Abstract
Vernalization, the promotion of flowering by a prolonged period of low temperature, results in repression of the floral repressor FLOWERING LOCUS C (FLC) and in early flowering. This repression bears the hallmark of an epigenetic event: the low expression state is maintained over many cell division cycles, but expression is derepressed in progeny. We show that the two stages of the response of FLC to vernalization, the repression of FLC and the maintenance of the repression during growth at normal temperatures after vernalization, are mediated through different regions of the FLC gene. Both promoter and intragenic regions are required for the responses. We also identify a 75-bp region in the FLC promoter that, in addition to intragenic sequences, is required for expression in nonvernalized plants.
INTRODUCTION
Vernalization, the promotion of flowering by a prolonged period of low temperature, is an important control of the time of flowering in plants from temperate regions (for reviews, see Michaels and Amasino, 2000; Sheldon et al., 2000a). In Arabidopsis, vernalization promotes flowering in late-flowering winter annual ecotypes and in some late-flowering mutants. The floral repressor FLOWERING LOCUS C (FLC) plays a role in the control of flowering time and is a key component of the response to vernalization (Michaels and Amasino, 1999, 2001; Sheldon et al., 1999, 2000b). Late-flowering ecotypes, such as Pitztal and San Feliu-2, have a high level of FLC expression, in contrast to the early-flowering ecotypes Landsberg erecta (Ler) and Columbia, which have a very low level of FLC expression (Sheldon et al., 1999; Rouse et al., 2002). Prolonged cold treatment of imbibed seeds of late-flowering ecotypes, and some late-flowering mutants, causes repression of FLC expression, and the vernalized plants flower early. The repression of FLC expression as a consequence of the cold treatment is maintained in all tissues throughout the development of the vernalized plant, but a high expression level is reestablished in the progeny of vernalized plants (Sheldon et al., 2000b).
The mechanisms by which prolonged exposure to low temperature is perceived by the germinating seed, and how this results in the repression of FLC expression and the promotion of flowering in the adult plant, are largely unknown. It is known that the degree of repression of FLC expression is proportional to the duration of the cold treatment and correlates with the extent of the promotion of flowering (Sheldon et al., 2000b). There is a substantial delay between the cold treatment of the germinating seed and the flowering of the adult plant, indicating that the induced vernalization response is transmitted mitotically during the development of the vernalized plant. As with FLC expression, the capacity to respond to vernalization is reset in the progeny of vernalized plants. Each generation requires vernalization to flower early.
The mitotic stability and the lack of heritability suggest an epigenetic basis for vernalization. An insight into this epigenetic mechanism has come from the recent cloning of the VERNALIZATION2 (VRN2) gene (Gendall et al., 2001). In the vrn2 mutant, FLC expression is downregulated in response to cold treatment, but instead of the low expression level being maintained during the life of the plant, expression increases as the plant develops. The mitotic stability of FLC repression, but not the initial decrease in FLC expression, is compromised in the vrn2 mutant, indicating that the repression of FLC by vernalization involves two components. VRN2 encodes a zinc finger protein with similarities to Polycomb group (PcG) proteins and may be involved in stabilizing a chromatin structure that represses FLC transcription and that is induced by cold treatment (Gendall et al., 2001).
In this article, we address the question of which regions of the FLC gene are important for nonvernalized expression and for the stable repression of expression by vernalization. We have identified a 75-bp region in the FLC promoter, containing three potential basic domain/Leu zipper (b-ZIP) binding motifs, that is necessary, in addition to intragenic sequences, for nonvernalized expression. Our results show that the sequences involved in the initial downregulation and those involved in the maintenance of repression by vernalization are different and require both promoter and intragenic segments.
RESULTS
A 6-kb Region of the FLC Gene Is Sufficient for the Initial Vernalization-Induced Downregulation and for the Maintenance of Repression
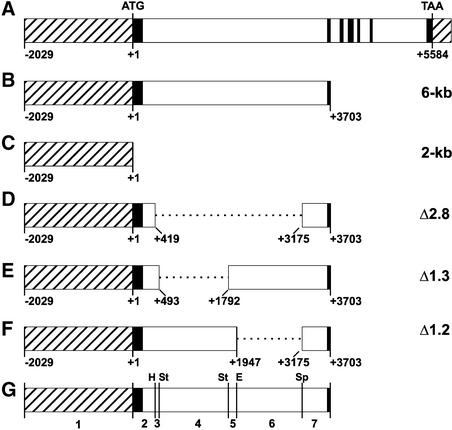
In nonvernalized plants of the C24 ecotype, FLC is expressed in most parts of the plant and during all stages of development (Sheldon et al., 1999). A 28-day cold treatment of imbibed C24 seeds, followed by growth at 23°C, results in a stable reduction of the level of FLC expression. We fused portions of the FLC gene to the β-glucuronidase reporter gene (GUS) to characterize the sequences required for this vernalization response. Figure 1A shows a diagram of the FLC genomic region, which includes seven exons and six introns, the first intron being 3.5 kb (Sheldon et al., 1999).
Figure 1.
Genomic Structure of FLC and FLC::GUS Reporter Constructs.
Coding and intron sequences are shown as black and white boxes, respectively. The 5′ and 3′ noncoding regions (including 5′ promoter regions) are shown as striped boxes. The start (ATG) and stop (TAA) codons are indicated. Nucleotide numbering is given below each construct, taking the A of the ATG as +1. The segment numbers referred to in the text are indicated at bottom. E, EcoRV; H, HindIII; Sp, SpeI; St, StyI.
(A) Diagram of the FLC gene.
(B) to (F) Diagrams of the FLC::GUS constructs. All constructs were fused to the GUS coding sequence and the 3′ octopine synthase terminator (Dolferus et al., 1994). The end points of the deletions in (D) to (F) are indicated below the diagrams of the constructs.
(B) 6-kb construct.
(C) 2-kb construct.
(D) Δ2.8 construct.
(E) Δ1.3 construct.
(F) Δ1.2 construct.
