Abstract
The mdx mouse, a mouse model of Duchenne muscular dystrophy, carries a loss-of-function mutation in dystrophin, a component of the membrane-associated dystrophin–glycoprotein complex. Unlike humans, mdx mice rarely display cardiac abnormalities and exhibit dystrophic changes only in a small number of heavily used skeletal muscle groups. By contrast, mdx:MyoD−/− mice lacking dystrophin and the skeletal muscle-specific bHLH transcription factor MyoD display a severe skeletal myopathy leading to widespread dystrophic changes in skeletal muscle and premature death around 1 year of age. The severely increased phenotype of mdx:MyoD−/− muscle is a consequence of impaired muscle regeneration caused by enhanced satellite cell self-renewal. Here we report that mdx:MyoD−/− mice developed a severe cardiac myopathy with areas of necrosis associated with hypertrophied myocytes. Moreover, heart tissue from mdx:MyoD−/− mice exhibited constitutive activation of stress-activated signaling components, similar to in vitro models of cardiac myocyte adaptation. Taken together, these results support the hypothesis that the progression of skeletal muscle damage is a significant contributing factor leading to development of cardiomyopathy.
Duchenne muscular dystrophy (DMD) is a common genetic disease resulting from mutations in the dystrophin protein, which lead to severe skeletal muscle wasting followed by premature death in early adulthood (reviewed in ref. 1). During the progression of DMD, the incidence of cardiomyopathy becomes increasingly prevalent such that >90% of patients eventually manifest significant cardiac defects (2–5). Subsequently, attendant cardiomyopathy has been suggested as a primary cause of death in a significant proportion of DMD patients (6, 7). However, the development of DMD cardiomyopathy may not be limited to a myocardial cell-autonomous loss of dystrophin, but may also be influenced by extrinsic parameters associated with disease progression. For example, in DMD the widespread dystrophic damage of skeletal muscle concurrent with postural adaptations may result in an increased hemodynamic load. Importantly, bloodflow adaptations are an established risk factor for the development of cardiomyopathy, independent of additional genetic alterations in cardiac tissue (8–10).
Some forms of dystrophy, such as X-linked cardiomyopathy and a proportion of Becker’s muscular dystrophy (BMD) patients, present with little or no skeletal muscle damage yet develop profound cardiomyopathies (4, 5). Although this disease originates with myocardial-specific loss of dystrophin, extrinsic hemodynamic parameters may also impact this developing cardiac pathology. Specifically, dystrophin deficiency may predispose the cardiomyocytes to structural damage, which becomes evident after long-term exposure to mechanical stress. As such, the extended volume and pressure loads associated with prolonged mobility in these patients may lead to the cumulative changes that are observed in the dystrophic myocardium (7, 11). Analogous to this potential work-related cardiac injury, dystrophin-deficient skeletal muscle fibers have been demonstrated to be highly susceptible to activity-induced (exercise) or work-related damage (12). Therefore, an understanding of the etiology of dystrophy-associated cardiomyopathies in general may remain incomplete before defining the degree of the involvement of extrinsic factor(s) in this pathology.
To date, the absence of appropriate animal models has clearly limited the investigation of DMD cardiomyopathy. The mdx mouse, which is the murine equivalent of DMD, lacks dystrophin as the result of a point mutation (13, 14), but rarely displays cardiac abnormalities and exhibits dystrophic changes in only a small number of heavily used skeletal muscle groups, for example, the diaphragm (15–16). The attenuated myopathy in mdx mice has been attributed in part to an enhanced regenerative capacity of skeletal muscle for that murine strain. However, other factors such as small mass of the animal, quadrapedal gait, short life span, and differential expression of compensatory proteins such as utrophin may also be involved (reviewed in ref. 17). The absence of cardiac pathology in the mdx mouse has not been adequately explained. Indeed, the attenuated skeletal muscle phenotype in mdx mice may protect the heart against the extrinsic influences that result from widespread muscle damage. Alternatively, it is possible that a related protein may functionally substitute for dystrophin in the myocardium of mdx mice. However, mdx mice that also lacked the dystrophin homologue utrophin failed to develop a uniform or extensive cardiomyopathy (18, 19). These observations suggested that the development of cardiomyopathy in dystrophic murine models may require precipitating events in addition to the cardiac autonomous loss of dystrophin and its structural homologues. Indeed, the dramatically reduced lifespan of these mice (≈15 weeks) may limit exposure to extrinsic perturbations, which may otherwise accelerate the cardiomyopathy.
