Abstract
Cell suspensions obtained from Nicotiana plumbaginifolia plants stably expressing the apoaequorin gene were used to analyze changes in cytosolic free calcium concentrations ([Ca2+]cyt) in response to elicitors of plant defenses, particularly cryptogein and oligogalacturonides. The calcium signatures differ in lag time, peak time, intensity, and duration. The intensities of both signatures depend on elicitor concentration and extracellular calcium concentration. Cryptogein signature is characterized by a long-sustained [Ca2+]cyt increase that should be responsible for sustained mitogen-activated protein kinase activation, microtubule depolymerization, defense gene activation, and cell death. The [Ca2+]cyt increase in elicitor-treated cells first results from a calcium influx, which in turns leads to calcium release from internal stores and additional Ca2+ influx. H2O2 resulting from the calcium-dependent activation of the NADPH oxidase also participates in [Ca2+]cyt increase and may activate calcium channels from the plasma membrane. Competition assays with different elicitins demonstrate that [Ca2+]cyt increase is mediated by cryptogein–receptor interaction.
INTRODUCTION
Calcium as a ubiquitous internal second messenger can regulate diverse cellular processes in plants, conveying signals received at the cell surface to the inside of the cell through spatiotemporal concentration changes that are decoded by an array of Ca2+ sensors (Trewavas and Malhó, 1998; Zielinski, 1998; Sanders et al., 1999; Reddy, 2001). Under resting conditions, the cytosolic free calcium concentration ([Ca2+]cyt) is low, between 100 and 200 nM (Bush, 1995), 104 times less than that in the fluid surrounding the cell and 104 to 105 less than that in the vacuolar compartment, which is an intracellular calcium store.
In plants, [Ca2+]cyt increase has been reported in response to various stimuli, including mechanical and low-temperature signals (Knight et al., 1991, 1992, 1996; van Der Luit et al., 1999), hypoosmotic shock (Taylor et al., 1996; Takahashi et al., 1997; Cessna et al., 1998), light (Shacklock et al., 1992; Baum et al., 1999), oxidative stress (Price et al., 1994; Pei et al., 2000), ozone (Clayton et al., 1999), hormones (Gilroy and Jones, 1992; McAinsh et al., 1992), Nod factors (Ehrhardt et al., 1996), and elicitors (Knight et al., 1991; Mithöfer et al., 1999; Blume et al., 2000). Thus, Ca2+ apparently mediates different cell-specific processes in plant growth, development, and defense responses. How this common Ca2+ signaling links different signals to so many diverse and specific responses has been an area of intense research for many years. There is now evidence that the temporal and spatial nature and the amplitude of [Ca2+]cyt changes (the calcium signature) caused by a given signal contribute to the specificity of the response (Trewavas, 1999; Knight, 2000). Moreover, the same signal induces different calcium signatures depending on the organ, the tissue, or the cell type in a tissue (McAinsh and Hetherington, 1998; Kiegle et al., 2000; Reddy, 2001). The origin of [Ca2+]cyt increase (extracellular medium, organelle types, and/or both) also may be important in the physiological response (Knight et al., 1996; van Der Luit et al., 1999).
Plants normally are subjected to a large variety of microorganisms, such as fungi, bacteria, and viruses, and they have developed molecular systems to perceive signal molecules from pathogenic or symbiotic microorganisms and to convert them into an adaptive response. Efficient mechanisms for resisting invading pathogens include the hypersensitive reaction and systemic acquired resistance (Dangl et al., 1996; Ryals et al., 1996). Elicitins secreted by Phytophthora species are 10-kD proteins with 74% sequence conservation that induce such defense systems in tobacco plants (Ricci et al., 1989; Bonnet et al., 1996). The mode of action of cryptogein, secreted by the oomycete Phytophthora cryptogea, has been investigated using tobacco (Nicotiana tabacum var Xanthi) cell cultures. The sequence of events triggered by cryptogein include its high-affinity binding on plasma membrane (PM) glycoprotein(s) (Wendehenne et al., 1995; Bourque et al., 1998, 1999), followed by the phosphorylation of a variety of proteins (Viard et al., 1994; Lecourieux-Ouaked et al., 2000). Manipulating the phosphorylation state of proteins by either staurosporine, a protein kinase inhibitor, or calyculin A, a protein phosphatase inhibitor, suppressed or mimicked cryptogein effects, respectively, establishing that reversible phosphorylation is a key process in the transduction pathway (Lecourieux-Ouaked et al., 2000).
Protein phosphorylation is followed by a large and sustained calcium influx (Tavernier et al., 1995) and subsequent calcium-dependent cellular responses, including (1) anion and K+ efflux (Blein et al., 1991; Pugin et al., 1997); (2) PM depolarization (Pugin et al., 1997); (3) activation of mitogen-activated protein kinases (MAPKs) (Lebrun-Garcia et al., 1998); (4) activation of a NADPH oxidase responsible for the transient production of active oxygen species (AOS) (Bottin et al., 1994; Pugin et al., 1997), cytosol acidification, and large changes in sugar metabolism (Pugin et al., 1997); (5) microtubule depolymerization (Binet et al., 2001); (6) phytoalexin synthesis (Milat et al., 1991); and, much later, (7) cell death (Binet et al., 2001). All of these effects were prevented when calcium influx was compromised either by a calcium chelator (EGTA) or a calcium surrogate (La3+). Moreover, decreasing the external Ca2+ concentration by adding EGTA or La3+ during treatments with cryptogein suppressed the biological effects of the elicitor, indicating that a sustained Ca2+ influx was necessary throughout the treatment. These results highlight the involvement of extracellular Ca2+ in this signaling process.
Taking into account the importance of [Ca2+]cyt in signal transduction, we investigated the [Ca2+]cyt changes in Nicotiana plumbaginifolia cells treated with different elicitors that induce (cryptogein) or do not induce (oligogalacturonides [OGs]) cell death and analyzed the origin of [Ca2+]cyt increase in relation to other events, including AOS production. We also investigated the physiological significance of cytosolic free-calcium increases. To address these questions, we used N. plumbaginifolia cells expressing aequorin in cytosol, the luminescence of which depends on free Ca2+ concentration. Our data indicate that cryptogein is typified by a biphasic [Ca2+]cyt signature compared with four other oligosaccharide elicitors: OGs, laminarin, chitopentaose, and lipopolysaccharides. We further demonstrate that [Ca2+]cyt increase depends on cryptogein interaction with its PM receptor and involves both calcium influx from the external medium and calcium mobilization from internal stores. Cryptogein-induced H2O2 production participates in this [Ca2+]cyt increase through plasma membrane channel activation. Our data also indicate that the sustained calcium increase in cryptogein-treated cells is responsible for sustained MAPK activation, defense gene activation, and cell death.
RESULTS
Aequorin-transformed N. plumbaginifolia cell suspensions were generated from leaves of transformed N. plumbaginifolia plants obtained by Knight et al. (1991). Before studying the variations of [Ca2+]cyt in response to elicitors, we determined the sensitivity of these cell suspensions to cryptogein. Our previous data had been obtained using cell suspensions from N. tabacum var Xanthi. Data shown in Table 1 indicate that although N. plumbaginifolia cells were less sensitive to cryptogein than N. tabacum cells, all of the responses induced by the elicitor were reproduced with similar response intensities and kinetics using 1 μM cryptogein instead 0.1 μM for N. tabacum. These data led us to consider the possibility that transformed N. plumbaginifolia cells were an appropriate material in which to investigate the cryptogein-induced variations of cytosolic free Ca2+.
Table 1.
Comparison of Cryptogein Effects in N. tabacum and Aequorin-Transformed N. plumbaginifolia Cell Suspensions
| Responses after 30 min of Treatment |
N. tabacum Cells Treated with 0.1 μM Cryptogein |
N. plumbaginifolia Cells Expressing Apoaequorin Treated with 1 μM Cryptogein |
|---|---|---|
| Ca2+ influx (nmol/g fresh wt.) | 140 ± 20 | 80 ± 30 |
| Extracellular alkalinization (pH change) | 1.45 ± 0.11 | 1.15 ± 0.08 |
| AOS production (nmol H2O2/g fresh wt.) | 1175 ± 126 | 1360 ± 144 |
| MAPK activation | Yes (50 and 46 kD) | Yes (50 and 46 kD) |
| Microtubule disruption | Yes | Yes |
Calcium influx was measured using 45Ca2+ as a tracer (Tavernier et al., 1995). Extracellular pH changes were measured in cell suspensions (Tavernier et al., 1995). AOS production was monitored using chemiluminescence of luminol (Bourque et al., 1998). In-gel kinase assays were performed to analyze MAPK activation (Lebrun-Garcia et al., 1998). Microtubules were examined using classic fluorescence techniques and confocal microscopy (Binet et al., 2001). All experiments were performed at least three times.