(G) Region contained within the 6-kb construct.
A construct containing 6 kb of FLC sequence (Figure 1B), including 2 kb of the promoter sequence, the first two exons, and intron I (Figure 1G, segments 1 to 7), was introduced into the C24 ecotype, and 18 single-locus, homozygous lines were examined for GUS activity pattern and staining intensity (Figure 2A). This construct directed expression in nonvernalized plants in a pattern similar to FLC expression in C24, as characterized by RNA gel blot and protein gel blot analyses (Sheldon et al., 1999; Rouse et al., 2002), suggesting that the 6-kb segment contains all of the necessary FLC regulatory regions. Figures 3A, 3C, and 3E show the typical nonvernalized staining pattern of this construct in 2-, 5-, and 15-day-old plants, respectively.
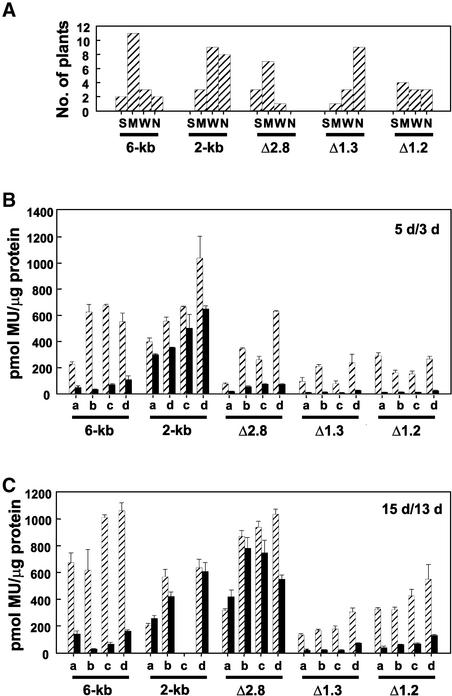
Figure 2.
Intragenic Sequences Are Important for Both the Cold-Induced Repression of FLC Expression and the Maintenance of Repression during Plant Growth at Room Temperature.
(A) Distribution of staining intensities for five FLC::GUS constructs: 6-kb, 2-kb, Δ2.8, Δ1.3, and Δ1.2. Each independent line was classified as strong (S), medium (M), weak (W), or no staining (N), as described in Methods. Strong, medium, and weak staining equates to >700, 350 to 700, and <350 pmol of 4-methyl umbelliferone (MU) per microgram of protein, respectively. All lines containing the 2-kb construct exhibited staining in cotyledons, and this expression was not included when determining relative staining intensities.
(B) Methylumbelliferyl-β-d-glucopyranoside activity data for four selected lines (a to d) for the five FLC::GUS constructs shown in (A) for 3-day-old vernalized (black bars) and 5-day-old nonvernalized (striped bars) seedlings. Error bars represent the standard error.
(C) Methylumbelliferyl-β-d-glucopyranoside activity data for up to four selected lines (a to d) for the five FLC::GUS constructs shown in (A) for 13-day-old vernalized (black bars) and 15-day-old nonvernalized (striped bars) plants. Error bars represent the standard error.
The 6-kb line c, the 2-kb line a, and the Δ2.8 line b are depicted in Figure 3.
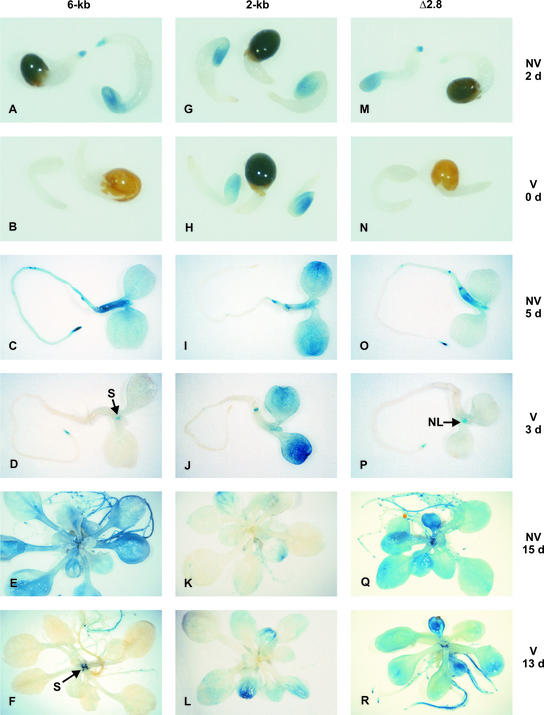
Figure 3.
Intragenic Regions Are Required for the Vernalization-Induced Downregulation and the Stable Repression of FLC::GUS Expression.
GUS staining of nonvernalized and vernalized C24 plants carrying the 6-kb (line c; Figure 2) ([A] to [F]), the 2-kb (line a; Figure 2) ([G] to [L]), and Δ2.8 (line b; Figure 2) ([M] to [R]) FLC::GUS constructs.
(A), (G), and (M) Seedlings after 1 day of cold treatment, stained after 2 days of growth at 23°C.
(B), (H), and (N) Seedlings after 28 days of cold treatment, stained at the end of cold treatment.
(C), (I), and (O) Seedlings after 1 day of cold treatment, stained after 5 days of growth at 23°C.
(D), (J), and (P) Seedlings after 28 days of cold treatment, stained after 3 days of growth at 23°C.
(E), (K), and (Q) Plants after 1 day of cold treatment, stained after 15 days of growth at 23°C.
(F), (L), and (R) Plants after 28 days of cold treatment, stained after 13 days of growth at 23°C.
The dark brown color of the seed coats in (A), (G), (M), and (H) is a consequence of the blue staining of the cotyledons within the seed coat. The seed coat itself shows no GUS activity. The 0- and 2-day-old seedlings, the 3- and 5-day-old seedlings, and the 13- and 15-day-old plants were photographed at ×50, ×20, and ×6.5 magnification, respectively. NL, nascent leaf; NV, nonvernalized; S, stipule; V, vernalized.