To distinguish between the effects of skeletal muscle damage vs. cardiac loss of dystrophin on the development of dystrophy-associated cardiomyopathy, we investigated cardiac postnatal growth and development in mice lacking both dystrophin and MyoD (designated mdx:MyoD−/− mice). Compound mutant mdx:MyoD−/− mice display a pronounced myopathic phenotype caused by a marked reduction in skeletal muscle regeneration because of impaired activity of satellite cells (17). As MyoD is not expressed in the heart, any myocardial changes evident in mdx:MyoD−/− mice would be directly attributable to the level of skeletal muscle damage. Here we report that mdx:MyoD−/− mice develop a progressive cardiomyopathy. Therefore, our results are consistent with the hypothesis that skeletal muscle damage is a crucial determinant in the progression of dystrophy-associated cardiomyopathy.
MATERIALS AND METHODS
Histological Analysis.
Hearts were removed and fixed in 10% formalin for 4–5 days, embedded in paraffin, sectioned at 10 μm and stained with haematoxylin-eosin or Masson trichrome (20). Preparation of tissue extracts: Hearts were removed from the mice, rapidly washed in three changes of cold PBS containing 100 mM NaF and 1.0 mM Na orthovanadate, sectioned into atrial and ventricular portions (atria discarded) and then flash-frozen in liquid N2. The frozen ventricle tissue was powdered and then lysed for 40 min on ice with a solution containing 50 mM Hepes (pH 7.5)/150 mM NaCl/1.0 mM EGTA/1.5 mM MgCl2/10 mM NaF/10 mM Na pyrophosphate/1.0 mM Na orthovanadate/10% glycerol/1% Triton X-100/10 μg/ml of the protease inhibitors phenylmethylsulfonyl fluoride, aprotinin, leupeptin, and pepstatin. Solubilized lysates were cleared by centrifugation, and protein content was determined.
Immunoprecipitation and Western Blot Analysis.
Immunoprecipitations (IPs) and Western blotting from the ventricle lysates were performed as previously described (21). Briefly, for IPs using the pan tyrosine phosphorylated antibody, PY20 (Transduction Laboratories, Lexington, KY), 250 μg of total protein was used with 5 μg PY20; for IPs using anti-p38 (Santa Cruz Biotechnology) or JNK-1 (PharMingen), 750 μg of total protein was used with 2 μg of anti-p38 and anti-JNK-1; all contained 25 μl protein G Sepharose beads (Pharmacia). Immunoprecipitation was carried out for 16 hr at 4°C. Precipitates were centrifuged and washed (three times) and were subjected to SDS/PAGE followed by transfer to Immobilon-P membranes (Millipore). Detection of proteins by Western blotting used the following dilutions for primary antibodies: p38, 1:500 (Santa Cruz); JNK-1 1:1000 (PharMingen); secondary 1:2000.
In Vitro Kinase Assays.
IPs with anti-p38 or anti-JNK-1 were performed as described above. Following this procedure, the precipitates were washed twice with kinase buffer containing 50 mM β-glycerophosphate/1 mM EGTA/1 mM Na vanadate/20 mM MgCl2/20 mM p-nitrophenylphosphate. Kinase reactions were then carried out in kinase buffer containing 5 μg of substrate (glutathione S-transferase-ATF2 for p38; glutathione S-transferase-cJun for JNK-1)/10 μCi [γ32P] ATP in a total volume of 30 μl. Reactions were allowed to proceed for 30 min at 30°C and then were terminated with the addition of 2× SDS loading buffer followed by SDS/PAGE. Gels were dried and exposed to film and/or subjected to phosphoimaging to quantitate the signal.
RESULTS AND DISCUSSION
Development of Cardiomyopathy in mdx:MyoD−/− Mice.
Mice lacking both MyoD and dystrophin display a marked increase in the severity of skeletal myopathy, leading to premature death around 12 months of age. MyoD-mutant muscle contains about a 2-fold increase in the number of satellite cells that undergo self-renewal rather than the normal proliferation and differentiation that occur during regeneration. Therefore, the inability of MyoD-deficient muscle to regenerate efficiently appears to result from increased myogenic stem cell self-renewal rather than progression through the myogenic program (17).
