Abstract
Gibberellins (GAs) are tetracyclic diterpenoids that are essential endogenous regulators of plant growth and development. GA levels within the plant are regulated by a homeostatic mechanism that includes changes in the expression of a family of GA-inactivating enzymes known as GA 2-oxidases. Ectopic expression of a pea GA 2-oxidase2 cDNA caused seed abortion in Arabidopsis, extending and confirming previous observations obtained with GA-deficient mutants of pea, suggesting that GAs have an essential role in seed development. A new physiological role for GAs in pollen tube growth in vivo also has been identified. The growth of pollen tubes carrying the 35S:2ox2 transgene was reduced relative to that of nontransgenic pollen, and this phenotype could be reversed partially by GA application in vitro or by combining with spy-5, a mutation that increases GA response. Treatment of wild-type pollen tubes with an inhibitor of GA biosynthesis in vitro also suggested that GAs are required for normal pollen tube growth. These results extend the known physiological roles of GAs in Arabidopsis development and suggest that GAs are required for normal pollen tube growth, a physiological role for GAs that has not been established previously.
INTRODUCTION
Gibberellins (GAs) are tetracyclic diterpenoids that are essential endogenous regulators of plant growth and development. GAs are involved in many aspects of plant development, including seed germination, trichome development, stem and leaf elongation, flower induction, anther development, and fruit and seed development (Langridge, 1957; Pharis and King, 1985; Ross et al., 1997; Yamaguchi et al., 1998; Kamiya and Garcia-Martinez, 1999; Hedden and Phillips, 2000). The identification and analysis of mutants with defects in either GA biosynthesis or GA response has been used to understand the GA biosynthesis and signal transduction pathways for a number of years. These mutants, particularly in Arabidopsis, pea, and maize, also have played a critical role in determining the physiological roles of GAs in vegetative tissues as well as in flower, seed, and fruit development (reviewed by Ross et al., 1997).
GAs are known to play various roles in reproductive development, depending on the species examined (Pharis and King, 1985). For example, GA application accelerates flowering in the facultative long-day plant Arabidopsis, particularly in short days (Langridge, 1957; Bagnall, 1992), and under short days in the laboratory, the severely GA-deficient Arabidopsis mutant ga1 is unable to flower (Koornneef and van der Veen, 1980; Wilson et al., 1992; Sun and Kamiya, 1994). The ga1 flowering phenotype can be suppressed by treatment with GA or by mutations in a negatively acting component of the GA response pathway, SPY (SPINDLY) (Jacobsen et al., 1996). In terms of flower development, it also has been suggested that GAs produced in the developing anthers are required for corolla development (Weiss et al., 1997). In cucumber, GA application promotes the development of male flowers, whereas in maize and castor bean, GAs promote female flowers (reviewed by Pharis and King, 1985). Maize plants with reduced GA levels or GA response possess anthers in the ear, an organ that contains developed female structures only in wild-type plants. In several plant species, including Arabidopsis and tomato, GA deficiency leads to male sterility as a result of abnormal anther development (Nester and Zeevaart, 1988; Goto and Pharis, 1999). GAs also are present in developing pollen after anthesis (Barendse et al., 1970; Mander et al., 1996), and numerous studies have reported an effect of GA application on pollen tube growth in vivo or in vitro (Bhandal and Malik, 1979; Viti et al., 1990; Setia et al., 1994; Kimura et al., 1996). Nevertheless, the known GA biosynthesis and GA response mutants identified in numerous species appear to have normal pollen function, although anther development often is perturbed. Consequently, a physiological role of GAs in pollen tube growth has not been established.
Analysis of GA-deficient mutants also has provided evidence that GAs are required for seed development, at least in some species. In tomato, Groot et al. (1987) used the GA-deficient gib1 mutant to suggest that GAs are required for normal seed growth. In pea, two alleles at the LH locus, lh-1 and lh-2, have been used to demonstrate that GAs produced in the embryo and/or endosperm are required for normal seed growth and survival (Swain et al., 1993, 1997). The LH locus is required for the three-step oxidation of ent-kaurene to ent-kaurenoic acid early in the GA biosynthetic pathway (Swain et al., 1997) and is likely to encode the enzyme ent-kaurene oxidase (Helliwell et al., 2001). Both the lh-1 and lh-2 alleles cause a reduction in endogenous GA levels in young seeds and reduce seed growth and final seed size. The more severe lh-2 mutation has a greater effect on seed growth rate and also causes an increased frequency of seed abortion, suggesting that a sufficient reduction in seed GA levels greatly decreases the probability that a seed will complete its development. Mutations in other pea GA biosynthesis genes have at most a minor effect on embryo/endosperm GA levels and do not obviously affect seed survival (Swain et al., 1995).
Despite the extensive analysis of GA physiology in a range of diverse plant species, the observation that not all pea GA mutants exhibit altered seed development demonstrates that individual GA mutants do not necessarily reveal all of the physiological processes that require GAs. Likely explanations include gene redundancy or the difficulty of identifying GA-related mutants that are either lethal, for example because of seed abortion, or difficult to recognize as GA related, because of unexpected or organ-specific phenotypes. Consequently, new physiological roles of active GAs may remain to be discovered in pea and other species. An alternative approach to understanding GA physiology and identifying these roles is to manipulate endogenous GA levels using transgenes that encode GA biosynthesis enzymes. For example, a role for GAs in promoting vegetative growth has been confirmed by manipulating the expression of a class of GA biosynthesis genes known as GA 20-oxidases (Coles et al., 1999). By contrast, a recently characterized class of GA-catabolizing enzymes, the GA 2-oxidases, are involved in the irreversible conversion of active GAs and their precursors to inactive forms and potentially can be used to reduce the endogenous levels of active GAs. cDNAs that encode 2-oxidases were isolated recently from runner bean, Arabidopsis, pea, and rice (MacMillan et al., 1997; Lester et al., 1999; Martin et al., 1999; Thomas et al., 1999; Sakamoto et al., 2001) and shown to perform oxidation reactions at carbon 2 of various biologically active and inactive GAs in vitro. In addition, several ESTs from lotus, Medicago, maize, soybean, and tomato, and sequences from the Arabidopsis and rice genome-sequencing projects, have been identified as putative GA 2-oxidases (Elliott et al., 2001).
Based on predicted amino acid sequences, two classes of 2-oxidases have been identified. Most of the GA 2-oxidases, including pea 2ox1 (encoded by the SLN locus), fall into one class, whereas pea 2ox2, a putative gene from Arabidopsis and an EST sequence from Medicago, fall into the second class (Elliott et al., 2001). The physiological significance, if any, of the two classes is not known, but 2ox2 differs from 2ox1 in its preference for GA substrates. When recombinant enzymes were expressed in Escherichia coli, 2ox1 converted GA1 to GA8, GA20 to GA29, and had weak activity for the 2-oxidation of GA29 to GA29 catabolite. Although 2ox2 was able to convert GA1 to GA8 and GA4 to GA34, GA20 was a poor substrate for this enzyme and 2ox2 did not convert GA29 to GA29 catabolite (Lester et al., 1999; J. Ross, personal communication).
In the present study, we ectopically expressed the pea 2ox2 cDNA in Arabidopsis to explore the physiological roles of GAs in plant growth and development. The 35S:2ox2 transgene caused seed abortion, extending and confirming previous results obtained with GA-deficient mutants of pea, suggesting that GAs play an essential role in seed development. In addition, the results suggest that GAs are required for normal pollen tube growth, a physiological role for GAs that has not been established previously.
RESULTS
Ectopic Expression of a Pea GA 2-Oxidase in Arabidopsis
Based on the comparison of predicted amino acid sequences and in vitro enzyme activity, the pea 2ox2 gene appears to represent a novel class of GA 2-oxidases (Elliott et al., 2001) that may play distinct physiological roles in regulating endogenous GA levels. In an attempt to discover new physiological roles for GAs, a construct designed to ectopically express the pea 2ox2 cDNA was introduced into Arabidopsis. For transformation, we used the Agrobacterium-mediated vacuum infiltration method (Ye et al., 1999), which relies on the survival of seeds containing transgenic embryos (usually hemizygous) and the postgermination identification of these seeds on selective medium. An unusual degree of difficulty in recovering transgenic lines containing the 35S:2ox2 construct was observed. Although other constructs were recovered with transformation efficiencies similar to those reported by other researchers (data not shown), ∼200,000 seeds from infiltrated plants were screened to obtain the 35S:2ox2 lines described in this article. One explanation for this problem is that the 35S:2ox2 transgene is in some way lethal during embryo or early seedling development. Based on the results described below, it is likely that the great majority of 35S:2ox2 seeds that were generated initially later aborted before they could be harvested and identified at the seedling stage.
