protein complexes associated with their cytoplasmic tails regulate the organization of actin-containing structures.27,28
Recent work has demonstrated that syndecans act as coreceptors, along with integrins, to regulate the organization of the actin cytoskeleton.29–31 Syndecans are a family of type I membrane proteins, with cytoplasmic and transmembrane domains that are highly conserved. Of the four members in the family, syndecans-3 and -4 are the predominant members that are in the anterior segment.32 Their extracellular domains bear multiple heparan sulfate glycosaminoglycan chains. Syndecans, like integrins, bind ECM proteins and regulate the organization of the actin cytoskeleton via association with downstream signaling molecules. Most of the signaling activity of syndecans can be linked, either directly or indirectly, to Rho family GTPase-mediated signaling pathways and involves interactions between the cytoplasmic domains of syndecans and signaling molecules like phosphatidylinositol 4,5-bisphosphate (PIP2) and protein kinase C (PKC).
In this study, we examined the structure of CLANs in HTM cells and the possible role that syndecans and integrins play in their formation. These studies show that α-actinin, syndecan-4, PIP2, and filamin are structural components of CLANs and the formation of these structures is regulated by a novel β1/β3 integrin signaling pathway.
Methods
Cell Culture
The A7-1 strain of HTM cells was obtained from Jon Polansky (University of California, San Francisco, CA). Cells were cultured in low-glucose DMEM (Sigma-Aldrich, St. Louis, MO), 15% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 2% l-glutamine (Sigma-Aldrich), 1% amphotericin B (Mediatech, Herndon, VA), and 0.05% gentamicin (Mediatech) + 1 ng/mL FGF-2 (Peprotech, Rocky Hill, NJ).33,34 In some experiments, confluent monolayers of HTM cells were cultured for either 3 or 10 days in the presence or absence of 500 nM DEX before being used in the spreading assay. DEX was added daily from a 500 μM stock solution stored at −20°C.
Cell-Spreading Assays
Confluent HTM monolayers, allowed to establish a stable morphology over a 7-day period,35 were serum-starved for 24 hours. Cells were serum starved to synchronize RhoA-mediated signaling pathways that regulate cell spreading and actin polymerization.36 Cells were then trypsin-treated and resuspended in serum-free DMEM containing 25 μg/mL cycloheximide. Both cycloheximide, which inhibits de novo protein synthesis, and serum-free medium were used in the assay to prevent unwanted signaling events mediated by serum matrix proteins and serum factors that would make it difficult to interpret the assay results. Among the serum components that would be problematic are the matrix proteins fibronectin, vitronectin,37–39 and lysophosphatidic acid.36 These serum components are known to activate integrin-specific signaling events. Cells were plated onto glass coverslips coated with poly-l-lysine (PL), fibronectin, vitronectin, type I collagen, type IV collagen, vascular cell adhesion molecule (VCAM)-1 or the III7–10, III12–14 or IIICS domains of fibronectin. All substrates except the fibronectin domains were at 20 nM in PBS. The fibronectin fragments were at 100 nM in PBS. Cells were allowed to spread for 3 hours and then fixed with −20°C methanol for 15 minutes or 4% p-formaldehyde plus 0.18% Triton X-100 for 30 minutes. Human fibronectin and the III7–10, III12–14, and IIICS domains were produced as described.40,41 Human vitronectin and human VCAM-1 were provided by Deane Mosher (University of Wisconsin, Madison, WI). Human type I collagen and bovine type IV collagen were obtained from Southern Biotechnology Associates, Inc. (Birmingham, AL), and Chemicon International, Inc. (Temecula, CA), respectively. In experiments with soluble antibodies, suspended cells were incubated for 10 minutes in the presence of 10 μg /mL mAb GAL-13 against β-galactosidase (Sigma-Aldrich), 10 μg /mL mAb B3B11 against β1 integrin (Chemicon International, Inc.), 8 μg /mL mAb AP-5 against β3 integrin (The Blood Center of Southeastern Wisconsin; Milwaukee, WI), or 6 μg /mL mAb JBS2 against α2β1 integrin (Chemicon International, Inc.) and then plated, in the presence of the same soluble antibodies, on PL-coated coverslips. In other assays, cells were spread, in the absence or presence of soluble mAb AP-5 against β3 integrin, on coverslips coated with either the various antibodies or matrix substrates just described. In experiments with antibodies used as coating substrates, glass coverslips were pre-coated with 1:100 unconjugated goat anti-mouse IgG (Jackson ImmunoResearch, Inc, West Grove, PA). In experiments examining the effects of DEX pretreatment on CLAN formation, cells were spread in the absence or presence of 500 nM DEX.
Immunofluorescence Microscopy
CLANs were labeled with either Alexa 488 phalloidin (Invitrogen, Carlsbad, CA) or polyclonal anti-F-actin (Sigma-Aldrich) together with antibodies against α-actinin (clone AT6.172; Sigma-Aldrich), syndecan-4 (clone 150.942), PIP2 (clone KT10; Assay Design, Inc., Ann Arbor, MI), filamin (clone TI10; Chemicon International, Inc.), Arp3 (clone 4; BD Biosciences, San Diego, CA), polyclonal anti-cortactin (Sigma-Aldrich), β1 integrin (clone B3B11), β3 integrin (clone 1, BD Biosciences), or glial fibrillary acidic protein (GFAP; clone G-A-5; Sigma-Aldrich). Anti-F-actin and anti-cortactin antibodies were detected using either Alexa 488-conjugated goat anti-rabbit IgG or Alexa 488-conjugated donkey anti-rabbit IgG. All monoclonal antibodies were detected using Alexa 546-conjugated goat anti-mouse IgG. All secondary antibodies and Hoechst 33342 were purchased from Invitrogen. Hoechst 33342 was used to localize nuclei. Fluorescence was observed with a microscope (Axioplan 2) equipped with a camera (Axiocam HR), and software (Axiovision 3.1; all from Carl Zeiss Meditec, Inc., Dublin, CA).
To quantitate the number of CLAN-positive cells (CPCs), five to seven low-power (200×) fluorescence images from each treatment group were captured. The total number of cells per image was counted and the percentage of CPCs per image was determined. Individual experiments were performed with duplicate determinations. The data presented in Figures 4, 6, 7, and 9 were each pooled from three to five experiments and represent the mean percentage of CPC ± SD. Statistical analysis comparing the different treatment groups was performed with a Student’s t-test.
Figure 4.
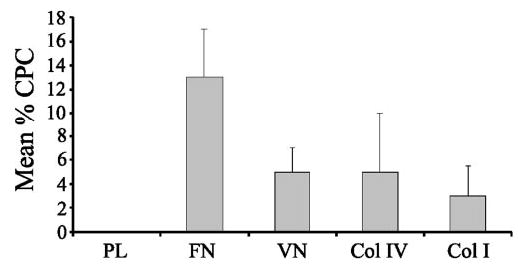
ECM substrates differentially induce CLAN formation in HTM cells. HTM cells were spread on poly-l-lysine (PL), fibronectin (FN), vitronectin (VN), type IV collagen (Col IV), or type I collagen (Col I) and labeled with phalloidin. The percentage of CPCs is shown as the mean ± SD. n = 2822–4206 cells/group.
Figure 6.
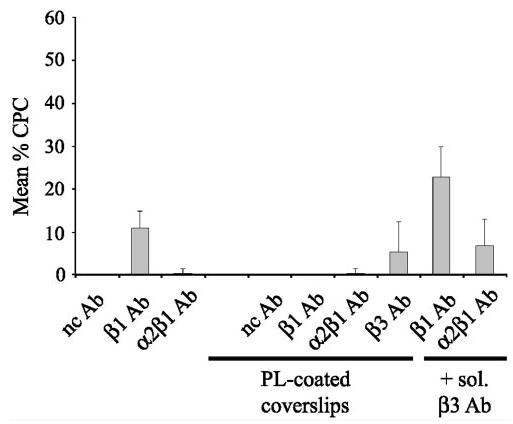
Histogram of CLAN formation induced by either immobilized or soluble integrin-activating antibodies. Cells were spread on coverslips, coated with immobilized β1 or α2β1 integrin-activating antibodies in the absence or presence of soluble β3 integrin-activating antibodies. Alternatively, cells were plated on coverslips coated with poly-l-lysine (PL) in the absence or presence of soluble integrin-activating antibodies. The percentage of CPCs is shown as the mean ± SD; n = 1196–3141 cells/group.