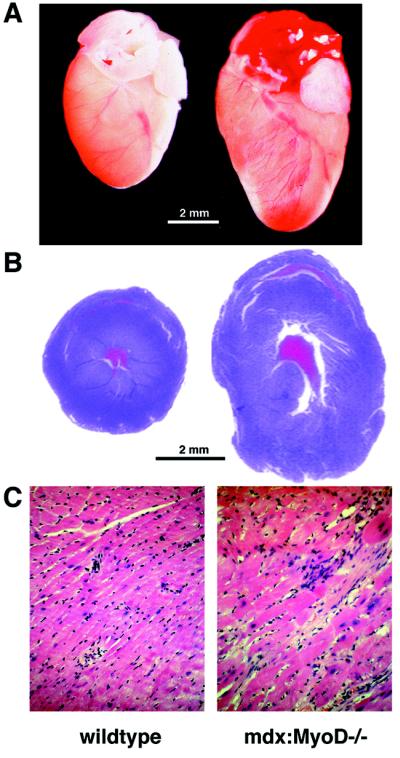
The mdx:MyoD−/− phenotype more closely resembles the myopathic changes evident in human DMD, and therefore may represent a more relevant model for investigating the attendant cardiac disease associated with dystrophy. To assess a potential cardiac phenotype, hearts of animals at various ages were weighed, fixed, serially sectioned, and stained with hematoxylin and eosin. Examination of 5-month-old mdx:MyoD−/− mice revealed examples of cardiac changes that resembled early-stage dilated cardiomyopathy. Histological analysis of sectioned hearts of severely affected mdx:MyoD−/− animals revealed an apparent increase in ventricular diameter without a change in the thickness of the ventricular wall (Fig. 1A). In addition, the ventricles of mdx:MyoD−/− hearts contained regions in which individual cardiac myocytes were substantially enlarged, a phenomenon not observed in wild-type (wt), mdx, or MyoD−/− hearts (Fig. 1B). Moreover, this cardiac myocyte hypertrophy was a more common feature in the left ventricle (four of five mice) than in the right ventricle (one of five mice) of mdx:MyoD−/− mice.
Figure 1.
Cardiac hypertrophy in mdx mice lacking MyoD. Hearts from 5-month-old mdx:MyoD−/− mice were compared with age-matched wt littermates. (A) Intact hearts of mdx:MyoD−/− mice appeared significantly larger than hearts from wt mice. (B) Hematoxylin-eosin stained sections of hearts (cut transversely at midventricle) revealed an increased ventricular diameter in mdx:MyoD−/− mice compared with wt. Although hearts were fixed ex vivo without diastole arrest, the apparent increase in ventricular diameter without concurrent changes in ventricular thickness suggests dilatation. We also observed marked cardiomyocyte hypertrophy and disarray in mdx:MyoD−/− hearts not present in wt mice (C). A and B were photographed at identical magnifications. (C, ×40.)
Both mdx and mdx:MyoD−/− hearts were significantly heavier than wt or MyoD−/− hearts (Table 1, P > 0.05). At 5 months of age, wt and MyoD−/− hearts weighed 156.8 mg (±9.7) and 153.8 mg (±4.7) respectively, whereas mdx hearts weighed 176.7 mg (±14.5) and mdx:MyoD−/− hearts weighed 183.4 mg (±7.3) (Table 1). Although the increased mass in mdx:MyoD mice relative to mdx mice was a reproducible trend, the sample size did not allow a determination of significance. However, the relative heart-weight to body-weight ratio of mdx:MyoD−/− mice was significantly increased compared with either parental strain or those of wt mice (Table 1, P < 0.01). The relative heart-weight ratio for wt, mdx, and MyoD−/− genotypes was comparable to published morphometric data for normal mice (22, 23). By contrast, the relative heart-weight ratio in mdx:MyoD−/− mice was similar to other models of murine cardiac myopathy/hypertrophy (23).
Table 1.