A 28-day cold treatment of imbibed seeds abolished GUS activity in seedlings just removed from the cold (Figure 3B), mirroring the response of the endogenous FLC gene and indicating that the 6-kb region containing segments 1 to 7 of the FLC gene is sufficient for the initial vernalization-induced downregulation of FLC.
We examined FLC::GUS activity at two additional time points after the cold treatment. Three days after removal from the cold, GUS activity was present in root tips and in stipules of seedlings at a lower level than in nonvernalized seedlings of a similar developmental stage, but not elsewhere within the seedlings (Figure 3D). Thirteen days after removal from the cold, a low level of GUS activity was present in roots and stipules (Figure 3F). We quantified GUS activity in nonvernalized and vernalized whole seedlings for four independent lines at two developmental stages (Figures 2B and 2C). GUS activity was reduced substantially in all lines early in development (Figure 2B), and this reduction was maintained as the plant developed further (Figure 2C). There were slight differences in the degree of response to the cold treatment between the lines, suggesting that chromosomal position effects may influence the ability of the transgene to be repressed by vernalization. However, for each line, the degree of repression was consistent for the two time points, suggesting that the degree of repression of FLC::GUS expression is maintained during development. The level of vernalization-induced FLC::GUS repression in lines containing the 6-kb construct is consistent with the level of repression of the endogenous FLC gene (reproducibly an ∼20-fold decrease in expression after a 28-day cold treatment).
Progeny of vernalized plants exhibited FLC::GUS activity identical to that of nonvernalized plants (data not shown), indicating that the vernalization-induced repression of FLC::GUS is not present in the next generation. Thus, segments 1 to 7 present in the 6-kb FLC region contain the sequences necessary and sufficient for the repression of FLC expression after vernalization, for the mitotic stability of expression level, and for derepression in progeny.
Promoter Sequences Alone Do Not Direct a Response to Vernalization
To define further the regions involved in the response to vernalization, transgenic plants containing smaller regions of the FLC gene fused to GUS were generated. Twenty single-locus homozygous lines that carried a construct (Figure 1C) containing 2 kb of FLC sequence (Figure 1G, segment 1) upstream of the ATG fused to GUS were generated. The staining pattern of a line containing this construct is shown in Figures 3G, 3I, and 3K, and the relative expression level of 1-day cold-treated (nonvernalized) plants for all 20 lines is given in Figure 2A. Expression of this construct differed from that of the 6-kb construct. GUS activity in 2- and 5-day-old nonvernalized seedlings was higher in cotyledons but weaker in roots than for the 6-kb construct. The expression in older plantlets was on average lower than for the 6-kb construct (Figures 2A and 3C).
In marked contrast to the 6-kb construct and the endogenous FLC gene, the pattern and level of expression of the transgene showed only a slight response to vernalization (Figures 2B, 2C, 3H, 3J, and 3L). Seedlings analyzed at 0, 3, or 13 days after removal from the cold showed little repression of FLC::GUS expression. In 3-day-old seedlings, there was a reduction in expression in the hypocotyl, but in other tissues, the expression level was unaffected by vernalization. Flowering time was decreased in the vernalized 2-kb construct plants, indicating that the vernalization treatment was successful and that the endogenous FLC level was reduced. These results indicate that the 2-kb promoter segment is not able to drive the response to cold treatment. Because the 6-kb construct is able to respond, the 4-kb region downstream of the ATG presumably is involved in the vernalization-induced reduction in FLC activity.
Intragenic Sequences Alone Cannot Confer a Response to Vernalization on a Heterologous Promoter
To assess whether the 4-kb FLC intragenic region alone can confer the ability to respond to vernalization, or whether promoter sequences also are required, we fused the 4-kb region (containing segments 2 to 7; Figure 1G) to a heterologous promoter, a 1.3-kb 35S promoter of Cauliflower mosaic virus (CaMV) (Figure 4A). Many of the lines had a high level of expression, as expected for the strong CaMV 35S promoter; however, several lines had weaker expression, similar in intensity to that of the 6-kb FLC::GUS lines. None of these lines showed an alteration in their level of expression in response to a 28-day cold treatment when examined either immediately after the end of the cold treatment (Figures 4B and 4D) or 8 days later (Figures 4C and 4E). Thus, the 4-kb exonI-intronI-exonII region alone is not sufficient to confer the downregulation of FLC::GUS expression in response to vernalization. This finding suggests that sequence elements within the FLC promoter (or the 5′ untranslated region) and within the 4-kb intragenic region both are required for the initial downregulation of the FLC gene.
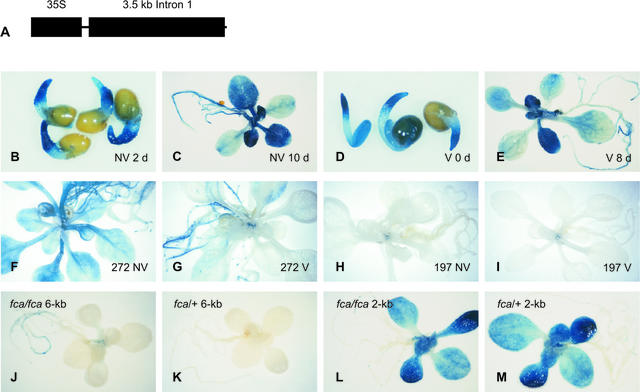
Figure 4.
Promoter Sequences Are Required for FLC::GUS Expression and Response to Vernalization, and Intragenic Sequences Are Important for the Regulation of FLC::GUS by FCA.
(A) Diagram showing a construct with the CaMV promoter fused to the 4-kb intragenic region of FLC. This cassette was fused to the GUS reporter gene and the 3′ octopine synthase terminator at the 3′ end. Black boxes represent the CaMV 35S promoter or the FLC intron I, as indicated; the thin line represents FLC coding regions.
(B) to (E) C24 lines containing the 35S::FLC::GUS transgene.
(B) Seedlings after 1 day of cold treatment (NV), stained after 2 days of growth at 23°C.