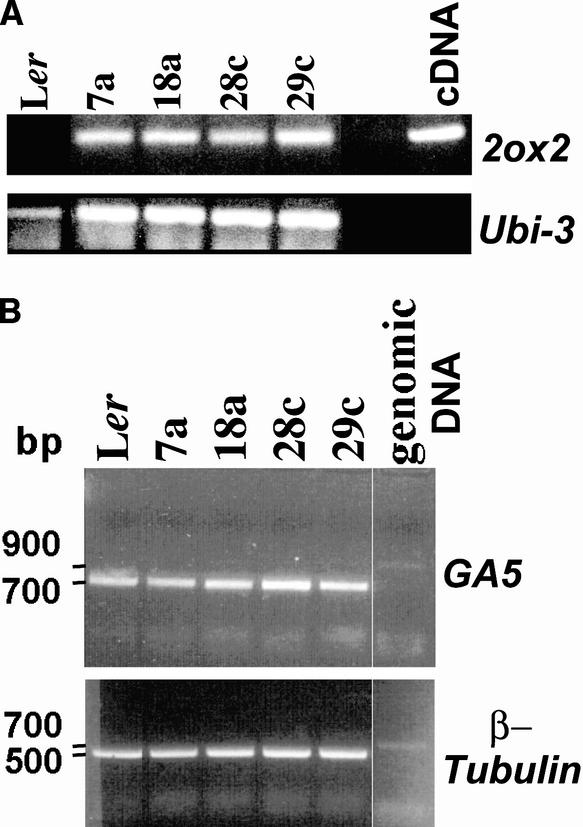
To confirm that the 35S:2ox2 lines express the 2ox2 gene, semiquantitative reverse transcriptase–mediated (RT) PCR using pea 2ox2-specific primers was performed on RNA isolated from young seedlings (Figure 1). This analysis revealed that all lines have very low expression of the 35S:2ox2 transgene, consistent with an inability to recover the majority of lines that would be expected to have high levels of expression as a result of the 35S promoter. Among the lines examined, line 29c has relatively high levels of 2ox2 expression compared with 35S:2ox2/28c seedlings, whereas lines 7a and 18a have intermediate levels of 2ox2 expression. As expected, the pea 2ox2 RNA was not detected in nontransgenic control plants. Although this analysis was semiquantitative, the difficulty of detecting 2ox2 RNA (see Methods) was consistent with the low expression of 2ox2 in the 35S:2ox2 lines examined.
Figure 1.
RT-PCR Analysis of 2ox2 and GA5 Expression.
(A) Semiquantitative RT-PCR analysis of 2ox2 transcript levels (top gel) in RNA from seedlings of nontransgenic control Ler and 35S:2ox2 lines in the Ler background. Pea 2ox2 cDNA was used as a positive control. RT-PCR (bottom gel) of UBIQUITIN3 (Ubi-3) transcripts, which were detected consistently in all of the plant samples used for the 2ox2 RT-PCR experiment.
(B) Semiquantitative RT-PCR analysis of GA5 (GA 20-oxidase) transcript levels (top gel) in RNA from seedlings of nontransgenic control Ler and 35S:2ox2 lines in the Ler background. RT-PCR (bottom gel) of β-tubulin transcripts, which were detected consistently in all of the plant samples used for the GA5 RT-PCR experiment.
The majority of transgenic lines obtained did not obviously display phenotypes generally associated with vegetative GA deficiency, such as small rosette size and reduced inflorescence internode elongation (data not shown), although one line did exhibit a dwarf phenotype. This line, 35S:2ox2/7a, exhibited reduced rosette size and inflorescence internode length compared with wild-type Landsberg erecta (Ler) plants, but this phenotype could not be rescued by GA3 application (data not shown). Therefore, the dwarf phenotype of this line may result from defects other than GA deficiency caused by insertion of the transgene into an Arabidopsis gene, although in terms of reproductive development, line 7a is consistent with the other 35S:2ox2 lines (see below).
The 35S:2ox2 Transgene Is Transmitted Poorly to the Next Generation
In pea, the preferential abortion of homozygous lh-2 seeds containing GA-deficient embryos and endosperm results in reduced transmission of the lh-2 allele to the next generation when LH lh-2 F1 plants are allowed to self-pollinate. Dwarf lh-2 lh-2 plants represent only ∼10% of the F2 progeny rather than the expected 25%, because many homozygous lh-2 seeds (∼60%) abort during development. Similarly, self-pollinated lh-2 plants exhibit seed abortion at various stages of seed development at a much higher frequency than occurs in wild-type plants (Swain et al., 1993). To determine whether the 35S:2ox2 transgene can cause similar phenotypes, the transmission of the 35S:2ox2 transgene into the progeny (T2) obtained from self-pollinated T1 lines was scored on the basis of resistance to kanamycin, the selection marker used for the identification of transformants. Of 12 independent transgenic 35S:2ox2-containing lines selected for analysis (see Methods), 7 (lines 7a, 18a, 28c, 29c, 42a, 44a, and 71a) exhibited a significant (P < 0.001) deficiency in the number of transgenic progeny (Figure 2). Three other lines segregated in agreement (P > 0.2) with a 3:1 resistant-to-sensitive ratio (data not shown), whereas two other lines appeared to contain complex or multiple transgene inserts that prevented further analysis. Subsequent analysis revealed that lines 18a and 44a appeared to contain more than one transgene locus, and these lines were not examined further in terms of reproductive development.
Figure 2.
The 35S:2ox2 Transgene Is Transmitted Poorly to the Next Generation.
Seeds from self-pollinated hemizygous 35S:2ox2 Arabidopsis plants were collected and scored as either transgenic (kanamycin-resistant seedlings; black sectors) or nontransgenic (kanamycin-sensitive seedlings; white sectors) by germinating on Murashige and Skoog (1962) medium containing 15 or 25 μg/mL kanamycin. For comparison, the circle marked 35S:GFP (green fluorescent protein) represents the ratio of transgenic to nontransgenic seedlings observed when heterozygous 35S:GFP plants containing a single transgene locus were allowed to self-pollinate. As expected if the 35S:GFP construct does not alter seed or pollen development, ∼75% of the progeny were transgenic. The total numbers of seeds analyzed were 605 for 35S:GFP (χ2 [3:1] = 0.005, P > 0.90) and 159 to 858 for each of the 35S:2ox2 lines (for all lines, P < 0.001 from the χ2 test for agreement with the expected 3:1 transgenic-to-nontransgenic ratio).
Several explanations for the observed deficiency of transgenic progeny are possible. Although a trivial explanation is that the nptII gene is silenced in a proportion of transgenic seedlings, subsequent analysis of individual lines eliminated this explanation (see below). An alternative explanation is that the 35S:2ox2 transgene impairs transmission through the male (pollen) and/or female (egg) gametes or, similar to the the lh-2 mutation, causes increased seed abortion of transgenic seeds.
The 35S:2ox2 Transgene Causes Seed Abortion
Based on the known consequence of GA deficiency for pea seed development, a likely explanation for the impaired transmission of the 35S:2ox2 transgene is preferential abortion of transgenic seeds compared with nontransgenic seeds. This hypothesis is supported by the presence of partially developed aborting seeds in the siliques of 35S:2ox2 plants (Figure 3 and data not shown) that are similar in appearance to the late-aborting seeds observed in the pods of the lh-2 mutant (Swain et al., 1993, 1995). This hypothesis also was tested by hand-pollinating flowers on hemizygous 35S:2ox2 plants with wild-type pollen. Consistent with impaired transmission through the female gamete and/or preferential abortion of 35S:2ox2 seeds, a significant (P < 0.001) deficiency of transgenic progeny was observed when lines 7a, 29c, and 42a were used as the female parent (Table 1). By contrast, the progeny from the cross between hemizygous 35S:2ox2/28c female and nontransgenic pollen segregated in agreement with a 1:1 resistant-to-sensitive ratio, suggesting that the transgene locus present in this line does not affect the female gamete or increase seed abortion (Table 1).
Figure 3.
Phenotypic Analysis of 35S:2ox2 Plants.
(A) 35S:2ox2 causes early and late seed abortion, as shown in a typical silique from a self-pollinated 35S:2ox2/29c plant. Only 3% of surviving seeds carry the 35S:2ox2 transgene.
(B) Siliques developing on self-pollinated homozygous 35S:2ox2/28c plants are only approximately half the size of siliques on self-pollinated wild-type Ler plants, and seeds are present only in the part of the silique nearest the stigma.
(C) A typical silique from a self-pollinated homozygous 35S:2ox2/28c plant showing a number of unfertilized ovules at the base of the silique, distal to the fertilized seed farthest from the stigma (arrow).
(D) The 35S:2ox2/28c pollen tube phenotype is partially rescued in the 35S:2ox2/28c spy-5 double mutant.
Table 1.