Figure 7.
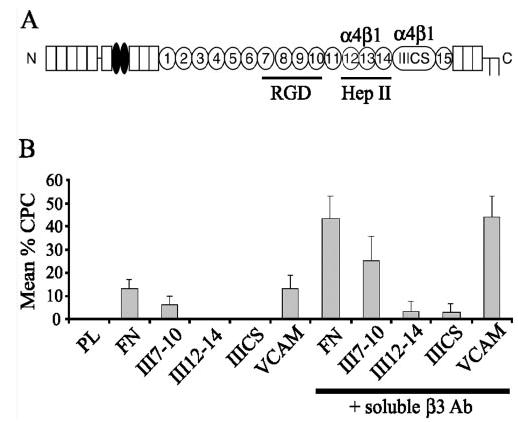
Histogram of the differential induction of CLAN formation on fibronectin, VCAM or fibronectin domains upon activation of β3 integrins. (A) Schematic of fibronectin monomer. Fibronectin is a dimer consisting of repeating sequences of type I (rectangles), II (ovals), and III (circles) repeats. The alternatively spliced IIICS domain lies in a variant region between the type III 14th and 15th repeats. The type III7–10 and type III12–14 domains are also indicated. (B) CLAN induction in HTM cells plated onto substrates of fibronectin, VCAM-1 or various domains of fibronectin in the presence or absence of soluble β3 integrin-activating antibodies. Cells were labeled with phalloidin. The percentage of CPCs is shown as the mean ± SD; n = 721–4206 cells/group.
Figure 9.
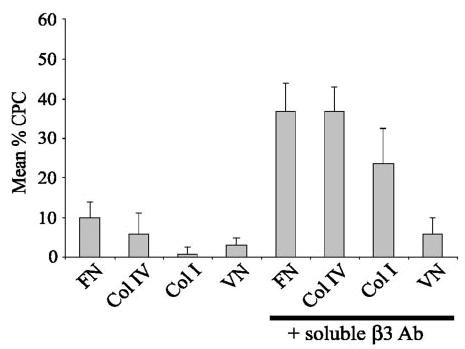
Activation of β3 integrin differentially enhances CLAN formation in HTM cells plated on vitronectin, types I or IV collagen. Cells were plated onto coverslips coated with either fibronectin (FN), type IV collagen (Col IV), type I collagen (Col I) or vitronectin (VN) in the presence or absence of soluble β3 integrin-activating antibody AP-5 and then labeled with phalloidin. The percentage of CPCs is shown as the mean ± SD; n = 1500–2008 cells/group.
RESULTS
Multimolecular Components within CLAN Vertices
To study CLAN formation in HTM cells, we developed a cell-spreading assay. This assay involved taking confluent cultures of HTM cells that had reached the quiescent stage described by Polansky et al.,33,34 and replating them at a subconfluent density for 3 hours on various immobilized substrates in the absence of DEX. On replating, these cells retain morphologic and biochemical features that are unique to the quiescent stage of HTM cells compared with proliferating cells.33,34 This assay was used instead of confluent monolayers, because it made it easy to visualize CLAN components and manipulate integrin-mediated signaling events that regulate actin polymerization. Both of these procedures would be difficult to do in DEX-treated confluent monolayers, especially since DEX-treatment induces expression of multiple ECM proteins35,43,44 in HTM cells that can activate any number of integrin-signaling pathways. Finally, it is significantly easier to quantitate CLAN formation in this assay than in DEX-treated confluent monolayers.
Using this assay, it was found that CLAN formation reached a maximum within 3 hours after plating (data not shown) and did not require a prolonged treatment with DEX as described elsewhere.8,9 Figure 1 shows that in addition to actin filaments, CLANs formed in HTM cells also contained multimolecular complexes at their vertices that we have named “vertisomes. ” One of the major components found in vertisomes was α-actinin (Fig. 1A), indicating that these actin structures were similar to those reported previously.19 In addition to α-actinin, vertisomes also labeled intensely for PIP2 (Fig. 1D) and syndecan-4 (Fig. 1G), two signaling molecules known to regulate actin polymerization.1,42,45–47 Lower levels of α-actinin, syndecan-4, and PIP2 were also found along actin filaments, suggesting that these proteins were also minor components of actin filaments in CLANs. Clearly, however, these three components were predominantly found within vertisomes. The localization of syndecan-4, PIP2, and α-actinin within CLAN vertices differed from that of the major actin-binding protein filamin.28 Double labeling for actin and filamin showed that filamin localized intensely along the entire length of the actin filaments as well as within the CLAN vertices (Fig. 1J).
Figure 1.
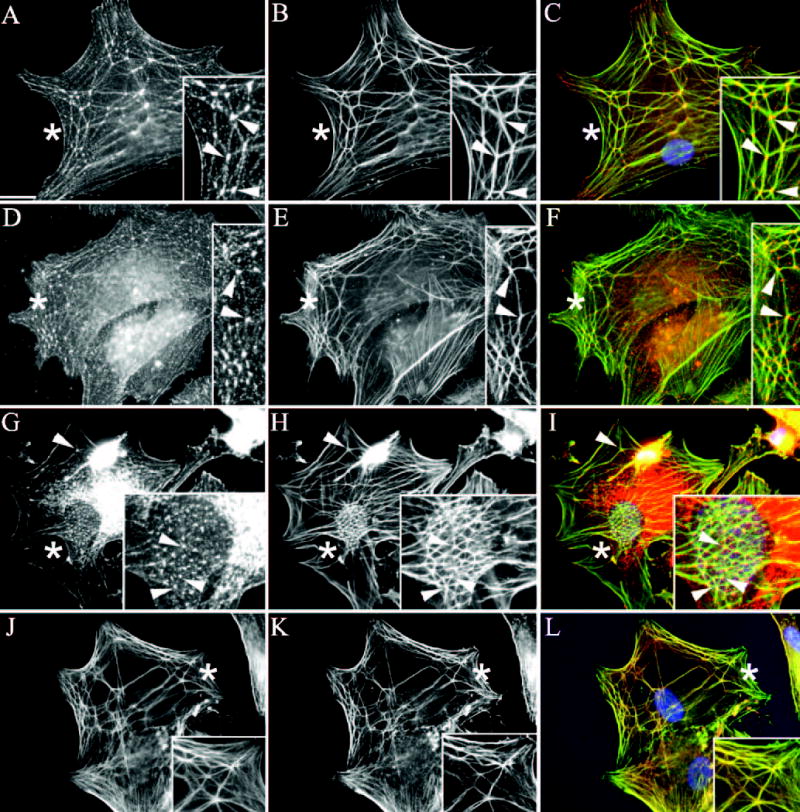
Colocalization of F-actin and various signaling molecules in CLANs formed in HTM cells spread on fibronectin. Cells were fixed in methanol and labeled with antibodies against F-actin (B, E, H, K) and α-actinin (A), PIP2 (D), syndecan-4 (G), or filamin (J). Arrowheads: points of colocalization of F-actin and α-actinin, PIP2, syndecan-4, or filamin. Merged images (C, F, I, L). Asterisks: Regions magnified in insets. Bar, 50 μm.