Specific Changes in [Ca2+]cyt in Response to Different Elicitors
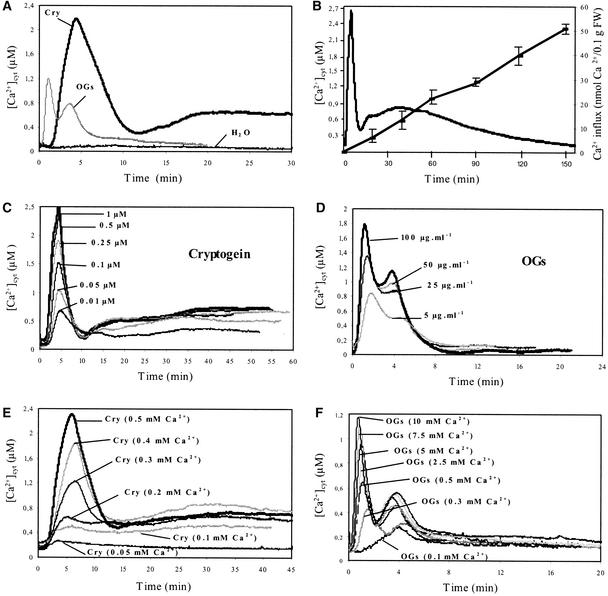
In control N. plumbaginifolia cells, the bioluminescence counts yielded resting [Ca2+]cyt values of 95 ± 25 nM (n = 10). At saturating concentrations, cryptogein or OGs induced a typical biphasic [Ca2+]cyt increase in N. plumbaginifolia cells, each characterized by specific intensity, kinetics, and duration (Figure 1A). In cryptogein-treated N. plumbaginifolia cells, a lag phase of 90 to 120 s preceded a 6-min transient and rapid [Ca2+]cyt increase, which peaked at 2.4 ± 0.17 μM (n = 10) after 5 min, and then decreased to 0.35 μM. The first peak was followed immediately by a second [Ca2+]cyt increase, which reached 0.75 ± 0.07 μM (n = 10) at 20 min after the beginning of the treatment and then decreased slowly but did not return to the background level even after ∼2.5 h of treatment. At the same time, Ca2+ influx, as monitored by 45Ca2+ accumulation, increased linearly within cryptogein-treated cells, reaching 51 ± 0.4 nmol Ca2+/0.1 g fresh weight (n = 3) after 2.5 h of treatment (Figure 1B).
Figure 1.
Changes in [Ca2+]cyt in Aequorin-Transformed Cells during Treatment with Cryptogein or OGs.
(A) Treatment with H2O, 1 μM cryptogein (Cry), or 100 μg/mL OGs.
(B) 45Ca2+ influx and [Ca2+]cyt changes in response to 1 μM cryptogein during a 3-h treatment.
(C) Dose-response relationships of [Ca2+]cyt in cryptogein-treated cells.
(D) Dose-response relationships of [Ca2+]cyt in OG-treated cells.
(E) Extracellular Ca2+ concentration dependence of cryptogein-induced [Ca2+]cyt increase.
(F) Extracellular Ca2+ concentration dependence of OG-induced [Ca2+]cyt increase.
Cryptogein treatment (1 μM) and OG treatment (100 μg/mL) were performed in medium containing different concentrations of Ca2+, as indicated. Data correspond to 1 representative experiment of 10 experiments performed. Mean values ± se are given in the text. FW, fresh weight.
After a lag phase of ∼20 s, OG treatment (Figure 1A) induced a first transient increase in [Ca2+]cyt, which peaked at 1.34 ± 0.35 μM (n = 10), within 60 to 90 s before decreasing. A second transient increase in [Ca2+]cyt occurred at 4 min after the beginning of treatment, with a maximum value of 0.9 ± 0.18 μM (n = 10). Then, [Ca2+]cyt returned to the resting value within 15 to 20 min. In cryptogein- or OG-treated cells, the magnitude of the Ca2+ response was dependent on the elicitor concentration (Figures 1C and 1D). For the first peak, the saturating concentration for cryptogein-induced [Ca2+]cyt increase was ∼500 nM, with a half-maximal effect at ∼100 nM. The magnitude and duration of the sustained second phase were similar for cryptogein concentrations between 100 and 500 nM, with 100 nM being the saturating concentration (Figure 1C). With OGs, the maximal [Ca2+]cyt increase was obtained using ∼100 μg/mL cell suspension (Figure 1D), and the intensities of both phases decreased equally with decreasing OG concentrations. Moreover, in elicitor-treated cells, the intensity of each peak of [Ca2+]cyt depended on the extracellular Ca2+ concentration. In cryptogein-treated cells, 0.5 mM extracellular Ca2+ triggered the maximal [Ca2+]cyt increases (Figure 1E), and extracellular Ca2+ concentrations >1 mM reduced both peaks (data not shown). In OG-treated cells, the Ca2+ concentration increase of the first peak was maximal with 10 mM extracellular calcium, whereas the second peak reached an optimal value with 0.5 mM extracellular calcium (Figure 1F).
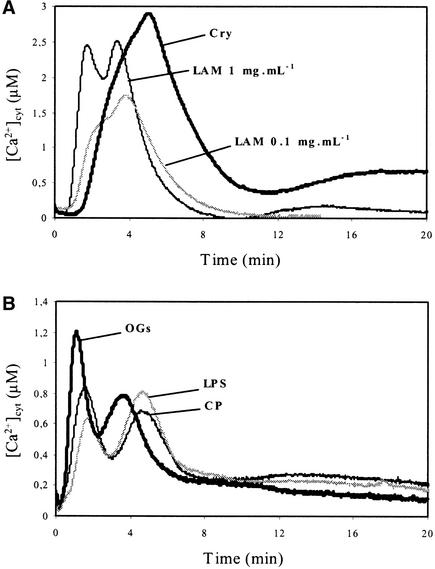
We sought to determine if oligosaccharide derivatives known to be elicitors are typified by the calcium signatures they induce. Laminarin (100 μg/mL cell suspension), a linear β-1,3-glucan from the brown alga Laminaria digitata (Klarzynski et al., 2000), induced a [Ca2+]cyt increase comparable to the first peak of the cryptogein [Ca2+]cyt responses, with a shorter lag time (60 s against 90 to 120 s with cryptogein) and a maximum [Ca2+]cyt increase reaching 1.62 ± 0.11 μM (n = 10). Using a 10-fold higher concentration, laminarin stimulated two distinct peaks in [Ca2+]cyt, at 2 and 4 min, respectively, with maxima reaching 2.51 ± 0.15 μM (n = 10) (Figure 2A). Chitooligosaccharides (chitopentaose) and lipopolysaccharides, both reported to be elicitors of defense responses in various plant species (Boller, 1995; Müller et al., 1998), yielded biphasic [Ca2+]cyt increases with kinetics and magnitudes comparable to those of OGs (Figure 2B).
Figure 2.
Effects of Different Elicitors on [Ca2+]cyt Changes in Aequorin-Transformed Cells.
(A) Treatment with 1 μM cryptogein (Cry) and 0.1 or 1 mg/mL laminarin (LAM).
(B) Treatment with 100 μg/mL OGs, 100 μg/mL lipopolysaccharides (LPS), or 50 μg/mL chitopentaoses (CP).
Data correspond to 1 representative experiment of 10 experiments performed. Mean values ± se are given in the text.
Receptor-Mediated [Ca2+]cyt Increases in Tobacco Cells Treated with Elicitins
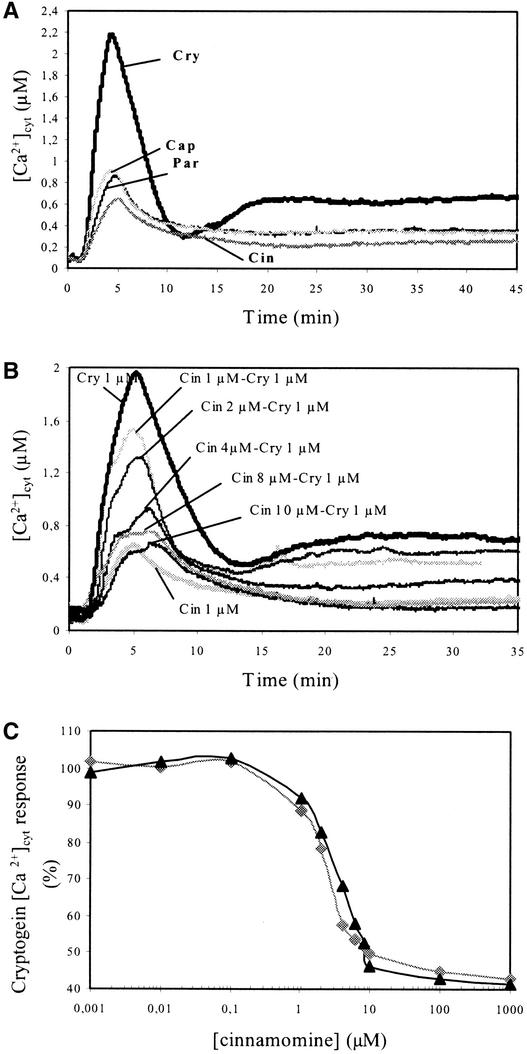
We previously demonstrated that four different elicitins (cryptogein, cinnamomin, parasiticein, and capsicein) bind to the same plasma membrane protein with comparable affinity (Bourque et al., 1998, 1999). All four elicitins triggered the same early events, but with different efficiencies. Regarding calcium influx, the relative potency was in the order cryptogein > parasiticein > capsicein > cinnamomin (Bourque et al., 1998). Here, we first monitored the [Ca2+]cyt increase induced by each elicitin in N. plumbaginifolia cells (Figure 3A). Used at the same concentration (1 μM), capsicein, parasiticein, and cinnamomin triggered transient [Ca2+]cyt increases that peaked at 0.92 ± 0.09 μM (n = 10), 0.87 ± 0.10 μM (n = 10), and 0.63 ± 0.08 μM (n = 10), respectively. Then, [Ca2+]cyt decreased slowly without reaching the basal [Ca2+]cyt level after 1 h of treatment ([Ca2+]cyt = 0.35 ± 0.04 μM with capsicein-treated cells, 0.35 ± 0.05 μM with parasiticein-treated cells, and 0.28 ± 0.04 μM with cinnamomin-treated cells). To determine whether the cryptogein-induced [Ca2+]cyt increases in N. plumbaginifolia cells depended on a preliminary interaction with plasma membrane binding sites, and taking into account the fact that elicitins bind on the same high-affinity sites, we monitored [Ca2+]cyt increases during competition assays in vivo using the most efficient (cryptogein) and the less efficient (cinnamomin) elicitins. As expected, increasing concentrations of cinnamomin (1 to 10 μM) decreased both cryptogein-induced [Ca2+]cyt increases, triggering a progressive shift from a cryptogein-induced [Ca2+]cyt signature toward a cinnamomin-induced [Ca2+]cyt signature (Figure 3B). The concentration of cinnamomin that induced 50% inhibition of the cryptogein Ca2+ response was ∼3 μM (Figure 3C).