The 35S:2ox2 Transgene Can Impair Transmission through the Female Parent
| Surviving Progenya
|
||||
|---|---|---|---|---|
| Female Parent (Hemizygous) | Male (Pollen) Parent | Transgenic | No Transgene | Transgenic (%)b |
| 35S:2ox2/7a | Ler | 3 | 30 | 9 |
| 35S:2ox2/28c | Ler | 58 | 62 | 48 |
| 35S:2ox2/29c | Ler | 3 | 45 | 6 |
| 35S:2ox2/42a | Columbia | 0 | 108 | 0 |
Seeds were collected and sown on Murashige and Skoog (1962) kanamycin plates to determine the presence or absence of the transgene.
Expected value is 50% transgenic for a transgene with no effect on transmission through the female parent.
Pollen Carrying the 35S:2ox2 Transgene Is Out-Competed by Wild-Type Pollen
An alternative explanation for the impaired transmission of the 35S:2ox2/28c transgene to the next generation is a defect in male gamete formation or pollen function. To test this hypothesis, wild-type flowers were pollinated with pollen from several hemizygous 35S:2ox2 lines, including 28c, so that the haploid male gametes were a mixture of transgenic and nontransgenic genotypes. A significant deviation (P < 0.001) from the expected ratio of 1:1 (resistant:sensitive) was observed when pollen from the hemizygous lines 7a, 28c, 29c, and 42a was crossed onto wild-type females (Table 2). For line 28c, this result, combined with the data in Table 1, indicates that the 35S:2ox2 transgene impairs transmission through the male gamete. The data from lines 7a, 29c, and 42a also are consistent with reduced transmission through the male gamete, although for these lines, seed abortion (see above) also would be expected to contribute to the observed deficiency of transgenic progeny.
Table 2.
The 35S:2ox2 Transgene Can Impair Transmission through the Male (Pollen) Parent
| Surviving Progenya
|
||||
|---|---|---|---|---|
| Female Parent | Male (Pollen) Parent (Hemizygous) | Transgenic | No Transgene | Transgenic (%)b |
| Ler | 35S:2ox2/7a | 3 | 21 | 13 |
| Ler | 35S:2ox2/28c | 18 | 68 | 21c |
| Ler | 35S:2ox2/29c | 0 | 407 | 0 |
| Columbia | 35S:2ox2/42a | 1 | 82 | 1 |
Seeds were collected and sown on Murashige and Skoog (1962) kanamycin plates to determine the presence or absence of the transgene.
Expected value is 50% transgenic for a transgene with no effect on transmission through the male or female parent.
Data in this row also are shown in Table 3.
To test the hypothesis that the 35S:2ox2 transgene impairs pollen function, another series of crosses was performed. Wild-type flowers were pollinated again with pollen from several hemizygous 35S:2ox2 lines, but in this case, the siliques were harvested in two separate parts: the “top” half containing the seeds nearest the stigma, and the “base” half containing seeds nearest the pedicel. If the impaired transmission of the 35S:2ox2 transgene through the male gamete is attributable to impaired germination or growth of 35S:2ox2 pollen compared with wild-type pollen, the majority of transgenic progeny resulting from these crosses should be found in the top half of the silique, because the pollen tube must elongate through the style toward the ovary, reaching ovules at the top of the ovary (silique) first. When the progeny of these crosses were examined (Table 3), this effect was observed for lines 28c, 7a, 42a, and 71a, suggesting that pollen tube growth is reduced or pollen grain germination is delayed by the 35S:2ox2 transgene. Line 29c could not be examined in this manner because no kanamycin-resistant progeny were obtained from crosses between wild-type Ler female and pollen from hemizygous 29c plants (Table 2). Based on the proportion of transgenic progeny recovered, line 28c has the least severe phenotype and line 29c has the most severe phenotype, consistent with the segregation data shown in Figure 2 and RT-PCR analysis of 2ox2 expression (Figure 1). The difference in the proportion of transgenic (kanamycin-resistant) progeny between the top and the base of the silique also argues against the hypothesis that silencing of the nptII gene accounts for the observed deficiency of transgenic progeny.
Table 3.
Pollen Carrying the 35S:2ox2 Transgene Is Out-Competed by Wild-Type Pollen
| Surviving Progenyb
|
|||||
|---|---|---|---|---|---|
| Female Parent | Male (Pollen) Parent (Hemizygous) | Silique Parta | Transgenic | No Transgene | Transgenic (%)c |
| Ler | 35S:2ox2/7a | Top | 14 | 89 | 14 |
| Base | 4 | 72 | 5 | ||
| Total | 18 | 161 | 11 | ||
| Ler | 35S:2ox2/28c | Top | 17 | 36 | 32 |
| Base | 1 | 32 | 3 | ||
| Total | 18 | 68 | 21 | ||
| Columbia | 35S:2ox2/42a | Top | 3 | 63 | 5 |
| Base | 1 | 60 | 2 | ||
| Total | 4 | 123 | 3 | ||
| Ler | 35S:2ox2/71a | Top | 6 | 18 | 25 |
| Base | 1 | 11 | 8 | ||
| Total | 7 | 29 | 19 | ||
Siliques were divided at harvest into two approximately equal parts: the top, nearest the stigma, and the base, farthest from the stigma. Pollen tubes must grow a greater distance to reach ovules in the base than in the top portion of the silique.
Seeds were collected and sown on Murashige and Skoog (1962) kanamycin plates to determine the presence or absence of the transgene.
Expected value is 50% transgenic for a transgene with no effect on transmission through the male or female parent.
The 35S:2ox2 Transgene Reduces Seed Number and Fruit Size in a Homoygous Line
The results described above can be explained most simply by the hypothesis that the 35S:2ox2 transgene impairs pollen tube growth and increases seed abortion in lines 7a, 29c, 42a, and 71a but only impairs pollen tube growth in the less severe line 28c. To analyze these phenotypes in more detail, progeny from self-pollinated hemizygous 35S:2ox2 plants were examined in an attempt to isolate plants homozygous for the different 35S:2ox2 transgene inserts. Consistent with the observation that the 35S:2ox2 transgene affects reproductive development, homozygous plants were isolated only for the weakest transgenic line, 35S: 2ox2/28c. Of 54 transgenic progeny examined initially, only 6 (11%) were homozygous based on their fruit phenotype (see below) and the observation that all progeny were resistant to kanamycin (data not shown). The remaining 48 plants had siliques similar to Ler fruit and segregated kanamycin-resistant and kanamycin-sensitive progeny in an ∼1:1 ratio (Figure 2), demonstrating that these plants were hemizygous for the 35S:2ox2/28c transgene. The observed segregation ratio does not agree with the expected ratio of 1:2 (homozygous:hemizygous) (P < 0.001) and provides further evidence that the disturbed segregation of the kanamycin resistance marker (Figure 2) is not simply a consequence of poor expression of the nptII gene. Internode elongation and rosette size of homozygous 28c plants were similar to those in wild-type Ler plants (data not shown).
To determine whether the 35S:2ox2 construct can alter active GA levels in vegetative tissues, two experiments were performed. In the first, endogenous GAs were measured in 17-day-old Ler and homozygous 35S:2ox2/28c plants grown under standard long-day conditions. The levels (ng/g dry weight) of GA1 and GA4, the major active GAs in Arabidopsis, were 0.57 (GA1) and 3.37 (GA4) in Ler plants compared with 0.34 (GA1) and 1.50 (GA4) in 35S:2ox2/28c plants. Consistent with the similar growth of the two genotypes, the relatively small magnitude of the observed differences (approximately twofold higher in Ler) would not be expected to have a clear effect on vegetative growth because of the log-linear relationship between active GA levels and vegetative growth (Ross et al., 1997). In the second experiment, the expression of the GA5 gene, which encodes a GA 20-oxidase required for GA biosynthesis (Xu et al., 1995), was determined, because it is well established that reduced GA levels or response in vegetative tissues leads to the upregulation of GA5 mRNA levels (Hedden et al., 1999). Consistent with the 35S:2ox2 transgene decreasing the levels of active GAs in vegetative tissues to a limited extent, increased GA5 expression was observed in homozygous 35S:2ox2/28c seedlings and to a lesser extent in hemizygous 35S:2ox2/18a and 35S:2ox2/29c plants (Figure 1). Although the observed magnitude of change in GA5 levels was not great, similar results were obtained in two additional experiments (data not shown).
In contrast to vegetative development, the silique size of self-pollinated homozygous 28c plants (4.0 ± 0.1 mm) was only approximately half that of wild-type Ler plants (11.0 ± 0.22 mm), and the siliques contained significantly (P < 0.001) fewer seeds (Figure 3B). Significantly, in the great majority (>95%) of fruit developing on self-pollinated homozygous 28c plants, all of the seeds were found at the end of the silique nearest the stigma, and the ovules in the basal part of the silique were unfertilized (Figures 3B and 3C). This result is consistent with the observation that the majority of transgenic seeds carrying the 35S:2ox2/28c transgene were present in the top half of the silique when pollen from hemizygous 35S:2ox2/28c plants was used to pollinate wild-type flowers (Table 3). The reduced seed set per fruit also caused delayed apical senescence, with homozygous 35S:2ox2/28c plants producing 27.1 ± 1.1 siliques on the main inflorescence compared with 11.9 ± 0.8 for Ler plants.