To determine whether CLANs were similar to other actin-branched structures, HTM cells were also labeled for the presence of Arp3 and cortactin which are normally found at actin branch points at the leading edge of spreading or motile cells.48 Figure 2 shows that, unlike other actin-branched structures, neither cortactin (Figs. 2A, 2B) nor Arp3 (Figs. 2C, 2D) localized to any part of the CLAN structure. Both proteins, however, localized to the leading edge of spreading HTM cells, consistent with previous reports. Thus, even though CLANs are actin-branched structures, they appear to be molecularly distinct from the branched actin networks found at the leading edge of cells. Cells labeled with a negative control antibody against GFAP did not show significant staining (not shown).
Figure 2.
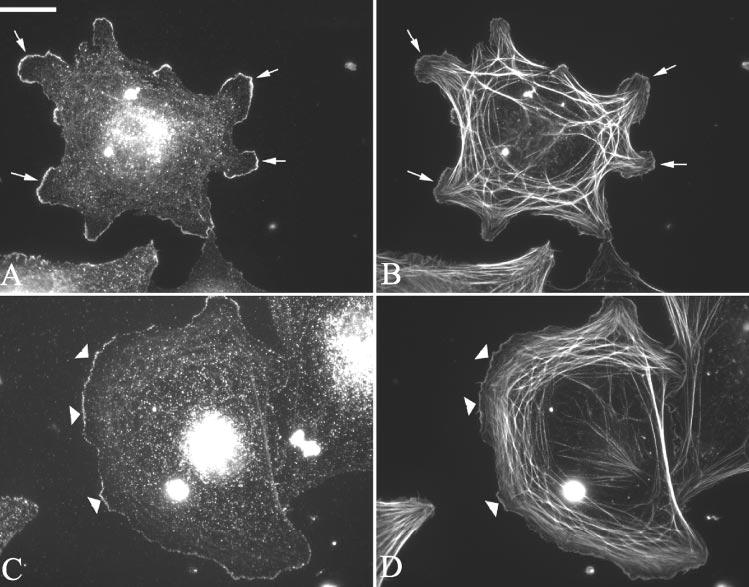
Localization of F-actin and cortactin or Arp3 in HTM cells spread on fibronectin. Cells were fixed in p-formaldehyde and labeled with phalloidin (B, D) and antibodies against either cortactin (A) or Arp3 (C). Arrows: points of colocalization of F-actin and cortactin. Arrowheads: points of colocalization of F-actin and Arp3. Note the absence of cortactin or Arp3 within the CLAN structures. Bar, 50 μm.
Presence of Syndecan-4, PIP2, and α-Actinin in Vertisomes Irrespective of Adsorbed Substrate
Immunofluorescence microscopy experiments were also performed on HTM cells spread on either vitronectin or type IV collagen, to determine whether the composition of vertisomes was dependent on interactions with the ECM. Double-labeling for F-actin and syndecan-4 demonstrated that, irrespective of the substrate that the cells were spread on, syndecan-4 was still prominently localized in vertisomes (Fig. 3). As before, weak syndecan-4 localization was also observed along the length of actin filaments and occasionally in focal adhesions regardless of whether cells possessed CLANs (not shown). The localization of PIP2 and α-actinin also remained the same, regardless of the adsorbed substrate (not shown) suggesting that the composition of vertisomes is not dependent on the adsorbed substrate.
Figure 3.
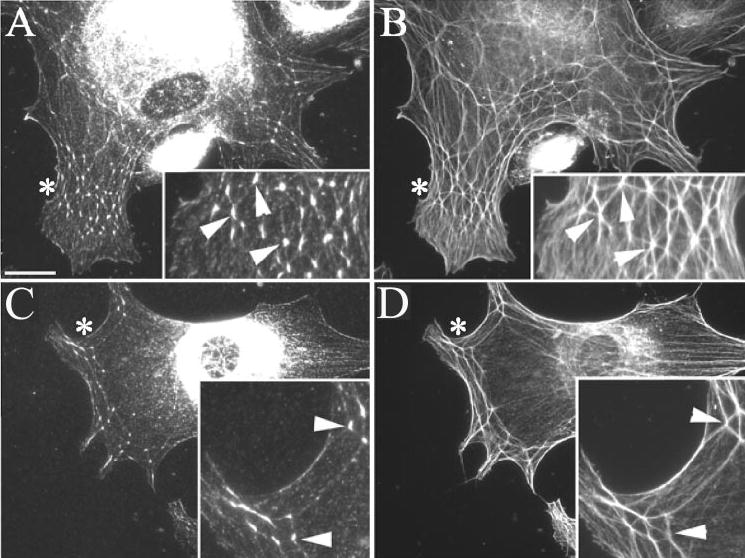
Colocalization of F-actin and syndecan-4 in CLANs formed in HTM cells spread on either vitronectin or type IV collagen. Cells spread on vitronectin (A, B) or type IV collagen (C, D) were processed as described in Figure 1. Arrowheads: points of colocalization of syndecan-4 (A, C) and F-actin (B, D). Asterisks: Regions magnified in insets. Bar,50 μm.
Differential Induction of CLANs in HTM Cells Spread on Integrin-Specific Substrates
Although the substrate did not affect the composition of CLANs, it affected the ability of HTM cells to induce CLAN formation. CLAN formation was found to be significantly greater (P < 0.0001) in cells plated on fibronectin than in cells plated on vitronectin, type IV collagen or type I collagen. As shown in Figure 4, approximately 13% of cells formed CLANs on fibronectin, whereas only 4% to 5% of cells formed CLANs on the other substrates. In contrast, CLANs were not observed in cells plated onto PL-coated coverslips (Figs. 5, 6). Thus, of the four substrates tested, fibronectin was the strongest inducer of CLAN formation. This suggests that regulation of CLAN formation is both integrin dependent and specific.
Figure 5.
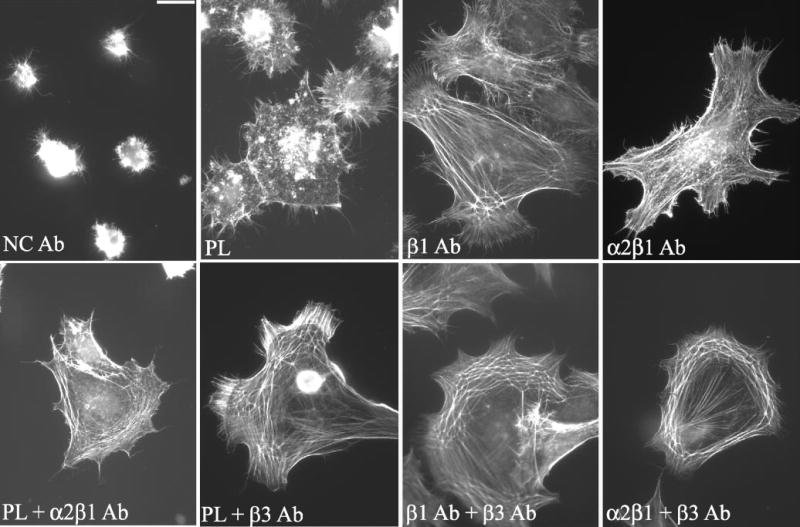
CLAN formation is triggered by specific integrins. Cells were plated on poly-l-lysine (PL), negative control (NC), β1 or α2β1 antibodies in the presence or absence of soluble α2β1- or β3-integrin–activating antibodies and then labeled with phalloidin. Bar, 50 μm.
To determine whether integrin signaling could activate CLAN formation, HTM cells were plated on specific integrin-activating antibodies in lieu of ECM proteins. These antibodies included an activating antibody that recognizes all β1 integrins, an antibody that recognizes all β3 integrins, and an antibody that recognizes α2β1 integrin, which is primarily a receptor for collagen. These antibodies have been shown to mediate cell attachment, spreading, and actin polymerization,49–53 indicating that they activate the same signaling events as ECM proteins. Thus, in addition to looking for CLAN formation, the abilities of these antibodies to mediate cell attachment, spreading, and actin polymerization were also used as criteria to evaluate the role of integrin signaling. As shown in Figure 5, cells plated onto the immobilized β1 and α2β1 integrin antibodies attached, spread, and polymerized actin, indicating that these antibodies were actively signaling to cells.