Figure 3.
Effects of Four Elicitins on Changes in [Ca2+]cyt in Aequorin-Transformed Cells and Competition Experiments using Cryptogein and Cinnamomin.
(A) Treatment with 1 μM cryptogein (Cry), 1 μM cinnamomin (Cin), 1 μM parasiticein (Par), or 1 μM capsicein (Cap).
(B) In vivo competition experiments with 1 μM cryptogein and increasing concentrations (0 to 10 μM) of cinnamomin.
(C) Inhibition of cryptogein-induced [Ca2+]cyt increase by increasing concentrations of cinnamomin during competition assays. The [Ca2+]cyt response induced by 1 μM cryptogein alone was referred to as 100%. The [Ca2+]cyt response after 5 min (gray diamonds) or 30 min (black triangles) of cotreatment was calculated from (B).
Data correspond to 1 representative experiment of 10 experiments performed. Mean values ± se are given in the text.
Mobilization of Extracellular and Intracellular Pools of Ca2+ in Cryptogein and OG Responses
Previous data indicated that both cryptogein and OGs induced a fast Ca2+ influx, which then triggered a wide array of responses (Mathieu et al., 1991; Tavernier et al., 1995; Pugin et al., 1997; Binet et al., 1998; Lebrun-Garcia et al., 1998; Lecourieux-Ouaked et al., 2000; Binet et al., 2001). Here, we investigated the involvement of extracellular Ca2+ and of Ca2+ from internal stores in elicitor-induced [Ca2+]cyt increases.
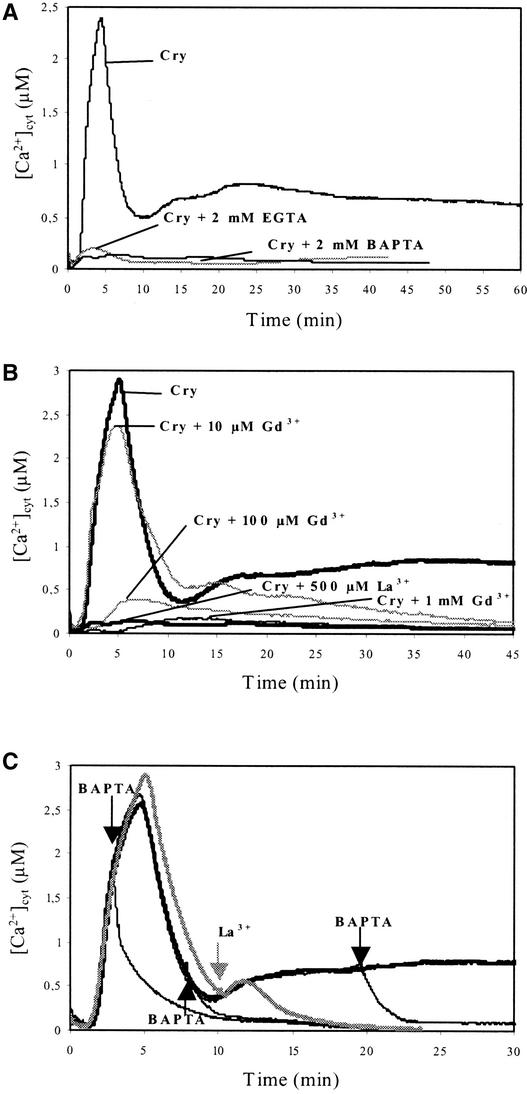
The addition of the calcium chelators 1,2-bis(o-aminophenoxy)ethane-N,N,N,N-tetraacetic acid (BAPTA; 2 mM) and EGTA (2 mM) in the extracellular medium suppressed [Ca2+]cyt increases induced by both cryptogein (Figure 4A) and OGs (Figure 5B). Similarly, the calcium surrogates Gd3+ (1 mM) and La3+ (0.5 mM), added before cryptogein treatment, suppressed both peaks of cytosolic free Ca2+ (Figure 4B). Moreover, the addition of BAPTA or La3+ during cryptogein treatment rapidly decreased [Ca2+]cyt to background levels independent of the time of addition (Figure 4C). These data indicate that this Ca2+ signature depends on a sustained Ca2+ influx from extracellular medium.
Figure 4.
Involvement of Extracellular Ca2+ in Cryptogein-Induced [Ca2+]cyt Increase in Aequorin-Transformed Cells.
(A) Effects of two Ca2+ chelators on changes in [Ca2+]cyt in cells treated with 1 μM cryptogein. EGTA (2 mM) or BAPTA (2 mM) was added at 10 min before cryptogein (Cry) treatment.
(B) Effects of the Ca2+ channel surrogates GdCl3 (Gd3+) and LaCl3 (La3+) on changes in [Ca2+]cyt in cells treated with cryptogein (1 μM). Gd3+ (10 μM to 1 mM) or La3+ (0.5 mM) was added at 10 min before cryptogein. The [Ca2+]cyt curve obtained with 1 μM cryptogein was used as a reference.
(C) Inhibition of [Ca2+]cyt increase by the addition of BAPTA or La3+ during cryptogein treatment (1 μM). BAPTA (2 mM) or La3+ (0.5 mM) was added as indicated by arrows. The cryptogein-induced [Ca2+]cyt response was monitored as a control (thick black line).
Data correspond to 1 representative experiment of 10 experiments performed.
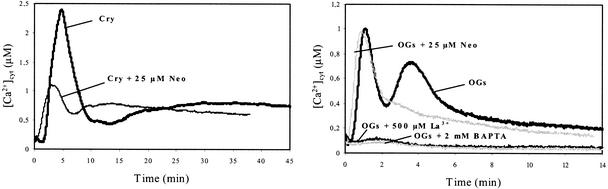
Figure 5.
Effects of Neomycin on [Ca2+]cyt Changes in Cryptogein- and OG-Treated Aequorin-Transformed Cells.
Cells were treated for 10 min with 25 μM neomycin (Neo) before the addition of 1 μM cryptogein (Cry) (A) or 100 μg/mL OGs (B). Data correspond to 1 representative experiment of 10 experiments performed.
Subsequently, we determined the possible involvement of intracellular stores in the cryptogein- and OG-induced increases of [Ca2+]cyt using neomycin, which inhibits phospholipase C and therefore the inositol 1,4,5-triphosphate (IP3)– mediated Ca2+ release (Alexandre et al., 1990; Berridge, 1993; Allen et al., 1995; Franklin-Tong et al., 1996). At first, the efficiency of neomycin in inhibiting Ca2+ release from internal stores was verified by measuring their effects on mastoparan-treated cells. Indeed, mastoparan is known to activate phospholipase C, IP3 production, and Ca2+ release from organelles in tobacco cells (Takahashi et al., 1998). Our results indicate that the mastoparan effect was prevented by neomycin (data not shown). In cryptogein-treated N. plumbaginifolia cells, neomycin (25 μM) reduced by ∼50% the intensity of the first peak of [Ca2+]cyt response and accelerated the peak of Ca2+ production between 5 and 3 min (Figure 5A). Assayed at 300 μM, neomycin was not more efficient at reducing the intensity of the first peak. In OG-treated cells, 25 μM neomycin clearly suppressed the second Ca2+ spike of the OG-induced [Ca2+]cyt response; the first spike was unaffected (Figure 5B). This result suggests that in OG-treated cells, the first [Ca2+]cyt increase, which peaked at ∼1 min, results from extracellular Ca2+ influx, whereas the second peak at 4 min probably results from Ca2+ from internal stores. In the same manner, in cryptogein-treated cells, the first transient [Ca2+]cyt increase, which peaked at 5 min, may correspond to two overlapping peaks of calcium from different origins: a first from external medium insensitive to neomycin, which peaked at ∼3 min and corresponding to ∼50% of the entire peak, and a second, inhibited by neomycin, that occurred later (between 3 and 8 min) and corresponding to Ca2+ from organelles. In cryptogein-treated cells, the second neomycin-insensitive sustained increase, which started after 15 min, could be attributable to the long-lasting influx of extracellular calcium.