To demonstrate that the restricted localization of fertilized seeds is caused by the effect of the 35S:2ox2 transgene on pollen function, rather than an effect of the transgene on the maternal part of the flower, three experiments were performed. In the first experiment, wild-type flowers were pollinated with pollen from homozygous 28c plants (i.e., all pollen carried the 28c transgene). Consistent with the 35S:2ox2 transgene affecting pollen development directly, fertilized seeds were observed only in the top half of the silique, even though the ovules and maternal tissues did not carry the transgene before fertilization (data not shown). In the second experiment, homozygous 28c flowers were pollinated with wild-type pollen, and seeds were observed throughout the silique, confirming that the presence of the 35S:2ox2 transgene in maternal tissues of the style or ovary does not detectably inhibit the function of wild-type pollen (Table 4). This result also is consistent with the presence of fertilized seeds throughout the siliques of self-pollinated hemizygous 35S:2ox2 plants, including lines 28c (data not shown) and 29c (Figure 3A). In the third experiment, two flowers on homozygous 35S:2ox2/28c plants were hand-pollinated first with 35S:2ox2/28c pollen (before anthesis) and on the next day with wild-type Ler pollen. Seeds were observed throughout the entire silique in both cases (data not shown), demonstrating that the previous presence of 35S:2ox2/28c pollen in the pistil does not significantly impair wild-type pollen function.
Table 4.
The spy-5 Mutation Can Partially Suppress the 35S:2ox2/28c Pollen Tube Phenotype
| Female Parent | Male (Pollen) Parent (Homozygous) | Silique Length (mm) | Fertilized Seedsa | Unfertilized Ovules at Silique Baseb |
|---|---|---|---|---|
| 35S:2ox2/28c | 35S:2ox2/28c | 4.40 | 1.6 ± 0.3 | 48.4 ± 4.9 |
| 35S:2ox2/28c | 28c spy-5c | 6.58 | 5.7 ± 1.8 | 18.8 ± 5.7 |
| 35S:2ox2/28c | Wild type (Ler) | 10.75 | 37.0 ± 10.5 | 5.0 ± 3.8 |
Plants were grown at day/night temperatures of 19/17°C to minimize the effects of the spy-5 mutation on anther development and pollen production. 35S:2ox2/28c flowers were emasculated and then hand pollinated.
Total number of fertilized seeds per silique.
Ovules not fertilized below the most distal fertilized seed (see Figure 3C).
35S:2ox2/28c spy-5 flowers produced less pollen than 35S:2ox2/28c and Ler flowers as a result of the effect of spy-5 on anther development.
To examine pollen grain germination, Ler and 35S:2ox2/28c flowers were self-pollinated by hand and pollen grain germination was visualized by scanning electron microscopy. At both 1 h (data not shown) and 5 h after pollination (Figure 4), no differences were observed in the size and germination of Ler or 35S:2ox2/28c pollen grains. Aniline blue staining of the callose in pollen tubes was used to examine pollen tube growth in pistils of self-pollinated Ler and 35S:2ox2/28c flowers. After 5 h (Figure 4), wild-type pollen tubes were clearly visible in the style, approaching the first ovule. By contrast, after 5 h, 35S:2ox2/28c pollen tubes had just begun to elongate, and few were visible in the style. After 24 h, wild-type pollen tubes had reached the farthest ovule, whereas only a small proportion of 35S:2ox2/28c pollen tubes were able to reach the ovules present near the pedicel at 48 h after pollination (data not shown). These findings suggest that 35S:2ox2 pollen tubes elongate more slowly than wild-type pollen tubes, consistent with the observation that transgenic 35S:2ox2 seeds generally are present only in the top half of the silique when homozygous or hemizygous 35S:2ox2 plants are used as the male parent (Figure 3, Table 3).
Figure 4.
Pollen Grain Germination and Pollen Tube Growth.
(A) Scanning electron microscopy images of germinated pollen grains on stigmas at 5 h after self-pollination of wild-type Ler and homozygous 35S:2ox2/28c flowers.
(B) Aniline blue staining at 5 h of wild-type Ler self-pollinated pistils (left), homozygous 35S:2ox2/28c self-pollinated pistils (center), and 35S:2ox2/28c pistils pollinated with 28c spy-5 pollen (right).
These results suggest that pollen tubes carrying the 35S:2ox2 transgene elongate more slowly so that fertilization of ovules, particularly those farthest from the stigmatic surface, by transgenic pollen is reduced greatly.
Partial Suppression of the 35S:2ox2/28c Pollen Tube Phenotype by spy-5
Because 2ox2 is a GA-deactivating enzyme (Lester et al., 1999), the simplest hypothesis to explain the pollen tube phenotype described above is that the growth of 35S:2ox2 pollen tubes is impaired as a result of a deficiency in biologically active GAs. One way to test this hypothesis is to determine if mutations that are known to increase GA response can suppress the 35S:2ox2/28c pollen tube defect. This approach cannot be used for all GA mutations because GA genes that either are not expressed or are genetically redundant in this organ will not alter pollen tube growth. In an attempt to avoid this constraint, an allele of the SPY locus was chosen because, of the known GA response mutants, only spy alleles are able to suppress the phenotypes caused by GA deficiency in developing flowers (Jacobsen and Olszewski, 1993; Swain et al., 2001). The SPY protein is a negative regulator of GA signal transduction, and spy mutants are able to partially suppress the phenotypes caused by GA deficiency in all plant organs examined to date (Jacobsen et al., 1996; Swain et al., 2001). The spy-5 mutation was used for this analysis because it is in the same genetic background as 35S:2ox2/28c (Ler), because it results from a single nucleotide substitution that changes one amino acid in the C terminus (Jacobsen et al., 1996), and because this allele does not appear to cause some of the putative non-GA phenotypes observed in severe spy mutants (Swain et al., 2001). In addition, severe spy mutants in the Ler background, such as spy-2 and spy-4 (Swain et al., 2001), are almost completely male sterile because of greatly reduced pollen production that is attributable, at least in part, to abnormal anther development. Although spy-5 plants also have slightly reduced male fertility (Wilson and Somerville, 1995), this phenotype was minimized by growing plants in relatively cool temperatures (see Methods) to partially restore spy mutant fertility (Jacobsen and Olszewski, 1993).
To characterize the effect of the spy-5 mutation on the 35S:2ox2/28c phenotype, three types of experiments were conducted. In the first, homozygous 35S:2ox2/28c flowers were pollinated with pollen carrying both the 35S:2ox2/28c transgene and spy-5, obtained from homozygous 35S: 2ox2/28c spy-5 plants. Aniline blue staining of pistils at 5 h after pollination (Figure 4B) revealed that 28c spy-5 pollen had elongated farther than the 35S:2ox2/28c SPY pollen. In the second experiment, a similar approach was taken, except that the fruit was allowed to develop to maturity. Consistent with the results shown in Figure 4, the spy-5 mutation was able to partially suppress the 35S:2ox2/28c pollen tube phenotype, causing an increase in final silique length and seeds per silique and decreasing the number of unfertilized ovules at the base of the silique compared with fruit developing on self-pollinated 35S:2ox2/28c plants (Table 4).
In the third experiment, fruit development was compared between homozygous plants allowed to self-pollinate naturally (Figure 5). Compared with wild-type Ler (Figures 5A and 5B), spy-5 siliques were of similar final length (0.2 < P < 0.3) but contained fewer fertilized seeds per silique (P < 0.001) and unfertilized ovules throughout both carpels (data not shown), consistent with previous reports of reduced male fertility in spy mutants (Jacobsen and Olszewski, 1993; Wilson and Somerville, 1995; Swain et al., 2001). Self-pollinated homozygous 35S:2ox2/28c plants possessed smaller siliques with fewer seeds than both Ler and spy-5 plants (P < 0.001). Self-pollinated plants homozygous for both the 35S: 2ox2/28c transgene and spy-5 displayed phenotypes consistent with partial suppression of the 35S:2ox2/28c pollen tube defect by loss of SPY function (Figures 3D and 5). The number of fertilized seeds per silique was increased (P < 0.001), and spy-5 also increased the final silique length (P < 0.001) in the 35S:2ox2/28c background. Note that the effect of spy-5 on silique length is not the result of parthenocarpy, because spy mutants do not display this phenotype (Vivian-Smith and Koltunow, 1999) (data not shown). In addition to silique length, one of the clearest phenotypes displayed by self-pollinated homozygous 35S:2ox2/28c plants was the presence of unfertilized ovules at the base of each carpel (Figure 3C). Compared with wild-type and spy-5 plants, self-pollinated homozygous 35S:2ox2/28c plants contained significantly (P < 0.001) more unfertilized ovules at the base of individual siliques, and this phenotype was rescued partially by spy-5 (Figure 5C). Together, these results suggest that 35S:2ox2/28c pollen tube elongation is increased in vivo by increased GA response.