(C) Plants after 1 day of cold treatment, stained after 10 days of growth at 23°C.
(D) Seedlings after 28 days of cold treatment (V), stained at the end of the cold treatment.
(E) Plants after 28 days of cold treatment, stained after 8 days of growth at 23°C.
(F) and (G) FLC::GUS construct with 272 bp of promoter plus the 4-kb intragenic region (272).
(H) and (I) FLC::GUS construct with 197 bp of promoter plus the 4-kb intragenic region (197).
Imbibed seeds were cold treated for either 1 day ([F] and [H]) or 28 days ([G] and [I]) and stained after 15 and 13 days of growth at 23°C, respectively.
(J) to (M) F1 plants of fca-1 lines containing either the 6-kb construct ([J] and [K]) or the 2-kb construct ([L] and [M]) crossed to either fca-1 (fca/fca) ([J] and [L]) or Ler (fca/+) ([K] and [M]).
Intron I Is Important for the Maintenance of the Vernalization-Induced Repression of FLC Expression
We made a construct with a 2.8-kb deletion extending over a large part of intron I (Figure 1D, Δ2.8). This construct, lacking segments 3 to 6 (Figure 1G), directed a nonvernalized pattern and level of expression similar to those of the 6-kb construct (Figures 2A, 3M, 3O, and 3Q); however, its response to vernalization was different. When 28-day cold-treated seedlings were examined immediately upon removal from the cold, FLC::GUS expression was absent (Figure 3N), indicating that the initial cold-induced downregulation occurred. However, after 13 days of growth at 23°C, there was little difference between the expression in vernalized and nonvernalized plants (Figures 2C, 3Q, and 3R). At an intermediate time point, 3 days after removal from the cold, expression in the vernalized Δ2.8 FLC::GUS seedlings was similar to that of the 6-kb construct in roots and stipules; in addition, GUS activity was found in the newly emerging true leaves (Figure 3P).
Thus, the Δ2.8 construct shows the initial repression of FLC::GUS, but the repression is short-lived and expression returns as the plant develops. As with other constructs, there was some variation in the response among lines (Figures 2B and 2C), suggesting an influence of the chromosomal location of the transgene on the maintenance of the repression.
Two constructs with smaller deletions of the intron sequence, one a 1.3-kb 5′ deletion (Δ1.3, deleting segment 4; Figures 1E and 1G) and the other a 1.2-kb 3′ deletion (Δ1.2, deleting segment 6; Figures 1F and 1G), were generated. Both constructs had a reduced level of expression in nonvernalized plants relative to the 6-kb and the Δ2.8 constructs (Figure 2). The reason for this is not clear, but presumably a balance of cis elements is required to direct full expression. Transgenic lines of both constructs showed the initial cold-induced repression of FLC::GUS expression (Figure 2B), and this repression was maintained in older plants (Figure 2C). Both constructs contain segments 3 and 5 that are deleted in the Δ2.8 construct (Figure 1G), suggesting either that these segments contain sequences capable of maintaining the vernalization-induced repression or that segments 4 or 6 can act redundantly to direct stable repression.
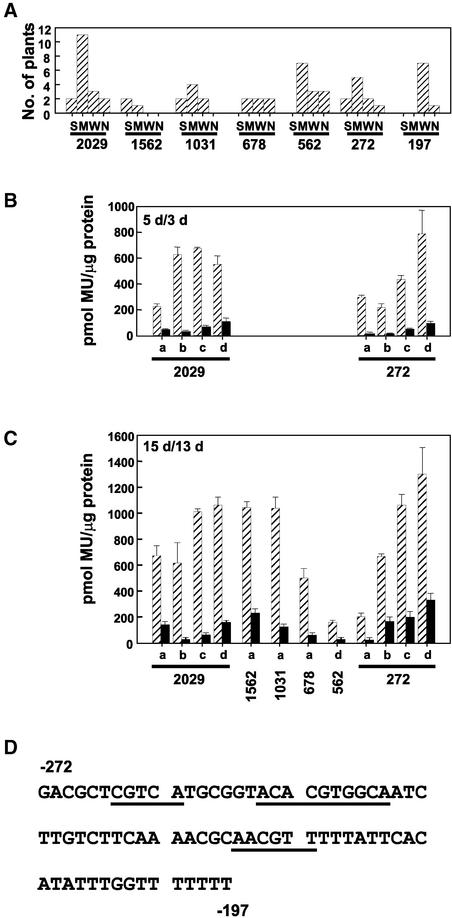
A 75-bp Promoter Sequence Is Important for Nonvernalized FLC Expression
We have established that promoter sequences in conjunction with intragenic regions are required for the stable downregulation of FLC in response to vernalization. We made a series of constructs containing the 4-kb intragenic region but with a range of deletions in the promoter. Constructs containing 2029, 1562, 1031, 678, 526, or 272 bp of FLC sequence upstream of the ATG all directed a similar pattern of expression (Figures 3E and 4F and data not shown). There was some variation in the expression level of the different constructs (Figure 5A). Constructs containing 678 and 526 bp of promoter generally had a lower level of expression than constructs with longer promoter regions or the construct containing 272 bp of promoter. This finding suggests that a positive regulatory element lies between −678 and −1031 bp and that a negative regulatory element may lie between −526 and −272 bp.
Figure 5.
A 75-bp Promoter Region That Is Important for FLC Expression Contains Three Potential b-ZIP Binding Sites.
(A) Distribution of staining intensities for seven FLC::GUS promoter deletion constructs. The length of FLC sequence (in bp), upstream of the ATG, present in the construct is given below each graph. Each construct also contained the 4-kb intragenic sequence. The construct with 2029 bp of promoter is the 6-kb construct in Figures 1 to 3. Except for the 197-bp construct, each independent line was classified as strong (S), medium (M), weak (W), or no staining (N), as described in Methods. Strong, medium, and weak staining equates to >700, 350 to 700, and <350 pmol of 4-methyl umbelliferone (MU) per microgram of protein, respectively, in (C). For the 197-bp construct, W indicates weak expression in stipules only, not in other tissues.