Relative heart mass in 5-month-old mice
| wt | MyoD−/− | mdx | mdx:MyoD−/− | |
|---|---|---|---|---|
| Absolute heart weight, mg | 156.8 ± 9.7 | 153.8 ± 4.7 | 176.7 ± 14.5 | 183.4 ± 7.3 |
| Relative heart weight, mg/g | 4.98 ± 0.41 | 4.92 ± 0.18 | 5.00 ± 0.26 | 6.57 ± 0.17* |
| Sample size | n = 5 | n = 6 | n = 5 | n = 7 |
Analysis of variance between mdx and mdx:MyoD−/− relative heart weights (Dunnett’s t-test). Error expressed as standard error of the mean.
, P < 0.01.
Taken together, the cardiac changes in mdx:MyoD−/− mice appear to resemble the cardiac adaptations associated with DMD cardiomyopathy (2–4). Importantly, MyoD is not expressed in the heart and plays no role in heart development. Moreover, the myopathic changes in the heart were not evident at times preceding the onset of dystrophic symptoms. Therefore, these results are consistent with the hypothesis that skeletal myopathy in DMD may contribute significantly to the development of cardiomyopathy.
Activation of SAPK and p38 Pathways in mdx:MyoD−/− Cardiomyopathy.
Recent in vitro studies have reported that activation of stress-activated protein kinases (SAPKs), specifically members of the cJun amino-terminal kinase family (JNKs) and the p38/HOG kinase family, occurs following myocyte hypertrophy (24–26). Activation of signal transduction pathways appears to provide the link between cardiac overload and subsequent changes in gene expression that mediate myocyte adaptation (27). Therefore, activation of JNK and p38 kinases constitutes an additional molecular criterion for defining cardiomyopathy.
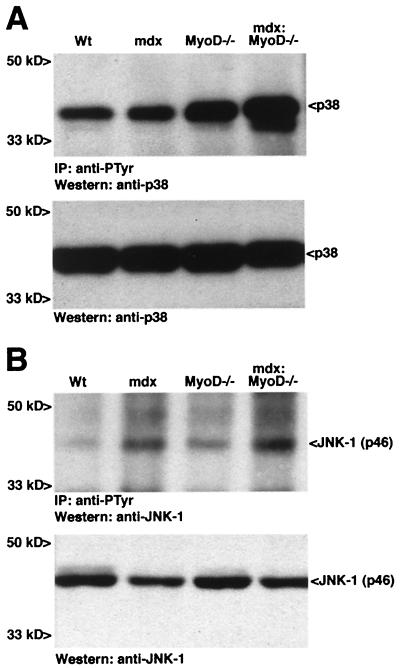
To investigate whether SAPK effectors were activated during the development of cardiomyopathy in mdx:MyoD−/− mice, we performed immunoblot analysis and in vitro kinase assays. To compare relative levels of tyrosine-phosphorylated activated kinase, protein lysates from ventricles were immunoprecipitated with an antiphosphotyrosine antibody and immunoblotted with antibodies reactive with JNK-1 and p38. Substantially increased levels of tyrosine phosphorylation on p38 were observed in mdx:MyoD−/− ventricles compared with ventricles extracted from 5-month-old mdx, MyoD−/− or wt mice (Fig. 2A). Phosphorylation of JNK-1 on tyrosine (p46 isoform) was increased similarly in lysates from mdx:MyoD−/− ventricles compared with those from wt ventricles (Fig. 2B). However, increased phosphorylation of JNK-1 on tyrosine (p46 isoform) was observed in ventricular lysates in both mdx and mdx:MyoD−/− mice (Fig. 2B). Thus, changes in JNK-1 activity did not appear to reflect solely the altered status of the myocardium. In addition, as detected by Western blot analysis, the levels of p38 and JNK-1 were similar in all samples, indicating that kinase expression was unaffected by the myopathy (see Fig. 2 A and B).
Figure 2.
Constitutive activation of SAPK effectors in ventricle protein lysates of 5-month-old wt, mdx, MyoD−/−, and mdx:MyoD−/− mice. Tyrosine phosphorylation of p38 (A) and JNK-1 (B) was assessed by immunoprecipitation with an antiphosphotyrosine antibody, followed by Western detection with anti-p38 or anti-JNK-1 (Upper). Lysates subjected to SDS/PAGE and Western blotting revealed equivalent amounts of p38 and JNK-1 within each genotype (Lower). Similar results were obtained in three independent experiments.