Figure 5.
Partial Suppression of the 35S:2ox2/28c Pollen Tube Phenotype by spy-5.
Plants were grown at day/night temperatures of 19/17°C to minimize the effects of the spy-5 mutation on anther development and pollen production. Data represent the largest fruit from the first 10 flowers on the main stem of at least 11 self-pollinated plants of each genotype.
(A) Final silique length.
(B) Mean number of fertilized seeds per silique.
(C) Mean number of unfertilized ovules at the base of each silique (see Figure 3C).
Changing GA Levels of Pollen Tubes Growing in Vitro
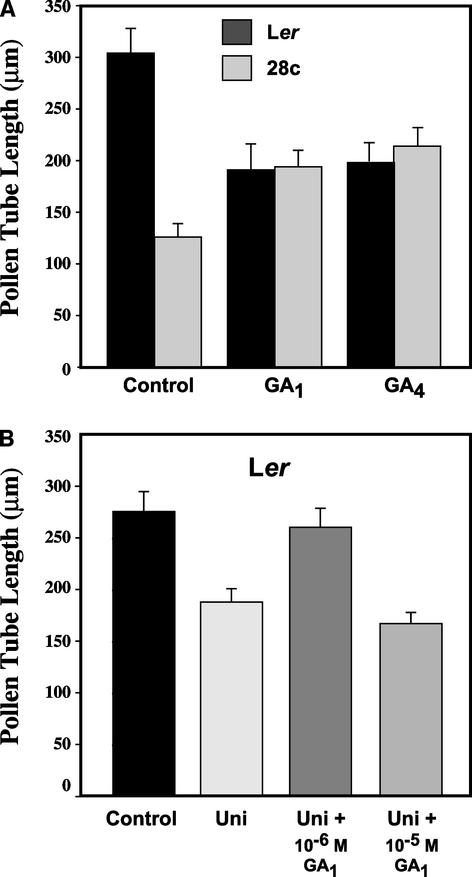
Depending on the species examined and the concentration used, GAs can promote, inhibit, or have no effect on pollen tube elongation in vitro (Bhandal and Malik, 1979; Viti et al., 1990; Setia et al., 1994). To further test the hypothesis that the reduced elongation of 35S:2ox2 pollen tubes is attributable to a deficiency in biologically active GAs, in vitro pollen tube growth assays were conducted with pollen from homozygous 28c and wild-type Ler flowers. Under these in vitro conditions, the majority of the wild-type and transgenic pollen grains germinated without GAs within the first 7 to 8 h of incubation (data not shown), consistent with the similar behavior of Ler and 35S:2ox2/28c pollen grains on stigmas (Figure 4). After 24 h of incubation, and in the absence of exogenous active GAs, 35S:2ox2/28c pollen tubes elongated only to approximately one-third the length of wild-type Ler pollen tubes (Figure 6A), consistent with the observed fruit phenotypes in vivo and the staining of pollen tubes with aniline blue (Figure 4). When 1 × 10−5 M GA1 or GA4 was present in the germination medium, the growth of wild-type and 35S:2ox2/28c pollen tubes was similar; the pollen tubes were longer than those of the 35S:2ox2/28c control but shorter than those of the wild-type control (Figure 6A). Higher concentrations of GA1 or GA4 (1 × 10−4 M) inhibited both 35S:2ox2/28c and wild-type pollen tube elongation compared with that of untreated controls (data not shown). Lower concentrations of GA1 or GA4 (1 × 10−6 and 1 × 10−7 M) did not inhibit pollen tube elongation but failed to rescue 28c pollen tube growth (data not shown). GA3 (1 × 10−5 M) inhibited wild-type pollen tube growth but did not rescue 28c pollen tube elongation (data not shown). The observed inhibition of wild-type pollen tube elongation by high concentrations of GA is consistent with the result of previous studies (Sidhu et al., 1986; Swamy and Khanna, 1991) in which high GA concentrations have been shown to inhibit wild-type pollen tube growth.
Figure 6.
Changing GA Levels of Pollen Tubes Growing in Vitro.
Pollen tube length after 24 h on pollen germination medium.
(A) Pollen from Ler or homozygous 35S:2ox2/28c plants was germinated in vitro with or without 1 × 10−5 M GA1 or GA4.
(B) Pollen from Ler was germinated in vitro in the presence of 1 × 10−5 M uniconazole (Uni), a chemical inhibitor of GA biosynthesis, with or without GA1.
An alternative approach to investigate the role of GAs in pollen tube growth is to use chemical inhibitors of GA biosynthesis such as uniconazole (Izumi et al., 1985). To confirm that GA deficiency can inhibit wild-type Ler pollen tube growth, pollen grains were incubated in the presence of 1 × 10−5 M uniconazole with or without GA1. Consistent with an essential role for GAs in pollen tube growth, and with the pollen phenotype of the 35S:2ox2 lines, uniconazole inhibited pollen tube elongation of wild-type Ler compared with the control (Figure 6B). The effect of uniconazole was overcome when 1 × 10−6 M GA1 also was included in the medium, demonstrating that this inhibitor alters pollen tube growth via its effects on GA biosynthesis. Similar to the results described above, a higher concentration of GA1 (1 × 10−5 M) failed to restore the inhibition of pollen tube growth by uniconazole, because this GA1 concentration is itself inhibitory (cf. Ler with or without 1 × 10−5 M GA1 in Figure 6A). The ability to overcome the effect of uniconazole with GA strongly suggests that uniconazole acts solely via its inhibitory effect on ent-kaurene oxidase, an early enzyme in the GA biosynthesis pathway. Further support for this conclusion is provided by the observation that pollen tubes carrying a 35S:GA3–green fluorescent protein construct, which is designed to ectopically express the ent-kaurene oxidase gene (Helliwell et al., 2001), which in turn encodes the target of uniconazole, are resistant to the effects of this inhibitor (S.M. Swain and D.P. Singh, unpublished results).
DISCUSSION
We used ectopic expression of the pea 2ox2 cDNA in Arabidopsis to explore the physiological roles of GAs in plant growth and development. Although the transgenic lines recovered were not phenocopies of plants with reduced vegetative GA levels, only lines with very low 2ox2 expression appeared to be recoverable. Most of the lines examined exhibited abortion of seeds at various stages of development and impaired pollen tube elongation. We believe that these phenotypes are consistent with GA deficiency in reproductive organs, although the small size of Arabidopsis seeds and pollen tubes precludes GA analysis.
GAs and Seed Development
Most of the hemizygous 35S:2ox2 lines exhibited a disturbed segregation ratio when allowed to self-pollinate (Figure 2), suggesting that there is an impaired transmission of the 35S:2ox2 transgene through the male or female gametophyte or that seeds are aborting. Swain et al. (1993) observed a significantly disturbed LH:lh-2 homozygote ratio in the F2 progeny of crosses between the seed GA-deficient lh-2 mutant and its wild-type progenitor (LH). This phenotype is caused by zygotic selection against the GA-deficient seeds containing homozygous lh-2 embryos that are more likely than wild-type seeds to abort during development. For the 35S:2ox2 lines 7a, 29c, 42a, and 71a, preferential abortion of transgenic seeds also appears to partially explain the disturbed segregation ratios. This conclusion is consistent with the presence of seeds in 35S:2ox2 siliques aborting both early and late (Figure 3A), crosses in which hemizygous 35S:2ox2 flowers were pollinated with wild-type pollen (Table 1), and the severely disturbed segregation ratios observed in the progeny of self-pollinated hemizygous 35S:2ox2 plants (Figure 2). Consistent with an essential role for GAs in Arabidopsis seed development, expression of the 2ox2 cDNA under the control of the seed-specific promoter FIS1 (fertilization-independent seed formation; Chaudhury et al., 1997) also causes seed abortion and reduces fruit size in Arabidopsis (D.P. Singh and S.M. Swain, unpublished results).
A physiological role for GAs in Arabidopsis seed development also is in agreement with previous results suggesting that GAs are required for normal seed growth in the gib1 tomato mutant (Groot et al., 1987) and in GA-deficient mutants of barley (Chandler, 1999). Increased abortion of seeds carrying the 35S:2ox2 transgene also can explain the low transformation efficiency observed for this construct using vacuum infiltration, because this method initially generates transgenic seeds that must successfully complete development before transgenic plants can be identified (Ye et al., 1999). Consequently, it is likely that the great majority of 35S:2ox2-expressing seeds aborted before they could be identified. GA application is not able to prevent lh-2 seed abortion, presumably because GA cannot move into the developing embryo/endosperm early in seed development (Swain et al., 1997). Similarly, the abortion of 35S:2ox2 and FIS1:2ox2 seeds also could not be prevented by GA application (data not shown).