Relationships between [Ca2+]cyt Increase and Other Upstream and Downstream Responses in Elicitor-Treated Cells
Relationships with Protein Phosphorylation and MAPK Activation
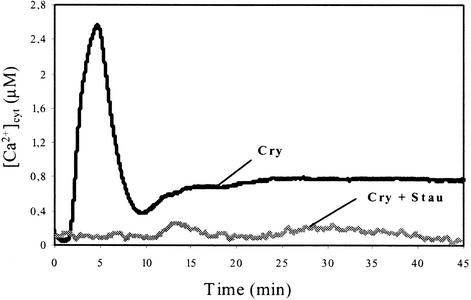
Previous studies have reported the involvement of protein phosphorylation/dephosphorylation in the early steps of cryptogein signal transduction and characterized the phosphorylated proteins (Viard et al., 1994; Tavernier et al., 1995; Lecourieux-Ouaked et al., 2000). The protein kinase inhibitor staurosporine inhibited all of the effects induced by cryptogein, whereas these effects were induced by calyculin A (Lecourieux-Ouaked et al., 2000), an inhibitor of plant protein phosphatases 1 and 2A. The present data indicate that the cryptogein-induced [Ca2+]cyt increase is inhibited completely by staurosporine (Figure 6).
Figure 6.
Effects of the Protein Kinase Inhibitor Staurosporine on Cryptogein-Induced [Ca2+]cyt Changes in Aequorin-Transformed Cells.
Staurosporine (2 μM) was added 10 min before challenging cells with 1 μM cryptogein (Cry + Stau). Data correspond to 1 representative experiment of 10 experiments performed.
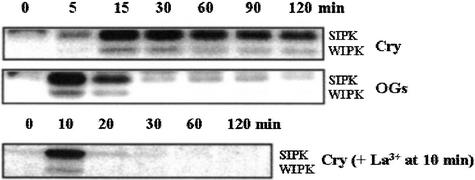
We previously reported a calcium influx–dependent activation of both MAPKs, salicylic-induced protein kinases (SIPKs), and wound-induced protein kinases (WIPKs), by cryptogein (Lebrun-Garcia et al., 1998). Here, we compared the kinetics of activation of SIPK and WIPK by cryptogein and OGs. Our data show a fast and sustained activation of MAPKs for at least 2 h in cryptogein-treated cells, whereas MAPK activation by OGs occurred early but did not exceed 15 min (Figure 7).
Figure 7.
Time Course Activation of Both MAPKs, SIPK and WIPK, by 1 μM Cryptogein in the Presence or Absence of 0.5 mM La3+ Added 10 min after the Beginning of the Treatment or by OGs (100 μg/mL).
In-gel kinase assays were performed using myelin basic protein in the gel as a substrate and 30 μg of protein from cell extracts. MAPK activity was revealed using a buffer containing 40 mM Hepes, pH 7.5, 0.1 mM EGTA, 20 mM MgCl2, 2 mM DTT, 25 μM ATP, and 0.37 to 0.925 MBq of γ-32P-ATP (Amersham). Then, the gels were exposed to X-Omat AR film (Kodak). Data correspond to one representative experiment of three experiments performed. Cry, cryptogein.
The same assays were performed in the presence of La3+ added 10 min after cryptogein treatment to check the involvement of the second sustained [Ca2+]cyt increase in MAPK activation. In these conditions, the activation of SIPK and WIPK was suppressed (Figure 7).
Relationships with H2O2 Production
Although in cryptogein-treated cells, most events depend on Ca2+ influx, this does not exclude the possibility that in a second step, Ca2+-dependent events amplify Ca2+ signaling by increasing Ca2+ influx and/or Ca2+ release from internal stores. In particular, H2O2 production from the Ca2+-dependent activation of a NADPH oxidase (Tavernier et al., 1995; Pugin et al., 1997) could trigger a Ca2+ influx, as reported previously (Price et al., 1994; Levine et al., 1996; Takahashi et al., 1998; Kawano and Muto, 2000; Pei et al., 2000).
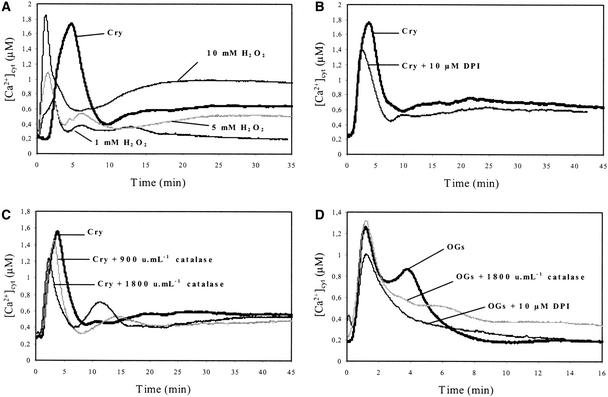
First, we investigated the effects of exogenous H2O2 on [Ca2+]cyt using aequorin-expressing cells. The addition of H2O2 (10 mM) induced a typical biphasic [Ca2+]cyt increase, with a first transient increase that peaked at ∼1 min ([Ca2+]cyt = 2.7 ± 0.22 μM; n = 10) and lasted 5 min and a second sustained increase that started after 10 min, peaked at ∼1 μM (Figure 8A), and lasted for at least 2 h (data not shown). The intensity of the entire response depended on H2O2 concentration assayed between 1 and 10 mM. H2O2-triggered [Ca2+]cyt increase was abated totally in the presence of La3+ and decreased very slightly in the presence of the intracellular Ca2+ release antagonist neomycin (data not shown). Together, these results suggested that the [Ca2+]cyt increase that followed H2O2 addition resulted mainly from extracellular Ca2+ influx.
Figure 8.
Involvement of H2O2 in Elicitor-Induced [Ca2+]cyt Changes in Aequorin-Transformed Cells.
(A) Effects of exogenous H2O2 (1 to 10 mM) on [Ca2+]cyt in cells compared with 1 μM cryptogein (Cry) treatment.
(B) Effects of diphenylene iodonium (DPI) on cryptogein-induced [Ca2+]cyt changes in cells. Cells were incubated with 10 μM diphenylene iodonium for 10 min before the addition of 1 μM cryptogein.
(C) Effects of catalase on cryptogein-induced [Ca2+]cyt changes in cells. Cells were treated for 15 min with catalase (900 and 1800 units/mL) before the addition of 1 μM cryptogein.
(D) Effects of diphenylene iodonium or catalase on OG-induced [Ca2+]cyt changes in cells. Cells were treated for 10 min with 10 μM diphenylene iodonium or incubated for 15 min with catalase (1800 units/mL) before the addition of OGs (100 μg/mL).
Data correspond to 1 representative experiment of 10 experiments performed. Mean values ± se are given in the text.
In a second step, we investigated the contribution of cryptogein-induced H2O2 production to the increase of [Ca2+]cyt. Cells were cotreated with cryptogein and diphenylene iodonium, an inhibitor of the mammalian neutrophil NADPH oxidase (Cross and Jones, 1986), which is known to inhibit cryptogein-induced AOS production (Pugin et al., 1997). Alternatively, cells were cotreated with cryptogein and catalase (900 to 1800 units/mL cell suspensions), which immediately consumed H2O2. The treatments with diphenylene iodonium (Figure 8B) and catalase (Figure 8C) had similar effects. In the absence of H2O2, the intensity of the first cryptogein-induced transient [Ca2+]cyt increase was reduced to ∼36% ± 7% (n = 10), and this increase peaked earlier (2.5 instead of 5 min), suggesting that the first cryptogein-induced [Ca2+]cyt increase resulted from at least two components; the second increase was caused by H2O2. The long-sustained [Ca2+]cyt increase in cryptogein-treated cells also was reduced in the presence of diphenylene iodonium or catalase (Figures 8B and 8C), indicating that H2O2 could participate in this sustained [Ca2+]cyt increase. In OG-treated cells, in which the transient [Ca2+]cyt increase clearly was dissociated into two peaks at 90 s and 4 min, respectively, diphenylene iodonium and catalase had similar effects, both compounds reducing the second peak (which was shown previously to be suppressed by neomycin), indicating that the corresponding [Ca2+]cyt increase was mediated by H2O2.
The [Ca2+]cyt increase in the presence of catalase (9 to 15 min in cryptogein-treated cells, and 5 to 8 min in OG-treated cells; Figures 8C and 8D) probably results from the release of O2. Indeed, catalase alone had no effect (data not shown).
Relationship with Gene Expression and Cell Death
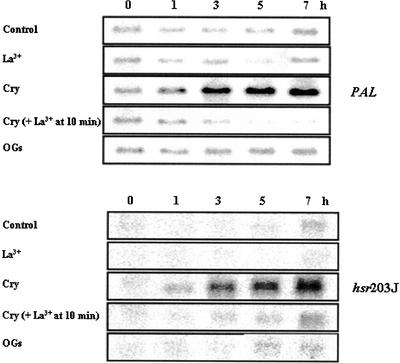
The PAL (Phe ammonia-lyase) gene encodes a key enzyme of the phenylpropanoid biosynthetic pathway (Dixon, 2001), and hsr203J expression has been related to the hypersensitive response (Marco et al., 1990; Pontier et al., 1994). Assuming that the second sustained [Ca2+]cyt increase, triggered by cryptogein but not by oligosaccharide elicitors, was a determinant for defense response expression and particularly the hypersensitive reaction, we compared the kinetics of accumulation of both gene transcripts in cryptogein-treated cell suspensions with and without lanthanum or BAPTA added after 10 min of cryptogein treatment and in OG-treated cells. The data are presented in Figure 9. Cell death was estimated under the same conditions.