Activation of the mitogen-activated protein kinase signaling pathway was also examined. Neither Erk1 nor Erk2 showed any increase in levels of tyrosine phosphorylation in lysates from mdx:MyoD−/− hearts (data not shown). Therefore, we conclude that activation of the stress-activated protein kinase signaling pathways are closely associated with the initial development of the mdx:MyoD−/− cardiac phenotype.
Cardiomyopathy in mdx:MyoD−/− Mice Is Progressive and Is Associated with the Development of Fibrosis.
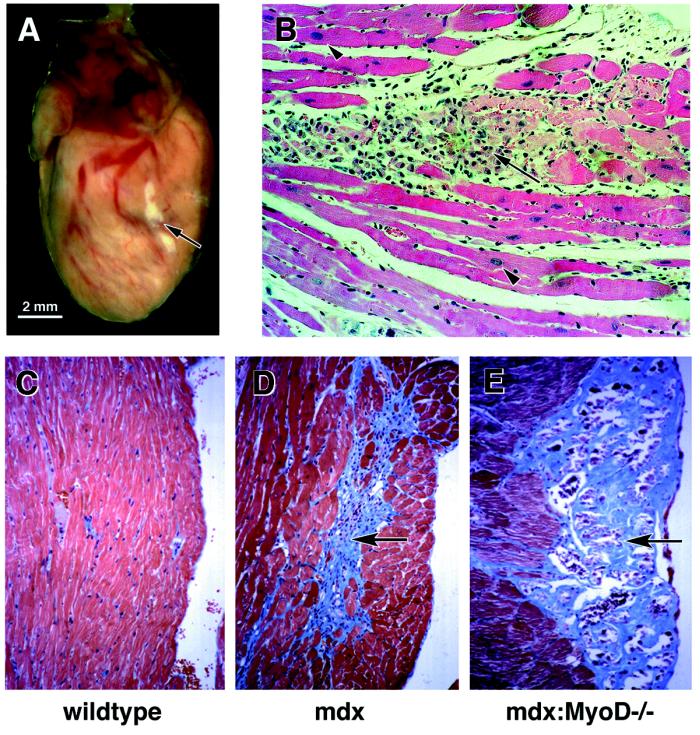
In DMD patients, myocardial changes are progressive, moving from a nonsymptomatic preclinical stage to sporadic cellular hypertrophy and eventual myopathy with widespread necrosis (2–5). If cardiac tissue from mdx:MyoD−/− mice recapitulates the course of the human disease, similar progressive changes should be evident. At 10 months of age (n = 13), >50% of mdx:MyoD−/− hearts examined displayed some visible evidence of fibrosis. However, in surviving 12-month-old mice (n = 15), >80% displayed extensive visible fibrotic regions (Fig. 3A). Therefore, we conclude that the development of cardiomyopathy in mdx:MyoD−/− mice was progressive in nature and thus appears analogous to the development of cardiomyopathy in DMD. In mdx:MyoD−/− mice, the visible cardiac fibrosis was found consistently in the left ventricle (13 of 13) but rarely in the right ventricle (3 of 13). Therefore, these observations combined with the preferred localization of cellular hypertrophy (see previous) suggest an accelerated left-heart involvement in the development of mdx:MyoD−/− cardiomyopathy. Although these data do not exclude right-heart involvement, the severe global myopathy in mdx:MyoD−/− mice is entirely consistent with hemodynamic alterations (increase left-ventricular afterload) that favor left-ventricular adaptation (8–10). Indeed, the progress of cardiac disease in both DMD and BMD patients presents with preferential left-ventricular dysfunction (28, 29). As the mdx:MyoD−/− mouse presents an analogous progressive cardiac phenotype, the use of this model may be an important resource in defining the molecular basis of dystrophy-associated cardiomyopathy.
Figure 3.
Advanced cardiac myopathy in older mdx:MyoD−/− mice. >50% of 10-month-old mdx:MyoD−/− mice exhibited visible fibrosis in the ventricular region (arrow in A and B). Masson trichrome-stained serial sections through mdx:MyoD−/− hearts revealed the fibrosis to be confined primarily to epicardial portions of the left ventricle (compare C and D to E) and in regions encompassed by hypertrophied myocytes (arrowhead in B). (B ×40; C–F ×20.)