(B) Methylumbelliferyl-β-d-glucopyranoside activity data for four selected lines (a to d) for the two FLC::GUS constructs with either 2029 or 272 bp of FLC promoter for 3-day-old vernalized (black bars) and 5-day-old nonvernalized (hatched bars) seedlings. Error bars represent the standard error.
(C) Methylumbelliferyl-β-d-glucopyranoside activity data for up to four selected lines (a to d) for the promoter deletion constructs in (A) for 13-day-old vernalized (black bars) and 15-day-old nonvernalized (hatched bars) plants. Error bars represent the standard error. The 272-bp line d is shown in Figure 4.
(D) Sequence of the 75-bp region with potential b-ZIP recognition motifs underlined.
Constructs containing between 2029 and 272 bp of promoter, in addition to the 4-kb intragenic region, exhibited strong repression of FLC::GUS activity in response to a 28-day cold treatment and showed developmental maintenance of the reduced expression (Figures 3G, 5B, and 5C). These results indicate that as little as 272 bp upstream of the ATG is sufficient, in combination with intragenic sequences, for the initial downregulation.
A construct containing 197 bp of sequence upstream of the ATG codon, in addition to the 4-kb intragenic region, directed much reduced expression in nonvernalized plants relative to longer promoter constructs (Figures 3H and 5A). There was no further reduction after vernalization (Figure 4I). The longest characterized FLC cDNA clone starts at −109 bp (Sheldon et al., 1999); thus, this construct contains a maximum of 88 bp upstream of the transcriptional start site. Comparison of the expression between the 197- and 272-bp constructs defines a 75-bp region (−272 to −197) as essential for nonvernalized expression. This 75-bp sequence contains three potential b-ZIP binding sites (Figure 5D), which have been characterized in other gene promoters as being involved in gene regulation by abscisic acid or light (Iwasaki et al., 1995; Terzaghi and Cashmore, 1995). FLC expression in response to applied abscisic acid (4 or 24 h of treatment) and a 1-h light induction was examined in 10-day-old plants of the C24 and Columbia ecotypes; however, there was little detectable effect of either treatment on FLC expression (data not shown).
Intragenic Regions Are Important for the Repression of FLC Expression by FCA
In addition to regulation by prolonged cold, FLC expression also is regulated by genes active in the autonomous pathway (Sheldon et al., 1999, 2000b). We investigated which sequences are required for the repression of FLC by one autonomous pathway gene, FCA. The late-flowering fca-1 mutant, in the Ler ecotype, has a higher level of FLC expression than wild-type Ler, indicating that the FCA gene product represses FLC expression (Sheldon et al., 1999; Rouse et al., 2002). However, the FLC expression level in fca-1 is substantially lower than the FLC level in C24. Consistent with this fact, the 6-kb FLC::GUS construct directed weak expression in fca-1. Homozygous fca-1 lines containing the 6-kb construct were crossed to both the wild-type Ler and the fca-1 mutant to create F1 progeny hemizygous for the FLC::GUS transgene, with either one or no wild-type FCA allele. Figures 4J and 4K show that expression of the 6-kb construct was abolished by one active FCA allele, indicating that the product of the dominant wild-type FCA allele may interact with sequences in the 6-kb region to repress FLC::GUS.
We also examined the effect of FCA activity on the 2-kb FLC::GUS construct. Homozygous T3 fca-1 lines containing the 2-kb FLC::GUS construct demonstrated an unexpectedly high level of GUS activity, higher than that of the same construct in C24. This high level of expression was not attributable to the fca mutation per se, because the same construct transformed into wild-type Ler also resulted in high levels of expression (data not shown). Similar levels of expression also were obtained when the 2-kb construct was introduced into the Columbia or Wassilewskija-2 ecotypes. F1 plants of crosses between fca-1 lines containing the 2-kb construct and either Ler or fca-1 were indistinguishable (Figures 4L and 4M), indicating that, with the 2-kb reporter construct, the wild-type allele of FCA had no effect on expression. These results indicate that the 4-kb intragenic region is required for the repression by FCA.
DISCUSSION
Different FLC Regulatory Regions Are Required for the Initial Vernalization-Induced Repression and for the Maintenance of the Repression
The FLC gene encodes a dosage-dependent repressor of flowering, with the level of FLC expression being a major determinant of the time of flowering in ecotypes and some mutants of Arabidopsis. The level of expression of FLC is regulated by genes that modulate flowering time in response to environmental cues, such as vernalization and photoperiod, as well as by genes that regulate flowering time independent of environmental controls, the autonomous pathway genes (Sheldon et al., 1999; Rouse et al., 2002). We have focused on the regions of the FLC gene required for the repression of FLC by vernalization, but in doing so, we also identified regions required for normal FLC expression. We have shown that different FLC genomic regions are required for the cold-induced downregulation of FLC expression, and for the maintenance of the low expression level at normal growth temperatures, after vernalization. These results support the analysis using the VRN2 gene that indicated that there are two components to the FLC vernalization response: the initial repression and the maintenance of repression (Gendall et al., 2001).
The poor response to cold treatment of the FLC::GUS reporter construct containing 2 kb of promoter sequence compared with the ability of the 6-kb construct, which also included 4 kb of intragenic sequence, to direct both the initial cold-induced repression and the mitotic maintenance of repression suggests that intragenic regions are important for the full vernalization response. The intragenic region alone is not sufficient to confer a vernalization response to a heterologous promoter. Together, these results suggest that sequences within both the promoter (or the 5′ untranslated region) and the intragenic region are required for the stable downregulation of FLC expression by cold.
The involvement of intragenic sequences in the regulation of MADS box genes also has been reported for the AGAMOUS (AG) gene (Sieburth and Meyerowitz, 1997). AG contains a large intron immediately 3′ to the MADS box, in a position similar to that of FLC. AG::GUS constructs containing solely promoter sequences have a widespread expression pattern, and sequences within the intron are required for the repression by regulatory factors that constrain AG expression to its correct domain (Sieburth and Meyerowitz, 1997).