GAs and Pollen Tube Growth
In addition to increased seed abortion, the reduced transmission of the transgenes present in lines 7a, 42a, and 71a also appears to be caused by impaired pollen function (Table 3). The disturbed segregation ratio observed when hemizygous 35S:2ox2/28c plants are allowed to self-pollinate appears to be entirely attributable to impaired transgene transmission through pollen (Tables 1 to 3). Genetic analysis suggests that this effect is caused by the action of the 35S:2ox2 transgene in elongating pollen tubes rather that an effect in sporophytic organs such as the anthers or pistil.
Because of the effects of the 35S:2ox2 transgene on reproductive development, we have been able to isolate only plants homozygous for the 35S:2ox2/28c transgene, consistent with the observation that it is the mildest of the 35S:2ox2 lines with an effect on reproductive development and has defects only in pollen function. Homozygous 28c plants have essentially normal shoot morphology, apart from the production of small siliques with fewer seeds when allowed to self-pollinate. In virtually all siliques, the seeds are present only toward the stigmatic end, where pollen first lands after pollination (Figure 3), and most of the ovules at the base are not fertilized.
The apparently normal germination of 35S:2ox2/28c pollen grains and the partial rescue of pollen tube elongation by GA application in vitro suggests that the 35S:2ox2 transgene affects pollen tube elongation by reducing endogenous GA levels. Defective pollen tube elongation caused by GA deficiency also can explain why transgenic pollen (from lines 7a, 42a, 71a, and 28c) is out-competed by pollen from wild-type plants. In the great majority of fruit on self-pollinated homozygous 35S:2ox2/28c plants, seeds are not present at the base of siliques. Presumably, the ovules at the base of the silique are not able to be fertilized when 35S:2ox2/28c pollen tubes arrive, consistent with the observation that wild-type ovules can be fertilized successfully only during a limited time after anthesis (Vivian-Smith and Koltunow, 1999). A role for GAs in pollen tube elongation is consistent with previous studies in which applied GAs have been shown to either promote or inhibit pollen tube elongation in vitro for numerous species depending on the GA concentration (Figure 6) (Bhandal and Malik, 1979; Viti et al., 1990; Setia et al., 1994) or to inhibit pollen tube growth in GA-treated pistils (Kimura et al., 1996). In addition to these previous experiments, we provide genetic evidence for a growth-promoting role of GAs in pollen tube growth in intact plants.
Exogenous GA partially restored the elongation of 35S:2ox2/28c pollen tubes in vitro (Figure 5). By contrast, treatment of intact 35S:2ox2/28c plants with GA did not appear to restore pollen tube growth, although fruit growth was increased (data not shown), consistent with the ability of GA to simulate parthenocarpy (Vivian-Smith and Koltunow, 1999). One likely problem with this type of experiment is that applied GA also stimulates pistil growth, which may impair fertilization by causing premature pistil elongation and effectively increasing the distance between the stigma and more distant ovules. A similar phenomenon may account for the reduced fertilization of the ovules farthest from the stigma in fruit of the parthenocarpic fwf (fruit without fertilization) mutant when fwf flowers are self-pollinated (Vivian-Smith et al., 2001).
Additional evidence for a role for GAs in pollen tube elongation is provided by the spy-5 mutant, which possesses increased GA response in vegetative tissues and flowers and can partially suppress the effects of GA deficiency in these organs (Wilson and Somerville, 1995). Genetic analysis (Figures 3D and 5) suggests that the growth of pollen tubes carrying both the 35S:2ox2/28c transgene and spy-5 is increased compared with 35S:2ox2/28c pollen tubes, suggesting that increased GA response can compensate partially for reduced GA levels in elongating pollen tubes. In addition to supporting a role of GAs in pollen tube development, this result also suggests a new role for the SPY protein in pollen tube elongation that has not been established previously. Targeted silencing of the 35S:2ox2/28c transgene using an antisense construct also increases pollen tube growth, suggesting that the pollen phenotype observed in 35S:2ox2/28c (and other 35S:2ox2 lines) is not the result of the mutation or silencing of an endogenous Arabidopsis gene (D.P. Singh and S.M. Swain, unpublished results). Our conclusion that GAs are required for pollen tube elongation is further strengthened by the effect of uniconazole on wild-type pollen tubes (Figure 6). The effect of uniconazole also suggests that de novo GA biosynthesis occurs in pollen tubes after germination, at least in vitro. Pollen tube elongation, at least in vitro, is unlike most other GA responses. For the majority of physiological processes requiring GAs, such as seed germination and stem growth, the response saturates at a particular GA concentration, and additional increases in the GA concentration have no further effect. By contrast, anther development (Jacobsen and Olszewski, 1993) and pollen tube growth (this article) require GAs, but superoptimal GA levels also are inhibitory.
GA mutants identified in pea, Arabidopsis, and other species have not been reported to exhibit an obvious pollen tube elongation phenotype (Swain and Olszewski, 1996; Swain et al., 1997). One explanation is that many of these mutants may have relatively mild effects on pollen tube elongation that cannot be identified easily. For example, a slight deficiency in the number of homozygous ga1 and ga2 progeny was observed when hemizygous GA1 ga1 or GA2 ga2 plants were allowed to self-pollinate (Koornneef and van der Veen, 1980). Although other explanations are possible, one hypothesis is that GA-deficient pollen tubes carrying ga1 or ga2 mutant alleles are slightly out-competed by wild-type pollen tubes. By contrast, other GA mutants segregate in agreement with a 3:1 dominant-to-recessive ratio, suggesting that pollen tube growth and seed development are at worst impaired only slightly. Therefore, it is likely that the majority of existing GA mutants do not clearly alter pollen tube growth or seed development because of the existence of gene families that encode redundant proteins. Problems with gametophytic/ embryo lethality would make the identification of GA mutants with defects in pollen tube growth difficult (Swain and Olszewski, 1996; Swain et al., 1997).
The transgenic 35S:2ox2 lines described here do not display the reduced vegetative growth phenotypes of existing mutants with reduced GA levels or response. By contrast, ectopic expression of a runner bean GA 2-oxidase produced dwarf Arabidopsis plants (Hedden et al., 1999) that did not show the pollen tube or seed abortion phenotypes observed in 35S:2ox2 plants. However, transgene expression in these lines appears to be silenced before reproductive development occurs, because bolt length or flower formation is not altered. Although the reason for this difference has not been determined, the simplest explanation is that we have been able to recover only lines with relatively low 2ox2 expression (Figure 1), because lines (i.e., seeds) with higher expression aborted before they could be recovered as transgenic seedlings. Reducing GA levels in vegetative tissues of transgenic plants also is made more difficult by the existence of “feedback regulation” (Hedden et al., 1999) of GA biosynthesis, in which the plants can compensate at least partially for perturbed GA metabolism by increasing the expression of the GA5 gene. Thus, the lack of an obvious dwarf phenotype in the 35S:2ox2 lines described here is a consequence of relatively normal vegetative GA levels caused by low 2ox2 expression combined with feedback regulation (Figure 1). Higher expression of the pea 2ox2 gene in vegetative tissues, more typical of the 35S promoter, may cause dwarfism.
In conclusion, overexpression of pea 2ox2 in Arabidopsis impairs pollen tube elongation and causes seed abortion. Examination of the spy-5 mutant and treatment of wild-type pollen tubes with an inhibitor of GA biosynthesis in vitro further supports a role for GAs in pollen tube growth. These results extend the known physiological roles of GAs in plant development. The transgenic lines generated during the course of this study also provide the potential to identify new GA-related genes in Arabidopsis that may have pollen tube- or seed-specific phenotypes.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana ecotypes Landsberg erecta (Ler) and Columbia, and the spy-5 mutant, were supplied by N.E. Olszewski (University of Minnesota, St. Paul). All seeds were stratified for 3 days at 4°C under dim light to aid germination. Plants were grown in growth rooms with 18 h of white fluorescent light (60 to 70 μmol·m−2·s−1 at pot top, 22°C) and 6 h of dark (20°C) on GroWool (GroWool Horticultural Systems, Girraween, NSW, Australia), 0.8% agar (Spectrum, New Brunswick, NJ) containing Murashige and Skoog (1962) salts (2.3 g/L; Sigma-Aldrich), and 1% (w/v) Suc, or individually in a peat-based soil mixture (Commonwealth Scientific and Industrial Research Organization–Plant Industry, Canberra, Australia) in the Arasystem (BetaTech, Gent, Belgium).