Histological analysis of Masson trichrome-stained sections revealed that the fibrotic areas were composed of necrotic myocytes associated with interstitial fibrosis. Areas of fibrosis were confined primarily to the epicardial region of the left ventricle (outer portion of myocardium) (Fig. 3 B and E). Interestingly, the necrotic tissue was observed consistently within regions containing hypertrophied myocytes (Fig. 3 B and E). In addition, we observed that myocyte hypertrophy appeared to precede necrosis (compare Figs. 1C, 3B and E). These data suggest that hypertrophied cardiac myocytes are more susceptible to damage. Indeed, in support of this hypothesis a recent study has demonstrated that G-protein-mediated signaling in cardiomyocytes results in a two-step phenotypic response, an initial hypertrophy followed by apoptotic cell death (30). Alternatively, cellular hypertrophy may also represent a response to local cardiac damage (31, 32). Clearly, verifying the origin and mechanism of cellular damage will be critical in understanding the pathology of this cardiomyopathy.
Differential Activation of JNK-1 and p38 in Early vs. Late Cardiomyopathy.
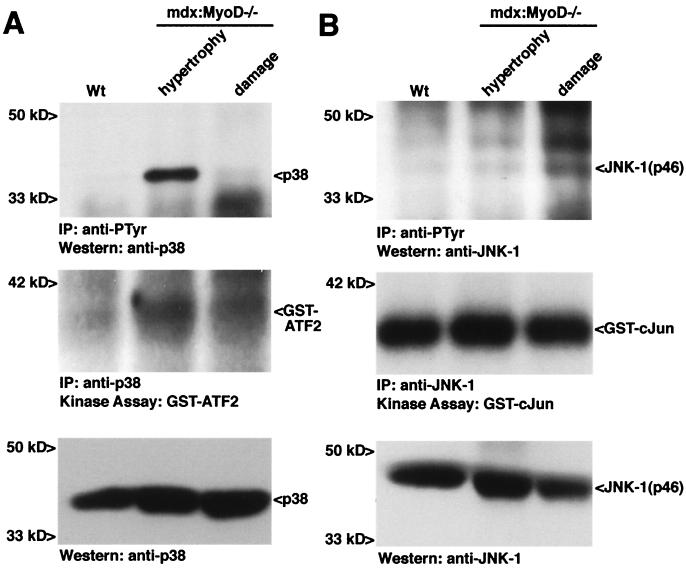
If the development of cellular hypertrophy and necrosis represents distinct stages in cardiomyopathy, we may observe differential activation of specific signaling cascades. Therefore, we compared the activation of the SAPK effectors JNK-1 and p38 in early- and late-stage disease as defined by age and the presence of fibrotic areas. In heart lysates from 10-month-old mdx:MyoD−/− mice with visible cardiac fibrosis, phosphorylation of p38 was dramatically reduced compared with the level observed in heart lysates from 7-month-old mdx:MyoD−/− mice with undamaged hearts (Fig. 4A). In addition, an in vitro kinase assay was performed with immunoprecipitated p38 by using a preferred substrate ATF2. In comparison to hypertrophy alone, immunoprecipitated p38 from damaged cardiac tissue was approximately 2-fold less effective at phosphorylating ATF2 (autoradiography of bands, wt = 1, hypertrophy = 3.0, damage = 1.8 arbitrary units) (Fig. 4A). Therefore, the increased activity of p38 during early-stage mdx:MyoD−/− disease suggests an early role for this kinase in the development of cardiac cell hypertrophy.
Figure 4.
Constitutive activation of SAPK effectors in wt, early (hypertrophy), and late (damage) mdx:MyoD−/− cardiomyopathy. Tyrosine phosphorylation of p38 (A) and JNK-1 (B) was assessed by immunoprecipitation with an antiphosphotyrosine antibody, followed by Western detection with anti-p38 or anti-JNK-1 (Top). p38 and JNK-1 were immunoprecipitated from these lysates and used in an in vitro kinase assay to test the ability of the proteins to phosphorylate an exogenous substrate (ATF2 for p38 and cJun for JNK-1) (Middle). Lysates subjected to SDS/PAGE followed by Western detection revealed equivalent amounts of p38 and JNK-1 within each genotype (Bottom). Similar results were obtained in independent experiments (three IP/Western blot analyses and two kinase assays). Note the IP:Western analysis in A revealing p38 phosphorylation was subjected to about a 10-fold shorter exposure than the experiment shown in Fig. 2A.