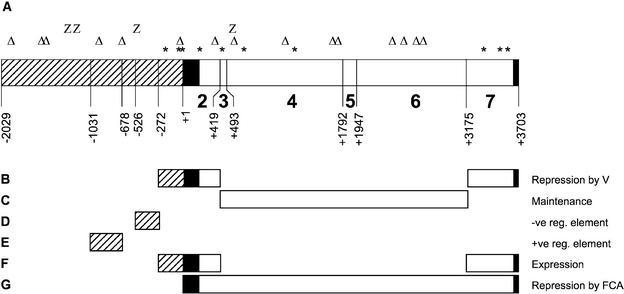
We have shown that as little as 272 bp upstream of the ATG, and the intragenic segments 2 and 7 (Figures 6A and 6B), are required for the cold-induced repression. Little is known about the mechanism by which the plant, or imbibed seed, perceives the prolonged cold treatment and how this results in the repression of FLC expression. Further analysis of the sequence elements, and their interacting factors, required for this repression may enable the molecular details of this repression to be revealed.
Figure 6.
Regulatory Regions of FLC.
(A) Representation of the FLC sequence contained within the 6-kb construct. GAGAG sites and potential PHO and Zeste recognition motifs are indicated (asterisk, triangle, and Z, respectively). FLC segment, nucleotide numbering, and shading are as in Figure 1.
(B) Regions sufficient for vernalization (V)-induced repression of FLC expression, as inferred from the Δ2.8 construct and the 272-bp promoter construct. A construct with 272 bp of promoter and with the 2.8-kb deletion from intron I was not tested for its response to vernalization.
(C) Region required for the maintenance of vernalization-induced repression. The entire region is not required, because partial deletions in the Δ1.2 and Δ1.3 constructs directed stable repression.
(D) Region containing potential negative regulatory elements.
(E) Region containing potential positive regulatory elements.
(F) Regions sufficient for expression in nonvernalized plants, as inferred from the Δ2.8 construct and the 272-bp promoter construct. The GUS activity in a construct with 272 bp of promoter and with the 2.8-kb deletion from intron I was not tested.
(G) The repression of FLC expression by FCA requires the 4-kb intragenic region. We have not determined whether the promoter sequence is required in addition to the intragenic region.
The lack of maintenance of the low expression state during the development of plants containing the Δ2.8 construct suggests that some part of the 2.8-kb intron region (Figure 6C) is critical for the maintenance of the vernalization-induced repression of FLC. Gendall et al. (2001) have shown that the VRN2 gene is required for the stable repression of FLC after vernalization, although it is not known if FLC is a direct target of VRN2. VRN2 has homology with the Drosophila PcG gene Suppressor of zeste 12 (Birve et al., 2001), implicating PcG-mediated repression as the mechanism for the stable repression of FLC (Gendall et al., 2001). The deletion of the 2.8-kb intron I segment may have disrupted the ability of a PcG complex to stably repress FLC expression.
In Drosophila, PcG proteins have been shown to form large complexes that interact with chromatin and function to stably repress the transcription of genes that were repressed initially by other factors (Brock and van Lohuizen, 2001). Three PcG proteins have been shown to have DNA binding ability: the GAGA factor, encoded by the Trithorax-like gene (Horard et al., 2000); the zinc finger–containing Pleiohomeotic protein (PHO; Brown et al., 1998); and the Zeste protein (Hur et al., 2002). These proteins appear to recruit other PcG proteins to chromatin; however, it is likely that these are not the only recruiting factors (Busturia et al., 2001).
It is not clear whether there are functional homologs of these proteins in Arabidopsis; however, it is intriguing that at least nine GAGAG elements (GAGA factor recognition motifs), in two clusters, are present in the FLC sequence contained within the 6-kb construct (Figure 6A). There are 15 potential recognition sites ([G/a]CCAT; Fritsch et al., 1999) for the Pleiohomeotic protein and four potential recognition motifs for Zeste ([T/C]GAG[T/C]G; Benson and Pirrotta, 1988) within the 6-kb region (Figure 6A). Some of these sites lie within the region deleted (segments 3 to 6; Figure 6A) in the Δ2.8 construct. If these elements are important for recruiting PcG complexes to chromatin in Arabidopsis, as they are in animal systems, deletion of these sites could explain the lack of stable repression in lines carrying this construct. Restoration of segments 3 and 4 in the Δ1.3 construct, and of segments 3, 5, and 6 in the Δ1.2 construct, restores a subset of the GAGA and PHO recognition motifs, possibly accounting for the restoration of the stability of repression. However, until functional relevance is demonstrated for these recognition motifs, such interpretations should be treated with caution. Alternatively, restoration of other unknown elements within these regions, or the alteration of spacing between the ends of the intron, may contribute to the restoration of the ability to maintain repression after vernalization.
All constructs that exhibited a stable repression of FLC::GUS expression during the development of the vernalized plant produced progeny with a level of FLC::GUS expression identical to that of a nonvernalized plant (data not shown), indicating that, as with the endogenous FLC gene, the expression of FLC::GUS was derepressed in progeny. The mechanism of derepression may be an inactivation or removal of one or more factors present in the repressive PcG complex during meiosis or at some stage in seed production. Alterations of histone tail modifications, such as acetylation and methylation, change the ability of the PcG complexes to be recruited to chromatin and are associated with alterations in stable expression states (Poux et al., 2002; Simon and Tamkun, 2002).