Figure 9.
Time Course Accumulation of Transcripts of PAL and hsr203J Genes in OG- and Cryptogein-Treated Cells in the Presence or Absence of 0.5 mM La3+ Added 10 min after the Beginning of the Treatment.
Treatment doses were 100 μg/mL OG and 1 μM cryptogein (Cry). RNA gel blot analysis was performed using 10 μg of total RNA per lane. Data correspond to one representative experiment of two experiments performed.
In cryptogein-treated cells, PAL and hsr203J mRNA levels increased as early as 1 h after treatment and persisted for at least 7 h. The addition of lanthanum at 10 min after treatment, before the second [Ca2+]cyt increase, suppressed the accumulation of both gene transcripts. In OG-treated cells, PAL and hsr203J mRNA levels did not change compared with control levels. As reported previously both by others (Darvill and Albersheim, 1984; Mathieu et al., 1991) and ourselves (Binet et al., 2001), OGs did not induce any cell death. Proportions of cells that died after cryptogein treatment were 13% ± 2%, 21.5% ± 3%, 31% ± 4%, and 72% ± 2% dead cells after 2.5, 5, 7.5, and 24 h, respectively. Control cell suspensions contained 2 to 4% dead cells during the 24-h assays. We reported previously that suppressing calcium influx by the addition of lanthanum before cryptogein treatment suppressed cell death (Binet et al., 2001). Here, our data indicate that the addition of 0.75 mM La3+ at 10 min after cryptogein addition also suppressed cell death. The proportion of dead cells after 5 h of culture was 3% ± 1%, 4% ± 2%, 21.5% ± 3%, and 5% ± 2% in control cells, control cells with 0.75 mM La3+, cryptogein-treated cells, and cryptogein-treated cell suspensions with lanthanum added at 10 min, respectively. Together, these results indicate that the sustained [Ca2+]cyt increase in cryptogein-treated cells is involved in cell death.
DISCUSSION
[Ca2+]cyt has been shown to play a key role in plant cell signal transduction. Particularly, the calcium signature of a given signal, characterized by its amplitude, duration, frequency, and location, was shown to encode a message that, after decoding by downstream effectors, contributes to the appropriate physiological response. The importance of [Ca2+]cyt signals in the control of response pathways is well established in animals and plants (Dolmetsch et al., 1997; De Koninck and Schulman, 1998; McAinsh and Hetherington, 1998; Trewavas and Malhó, 1998; Allen et al., 2000). Cell suspensions, obtained from N. plumbaginifolia plants stably expressing the apoaequorin gene, were used to monitor and analyze [Ca2+]cyt changes in response to elicitors and to investigate their origin and role. It was reported that the binding affinity and kinetic parameters of the aequorin protein could be altered by changes in intracellular monovalent ion concentration (Cessna et al., 2001). Thus, the [Ca2+]cyt levels calculated by this method should be considered as estimates rather than exact values.
Cryptogein and Oligosaccharide Elicitors Trigger Specific [Ca2+]cyt Signatures
Cryptogein and OGs trigger typical calcium signatures that differ in both kinetics (lag time, peak time, and duration) and peak intensities (Figure 1A). All three of the other oligosaccharide elicitors—laminarin, a β-1,3-glucan from the brown alga Laminaria digitata (Klarzynski et al., 2000), and chitooligosaccharides and lipopolysaccharides from Pseudomonas (Boller, 1995; Müller et al., 1998)—induced a signature resembling the OG response (Figure 2). The intensity of both cryptogein and OG signatures depends on elicitor concentration (Figures 1C and 1D) and on extracellular calcium concentration (Figures 1E and 1F). In cryptogein-treated cells, both [Ca2+]cyt increases became larger with increasing extracellular calcium concentrations; 0.5 and 0.2 mM extracellular calcium saturated the response corresponding to the first transient peak and the second sustained phase, respectively (Figure 1E). Extracellular calcium concentrations >1 mM reduced the intensity of the first peak (data not shown).
This effect of high extracellular calcium concentrations is not the result of an inhibition of calcium influx, which increased linearly with increasing concentrations of extracellular calcium to 5 mM (data not shown). In OG-treated cells, the extent of the first [Ca2+]cyt increase became larger with increasing extracellular calcium concentrations, 10 mM saturating this response, whereas the second [Ca2+]cyt increase was optimal with 0.5 mM. Together, these data (Figures 1A, 1E, and 1F) suggest that cells activate new membrane components able to remove calcium from the cytosol when [Ca2+]cyt approaches values greater than ∼2.4 μM. On the other hand, comparison of [Ca2+]cyt and calcium influx (Figures 1A and 1B) indicates that a very low proportion of the calcium entering the cells is free. Indeed, considering that 1 g fresh weight of cells corresponds to ∼1 mL (Pugin et al., 1997), only 0.9% of the calcium that had penetrated the cells after 30 min of treatment was free in cytosol. This value decreased to 0.05% after 2.5 h. This observation highlights the ability of cells to buffer and store calcium in organelles and, particularly, the efficiency of vacuolar and endoplasmic reticulum Ca2+-ATPases and Ca2+/H+ antiporters. The efficiency with which cells maintain low [Ca2+]cyt for protracted periods demonstrates the functionality of the tonoplast and agrees with the long viability of cells (∼10% dead cells after 2.5 h of treatment) despite a large calcium influx and potentially damaging cellular events occurring from the first 5 min, including AOS production, anion efflux, PM depolarization, changes in sugar metabolism, and cytoskeleton depolymerization (see Introduction). These results also demonstrate that the prolonged and reversible phase of calcium response, which lasted 2 h (Figure 1B), is not a result of cell death.
Implication of Extracellular and Intracellular Pools of Ca2+ in Elicitor Responses and Involvement of H2O2
Using calcium chelators (EGTA and BAPTA) and calcium surrogates (La3+ and Gd3+), we have shown that elicitor-induced [Ca2+]cyt increases depend on a sustained calcium influx from external medium (Figure 4). This calcium influx then triggers calcium efflux from organelles, probably through IP3-activated calcium channels, as demonstrated indirectly by inhibiting the release of calcium from internal stores with neomycin (Figure 5). Calcium channels located on vacuolar and endoplasmic reticulum membranes (White, 2000), ligand gated by IP3 (Schumaker and Sze, 1987; Alexandre et al., 1990), are involved in many physiological processes in plants (Sanders et al., 1999), especially in abscisic acid signaling (Wu et al., 1997; Leckie et al., 1998) and plant defense (Durner et al., 1998; Mithöfer et al., 1999; Blume et al., 2000; Klessig et al., 2000).
We continued the analysis of the cryptogein calcium signature by determining the role of cryptogein-induced transient H2O2 production, which was detected after 3 min of treatment and peaked between 15 and 20 min (Lecourieux-Ouaked et al., 2000). H2O2 was shown to trigger calcium influx in tobacco (Price et al., 1994; Takahashi et al., 1998; Kawano and Muto, 2000), and [Ca2+]cyt increase was reported to be involved in AOS-mediated cell death (Levine et al., 1996). Moreover, recent studies have reported that abscisic acid action was mediated by H2O2-activated calcium channels in the PM of Arabidopsis guard cells (Pei et al., 2000). Our data indicate that exogenous H2O2 induced a biphasic [Ca2+]cyt increase (Figure 8A) that could be attributable to an influx of extracellular Ca2+. This [Ca2+]cyt increase was suppressed by EGTA or La3+ but was not affected by neomycin (data not shown). Nevertheless, we do not exclude the contribution of neomycin-insensitive calcium release from internal stores as a result of H2O2 in addition to calcium influx. We also show that H2O2 produced in response to elicitors participates in the [Ca2+]cyt increase. Indeed, NADPH oxidase inhibition (suppression of both O2.− and H2O2) or H2O2 consumption in cryptogein- or OG-treated cells reduced or suppressed a peak of [Ca2+]cyt increase. In both signatures, this peak corresponds kinetically to a peak that is reduced or suppressed in the presence of neomycin. Nevertheless, H2O2 produced by cells should not activate IP3-regulated channels from internal stores, as indicated by assays with exogenous H2O2 and neomycin.