By contrast to p38, phosphorylation of JNK-1 on tyrosine was elevated in damaged mdx:MyoD−/− hearts (Fig. 4B). In an in vitro kinase assay, immunoprecipitated JNK-1 phosphorylated a preferred transcription factor target cJun to similar levels in lysates from wt, hypertrophied, and damaged hearts (autoradiography of bands 1, 1.4, and 1.1, respectively) (Fig. 4B). However, JNK-1 activates proteins ranging from non-cJun transcription factors to ICE-CED-like proteases (33, 34) and the four isoforms JNK-1 vary widely in the ability to activate cJun (33). Therefore, activation of JNK-1 may have profound consequences for cardiac myocyte homeostasis independent of cJun phosphorylation.
Taken together, these data support the hypothesis that systemic changes originating from widespread skeletal muscle damage are a significant contributing factor to the development of cardiomyopathy in the mdx:MyoD−/− mouse. Moreover, it is possible that skeletal muscle damage may not be the only precipitating event that induces the cardiac damage in the mdx:MyoD−/− mouse. Indeed, as the loss of dystrophin reduces the structural stability of muscle fibers, the normal cardiac workloads associated with prolonged mobility may also contribute to cardiomyocyte damage. In addition, other factors may influence the progress of the myopathy, separate from or in conjunction with hemodynamic loads. For example, damaged skeletal muscle may release unidentified factors that affect myocardial integrity. Specifying the origin of this secondary effect will be an important step in understanding the etiology of the mdx:MyoD−/− model, which in turn may impact our understanding of human dystrophy-associated cardiomyopathy.
The present study implicates a role for the kinases p38 and JNK-1 in the development of dystrophy-associated cardiomyopathy. Nevertheless, activation of alternate signaling cascades may also contribute to this phenotype. For example, the calcium-dependent phosphatase calcineurin induces the translocation of the transcription factor NF-AT3 to the nucleus, where it synergistically activates transcription by binding the transcription factor GATA-4. Consequently, this pathway is believed to play an important role in the development of cardiac hypertrophy (35). However, whether the calcineurin-NF-AT signaling pathway plays a role in the development of cardiomyopathy in mdx:MyoD−/− mice remains to be addressed.
The mdx:MyoD−/− mouse represents the most accurate murine model of DMD cardiomyopathy that is currently available (the pathology develops without additional cardiac-specific gene alterations). Clearly, the mdx:MyoD−/− mouse will be a critical resource for investigating cardiac disease progression associated with dystrophy. Indeed, the observed activation of specific protein kinase signaling pathways suggests that selective in vivo modulation of these kinases may provide an approach to attenuate this cardiac phenotype.
Acknowledgments
We thank Drs. John Hassell, David Picketts, and Ron Worton for helpful discussions. L.A.M. was a Postdoctoral Fellow of the Medical Research Council of Canada. M.A.R. is a Research Scientist of the National Cancer Institute of Canada and a member of the Canadian Genetic Disease Network of Excellence. This work was supported by grants from the National Institutes of Health and the Muscular Dystrophy Association to M.A.R.