Correct Expression in Nonvernalized Plants Requires Promoter and Intragenic Sequences
Analysis of the promoter requirements for expression (in the context of the 4-kb intragenic region) revealed several possible regulatory regions. There appears to be a negative regulatory region (Figure 6D) as well as a positive regulatory region (Figure 6E). The FLC sequence that is required for normal expression in C24 includes 272 bp of promoter and 5′ untranslated region and segments 2 and 7 of the intragenic region (Figure 6F). The 75-bp region within the promoter that is critical for FLC expression contains a number of motifs that may be important for FLC regulation, including b-ZIP binding motifs that are similar to elements known to be involved in the control of gene expression by abscisic acid or by light (Iwasaki et al., 1995; Terzaghi and Cashmore, 1995). However, at the whole seedling level, there appears to be little effect on FLC expression by treatment with abscisic acid or by light induction. A role of abscisic acid in floral repression has been suggested (Chandler et al., 2000), but abscisic acid appears not to play a role in the vernalization response (Liu et al., 2002). Additional experiments are required to assess the importance of these motifs and their interacting factors.
In Ler and in two other ecotypes, the 2-kb FLC::GUS construct had a higher expression level than the 6-kb construct and the endogenous FLC gene. Therefore, in these ecotypes, the intragenic region of FLC modifies the expression directed by the promoter sequence, analogous to the action of the AG intragenic sequence (Sieburth and Meyerowitz, 1997). In C24 plants, the 2-kb promoter construct directed a lower level of expression than the 6-kb construct. It is not clear why this effect is different from that of the other three ecotypes, but presumably, ecotype-specific alleles of regulatory genes are involved.
FCA repression of FLC expression, like repression by vernalization, requires the 4-kb intragenic region (Figure 6G). This repression may be direct, or intermediary genes may be involved. Because FCA contains RNA binding domains and its regulation of FLC requires sequences that include an intron, it is tempting to speculate that it is involved in the post-transcriptional processing of the FLC transcript. We have not investigated whether the FLC promoter region also is required for repression by FCA.
FLC expression is under the control of many loci, which function to either upregulate or downregulate expression, resulting in a fine-tuning of the FLC expression level. The regulation of FLC expression is important for determining the flowering time of the plant and ensures that flowering occurs at the most advantageous time. With the FLC::GUS constructs, we have the tools to investigate how these genes regulate FLC; ultimately, a map of the genetic controls of FLC expression may be elucidated.
METHODS
FLC::β-Glucuronidase Gene Fusion Constructs
FLC::β-Glucuronidase (GUS) gene fusion constructs were generated by introducing FLC gene sequences from the C24 ecotype upstream of the GUS::3′ octopine synthase terminator cassette in the pHW9 vector (Dolferus et al., 1994) using a combination of subcloning and PCR approaches. All PCR products, and the subcloning junction sites, were checked by sequencing. The FLC::GUS::3′ octopine synthase cassettes were subcloned into the binary vector pBin19 (Bevan, 1984) for plant transformation. The FLC sequences present in the constructs are indicated in Figure 1. The final codon of exon II was altered from TTG to ATG using a PCR approach to create an NcoI site, allowing a translational fusion with the NcoI site at the ATG of the GUS gene. The lengths of promoter fragments for the promoter deletion series constructs are given in Figure 5. For the 35S::FLC::GUS construct, the 2029-bp FLC promoter in the 6-kb construct was replaced by the 1.3-kb promoter of Cauliflower mosaic virus from pART7 (Gleave, 1992). Full details of the constructs are available on request.
Plant Material, Plant Transformation, and Growth Conditions
Arabidopsis thaliana ecotypes and the fca-1 mutant were obtained from the ABRC (Ohio State University, Columbus). Binary vectors were introduced into Agrobacterium tumefaciens GV3101 by triparental mating (Sheldon et al., 1999), and transformants were generated using the floral dip method (Clough and Bent, 1998). T2 lines that segregated ∼3:1 for the NPTII selection marker, indicating that they contained a single locus of T-DNA insertion, were identified, and homozygous T3 progeny were obtained. PCR, using construct-specific primers, was used to confirm the presence of the appropriate transgene in each line.
Analyses of transgenic lines were performed on homozygous T3 plants. T3 seeds were sown in vitro as described (Sheldon et al., 1999) and were cold treated (at 5 ± 1°C) in dim light for 1 day (nonvernalized) or 28 days (vernalized) before growth under cool-white fluorescent lights with a 16-h photoperiod at 23°C.
Genetic crosses were performed with the transgenic plant as the pollen donor. Successful crosses were identified by kanamycin-resistant F1 plants. The results presented in Figures 4J to 4M were confirmed in crosses of independent lines.
GUS Analysis
GUS activity in transgenic plants was revealed by incubation in 100 mM NaPO4, pH 7.2, 2.5 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (BioVectra, Charlottetown, Canada), 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, 10 mM Na2EDTA, 0.3% Triton X-100, and 10% methanol. Plant tissue was vacuum infiltrated before incubation at 37°C for 20 h. After staining, chlorophyll was cleared from the samples by dehydration through an ethanol series. Vernalized plants were stained at 0, 3, and 13 days after removal from the cold. Nonvernalized plants were stained at comparable stages of development, usually 2, 5, and 15 days old, respectively. All independent lines for each construct that were included in the analysis had very similar patterns of expression. Staining in roots, hypocotyls, cotyledons, leaves, and stipules of 10-day-old plants was scored as 0 (no staining) to 3 (strong staining) for each T3 line; these scores were summarized to give a measure of staining intensity within the plant as a whole of strong, medium, weak, or no staining. Photography was performed using a Zeiss MC80-DX microscope with a MC80-DX camera (Jena, Germany).
Quantitation of GUS activity was achieved using the substrate methylumbelliferyl-β-d-glucopyranoside (Diagnostic Chemicals, Charlottetown, Canada) and measuring the fluorescence of the product with excitation and emission wavelengths of 365 and 455 nm, respectively. Protein was extracted from plants and methylumbelliferyl-β-d-glucopyranoside assays were performed essentially as described by Jefferson (1987), with end point measurements taken at 8 h (product accumulation was linear between 0 and 18 h). Plants were harvested at 3 and 13 days after removal from the cold for vernalized plants and at comparable developmental stages for nonvernalized plants. Two biological replicates for each line were assayed, and each assay was replicated at least twice. The fluorescence values were normalized against protein concentration, as determined using Bradford protein assays (Bio-Rad), according to the manufacturer's instructions.
cis Element Analysis
Identification of potential regulatory motifs was performed using the PLACE database (Higo et al., 1999) and the PlantCARE database (Lescot et al., 2002).