Plant Transformation
The pea 2ox2 cDNA encoding a full-length protein was cloned at the SalI and SacI sites of the pOCA28 vector (Olszewski et al., 1988) to generate the 35S:2ox2 construct. Standard molecular techniques were followed for DNA manipulations (Sambrook et al., 1989).
Agrobacterium tumefaciens GV3101 (a gift from J. Botella, Department of Botany, University of Queensland, Australia) was transformed with constructs in the pOCA vector (Olszewski et al., 1988; Robertson et al., 1998) by electroporation (Shen and Forde, 1989). The constructs were introduced into Arabidopsis using the Agrobacterium-mediated vacuum infiltration method (Ye et al., 1999). Putative transformants containing the 35S:2ox2 construct were selected on 0.8% agar (as described above) and kanamycin (15 or 25 μg/mL) for 10 to 15 days.
To generate additional independent lines and to confirm that the low numbers of 35S:2ox2 T1 plants recovered were a result of this particular transgene rather than a general problem with Arabidopsis transformation, wild-type Arabidopsis plants also were infiltrated with a mixture of Agrobacterium containing a pOCA28-derived plasmid designed to express either 2ox2 or green fluorescent protein under the control of the 35S promoter. Large numbers of transformants were recovered in this experiment, with the majority expected to contain the 35S:green fluorescent protein transgene. Forty-six lines were selected for further analysis, and from these, four lines (lines 28c, 29c, 42a, and 44a) were identified as exhibiting a deficiency of transgenic progeny in the next generation (Figure 2). PCR analysis of genomic DNA from these lines using primers specific for the pea 2ox2 cDNA was used to confirm the presence of the 35S:2ox2 transgene in all of these lines (data not shown). Lines 7a, 18a, 28c, and 29c were isolated in the Ler background, and lines 42a, 44a, and 71a were isolated in Columbia. Lines 7a, 28c, 29c, 42a, and 71a were analyzed in greatest detail, and all exhibited a consistent phenotype over several generations.
PCR and Reverse Transcriptase–Mediated PCR Analyses
Total RNA was isolated using Trizol reagent (Gibco BRL) according to the manufacturer's instructions with some modifications. First-strand cDNA was synthesized in 20.0-μL reaction volumes using 5.0 μg of total RNA with the SuperScript First–Strand Synthesis System for reverse transcriptase–mediated PCR (Gibco BRL). Subsequently, 5.0 μL of the first-strand reaction was used for 2ox2 cDNA amplification, whereas only 2.0 μL was used for the amplification of a fragment of the UBIQUITIN3 (UBQ3) cDNA (Norris et al., 1993) in 50.0-μL PCR volumes. The primer pair used for PCR was F2ox2/3 (5′-TACAATGGTAGTGCCTTCTCCAACTTCC-3′) and R2ox2/4 (5′-TTC-AAGTGTCATAGAGAATTGAG-3′). The PCR conditions were 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, for 40 cycles. An initial denaturation step at 94°C for 2 min was included before the commencement of the first cycle. The primers, Taq polymerase (Promega), and other components were used at the concentrations recommended by the manufacturer (Promega). The 2ox2 PCR product was obtained only after 40 cycles of amplification, and no product was obtained after either 25 or 30 cycles (Figure 1 and data not shown), suggesting that the expression of 2ox2 in these transgenic lines is very low. For each plant sample, Arabidopsis UBQ3 or β-tubulin transcripts also were amplified as a constitutive expression control. The primer pairs used for UBQ3 and β-tubulin PCR were FUBQ3 (5′-CTCTCCCAAAGCCTAAAGCGA-3′) and RUBQ3 (5′-GTCGAC-TCCTTTTGAATGTTGTA-3′) and β-tubF2 (5′-GGACACTACGCTGAAGGTGCTGAG-3′) and β-tubR3 (5′-GGCTCTGTATTGCTGTGA-TCCGCG-3′), which flank an intron and produced different sized bands from genomic DNA and cDNA. GA5 (gibberellin [GA] 20-oxidase) reverse transcriptase–mediated PCR and amplification were performed as described by Xu et al. (1995). No product was obtained with 2ox2, UBQ3, GA5, or β-tubulin primers when RNA that had not been transcribed was used as a template.
GA Determinations
GAs were quantified using gas chromatography–selected ion monitoring with dideuterated internal standards. After harvest and lyophilization, samples were extracted in 100 mL of 80% methanol for 24 h along with a range of 2H-GA standards (provided by L.N. Mander, Australian National University, Canberra, Australia). Purification by ethyl acetate partitioning, QAE Sephadex, and C18 SepPak was according to Green et al. (1997). HPLC separation was the same as the initial HPLC step described by Gocal et al. (1999) with appropriate fractions pooled, dried, and derivatized for analysis by gas chromatography–selected ion monitoring.
Pollen Tube Growth Assays and Aniline Blue Staining
Aniline blue staining was performed as described by Ryan et al. (1998) with some modifications. The anthers were removed (at anthesis) and used to brush pollen onto the stigma until it was completely covered with pollen. The pistil was dissected at 5 h after pollination and fixed in 3:1 ethanol:acetic acid for 30 min and then softened in 1 M NaOH overnight at room temperature. The pistils were washed three times in sterile distilled water and stained with 0.1% aniline blue in K3PO4 buffer, pH 8.5, for >2 h. The pistils were rinsed briefly in buffer and mounted in 80% glycerol. The samples were observed under UV epifluorescence.
Pollen tube growth assays were performed according to the method described by Taylor et al. (1998). GAs or uniconazole were dissolved in an appropriate volume of pollen germination medium to obtain the desired concentration. Pollen tube lengths were measured using an Olympus BH-2 compound microscope (Tokyo, Japan).
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We are indebted to John Ross for providing the pea 2ox2 cDNA, Andrew Poole and Sue Allen for analysis of endogenous GA levels, Jimmy Botella for Agrobacterium strain GV3101, Rod King for pure GAs, Richard Storey for scanning electron microscopy images, and Peter Waterhouse and Masumi Robertson for vectors as well as useful and critical discussions. For epifluorescence photography of aniline blue–stained pistils, we thank Celia Miller and Rosemary White (Commonwealth Scientific and Industrial Research Organization–Plant Industry, Canberra). We also thank Amanda Walker and Ayalsew Zerihun for their advice and suggestions.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.003046.
References
- Bagnall, D.J. (1992). Control of flowering in Arabidopsis thaliana by light, vernalization, and gibberellins. Aust. J. Plant Physiol. 19, 401–409. [Google Scholar]
- Barendse, G.W.M., Rodrigues-Pereira, A.J., Berkers, P.A., Driessen, F.M., van Eyden-Emons, A., and Linskens, H.F. (1970). Growth hormones in pollen, styles and ovaries of Petunia hybrida and Lilium species. Acta Bot. Neerl. 19, 175–186. [Google Scholar]
- Bhandal, I.S., and Malik, C.P. (1979). Effect of gibberellic acid, (2-chloroethyl)phosphoric acid, actinomycin-D and cycloheximide on the activity and leaching of some hydrolases in pollen suspension cultures of Crotalaria juncea. Physiol. Plant. 45, 297–300. [Google Scholar]
- Chandler, P.M. (1999). Gibberellins and the regulation of growth in barley. In Abstracts ComBio 1999: Sym 01-01. Canberra, Australia.