Moreover, in cryptogein- or OG-treated cells, an additional Ca2+ influx could result from PM depolarization (Mathieu et al., 1991; Pugin et al., 1997). Voltage-dependent Ca2+ channels activated by membrane depolarization were identified on the PM (Pineros and Tester, 1997; White, 2000). Activation of voltage-dependent cation channels was reported in response to various signals, including blue and red light (Spalding and Cosgrove, 1989; Ermolayeva et al., 1996), Nod factors (Ehrhardt et al., 1992; Yokoyama et al., 2000), and fungal elicitors (Kuchitsu et al., 1993). In cryptogein-treated cells, the major calcium influx did not result from PM depolarization. Calcium influx occurred upstream and triggered anion efflux and PM depolarization (Pugin et al., 1997). Nitric oxide also may be involved in [Ca2+]cyt increase. Cryptogein induced a very fast production of nitric oxide in tobacco cells (Foissner et al., 2000), and nitric oxide has been reported to trigger (1) calcium influx through cyclic GMP–dependent Ca2+ channels, (2) calcium release through cyclic ADPribose–dependent Ca2+ channels, and (3) direct activation of the ryanodine channel by S-nitrosylation in animal cells (reviewed by Wendehenne et al., 2001). Recent data highlight the involvement of cyclic nucleotide-gated ion channels (CNGC) in plant defense. Interestingly, the absence of functional AtCNCG2, a plasma membrane CNGC permeable to Ca2+ (Clough et al., 2000), characterizes the Arabidopsis dnd1 mutant, which fails to produce the hypersensitive reaction in response to avirulent Pseudomonas syringae but expresses systemic acquired resistance constitutively (Yu et al., 1998).
[Ca2+]cyt Increase Is Mediated by Cryptogein–Receptor Interaction
We sought to determine whether the cryptogein-induced [Ca2+]cyt increase depends on receptor interaction, taking advantages of four different elicitins that bind with comparable affinities to the same binding sites (Bourque et al., 1999). These triggered the same effects but with different magnitudes (Bourque et al., 1998). Competition assays in vivo using the most efficient elicitin (cryptogein) and increasing concentrations of the less efficient elicitin (cinnamomin) (Figure 3A) revealed a shift of the cryptogein calcium signature toward the cinnamomin calcium signature (Figure 3B). A cinnamomin concentration of 3 μM inhibited 50% of the cryptogein Ca2+ response (Figure 3C), consistent with the data reported by Bourque et al. (1998) and indicating that the concentration of elicitins inhibiting 50% of the effects of cryptogein in competition assays in vivo was approximately twice the concentration of cryptogein used. These results indicate that the typical [Ca2+]cyt increase induced by cryptogein-treated cells depends on specific interactions with the high-affinity binding sites characterized previously (Bourque et al., 1999). In the same manner, [Ca2+]cyt changes in parsley cells in response to the Phytophthora sojae–derived oligopeptide elicitor Pep-13 were shown to be receptor mediated (Blume et al., 2000). Inhibition of [Ca2+]cyt increase by the protein kinase inhibitor staurosporine (Figure 6) indicates that protein phosphorylation occurs upstream of the activation of PM calcium channels and could involve the receptor or associated proteins.
Effects of the Sustained [Ca2+]cyt Increase in Cryptogein-Treated Cells
We reported previously a fast microtubule depolymerization in cryptogein-treated cells, whereas OGs had no effects. We also established that microtubule depolymerization was related to the intensity of calcium influx and to cell death (Binet et al., 2001). The relationship between the intensity of calcium influx, microtubule depolymerization, and cell death is reinforced by the present data, which show that laminarin, chitopentaose, and lipopolysaccharide, which did not trigger any sustained [Ca2+]cyt (Figure 2), did not induce either microtubule depolymerization or cell death after 24 h of treatment (data not shown).
Our data demonstrate the involvement of the cryptogein-induced [Ca2+]cyt increase in the sustained activation of MAPKs, in the accumulation of transcripts corresponding to a defense gene (PAL) and to a gene associated with the hypersensitive cell death (hsr203J), and in cell death. Indeed, suppression of the sustained [Ca2+]cyt increase in cryptogein-treated cells by the addition of lanthanum at 10 min after the beginning of the treatment suppressed the activation of both MAPKs (Figure 7), the accumulation of transcripts corresponding to PAL and hsr203J (Figure 9), and cell death. Interestingly, the SIPK/WIPK signaling cascade also has been reported to be involved in cryptogein-induced hypersensitive reaction activation (Zhang et al., 2000; Yang et al., 2001). The sustained activation of SIPK/WIPK caused by the maintained [Ca2+]cyt increase might be explained by the continuous activation of the MAPK cascade and/or by the inhibition of negative regulators, including the protein phosphatase 2C–type phosphatase MP2C, which has been described as a negative regulator of SIPK/WIPK activation (Meskiene et al., 1998), and silenced by high [Ca2+]cyt (Baudouin et al., 1999).
Thus, cryptogein receptor-mediated increase in [Ca2+]cyt in N. plumbaginifolia cells depends on protein phosphorylation and involves successive influxes of extracellular calcium, probably through different types of PM calcium-permeable channels, and a release of calcium from intracellular stores. Activation of these calcium fluxes leads to a typical and well-defined calcium signature decoded by downstream effectors that, in conjunction with other second messengers, initiate a specific signaling cascade that leads to the appropriate physiological responses, hypersensitive reaction and systemic acquired resistance. The sustained increase that characterized the cryptogein signature, compared with oligosaccharide elicitors, may be involved in the hypersensitive reaction and cell death. Using different elicitors and tobacco cells, our research is now focused on the analysis of Ca2+ concentration changes in the nucleus and on the identification of downstream effectors such as protein kinases and transcriptional regulators.
METHODS
Aequorin-Transformed Tobacco Cells
Transformed Nicotiana plumbaginifolia plants (line MAQ2.4) expressing apoaequorin (Knight et al., 1991) were used to generate dark-grown cell suspensions as described previously (Chandra et al., 1997). Eight milliliters of aequorin-transformed cells was transferred to 100 mL of fresh liquid Chandler's medium (Chandler et al., 1972) every 8 days and maintained in dark-grown suspension by continuous shaking (150 rpm at 24°C). Transgenic tobacco (Nicotiana tabacum) cell suspensions behaved similarly to the untransformed N. plumbaginifolia cultures with respect to phenotype and growth kinetics. Before functional aequorin reconstitution and elicitor treatments, 8-day-old transgenic tobacco cell suspensions were collected and washed by filtration with a suspension buffer (175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM Hepes adjusted to pH 5.75). Cells were resuspended in suspension buffer to give a final concentration of 0.1 g fresh weight/mL. In vivo reconstitution of aequorin was performed by the addition of 2 μL of coelenterazine (5 mM stock solution in methanol) to 10 mL of cell suspension for at least 3 h in the dark (150 rpm at 24°C).
Cell viability was assayed using the vital dye neutral red as described by Binet et al. (2001). Cells (1 mL) were washed with 1 mL of a solution containing 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM Hepes, pH 7.0, and then incubated for 5 min in the same solution supplemented with neutral red to a final concentration of 0.01%. Cells that did not accumulate neutral red were considered dying. At least 500 cells were counted for each treatment. The experiment was repeated three times.
Products
Elicitins were purified according to Bonnet et al. (1996) and were a gift from M. Ponchet (Institut National de la Recherche Agronomique, Antibes, France). Purified oligogalacturonides were a gift from M.A. Rouet-Mayer (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) and were used as a mixture of oligomers with degrees of polymerization ranging from 7 to 20. Laminarin, a linear β-1,3-glucan (degrees of polymerization from 25 to 30) from Laminaria digitata, was generously provided by Bernard Kloareg (Centre National de la Recherche Scientifique–Goëmar, Roscoff, France). Lipopolysaccharides (from Pseudomonas aeruginosa) were obtained from Sigma-Aldrich, and penta-N-acetylchitopentaose was obtained from Seikagaku America (Falmouth, MA). Coelenterazine was supplied by Calbiochem. Other chemicals were purchased from Sigma-Aldrich. When used, DMSO did not exceed a final concentration of 0.1%.
Aequorin Luminescence Measurements and Calibration
Bioluminescence measurements were made using a digital luminometer (Lumat LB9507; Berthold, Bad Wildbad, Germany). Cell culture aliquots (250 μL) were transferred carefully to a luminometer glass tube, and the luminescence counts were recorded continuously at 1-s intervals (recorded as relative light units per second) and exported simultaneously (using Win Term software; Berthold) into Excel version 5.0 on a computer. Inhibitors, chelators, and control solvents were added at 15 min before elicitor treatments, with each volume of treatment not exceeding 1% of the cell aliquot volume.
In reconstituted aequorin control cells, the bioluminescence emission during a complete discharge of aequorin (with excess Ca2+) was in the range of 1 to 2 × 105 relative light units·s−1·mg−1 fresh weight. Approximately 40% of the reconstituted aequorin was consumed after 1 h of cryptogein treatment (1 μM), against <5% after 1 h of oligogalacturonide treatment (50 μg/mL cell suspension).
At the end of each experiment, residual functional aequorin was quantified by adding 300 μL of lysis buffer (10 mM CaCl2, 2% Nonidet P-40, and 10% ethanol) and monitoring the resulting increase in luminescence (until recordings returned to basal levels). Luminescence data transformation into cytosolic Ca2+ concentration was performed using the equation established by Allen et al. (1977), [Ca2+] = {(L0/Lmax)1/3 + [KTR(L0/Lmax)1/3] − 1}/{KR − [KR(L0/Lmax)1/3]}, with KR and KTR values of 2 × 106 and 55 M−1, respectively, as calculated by van Der Luit et al. (1999) using native coelenterazine and the specific aequorin isoform we used in these experiments at 22°C (our cell line was generated from MAQ2.4 transgenic plants, as used by these authors). In this equation, L0 is the luminescence intensity per second and Lmax is the total amount of luminescence present in the entire sample during the course of the experiment. Statistics on the magnitude of [Ca2+]cyt peaks are given in the text as means ± se.