Cell spreading and CLAN formation, however, varied on the different integrin antibody substrates, which suggests that activation of integrins could trigger CLAN formation, but the degree of CLAN formation may be integrin specific. Quantification of the number of CPCs verified this idea. As shown in Figure 6, the level of CLAN formation in cells spread on the β1-specific antibody, B3B11, was comparable to that observed on whole fibronectin (11%) indicating that β1 integrins could regulate CLAN formation (Fig 6). The induction of CLAN formation on the β1-specific antibody, B3B11, however, was significantly greater (P < 0.0001) than CLAN formation in cells plated on PL or α2β1 integrin antibodies. Thus, CLAN formation appeared to be integrin specific. CLAN formation could not be determined in cells spread on the β3 integrin-specific antibody AP-5, because few cells even bound to this antibody when it was adsorbed to glass coverslips (not shown).
Because previous studies have shown that the β3 activating antibody AP-5 was more effective as a soluble ligand,53 the experiments were repeated to see whether soluble β3 antibody could activate CLAN formation. For comparison purposes, we also examined the ability of soluble β1 antibody B3B11 and soluble α2β1 antibody JBS2 to activate CLAN formation. For these studies, soluble activating integrin antibodies were added to cells plated onto PL-coated coverslips. As shown in Figure 6, when soluble β3 antibody was added to cells plated on PL-coated coverslips, the soluble β3 antibody not only enhanced spreading, but CLAN formation was observed in 5.5% of the cells (Figs. 5, 6) indicating that activation of β3 integrins could also promote CLAN formation. The opposite, however, was observed when soluble β1 antibody B3B11 (data not shown; Fig. 6) or soluble α2β1 antibody JBS2 (Figs. 5, 6) were added to cells on PL-coated coverslips. In these cases CLAN formation was not induced. Presumably, these antibodies need to be immobilized to induce spreading and/or subsequent CLAN formation in HTM cells.
Cells plated on PL-coated coverslips in the presence of soluble α2β1 antibody demonstrated enhanced spreading and actin polymerization relative to cells plated in the absence of soluble antibodies even though CLAN formation was not induced. This suggests that the ability to induce cell spreading and actin polymerization alone is not sufficient to trigger CLAN formation. Soluble negative control antibodies did not have an effect on either CLAN formation or cell spreading (Fig. 5).
Enhanced CLAN Formation in Response to Combined β1/β3 Integrin Signaling
Because many integrin-mediated signaling events involve co-signaling between different integrins, we next examined whether activation of both β1 and β3 integrins could enhance CLAN formation. For this study, HTM cells were plated onto immobilized β1 antibody B3B11 or α2β1 antibody JBS2 in the presence of soluble β3 antibody AP-5. As shown in Figure 5, cells plated on either the β1 or α2β1 activating antibodies in the presence of the soluble β3 antibody were able to spread and form CLANs. The percentage of CPCs on the β1 antibody substrate significantly increased (from 11% to 23%; P < 0.0001) when soluble β3 integrin antibody was added (Fig. 6). CLAN formation on the α2β1 antibody also increased from negligible to 7%. This increase in CLAN formation, however, may be due to the β3 antibody alone, since the percentage of CPCs in the presence of β3 and α2β1 antibodies is not significantly different from that observed with just the β3 antibody. Thus, although β1 integrin signaling by itself is sufficient to induce CLAN formation, combined β1 and β3 integrin signaling enhances CLAN formation in spreading HTM cells.
We then determined whether activation of β3 integrins could induce CLAN formation in HTM cells spread on specific β1 integrin binding ECM ligands. In addition to fibronectin, three integrin binding domains of fibronectin were examined (Fig. 7A). These domains include the III7–10 domain, which contains the RGD sequence in fibronectin54 and is the major β1 integrin-binding site in fibronectin.55,56 The III12–14 domain, which contains an α4β1 integrin-binding site54 that functions independently of its syndecan binding activity in HTM cells,55 and the IIICS domain which binds α4β1 integrins.57 VCAM-1, which is a strong activator of α4β1 integrin58 was also used.
As shown in Figure 7B, none of the three fibronectin domains induced CLAN formation as well as intact fibronectin. Of the three domains tested, the III7–10 domain performed best, with 6% of the cells forming CLANs. In contrast, none of the cells plated on the III12–14 or IIICS domains spread or formed CLANs (Figs. 7B, 8). The absence of any cell spreading on the III12–14 and IIICS domains suggests that the α4β1 integrin is incapable of promoting spreading in HTM cells. However, cell spreading and CLAN formation, identical with that observed on fibronectin (13%), were observed when cells were plated on VCAM-1 (Fig. 8). Thus, α4β1 integrins were as potent as other β1 integrins in inducing CLAN formation (Fig. 7B).
Figure 8.
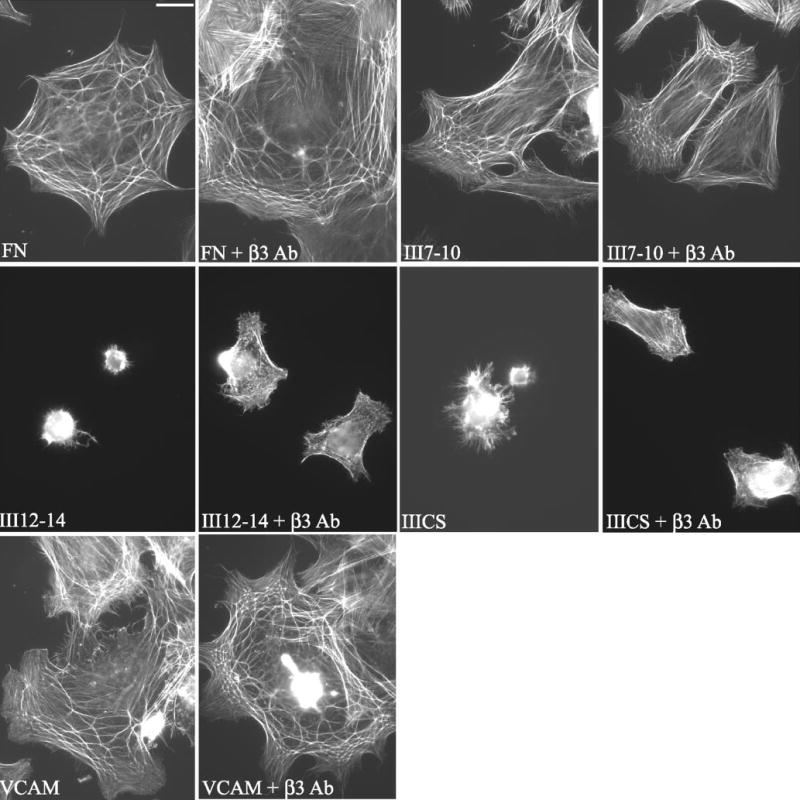
CLAN formation is enhanced by activation of β3 integrins in HTM cells plated on fibronectin, VCAM, or fibronectin domains. Cells were spread on fibronectin (FN), VCAM-1, or the III7–10, III12–14, or IIICS domains of fibronectin in the absence or presence of activating β3 antibodies and then labeled with phalloidin. Fibronectin fragments are defined in the Figure 7 legend. Bar, 50 μm.
Activation of β3 integrins enhanced CLAN formation in cells, regardless of which integrin ligand was used as the substrate. As shown in Figure 7B, CLAN formation increased threefold in cells spread on either fibronectin (43%) or VCAM-1 (44%) if the β3 integrin antibody was present. Similarly, there was a fourfold increase in CPCs when cells were spread on the III7–10 fibronectin domain (to 25%) in the presence of solubleβ3 antibody. A slight increase in cell spreading and CLAN formation (3%– 4%) was also observed on the III12–14 and IIICS domains when the soluble β3 antibody was present (Fig. 8). For each substrate, CLAN formation was significantly greater in the presence of soluble β3 antibody than in its absence (P < 0.0001).