ABBREVIATIONS
- DMD
Duchenne muscular dystrophy
- BMD
Becker’s muscular dystrophy
- IP
immunoprecipitation
- wt
wild-type
- SAPKs
stress-activated protein kinases
- JNK
cJun amino-terminal kinase family
References
- 1.Emery A E H. Duchenne Muscular Dystrophy. 2nd Ed. Oxford: Oxford Univ. Press; 1993. [Google Scholar]
- 2.Frankel K A, Rosser R J. Hum Pathol. 1976;7:375–386. doi: 10.1016/s0046-8177(76)80053-6. [DOI] [PubMed] [Google Scholar]
- 3.Nigro G, Comi L, Politano L, Bain R J I. Int J Cardiol. 1990;26:271–277. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 4.Politano L, Nigro V, Nigro G, Petretta V R, Passamano L, Papparella S, DiSomma S, Comi L I. J Am Med Assoc. 1996;275:1335–1338. [PubMed] [Google Scholar]
- 5.Cox G F, Kunkel L M. Curr Opin Cardiol. 1997;12:329–343. [PubMed] [Google Scholar]
- 6.Mukoyama M, Kondo K, Hizawa K, Nishitani H. J Neurol Sci. 1987;81:155–158. doi: 10.1016/0022-510x(87)90092-x. [DOI] [PubMed] [Google Scholar]
- 7.Melacini P, Vianello A, Villanova C, Fanin M, Mioran M, Angelini C, Della Volta S. Neuromuscul Disord. 1996;6:367–376. doi: 10.1016/0960-8966(96)00357-4. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz K, Boheler K R, de la Bastie D, Lompre A M, Mercadier J J. Am J Physiol. 1992;262:R364–R369. doi: 10.1152/ajpregu.1992.262.3.R364. [DOI] [PubMed] [Google Scholar]
- 9.Katz, A. M. (1995) Eur. Heart J. 16, Suppl. O, 110–114. [DOI] [PubMed]
- 10.Hudlicka O, Brown M D. J Vasc Res. 1996;33:266–287. doi: 10.1159/000159155. [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Kawai H, Akaike M, Adachi K, Nishida Y, Saito S. Am Heart J. 1996;132:642–647. doi: 10.1016/s0002-8703(96)90250-1. [DOI] [PubMed] [Google Scholar]
- 12.Petrof B J. Mol Cell Biochem. 1998;179:111–123. doi: 10.1023/a:1006812004945. [DOI] [PubMed] [Google Scholar]
- 13.Bulfield G, Siller W G, Wight P A L, Moore K J. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sicinski P, Geng Y, Ryder-Cook A S, Barnard E A, Darlison M G, Barnard P J. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 15.Torres L F, Duchen L W. Brain. 1987;110:269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- 16.Coulton G R, Morgan J E, Partridge T A, Sloper J C. Neuropathol Appl Neurobiol. 1988;14:53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 17.Megeney L A, Kablar B, Garrett K, Anderson J E, Rudnicki M A. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- 18.Deconinck A E, Rafael J A, Skinner J A, Brown S C, Potter A C, Metzinger L, Watt D J, Dickson J G, Tinsley J M, Davies K E. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 19.Grady R M, Teng H, Nichol M C, Cunningham J C, Wilkinson R S, Sanes J R. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 20.Bancroft J D, Stevens A. Theory and Practice of Histological Techniques. Edinburgh: Churchill Livingstone; 1990. [Google Scholar]
- 21.Megeney L A, Perry R L S, LeCouter J E, Rudnicki M A. Dev Genet (Amsterdam) 1996;19:139–145. doi: 10.1002/(SICI)1520-6408(1996)19:2<139::AID-DVG5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Barth E, Stammler G, Speiser B, Schaper J. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 23.Graham B H, Waymire K G, Cottrell B, Trounce I A, MacGregor G R, Wallace D C. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 24.Bogoyevitch M A, Ketterman A J, Sugden P H. J Biol Chem. 1995;270:29710–29717. doi: 10.1074/jbc.270.50.29710. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez M T, Sah V P, Zhao X-L, Hunter J J, Chien K R, Brown J H. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 26.Zechner D, Thuerauf D J, Hanford D S, McDonough P M, Glembotski C C. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadoshima J, Izumo S. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 28.Quinlaven R M, Lewis P, Marsden P, Dundas R, Robb S A, Baker E, Maisey M. Neuromuscul Disord. 1996;6:237–246. doi: 10.1016/0960-8966(96)00007-7. [DOI] [PubMed] [Google Scholar]
- 29.Hoogerward E M, de Voogt W G, Wilde A, van der Wouw P A, Bakker E, van Ommen G J, de Visser M. J Neurol. 1997;244:657–663. doi: 10.1007/s004150050163. [DOI] [PubMed] [Google Scholar]
- 30.Adams J W, Sakata Y, Davies M G, Sah V P, Wang Y, Liggett S B, Chien K R, Brown J H, Dorn G W. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeffer M A, Braunwald E. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 32.Olivetti G, Melissari M, Capasso J M, Anversa P. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Derijard B, Davies R J. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 34.Seimiya H, Mashima T, Toho M, Tsuruo T. J Biol Chem. 1997;272:4631–4636. doi: 10.1074/jbc.272.7.4631. [DOI] [PubMed] [Google Scholar]
- 35.Molkentin J D, Lu J-R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]