Except for diphenylene iodonium and catalase, all reagents used were tested previously on lysates containing recombinant aequorin to examine the direct effects of the reagent on aequorin luminescence (Haley et al., 1995; Sedbrook et al., 1996; Knight et al., 1996; Chandra et al., 1997; Sinclair and Trewavas, 1997; Blume et al., 2000). We tested diphenylene iodonium and catalase. None of these products interfered with aequorin luminescence.
RNA Gel Blot Analysis
Total RNA was isolated from tobacco cell suspensions using Trizol reagent (Gibco BRL) as described by the supplier. RNA gel blot analysis was performed using 10 μg of total RNA per lane, separated on 1.2% agarose gels containing 1.1% formaldehyde. The gel was blotted to a nylon membrane (Hybond-XL; Amersham) and cross-linked by UV light. The probes for hybridization were labeled by random priming using the Ready-To-Go DNA Labeling Beads kit (without dCTP) from Amersham. The membrane was hybridized to the probes at 65°C and washed for 5 min with 2 × SSC at room temperature (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate), once for 30 min at 65°C with 0.5% SDS and 2 × SSC, and subsequently with 0.1 × SSC for 30 min at room temperature. The membrane was exposed to a PhosphorImager screen, analyzed with a PhosphorImager Storm 880 (Molecular Dynamics, Sunnyvale, CA), and exposed to X-Omat AR film (Kodak).
In-Gel Kinase Assay
This technique was performed using the protocol described by Lebrun-Garcia et al. (1998) without modifications.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We are grateful to M.R. Knight and A.J. Trewavas for the N. plumbaginifolia line MAQ2.4. We thank M. Ponchet for the gift of cryptogein and Y. Marco for the hsr203J cDNA clone. We thank D. Wendehenne and A. Lebrun-Garcia for critical reading of the manuscript and A. Chiltz for in-gel kinase assays. We are grateful to K. Gould for reviewing the English manuscript. This work was supported by the Institut National de la Recherche Agronomique (INRA), Ministère de L'Enseignement Supérieur et de la Recherche, and the Conseil Régional de Bourgogne. D.L. was supported by a grant from the INRA and the Conseil Régional de Bourgogne.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.005579.
References
- Alexandre, J., Lassalles, J.P., and Kado, R.T. (1990). Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5-triphosphate. Nature 343, 567–570. [Google Scholar]
- Allen, D.G., Blinks, J.R., and Prendergast, F.G. (1977). Aequorin luminescence: Relation of light emission to calcium concentration. A calcium-independent component. Science 195, 996–998. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Chu, S.P., Schumacher, K., Shimazaki, C.T., Vafeados, D., Kemper, A., Hawke, S.D., Tallman, G., Tsien, R.Y., Harper, J.F., Chory, J., and Schroeder, J.I. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289, 2338–2342. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Muir, S.R., and Sanders, D. (1995). Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic-ADP-ribose. Science 268, 735–737. [DOI] [PubMed] [Google Scholar]
- Baudouin, E., Meskiene, I., and Hirt, H. (1999). Short communication: Unsaturated fatty acids inhibit MP2C, a protein phosphatase 2C involved in the wound-induced MAP kinase pathway regulation. Plant J. 20, 343–348. [DOI] [PubMed] [Google Scholar]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc. Natl. Acad. Sci. USA 96, 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, M.J. (1993). Inositol triphosphate and calcium signalling. Nature 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Binet, M.N., Bourque, S., Lebrun-Garcia, A., Chiltz, A., and Pugin, A. (1998). Comparison of the effects of cryptogein and oligogalacturonides on tobacco cells and evidence of different forms of desensitization induced by these elicitors. Plant Sci. 137, 33–41. [Google Scholar]
- Binet, M.N., Humbert, C., Lecourieux, D., Vantard, M., and Pugin, A. (2001). Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol. 125, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blein, J.P., Milat, M.L., and Ricci, P. (1991). Responses of cultured tobacco cells to cryptogein, a proteinaceous elicitor from Phytophthora cryptogea: Possible plasmalemma involvement. Plant Physiol. 95, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume, B., Nürnberger, T., Nass, N., and Scheel, D. (2000). Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12, 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. (1995). Chemoperception of microbial signals in plant cells. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 189–214. [Google Scholar]
- Bonnet, P., Bourdon, E., Ponchet, M., Blein, J.P., and Ricci, P. (1996). Acquired resistance triggered by elicitins in tobacco and others plants. Eur. J. Plant Pathol. 102, 181–192. [Google Scholar]
- Bottin, A., Véronési, C., Pontier, D., Esquerré-Tugayé, M.T., Blein, J.P., Rustérucci, C., and Ricci, P. (1994). Differential responses of tobacco cells to elicitors from two Phytophthora species. Plant Physiol. Biochem. 32, 373–378. [Google Scholar]
- Bourque, S., Binet, M.N., Ponchet, M., Pugin, A., and Lebrun-Garcia, A. (1999). Characterization of the cryptogein binding sites on plant plasma membranes. J. Biol. Chem. 274, 34699–34705. [DOI] [PubMed] [Google Scholar]
- Bourque, S., Ponchet, M., Binet, M.N., Ricci, P., Pugin, A., and Lebrun-Garcia, A. (1998). Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 118, 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, D.S. (1995). Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 95–122. [Google Scholar]
- Cessna, S.G., Chandra, S., and Low, P.S. (1998). Hypo-osmotic shock of tobacco cells stimulates calcium fluxes deriving first from external and then internal calcium stores. J. Biol. Chem. 273, 27286–27291. [DOI] [PubMed] [Google Scholar]
- Cessna, S.G., Messerli, M.A., Robinson, K.R., and Low, P.S. (2001). Measurement of stress-induced Ca2+ pulses in single aequorin-transformed tobacco cells. Cell Calcium 30, 151–156. [DOI] [PubMed] [Google Scholar]
- Chandler, M.T., Tandeau de Marsac, N., and Kouchkovsky, Y. (1972). Photosynthetic growth of tobacco cells in liquid suspension. Can. J. Bot. 50, 2265–2270. [Google Scholar]
- Chandra, S., Stennis, M., and Low, P.S. (1997). Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J. Biol. Chem. 272, 28274–28280. [DOI] [PubMed] [Google Scholar]
- Clayton, H., Knight, M.R., Knight, H., McAinsh, M.R., and Hetherington, A.M. (1999). Dissection of the ozone-induced calcium signature. Plant J. 17, 575–579. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., Fengler, K.A., Yu, I.-C., Lippok, B., Smith, R.K., and Bent, A.F. (2000). The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 97, 9323–9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, A.R., and Jones, O.T.G. (1986). The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils: Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J.F., Robert, A.D., and Richberg, M.H. (1996). Death don't have no mercy: Cell death programs in plant-microbe interaction. Plant Cell 8, 1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill, A., and Albersheim, P. (1984). Phytoalexins and their elicitors: A defense against microbial infection in plants. Annu. Rev. Plant Physiol. 35, 243–275. [Google Scholar]
- De Koninck, P., and Schulman, H. (1998). Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230. [DOI] [PubMed] [Google Scholar]
- Dixon, A.D. (2001). Natural products and plant disease resistance. Nature 411, 843–847. [DOI] [PubMed] [Google Scholar]
- Dolmetsch, R.E., Lewis, R.S., Goodnow, C.C., and Healy, J.I. (1997). Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858. [DOI] [PubMed] [Google Scholar]
- Durner, J., Wendehenne, D., and Klessig, D.F. (1998). Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95, 10328–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Atkinson, E.M., and Long, S.R. (1992). Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 256, 998–1000. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Wais, R., and Long, S.R. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85, 673–681. [DOI] [PubMed] [Google Scholar]
- Ermolayeva, E., Holmeyer, H., Johannes, E., and Sanders, D. (1996). Calcium-dependent membrane depolarisation activated by phytochrome in the moss Physcomitrella patens. Planta 199, 352–358. [Google Scholar]
- Foissner, I., Wendehenne, D., Longteraals, P., and Durner, J. (2000). In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 23, 817–824. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong, V.E., Drobak, B.K., Allan, A.C., Watkins, P.A.C., and Trewavas, A.J. (1996). Growth of pollen tubes of Papaver rhoeas is regulated by a slow-moving calcium wave propagated by inositol 1,4,5-triphosphate. Plant Cell 8, 1305–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy, S., and Jones, R.L. (1992). Gibberellic acid and abscisic acid co-ordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc. Natl. Acad. Sci. USA 89, 3591–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, A., Russell, A.J., Wood, N., Allan, A.C., Knight, M., Campbell, A.K., and Trewavas, A.J. (1995). Effects of mechanical signaling on plant cell cytosolic calcium. Proc. Natl. Acad. Sci. USA 92, 4124–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, T., and Muto, S. (2000). Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. J. Exp. Bot. 51, 685–693. [PubMed] [Google Scholar]
- Kiegle, E., Moore, C.A., Haseloff, J., Tester, M.A., and Knight, M.R. (2000). Cell-type-specific calcium response to drought, salt and cold in the Arabidopsis root. Plant J. 23, 267–278. [DOI] [PubMed] [Google Scholar]