Activation of β3 integrins also enhanced CLAN formation on type IV or type I collagen (Figs. 9, 10), almost to levels observed on fibronectin. In the absence of β3 integrin activation, both collagens were weak inducers of CLAN formation compared with fibronectin (Fig. 4). In the presence of soluble β3 antibody, however, CLAN formation significantly increased sixfold from 6% to 37% (P < 0.0001) on type IV collagen and 23.5-fold from 1% to 23.5% (P < 0.0001) on type I collagen. This is significant, given that collagens are a major part of the ECM in the trabecular meshwork.59
Figure 10.
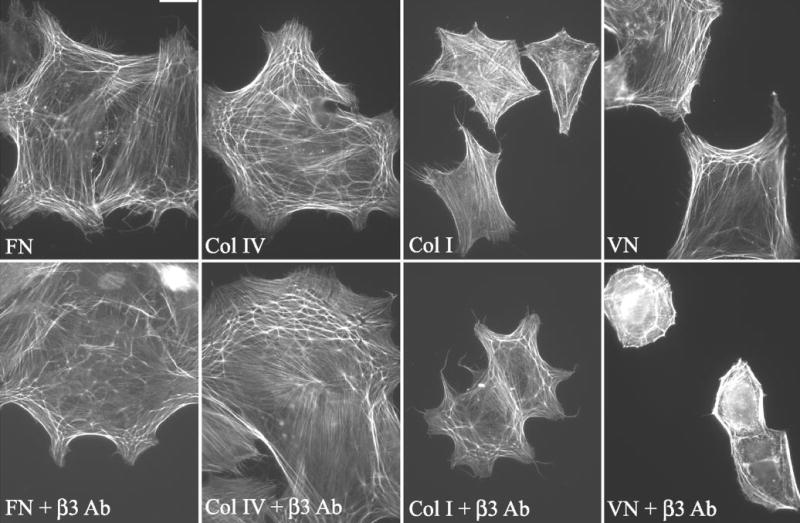
CLAN formation in HTM cells spread on type I or IV collagen or vitronectin in the presence or absence of soluble β3-integrin–activating antibodies. Cells were spread on either fibronectin (FN), type IV collagen (Col IV), type I collagen (Col I), or vitronectin (VN) and then labeled with phalloidin. Bar, 50 μm.
In contrast to the effect observed in cells plated on collagen, there was only a twofold increase in CPCs when they were plated on vitronectin in the presence of soluble β3 antibody compared with vitronectin alone (Fig. 9). Nevertheless, this represented a statistically significant (P < 0.0023) increase in CLAN formation, despite the fact that overall cell spreading was impaired when cells were plated on vitronectin in the presence of the soluble β3 antibody AP-5 rather than vitronectin alone (Fig. 10). This reduction in cell spreading probably reflects competition between vitronectin and the β3 antibody for binding to the β3 integrin.
Effect of DEX on CLAN Formation in Spread Cells
Because DEX treatment has been reported to induce CLANs,8,9,14,15 we next examined whether DEX treatment had any effect on CLAN formation in cells plated on fibronectin-coated coverslips. Pretreatment with 500 nM DEX before use in the spreading assay resulted in only minor differences, at best, in the number of CPCs. After 3 days of DEX treatment, only a 1.4-fold increase in CPCs was observed (26% ± 7%) compared with untreated cells (19% ± 4.5%). Longer pretreatments with DEX for 10 days resulted in even smaller increases in CLAN formation. In this instance, 12% ± 3% of the cells treated with DEX formed CLANs compared with 10.5% ± 6.5% of the untreated cells.
Likewise, DEX pretreatment did not have any effect on CLAN formation in cells plated on fibronectin-coated coverslips in the presence of the β3 activating mAb AP-5. In the absence of DEX, 45% ± 8% of cells formed CLANs if the β3 integrin was activated. If cells were treated with 500 nM DEX for 3 days before the spreading assay and then plated onto fibronectin-coated coverslips in the presence of mAb AP-5, 43% ± 10% of the cells formed CLANs. Similar results were observed in cells plated and spread onto VCAM-coated coverslips in the absence or presence of mAb AP-5 (not shown). Thus, CLAN formation appeared to be largely dependent on integrin-induced signaling events rather than a separate DEX-induced mechanism.
β1 and β3 Integrins Absent from Vertisomes
Because CLAN formation could be induced by β1 and β3 integrin activation, we examined whether these two integrins colocalized within vertisomes or any other part of the CLAN structure. As shown in Figure 11, neither β1 nor β3 integrins were found within vertisomes or along the actin filaments in the CLANs. β1 Integrins were found in focal adhesions in the presence (Figs. 11A, 11B) or absence (data not shown) of the β3 integrin-activating mAb AP-5. In contrast, β3 integrins were found only in focal adhesions in the presence of activating mAb AP-5 (Figs. 11C, 11D) suggesting that activation of β3 integrins was responsible for driving the β3 integrin into focal adhesions. In some instances, actin filaments in CLANs appeared to terminate in β1- or β3-positive focal adhesions19,21,23 (Figs. 11A, 11C, respectively) suggesting that CLANS could be connected to focal adhesions, although they generally do not appear to originate from these structures.
Figure 11.
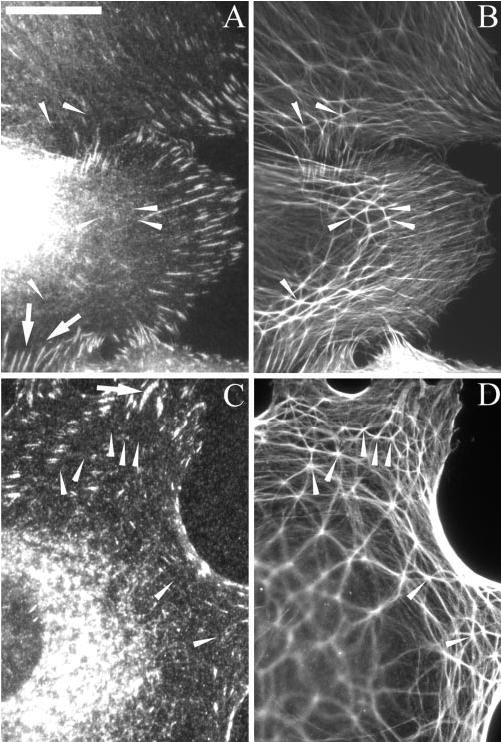
Localization of F-actin and β1 and β3 integrins in CPCs spread on fibronectin. Cells spread in the presence of the soluble β3 integrin mAb AP-5 were fixed with methanol and labeled with polyclonal anti-F-actin (B, D) and either mAb B3B11 against β1 (A) or mAb AP-5 against β3 (C) integrin. For cells not incubated with AP-5, mAb clone 1 was used to localize β3 integrin in fixed cells (not shown). Arrows: β1 (A) or β3 (C) integrin-positive focal adhesions. Arrowheads: CLAN vertices where vertisomes were found (see Figs. 1, 3). Note that β1 or β3 integrins were absent from the CLAN structures. Arrows: focal adhesions into which actin filaments from CLANs are inserted. Bar, 50 μm.
DISCUSSION
In this study, CLANs appeared to be molecularly distinct actin-branched networks that had a unique localization and were regulated by an Arp2/3-independent pathway. CLANs, unlike other actin-branched structures, did not contain a signaling complex composed of Arp3 or cortactin at their branch points, but instead contained syndecan-4, α-actinin, filamin, and PIP2. The absence of Arp3 and cortactin from vertisomes not only suggests that CLANs are structurally different from other actin-branched networks but that the regulation of their assembly is via an Arp2/3-independent pathway. CLANs also differ from other branched actin networks, in that they tend to be near the apical plasma membrane within the spreading lamellipodia rather than at the leading edge of spreading or motile cells.18,20–22
CLANs formed in spread HTM cells appeared to be structurally similar to and to contain the same molecular components as CLANs formed in DEX-treated confluent HTM monolayers (Filla MS, Kaufman PL, Peters DM, unpublished results, 2004). Specifically, vertisomes in both spreading cells and in DEX-treated monolayers contained α-actinin, syndecan-4, and PIP2. In addition, filamin localized along the entire length of the actin filaments within CLANs in both cases. Given that the cell-spreading assay described in this report did not appear to alter the molecular composition of CLANs, it is likely that the mechanisms involved in forming CLANs are similar, whether they are present in DEX-treated monolayers or in spreading cells.