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We thank Jean Finnegan and Dean Rouse for discussions.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.004564.
References
- Benson, M., and Pirrotta, V. (1988). The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: Implications for transvection and gene regulation. EMBO J. 12, 3907–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan, M. (1984). Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 12, 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birve, A., Sengupta, A.K., Beuchle, D., Larsson, J., Kennison, J.A., Rasmunson-Lestander, A., and Mueller, J. (2001). Su(z)12, a novel Drosophila Polycomb group gene that is conserved in vertebrates and plants. Development 128, 3371–3379. [DOI] [PubMed] [Google Scholar]
- Brock, H.W., and van Lohuizen, M. (2001). The Polycomb group: No longer an exclusive club? Curr. Opin. Genet. Dev. 11, 175–181. [DOI] [PubMed] [Google Scholar]
- Brown, J.L., Mucci, D., Whitely, M., Dirksen, M.-L., and Kassis, J.A. (1998). The Drosophila Polycomb group gene pleiohomeotic encodes a sequence specific DNA binding protein with homology to the multifunctional mammalian transcription factor YY1. Mol. Cell 1, 1057–1064. [DOI] [PubMed] [Google Scholar]
- Busturia, A., Lloyd, A., Bejarano, F., Zavortink, M., Xin, H., and Sakonju, S. (2001). The MCP silencer of the Drosophila Abd-B gene requires Pleiohomeotic and GAGA factor for the maintenance of repression. Development 128, 2163–2173. [DOI] [PubMed] [Google Scholar]
- Chandler, J., Martinez-Zapater, J.M., and Dean, C. (2000). Mutations causing defects in the biosynthesis and response to gibberellins, abscisic acid and phytochrome B do not inhibit vernalization in Arabidopsis fca-1. Planta 210, 677–682. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dolferus, R., Jacobs, M., Peacock, W.J., and Dennis, E.S. (1994). Differential interactions of promoter elements in stress response of the Arabidopsis Adh gene. Plant Physiol. 105, 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch, C., Brown, J.L., Kassis, J.A., and Mueller, J. (1999). The DNA-binding Polycomb group protein Pleiohomeotic mediates silencing of a Drosophila homeotic gene. Development 126, 3905–3913. [DOI] [PubMed] [Google Scholar]
- Gendall, A.R., Levy, Y.Y., Wilson, A., and Dean, C. (2001). The VERNALIZATION2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107, 525–535. [DOI] [PubMed] [Google Scholar]
- Gleave, A.P. (1992). A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20, 1203–1207. [DOI] [PubMed] [Google Scholar]
- Higo, K., Ugawa, Y., Iwamoto, M., and Korenaga, T. (1999). Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 27, 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horard, B., Tatout, C., Poux, S., and Pirrotta, V. (2000). Structure of a Polycomb response element and in vitro binding of Polycomb group complexes containing GAGA factor. Mol. Cell. Biol. 20, 3187–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur, M.-W., Laney, J.D., Jeon, S.-H., Ali, J., and Biggin, M.D. (2002). Zeste maintains repression of Ubx transgenes: Support for a new model of Polycomb repression. Development 129, 1339–1343. [DOI] [PubMed] [Google Scholar]
- Iwasaki, T., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1995). Identification of a cis-regulatory region of a gene in Arabidopsis thaliana whose induction by dehydration is mediated by abscisic acid and requires protein synthesis. Mol. Gen. Genet. 247, 391–398. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A. (1987). Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 5, 387–405. [Google Scholar]
- Lescot, M., Déhais, P., Thijs, G., Marchal, K., Moreau, Y., Van de Peer, Y., Rouzé, P., and Rombauts, S. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Gilmour, S.J., Thomashow, M.F., and van Nocker, S. (2002). Cold signalling associated with vernalization in Arabidopsis thaliana does not involve CBF1 or abscisic acid. Physiol. Plant. 114, 125–134. [DOI] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (2000). Memories of winter: Vernalization and the competence to flower. Plant Cell Environ. 23, 1145–1153. [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (2001). Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13, 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poux, S., Horard, B., Sigrist, C.J.A., and Pirrotta, V. (2002). The Drosophila Trithorax protein is a coactivator required to prevent re-establishment of Polycomb silencing. Development 129, 2483–2493. [DOI] [PubMed] [Google Scholar]
- Rouse, D.T., Sheldon, C.C., Bagnall, D.J., Peacock, W.J., and Dennis, E.S. (2002). FLC, a repressor of flowering, is regulated by genes in different inductive pathways. Plant J. 29, 183–191. [DOI] [PubMed] [Google Scholar]
- Sheldon, C.C., Burn, J.E., Perez, P.P., Metzger, J., Edwards, J.A., Peacock, W.J., and Dennis, E.S. (1999). The FLF MADS box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, C.C., Finnegan, E.J., Rouse, D.T., Tadege, M., Bagnall, D.J., Helliwell, C.A., Peacock, W.J., and Dennis, E.S. (2000. a). The control of flowering by vernalization. Curr. Opin. Plant Biol. 3, 418–422. [DOI] [PubMed] [Google Scholar]
- Sheldon, C.C., Rouse, D.T., Finnegan, E.J., Peacock, W.J., and Dennis, E.S. (2000. b). The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC). Proc. Natl. Acad. Sci. USA 97, 3753–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth, L.E., and Meyerowitz, E.M. (1997). Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, J.A., and Tamkun, J.W. (2002). Programming off and on states in chromatin: Mechanisms of Polycomb and Trithorax group complexes. Curr. Opin. Genet. Dev. 12, 210–218. [DOI] [PubMed] [Google Scholar]
- Terzaghi, W.B., and Cashmore, A.R. (1995). Light regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 445–474. [Google Scholar]