- Klarzynski, O., Plesse, B., Joubert, J.M., Yvin, J.C., Kopp, M., Kloareg, B., and Fritig, B. (2000). Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124, 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig, D.F., et al. (2000). Nitric oxide and salicylic acid signaling in plant defenses. Proc. Natl. Acad. Sci. USA 97, 8849–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H. (2000). Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 195, 269–324. [DOI] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8, 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526. [DOI] [PubMed] [Google Scholar]
- Knight, M.R., Smith, S.M., and Trewavas, A.J. (1992). Wind-induced plant motion immediately increases cytosolic calcium. Proc. Natl. Acad. Sci. USA 89, 4967–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchitsu, K., Kikuyama, M., and Shibuya, N. (1993). N-Acetyl-chito-oligosaccharides, biotic elicitors for phytoalexin production, induce transient membrane depolarization in suspension-cultured rice cells. Protoplasma 174, 79–81. [Google Scholar]
- Lebrun-Garcia, A., Ouaked, F., Chiltz, A., and Pugin, A. (1998). Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 15, 773–781. [DOI] [PubMed] [Google Scholar]
- Leckie, C.P., McAinsh, M.R., Allen, G.J., Sanders, D., and Hetherington, A.M. (1998). Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95, 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux-Ouaked, F., Pugin, A., and Lebrun-Garcia, A. (2000). Phosphoproteins involved in the signal transduction of cryptogein, an elicitor of defense reactions in tobacco. Mol. Plant-Microbe Interact. 13, 821–829. [DOI] [PubMed] [Google Scholar]
- Levine, A., Pennell, R.I., Alvarez, M.E., Palmer, R., and Lamb, C. (1996). Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 6, 427–437. [DOI] [PubMed] [Google Scholar]
- Marco, Y., Raguesh, F., Godiard, L., and Froissard, D. (1990). Transcriptional activation of 2 classes of genes during hypersensitive reaction of tobacco leaves infiltrated with an incompatible isolate of the phytopathogenic bacterium Pseudomonas solanacearum. Plant Mol. Biol. 15, 145–154. [DOI] [PubMed] [Google Scholar]
- Mathieu, Y., Kurkdijan, A., Xia, H., Guern, J., Koller, A., Spiro, M., O'Neill, M., Albersheim, P., and Darvill, A. (1991). Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J. 1, 333–343. [DOI] [PubMed] [Google Scholar]
- McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1992). Visualizing changes in cytosolic Ca2+ during the response of stomatal guard cells to abscisic acid. Plant Cell 4, 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M.R., and Hetherington, A.M. (1998). Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 3, 32–36. [Google Scholar]
- Meskiene, I., Bogre, L., Glaser, W., Balog, J., Brandstotter, M., Zwerger, K., Ammerer, G., and Hirt, H. (1998). MP2C, a plant protein phosphatase 2C, functions as a negative regulator of activated protein kinase pathways in yeast and plants. Proc. Natl. Acad. Sci. USA 95, 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milat, M.L., Ricci, P., and Blein, J.P. (1991). Capsidiol and ethylene production by tobacco cells in response to cryptogein. Phytochemistry 30, 2171–2173. [Google Scholar]
- Mithöfer, A., Ebel, J., Bhagwat, A.A., Boller, T., and Neuhaus-Url, G. (1999). Transgenic aequorin monitors cytosolic transients in soybean cells challenged with β-glucan or chitin elicitors. Planta 207, 566–574. [Google Scholar]
- Müller, P., Zähringer, U., and Rudolph, K. (1998). Induced resistance by bacterial lipopolysaccharides (LPS). In Proceedings of the Ninth International Conference, Centre for Advanced Study in Botany, Plant Pathogenic Bacteria. A. Mahadevan, ed (Madras, India: University of Madras), pp. 569–575.
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, A.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Pineros, M., and Tester, M. (1997). Characterization of the high-affinity verapamil binding site in a plant plasma membrane Ca2+-selective channel. J. Membr. Biol. 157, 139–145. [DOI] [PubMed] [Google Scholar]
- Pontier, D., Godiard, L., Marco, Y., and Roby, D. (1994). hsr203J, a tobacco gene whose activation is rapid, highly localized and specific for incompatible plant/pathogen interactions. Plant J. 5, 507–521. [DOI] [PubMed] [Google Scholar]
- Price, A.H., Taylor, A., Ripley, S.J., Griffiths, A., Trewavas, A.J., and Knight, M.R. (1994). Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin, A., Frachisse, J.M., Tavernier, E., Bligny, R., Gout, E., Douce, R., and Guern, J. (1997). Early events induced by the elicitor cryptogein in tobacco cells: Involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell 9, 2077–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A.S.N. (2001). Calcium: Silver bullet in signalling. Plant Sci. 160, 381–404. [DOI] [PubMed] [Google Scholar]
- Ricci, P., Bonnet, P., Huet, J.C., Sallantin, M., Beauvais-Canté, F., Bruneteau, M., Billard, V., Michel, G., and Pernollet, J.C. (1989). Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur. J. Biochem. 183, 555–563. [DOI] [PubMed] [Google Scholar]
- Ryals, J.A., Neuenschwander, U.H., Willits, M.G., Molina, A., Steiner, H.-Y., and Hunt, M.D. (1996). Systemic acquired resistance. Plant Cell 8, 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D., Brownlee, C., and Harper, J.F. (1999). Communicating with calcium. Plant Cell 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker, K.S., and Sze, H. (1987). Inositol 1,4,5-triphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J. Biol. Chem. 262, 3944–3946. [PubMed] [Google Scholar]
- Sedbrook, J.C., Kronebusch, P.J., Borisy, G.G., Trewavas, A.J., and Masson, P.H. (1996). Transgenic aequorin reveals organ-specific Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 111, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacklock, P.S., Read, N.D., and Trewavas, A.J. (1992). Cytosolic free calcium mediates red-light induced photomorphogenesis. Nature 358, 753–755. [Google Scholar]
- Sinclair, W., and Trewavas, A.J. (1997). Calcium in gravitropism: A re-examination. Planta 203 (suppl. 1), 85.–90. [DOI] [PubMed] [Google Scholar]
- Spalding, E.P., and Cosgrove, D.J. (1989). Large plasma membrane depolarisation precedes rapid blue-light-induced growth inhibition in cucumber. Planta 178, 407–410. [PubMed] [Google Scholar]
- Takahashi, K., Isobe, M., and Muto, S. (1997). An increase in cytosolic calcium ion concentration precedes hypoosmotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett. 401, 202–206. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Isobe, M., and Muto, S. (1998). Mastoparan induces an increase in cytosolic calcium ion concentration and subsequent activation of protein kinases in tobacco suspension culture cells. Biochim. Biophys. Acta 1401, 339–346. [DOI] [PubMed] [Google Scholar]
- Tavernier, E., Wendehenne, D., Blein, J.P., and Pugin, A. (1995). Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 109, 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A.R., Manison, N.F.H., Fernandez, C., Wood, J., and Brownlee, C. (1996). Spatial organization of calcium signalling involved in cell volume control in the Fucus rhizoid. Plant Cell 8, 2015–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas, A.J. (1999). Le calcium, c'est la vie: Calcium makes waves. Plant Physiol. 120, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas, A.J., and Malhó, R. (1998). Ca2+ signalling in plant cells: The big network! Curr. Opin. Plant Biol. 1, 428–433. [DOI] [PubMed] [Google Scholar]
- van Der Luit, A.H., Olivari, C., Haley, A., Knight, M.R., and Trewavas, A.J. (1999). Distinct signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiol. 121, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard, M.P., Martin, F., Pugin, A., Ricci, P., and Blein, J.P. (1994). Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 104, 1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne, D., Binet, M.N., Blein, J.P., Ricci, P., and Pugin, A. (1995). Evidence for specific high-affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 374, 203–207. [DOI] [PubMed] [Google Scholar]
- Wendehenne, D., Pugin, A., Klessig, D.F., and Durner, J. (2001). Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 6, 177–183. [DOI] [PubMed] [Google Scholar]
- White, P.J. (2000). Calcium channels in higher plants. Biochim. Biophys. Acta 1465, 171–189. [DOI] [PubMed] [Google Scholar]
- Wu, Y., Kuzma, J., Marechal, E., Graeff, R., Lee, H.C., Foster, R., and Chua, N.-H. (1997). Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278, 2126–2130. [DOI] [PubMed] [Google Scholar]
- Yang, K.-Y., Liu, Y., and Zhang, Z. (2001). Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 98, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama, T., Kobayashi, N., Kouchi, H., Minamisawa, K., Kaku, H., and Tsuchiya, K. (2000). A lipochito-oligosaccharide, Nod factor, induces transient calcium influx in soybean suspension-cultured cells. Plant J. 22, 71–78. [DOI] [PubMed] [Google Scholar]
- Yu, I.-C., Parker, J., and Bent, A. (1998). Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc. Natl. Acad. Sci. USA 95, 7819–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Liu, Y., and Klessig, D.F. (2000). Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J. 23, 339–347. [DOI] [PubMed] [Google Scholar]
- Zielinski, R.E. (1998). Calmodulin and calmodulin-binding proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 697–725. [DOI] [PubMed] [Google Scholar]