The localization of a multimolecular complex of syndecan-4, α-actinin, filamin, and PIP2 in vertisomes suggests that this complex functions either to stabilize the CLAN structure or to regulate CLAN formation at the apical cell membrane.21,22 Both filamin and α-actinin cross-link actin filaments28 and thus could have structural roles within CLANs. α-Actinin also interacts with syndecan-4,60 and because syndecan-4 is a transmembrane protein, this interaction could function as a way to attach CLANs to the cell membrane. In addition, both syndecan-4 and PIP2 have been shown to activate signaling pathways that regulate the organization of actin filaments. In particular, they appear to activate members of the Rho family, which organize the formation of actin stress fibers in focal adhesions. Members of the syndecan family can also interact with CASK and ezrin.61–63 CASK–protein 4.1 complexes promote assembly of actin filaments in brain extracts,64 and it has been proposed that a syndecan-4, CASK, and protein 4.1 complex directs the arrangement of F-actin in nonneuronal cells.65 Thus, both syndecan-4 and PIP2, could play a role in regulating the organization of CLANs. Any regulatory functions of this complex would not necessarily preclude vertisomes from also participating in a structural role.
Despite earlier suggestions that CLANs may be precursors to stress fiber formation,19 they appear to be structurally distinct from mature stress fibers. Stress fibers originate from focal adhesions on the basal cell surface,18,20–22 whereas very few of the actin filaments in CLANs terminate at focal adhesions. In addition, CLANs are localized on the apical cell surface. Finally, vertisomes appear to be distinct from focal adhesions, even though they both contain α-actinin, syndecan-4, and PIP2. Focal adhesions contained β1 and β3 integrins, whereas vertisomes did not.
To the best of our knowledge, this is the first report of any syndecan being associated with actin branch points, and it represents a novel role for syndecan-4. Syndecan-4 is best known as a focal adhesion protein, where it associates with protein kinase Cα and PIP245,47,66,67 to activate the Rho GTPase signaling pathway and trigger the formation of actin stress fibers.46 In fibroblasts, the major ligand responsible for binding and activating the syndecan-4 mediated signaling pathways is the III12–14 (Hep II) domain of fibronectin.30 Whether interactions between the Hep II domain and syndecan-4 are also involved in CLAN formation is still unclear. In this study, the HepII domain, as an adsorbed substrate, failed to promote CLAN formation suggesting that either another syndecan-4 ligand may be involved or interactions with the extracellular domain of syndecan-4 is not necessary for CLAN formation. Clearly, additional studies are needed to evaluate the signaling role of syndecan-4 and its ligands in CLAN formation.
Even though integrins are not part of CLANs, they appear to play an integral role in regulating CLAN formation. β1 Integrins appear to be responsible for most CLANs, since HTM cells plated on β1 ligands, such as fibronectin or VCAM, showed the highest percentage of CPCs compared with cells plated on the β3 ligand, vitronectin. In addition, only cells plated on adsorbed β1 integrin antibodies formed CLANs, whereas cells plated on β3 integrin antibody failed to bind let alone assemble a CLAN. Given that integrins regulate CLAN formation, but are absent from them, it is likely that CLAN formation is regulated by an integrin-mediated inside-out signaling pathway.68 This is consistent with the observation that the Hep II domain of fibronectin, which is an extracellular ligand for syndecan-4, failed to induce CLAN formation.
The degree to which CLAN formation can be activated may depend on the α subunit of the β1 integrin heterodimer. This possibility was suggested by the differences in the percentage of CPCs that resulted when HTM cells are plated on different β1 integrin substrates. CLAN formation on type I or IV collagen was weak at best, and direct activation of the α2β1 collagen receptor induced little, if any, CLAN formation. In contrast, CLAN formation on fibronectin or VCAM-1, which use α5β1 and α4β1 integrins, respectively,55,58 was approximately three times higher than that on the collagens. The specific regulation of an integrin function by the integrin α-subunit is consistent with the role of the α subunit in regulating cell migration, invasion, proliferation, differentiation, adhesion, and intracellular calcium levels.69–71 Clearly, the specificity of the α-subunit is not the whole story. The III12–14 and IIICS domains of fibronectin failed to promote CLAN formation as well as VCAM-1, despite the fact that all these ligands bind and activate α4β1 integrin. Because VCAM-1 has a higher affinity for α4β1 integrin relative to the other two fibronectin domains,72 it is likely that the affinity and/or activation state of the integrin is also essential in regulating CLAN formation.
The mechanism through which β1 and β3 integrin cooperative signaling could regulate CLAN formation can only be speculated on at this point. One possibility is that β1 activation, in addition to inducing a basal level of CLAN formation, regulates the activation state of the β3 integrin.73 Thus, the addition of the β3 antibody alone does not lead to significant CLAN formation in the absence of β1 activation, either by a specific antibody or a β1-binding peptide or protein. Another possibility is that there is a differential activation of Rac and Rho by β1 and β3 integrins, respectively, which leads to the enhanced formation of CLANs when both signaling pathways are activated. Differential regulation of Rho family GTPases by β1 and β3 integrins has been reported in other cell types.74,75
Activation of integrin-specific pathways that modulate the actin architecture and contractility are likely to occur after steroid treatments and during aging, and such changes could have a profound effect on IOP. Glucocorticoids have been shown to increase or alter fibronectin deposition in the matrix of cultured TM cells44,76 and cultured anterior segments.12 Increased fibronectin levels have also been reported in the meshworks of glaucomatous eyes,77–79 although clearly not all glaucomatous eyes show an increase in the level of fibronectin compared with normal eyes.80 In addition, the expression of integrins and/or their ligands can be regulated by steroid treatments as well as by age-related changes in the ECM.7–9,12,15,35,44,76,81 Thus, it is possible that increased fibronectin production and/or integrin expression by glaucomatous or steroid-treated TM cells contribute to glaucoma by promoting the formation of aberrant actin structures such as CLANs. The fact that steroid treatment did not dramatically increase the number of CPCs observed when cells were plated and spread on a fibronectin or VCAM substrate in the absence or presence of an activating β3 antibody further supports the idea that the effects of steroids on CLAN formation are mediated by signaling pathways regulated by integrins and/or their ligands.
It remains unclear at present, however, whether CLANS would be involved in the regulation of aqueous outflow in general and in the pathophysiology of steroid-induced glaucoma or POAG in particular. One may speculate that CLANs represent, or could lead to, a “stiffening” of the actin cytoskeleton and, consequently, increased rigidity of overall meshwork architecture so as to increase outflow resistance (i.e., the converse of the proven ability of contractility-inhibiting small molecules)2,82–84 and overexpressed proteins85–88 to decrease resistance. It remains to be shown, however, that there is a direct correlation between CLAN formation and alterations in outflow facility.
In conclusion, the data suggest that specific integrin-signaling pathways involving β1 and β3 integrins regulate CLAN formation. Understanding how specific integrin pathways regulate CLAN formation may be useful in understanding how integrin signaling regulates the activity of the cytoskeleton and hence the cytoarchitecture of the TM.
Footnotes
Disclosure: M.S. Filla, None; A. Woods, None; P.L. Kaufman, None; D.M. Peters, None
Supported by National Eye Institute Grants EY06665-01, EY12515 (DMP), and EY02698 (PLK), NIH grant GM50194 (AW), Glaucoma Research Foundation, and Research to Prevent Blindness.