- Chaudhury, A.M., Ming, L., Miller, C., Craig, S., Dennis, E.S., and Peacock, W.J. (1997). Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94, 4223–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles, J.P., Phillips, A.L., Croker, S.J., Garcia-Lepe, R., Lewis, M.J., and Hedden, P. (1999). Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J. 17, 547–556. [DOI] [PubMed] [Google Scholar]
- Elliott, R.C., Ross, J.J., Smith, J.J., Lester, D.R., and Reid, J.B. (2001). Feed-forward regulation of gibberellin deactivation in pea. J. Plant Growth Regul. 20, 87–94. [Google Scholar]
- Gocal, G.F.W., Poole, A.T., Gubler, F., Watts, R.J., Blundell, C., and King, R.W. (1999). Long-day up-regulation of a GAMYB gene during Lolium temulentum inflorescence formation. Plant Physiol. 119, 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, N., and Pharis, R.P. (1999). Role of gibberellins in the development of floral organs of gibberellin-deficient mutant, ga1-1, of Arabidopsis thaliana. Can. J. Bot. 77, 944–954. [Google Scholar]
- Green, L.S., Faergestad, E.M., Poole, A., and Chandler, P.M. (1997). Grain development mutants of barley: α-Amylase production during grain maturation and its relation to endogenous gibberellic acid content. Plant Physiol. 114, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot, S.P.C., Bruinsma, J., and Karssen, C.M. (1987). The role of endogenous gibberellins in seed and fruit development of tomato: Studies with a gibberellin-deficient mutant. Physiol. Plant. 71, 184–190. [Google Scholar]
- Hedden, P., and Phillips, A.L. (2000). Manipulation of hormone biosynthetic genes in transgenic plants. Curr. Opin. Biotechnol. 11, 130–137. [DOI] [PubMed] [Google Scholar]
- Hedden, P., Phillips, A.L., Coles, J.P., Thomas, S., Appleford, N., Ward, D., Beale, M., and Lenton, J. (1999). Gibberellin biosynthesis: Genes, regulation and genetic manipulation. RIKEN Rev. 21, 29–30. [Google Scholar]
- Helliwell, C.A., Chandler, P.M., Poole, A., Dennis, E.S., and Peacock, W.J. (2001). The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc. Natl. Acad. Sci. USA 98, 2065–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi, K., Kamiya, Y., Sakurai, A., Oshio, H., and Takahashi, N. (1985). Studies of sites of action of a new plant growth retardant (E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-trizol-1-yl)-penten-3-ol (S-3307) and comparative effects of its stereoisomers in a cell free system Cucurbita maxima. Plant Cell Physiol. 29, 821–827. [Google Scholar]
- Jacobsen, S.E., Binkowski, K.A., and Olszewski, N.E. (1996). SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc. Natl. Acad. Sci. USA 93, 9292–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, S.E., and Olszewski, N.E. (1993). Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5, 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, Y., and Garcia-Martinez, J.L. (1999). Regulation of gibberellin biosynthesis by light. Curr. Opin. Plant Biol. 2, 398–403. [DOI] [PubMed] [Google Scholar]
- Kimura, P.H., Okomoto, G., and Hirano, K. (1996). Effects of gibberellic acid and streptomycin on pollen germination and ovule and seed development in Muscat Bailey A. Am. J. Enol. Vitic. 47, 152–156. [Google Scholar]
- Koornneef, M., and van der Veen, J.H. (1980). Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L) Heynh. Theor. Appl. Genet. 58, 257–263. [DOI] [PubMed] [Google Scholar]
- Langridge, J. (1957). Effect of day-length and gibberellic acid on the flowering of Arabidopsis. Nature 180, 36–37. [Google Scholar]
- Lester, D.R., Ross, J.J., Smith, J.J., Elliott, R.C., and Reid, J.B. (1999). Gibberellin 2-oxidation and the SLN gene of Pisum sativum. Plant J. 19, 1–9. [DOI] [PubMed] [Google Scholar]
- MacMillan, J., Ward, D.A., Phillips, A.L., Sanchez-Beltran, M.J., Gaskin, P., Lange, T., and Hedden, P. (1997). Gibberellin biosynthesis from gibberellin A12-aldehyde in endosperm and embryos of Marah macrocarpus. Plant Physiol. 113, 1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander, L.N., Owen, D.J., Croker, S.J., Gaskin, P., Hedden, P., Lewis, M.J., Talon, M., Gage, D.A., Zeevaart, J.A., Brenner, M.L., and Sheng, C. (1996). Identification of three C20-gibberellins: GA97 (2 beta-hydroxy-GA53), GA98 (2 beta-hydroxy-GA44) and GA99 (2 beta-hydroxy-GA19). Phytochemistry 43, 23–28. [DOI] [PubMed] [Google Scholar]
- Martin, D.N., Proebsting, W.M., and Hedden, P. (1999). The SLENDER gene of pea encodes a gibberellin 2-oxidase. Plant Physiol. 121, 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nester, J.E., and Zeevaart, J.A.D. (1988). Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am. J. Bot. 75, 45–55. [Google Scholar]
- Norris, S.R., Meyer, S.E., and Callis, J. (1993). The intron of Arabidopsis thaliana polyubiquitin genes is conserved in location and is a quantitative determinant of chimeric gene expression. Plant Mol. Biol. 21, 895–906. [DOI] [PubMed] [Google Scholar]
- Olszewski, N.E., Martin, F.B., and Ausubel, F.M. (1988). Specialized binary vector for plant transformation: Expression of the Arabidopsis thaliana AHAS gene in Nicotiana tabacum. Nucleic Acids Res. 16, 10765–10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis, R.P., and King, R.W. (1985). Gibberellins and reproductive development in seed plants. Annu. Rev. Plant Physiol. 36, 517–568. [Google Scholar]
- Robertson, M., Swain, S.M., Chandler, P.M., and Olszewski, N.E. (1998). Identification of a negative regulator of gibberellin action, HvSPY, in barley. Plant Cell 10, 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, J.J., Murfet, I.C., and Reid, J.B. (1997). Gibberellin mutants. Physiol. Plant. 100, 550–560. [Google Scholar]
- Ryan, E., Grierson, C.S., Cavell, A., Steer, M., and Dolan, L. (1998). TIP1 is required for both tip growth and non-tip growth in Arabidopsis. New Phytol. 138, 49–58. [Google Scholar]
- Sakamoto, T., Kobayashi, M., Hironori, I., Tagiri, A., Kayano, T., Hiroshi, T., Iwahori, S., and Matsuoka, M. (2001). Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol. 125, 1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Setia, N., Setia, R.C., and Chabra, N. (1994). Interactive effects of growth hormones and calcium antagonists on germination and tube elongation of groundnut pollen. Plant Cell Incompatibility Newsletter 26, 70–80. [Google Scholar]
- Shen, W.-J., and Forde, B.G. (1989). Efficient transformation of Agrobacterium sp. by high voltage electroporation. Nucleic Acids Res. 17, 8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu, R.K., Basra, A.S., and Malik, C.P. (1986). Hormonal effects on tube elongation, 14CO2 fixation and phosphoenolpyruvate carboxylase activity in Amaryllis pollen: Promotion by abscisic acid. Plant Growth Regul. 4, 293–298. [Google Scholar]
- Sun, T.-p., and Kamiya, Y. (1994). The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthase A of gibberellin biosynthesis. Plant Cell 6, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain, S.M., and Olszewski, N.E. (1996). Genetic analysis of gibberellin signal transduction. Plant Physiol. 112, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain, S.M., Reid, J.B., and Kamiya, Y. (1997). Gibberellins are required for embryo and seed development in pea. Plant J. 12, 1329–1338. [Google Scholar]
- Swain, S.M., Reid, J.B., and Ross, J.J. (1993). Seed development in Pisum: The lhi allele reduces gibberellin levels in developing seeds, and increases seed abortion. Planta 191, 482–488. [Google Scholar]
- Swain, S.M., Ross, J.J., Reid, J.B., and Kamiya, Y. (1995). Gibberellins and pea seed development: Expression of the lhi, ls and le5839 mutations. Planta 191, 426–433. [Google Scholar]
- Swain, S.M., Tseng, T.-s., and Olszewski, N.E. (2001). Altered expression of SPINDLY affects gibberellin response and plant development. Plant Physiol. 126, 1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy, A.V.S.R., and Khanna, V.K. (1991). Effect of some growth regulators on in vitro pollen germination and tube growth in Cicer. International Chickpea Newsletter 25, 32–34. [Google Scholar]
- Taylor, P.E., Glover, J.A., Lavithis, M., Craig, S., Singh, M.B., Knox, R.B., Dennis, E.S., and Chaudhury, A.M. (1998). Genetic control of male fertility in Arabidopsis thaliana: Structural analyses of postmeiotic developmental mutants. Planta 205, 492–505. [DOI] [PubMed] [Google Scholar]
- Thomas, S.G., Phillips, A.L., and Hedden, P. (1999). Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. USA 87, 7983–7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viti, R., Bartolini, S., and Vitagliano, C. (1990). Growth regulators on pollen germination in olive. Acta Hortic. 286, 227–230. [Google Scholar]
- Vivian-Smith, A., and Koltunow, A. (1999). Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol. 121, 437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian-Smith, A., Luo, M., Chaudhury, A., and Koltunow, A. (2001). Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128, 2321–2331. [DOI] [PubMed] [Google Scholar]
- Weiss, D., Izhaki, A., Ben-Nissan, G., Dagan, Y., and Borochov, A. (1997). GA-induced gene expression in Petunia flowers. Plant Physiol. 114 (suppl.), S160.. [Google Scholar]
- Wilson, R.N., Heckman, J.W., and Somerville, C.R. (1992). Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 100, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R.N., and Somerville, C.R. (1995). Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol. 108, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y.L., Li, L., Wu, K., Peeters, A.J.M., Gage, D.A., and Zeevaart, J.A.D. (1995). The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: Molecular cloning and functional expression. Proc. Natl. Acad. Sci. USA 92, 6640–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, S., Smith, M.W., Brown, R.G., Kamiya, Y., and Sun, T.P. (1998). Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell 10, 2115–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, G.N., Stone, D., Pang, S.Z., Creely, W., Gonzalez, K., and Hinchee, M. (1999). Arabidopsis ovule is the target for Agrobacterium in planta vacuum infiltration transformation. Plant J. 19, 249–257. [DOI] [PubMed] [Google Scholar]