References
- 1.Hilpela PV, Vartiaininen MK, Lappalainen P. Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr Topics Microbiol Immunol. 2004;282:117–163. doi: 10.1007/978-3-642-18805-3_5. [DOI] [PubMed] [Google Scholar]
- 2.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- 3.Thieme H, Nuskovski M, Nass JU, et al. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and Rho-A. Invest Ophthalmol Vis Sci. 2000;41:4240–4246. [PubMed] [Google Scholar]
- 4.Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- 5.Epstein DL, Rowlette L, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- 6.Gills JP, Roberts BC, Epstein DL. Microtubule disruption leads to cellular contraction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:653–658. [PubMed] [Google Scholar]
- 7.Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–667. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 8.Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993;12:783–793. doi: 10.3109/02713689309020383. [DOI] [PubMed] [Google Scholar]
- 9.Clark AF, Wilson K, McCartney MD, et al. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- 10.Knepper PA, Collins JA, Frederick R. Effects of dexamethasone, progesterone, and testosterone on IOP and GAGs in the rabbit eye. Invest Ophthalmol Vis Sci. 1985;26:1093–1100. [PubMed] [Google Scholar]
- 11.Kass M, Cheetham J, Duzman E, Burke PJ. The ocular hypertensive effect of 0.25% fluorometholone in corticosteroid responders. Am J Ophthalmol. 1986;102:159–163. doi: 10.1016/0002-9394(86)90137-6. [DOI] [PubMed] [Google Scholar]
- 12.Clark AF, Wilson K, de Kater AW, Allingham RR, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest Ophthalmol Vis Sci. 1995;36:478–489. [PubMed] [Google Scholar]
- 13.Gelatt KN, Mackay EO. The ocular hypertensive effects of topical 0.1% dexamethasone in beagles with inherited glaucoma. J Ocular Pharmacol Ther. 1998;14:57–66. doi: 10.1089/jop.1998.14.57. [DOI] [PubMed] [Google Scholar]
- 14.Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskel. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 15.Clark AF, Lane D, Wilson K, Miggans ST, McCartney MD. Inhibition of dexamethasone-induced cytoskeletal changes in cultured human trabecular meshwork cells by tetrahydrocortisol. Invest Ophthalmol Vis Sci. 1996;37:805–813. [PubMed] [Google Scholar]
- 16.Clark AF, Miggans ST, Wilson K, Browder S, McCarthy MD. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–188. [PubMed] [Google Scholar]
- 17.Liu X, Wu Z, Sheibani N, et al. Low dose latrunculin-A inhibits dexamethasone-induced changes in the actin cytoskeleton and alters extracellular matrix protein expression in cultured human trabecular meshwork cells. Exp Eye Res. 2003;77:181–188. doi: 10.1016/s0014-4835(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 18.Gordon WE, 3rd, Bushnell A. Immunofluorescent and ultrastructural studies of polygonal microfilament networks in respreading non-muscle cells. Exp Cell Res. 1979;120:335–348. doi: 10.1016/0014-4827(79)90393-8. [DOI] [PubMed] [Google Scholar]
- 19.Lazarides E. Actin, α-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976;68:202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanger JM, Mittal B, Pochapin M, Sanger JW. Observations of microfilament bundles in living cells microinjected with fluorescently labelled contractile proteins. J Cell Sci Suppl. 1986;5:17–44. doi: 10.1242/jcs.1986.supplement_5.2. [DOI] [PubMed] [Google Scholar]
- 21.Ireland GW, Sanders EJ, Voon FC, Wakely J. The ultrastructure of polygonal networks in chick embryonic cells in vitro. Cell Biol Int. 1983;7:679–688. doi: 10.1016/0309-1651(83)90196-0. [DOI] [PubMed] [Google Scholar]
- 22.Mochizuki Y, Furukawa K, Mitaka T, Yokoi T, Kodama T. Polygonal networks, “geodomes”, of adult rat hepatocytes in primary culture. Cell Biol Int. 1988;12:1–7. doi: 10.1016/0309-1651(88)90105-1. [DOI] [PubMed] [Google Scholar]
- 23.Ireland GW, Voon FC. Polygonal networks in living chick embryonic cells. J Cell Sci. 1981;52:55–69. doi: 10.1242/jcs.52.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Lin ZX, Holtzer S, Schultheiss T, et al. Polygons and adhesion plaques and the disassembly and assembly of myofibrils in cardiac myocytes. J Cell Biol. 1989;108:2355–2367. doi: 10.1083/jcb.108.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 26.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 27.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 28.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–2333. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couchman JR, Woods A. Syndecan-4 and integrins: combinatorial signaling in cell adhesion. J Cell Sci. 1999;112:3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]
- 30.Couchman JR, Chen L, Woods A. Syndecans and cell adhesion. Int Rev Cytol. 2001;207:113–150. doi: 10.1016/s0074-7696(01)07004-8. [DOI] [PubMed] [Google Scholar]
- 31.Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filla MS, David G, Weinreb RN, Kaufman PL, Peters DP. Distribution of syndecans 1–4 within the anterior segment of the human eye: expression of a variant syndecan-3 and matrix associated syndecan-2. Exp Eye Res. 2004;79:61–74. doi: 10.1016/j.exer.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–1049. [PubMed] [Google Scholar]
- 34.Polansky JR, Weinreb R, Alvarado JA. Studies on human trabecular cells propagated in vitro. Vis Res. 1981;21:155–160. doi: 10.1016/0042-6989(81)90151-6. [DOI] [PubMed] [Google Scholar]
- 35.Filla MS, Lui X, Nguyen TD, et al. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002;43:151–161. [PubMed] [Google Scholar]
- 36.Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis. 2003;9:329–336. [PubMed] [Google Scholar]
- 37.Mosesson MW, Umfleet RA. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Cell Sci. 1970;245:5728–5736. [PubMed] [Google Scholar]
- 38.Steele JG, Johnson G, Underwood PA. Role of serum vitronectin and fibronectin in adhesion of fibroblasts following seeding onto tissue culture polystyrene. J Biomed Mater Res. 1992;26:861–884. doi: 10.1002/jbm.820260704. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki S, Oldberg A, Hayman EG, Pierschbacher MD, Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA: similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985;4:2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aukhil I, Joshi P, Yan Y, Erickson HP. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Cell Sci. 1993;268:2542–2553. [PubMed] [Google Scholar]
- 41.Santas AJ, Peterson JA, Halbleib JL, et al. Alternative splicing of the IIICS domain in fibronectin governs the role of the heparin II domain in fibrillogenesis and cell spreading. J Biol Chem. 2002;277:13650–13658. doi: 10.1074/jbc.M111361200. [DOI] [PubMed] [Google Scholar]
- 42.Longley RL, Woods A, Fleetwood A, et al. Control of morphology, cytoskeleton and migration by syndecan-4. J Cell Sci. 1999;112:3421–3431. doi: 10.1242/jcs.112.20.3421. [DOI] [PubMed] [Google Scholar]
- 43.Yun AJ, Murphy CG, Polansky JR, Newsome DA, Alvarado JA. Proteins secreted by human trabecular cells: glucocorticoid and other effects. Invest Ophthalmol Vis Sci. 1989;30:2012–2022. [PubMed] [Google Scholar]
- 44.Zhou LL, Li YH, Yue B. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med. 1998;1:339–346. [PubMed] [Google Scholar]
- 45.Oh ES, Woods A, Lim ST, Theibert AW, Couchman JR. Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4,5-bisphosphate coordinately regulate protein kinase C activity. J Biol Chem. 1998;273:10624–10629. doi: 10.1074/jbc.273.17.10624. [DOI] [PubMed] [Google Scholar]
- 46.Saoncella S, Echtermeyer F, Denhez F, et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Nat Acad Sci USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods A, Couchman JR. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 1994;5:183–192. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver AM, Young ME, Lee W-L, Cooper JA. Integration of signals to the Arp2/3 complex. Curr Opin Cell Biol. 2003;15:23–30. doi: 10.1016/s0955-0674(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 49.Wilkins JA, Li A, Ni H, Stunpack DG, Shen C. Control of β1 integrin function: localization of stimulatory epitopes. J Biol Chem. 1996;271:3046–3051. [PubMed] [Google Scholar]
- 50.Coppolino M, Leung-Hagesteijn C, Dedhar S, Wilkins J. Inducible interaction of integrin α2β1 with calreticulin: dependence on the activation state of the integrin. J Biol Chem. 1995;270:23132–23138. doi: 10.1074/jbc.270.39.23132. [DOI] [PubMed] [Google Scholar]
- 51.Schreider C, Peignon G, Thenet S, Chambaz J, Pincon-Raymond M. Integrin-mediated functional polarization of Caco-2 cells through E-cadherin–actin complexes. J Cell Sci. 2002;115:543–552. doi: 10.1242/jcs.115.3.543. [DOI] [PubMed] [Google Scholar]
- 52.Ho WC, Heinemann C, Hangan D, et al. Modulation of in vivo migratory function of α2β1 integrin in mouse liver. Mol Biol Cell. 1997;8:1863–1875. doi: 10.1091/mbc.8.10.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelletier AJ, Kunicki T, Ruggeri ZM, Quaranta V. The activation state of the integrin αIIbβ3 affects outside-in signals leading to cell spreading and focal adhesion kinase phosphorylation. J Biol Chem. 1995;270:18133–18140. doi: 10.1074/jbc.270.30.18133. [DOI] [PubMed] [Google Scholar]
- 54.Mould AP, Humphries MJ. Identification of a novel recognition sequence for the integrin alpha 4 beta 1 in the COOH-terminal heparin-binding domain of fibronectin. EMBO J. 1991;10:4089–4095. doi: 10.1002/j.1460-2075.1991.tb04985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson JA, Sheibani N, David G, Garcia-Pardo A, Peters DM. Heparin II domain of fibronectin uses α4β1 integrin to control focal adhesion and stress fiber formation, independent of syndecan-4. J Biol Chem. 2005;280:6915–6922. doi: 10.1074/jbc.M406625200. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L, Zhang S, Yue BY. Adhesion of human trabecular meshwork cells to extracellular matrix proteins: Roles and distribution of integrin receptors. Invest Ophthalmol Vis Sci. 1996;37:104–113. [PubMed] [Google Scholar]
- 57.Mould AP, Komoriya A, Yamada KM, Humphries MJ. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin α4β1: inhibition of α4β1 function by RGD peptide homologues. J Biol Chem. 1991;266:3579–3585. [PubMed] [Google Scholar]
- 58.Elices MJ, Osborn L, Takada Y, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 59.Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Sur Ophthalmol. 1996;40:379–390. doi: 10.1016/s0039-6257(96)80066-x. [DOI] [PubMed] [Google Scholar]
- 60.Greene DK, Tumova S, Couchman JR, Woods A. Syndecan-4 associates with α-actinin. J Biol Chem. 2003;278:7617–7623. doi: 10.1074/jbc.M207123200. [DOI] [PubMed] [Google Scholar]
- 61.Cohen AR, Woods DF, Marfatia SM, et al. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grootjans JJ, Reekmans G, Ceulemans H, David G. Syntenin-syndecan binding requires syndecan-synteny and the co-operation of both PDZ domains of syntenin. J Biol Chem. 2000;275:19933–19941. doi: 10.1074/jbc.M002459200. [DOI] [PubMed] [Google Scholar]
- 63.Granes F, Urena JM, Rocamora N, Vilaro S. Ezrin links syndecan-2 to the cytoskeleton. J Cell Sci. 2000;113:1267–1276. doi: 10.1242/jcs.113.7.1267. [DOI] [PubMed] [Google Scholar]
- 64.Biederer T, Sudhof TC. CASK and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- 65.Bass MD, Humphries MJ. Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem J. 2002;368:1–15. doi: 10.1042/BJ20021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oh ES, Woods A, Couchman JR. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C. J Biol Chem. 1997;272:11805–11811. doi: 10.1074/jbc.272.18.11805. [DOI] [PubMed] [Google Scholar]
- 67.Oh ES, Woods A, Couchman JR. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J Biol Chem. 1997;272:8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- 68.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 69.Keely PJ, Rusyn EV, Cox AD, Parise LV. R-Ras signals through specific integrin alpha cytoplasmic domains to promote migration and invasion of breast epithelial cells. J Cell Biol. 1999;145:1077–1088. doi: 10.1083/jcb.145.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwartz MA, Denninghoff K. Alpha v integrins mediate the rise in intracellular calcium in endothelial cells on fibronectin even though they play a minor role in adhesion. J Biol Chem. 1994;269:11133–11137. [PubMed] [Google Scholar]
- 72.Mould AP, Askari JA, Craig SE, et al. Integrin α4β1-mediated melanoma cell adhesion and migration on vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced IIICS region of fibronectin. J Biol Chem. 1994;269:27224–27230. [PubMed] [Google Scholar]
- 73.Ly DP, Zazzali KM, Corbett SA. De novo expression of the integrin α5β1 regulates αvβ3-mediated adhesion and migration on fibrinogen. J Biol Chem. 2003;278:21878–21885. doi: 10.1074/jbc.M212538200. [DOI] [PubMed] [Google Scholar]
- 74.Miao H, Li S, Hu YL, et al. Differential regulation of Rho GTPases by β1 and β3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J Cell Sci. 2002;115:2199–2206. doi: 10.1242/jcs.115.10.2199. [DOI] [PubMed] [Google Scholar]
- 75.Danen EHJ, van Rheenen J, Franken W, et al. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–528. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steely HT, Browder SL, Julian MB, et al. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- 77.Rodrigues MM, Katz SI, Foidart JM, Spaeth GL. Collagen, factor VIII antigen, and immunoglobulins in the human aqueous drainage channels. Ophthalmology. 1980;87:337–345. doi: 10.1016/s0161-6420(80)35242-1. [DOI] [PubMed] [Google Scholar]
- 78.Babizhayev MA, Brodskaya MW. Immunohistochemical monitoring of the effect of a synthetic fibronectin-like peptide (Arg-Gly-Asp) on the age-related changes in the isolated human corneoscleral tissue of glaucomatous eyes. Mech Ageing Dev. 1993;72:1–12. doi: 10.1016/0047-6374(93)90126-c. [DOI] [PubMed] [Google Scholar]
- 79.Babizhayev MA, Brodskaya MW. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mech Ageing Dev. 1989;47:145–157. doi: 10.1016/0047-6374(89)90017-1. [DOI] [PubMed] [Google Scholar]
- 80.Hann CR, Springett MJ, Wang X, Johnson DH. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic Res. 2001;33:314–324. doi: 10.1159/000055687. [DOI] [PubMed] [Google Scholar]
- 81.Dickerson JE, Jr, Steely HT, Jr, English-Wright SL, Clark AF. The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp Eye Res. 1998;66:731–738. doi: 10.1006/exer.1997.0470. [DOI] [PubMed] [Google Scholar]
- 82.Tian B, Brumback LC, Kaufman PL. ML-7, chelerythrine and phorbol ester increase outflow facility in the monkey eye. Exp Eye Res. 2000;71:551–566. doi: 10.1006/exer.2000.0919. [DOI] [PubMed] [Google Scholar]
- 83.Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol. 1998;116:633–643. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- 84.Honjo M, Tanihara H, Inatani M, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- 85.Vittitow JL, Garg R, Rowlette LL, et al. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol Vis. 2002;8:32–44. [PubMed] [Google Scholar]
- 86.Rao PV, Deng P, Maddala R, et al. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005;11:288–297. [PubMed] [Google Scholar]
- 87.Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- 88.Gabelt BT, Hu Y, Vittitow JL, et al. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res. Published online, January 25, 2006. [DOI] [PubMed]


