INTRODUCTION
Polypeptide hormones (extracellular signals) have been recognized for many years as important regulatory molecules in animals and yeast. Beginning with the discovery of insulin in 1922 (Banting and Best, 1922), polypeptides have been established as signals that regulate a broad spectrum of physiological processes. Until 1991, however, plants were not known to utilize polypeptides as regulatory molecules; instead they were thought to use only small organic phytohormones, including auxins, cytokinins, gibberellins, ethylene, abscisic acid (Kende and Zeevaart, 1997), and more recently, brassinolides (Mandava, 1988).
The discoveries of polypeptide signals for plant defense, growth, and development over the past decade have heralded the beginning of a new and growing field of polypeptide signaling in plants. Here, we review the currently known polypeptides from plant origins that act as signaling molecules. Where known, the receptor proteins with which the polypeptides interact are discussed, as well as their functional roles in polypeptide/receptor–mediated signaling cascades. This article is not meant to be a comprehensive review of the underlying biology of systems in which polypeptide signaling is found, and our discussion of the relevant research areas emphasizes the biochemical and structural features of polypeptide signals and their receptors.
SYSTEMINS
Tomato Systemin
The systemin polypeptide was discovered during a search for the systemic wound signal that regulates the expression of defensive genes in tomato leaves in response to insect attacks or other severe mechanical wounding (Pearce et al., 1991). Several reviews concerning tomato systemin have been written in recent years (Schaller and Ryan, 1995; Ryan, 1998, 2000; Ryan and Pearce, 1998; Schaller, 1999), and the structural and functional data relevant to polypeptide signaling, along with more recent developments, are summarized below.
Tomato and potato plants respond to insect attacks by releasing a signal or signals from the wound site that cause the synthesis and accumulation of proteinase inhibitors in both wounded leaves and distal, unwounded leaves (Green and Ryan, 1972). A search for the proteinase inhibitor-inducing factor over the next 20 years led to the isolation of a polypeptide signaling molecule (Pearce et al., 1991; Ryan, 1998). The polypeptide is composed of 18 amino acids with the sequence +AVQSKPPSKRDPPKMQTD−, and because of its association with systemic signaling, it is called systemin. Systemin induces the synthesis of proteinase inhibitors in leaves of young excised tomato plants when supplied at low nanomolar levels through their cut petioles (Pearce et al., 1991). The potency is in the same range as that found for the activity of various polypeptide hormones in animals.
A synthetic 14C-labeled systemin was mobile when applied to wound sites, and its movement in the phloem correlates with the movement of the systemic signal that is produced in response to wounding, that is, ∼3 cm/hr (Nelson et al., 1983; Pearce et al., 1991; Narvaez-Vasquez et al., 1995). Prosystemin is found in the vascular bundles of tomato petioles (Jacinto et al., 1997). The mechanism for the movement of systemin in the phloem is not understood, but for systemin to have long-range signaling effects, an amplification of the signal most likely occurs. The presence of prosystemin in phloem parenchyma cells (J. Narvaez-Vasquez and C.A. Ryan, unpublished data) suggests that an interplay between the phloem and its surrounding cells may contribute to the signaling mechanism.
Systemin contains several charged amino acids as well as two proline doublets, at positions 6 and 7 and at 12 and 13. Only weak structural features are present in aqueous solutions of systemin, analyzed using nuclear magnetic resonance spectroscopy (Russell et al., 1992), but circular dichroism spectra reveals a poly(l-proline) II, 31 helix secondary structure in the central region of the polypeptide (Toumadje and Johnson, 1995). The polyproline helix is known to provide a kink in proteins (Ferris et al., 2001) and may provide a structure in systemin that is important to its recognition by the systemin receptor (discussed below).
Sequential alanine substitution at each of the 18 amino acids of systemin has variable effects on activity. A Thr to Ala substitution at position 17 eliminates activity (Pearce et al., 1993a) and creates a powerful antagonist of native systemin. Removal of the C-terminal Asp of systemin abolishes activity, and this analog is also an antagonist of systemin. Substitution of Pro12 and Pro13 residues with Ala severely reduces the activities of the analogs compared with that of native systemin.
The Ala substitution experiments, together with the deletion experiments, indicated that the 14 amino acids at the N terminus are likely involved in receptor binding, whereas the MQTD sequence at the C terminus has a direct role in signaling (Pearce et al., 1993a). This conclusion is supported by experiments in which binding of a systemin analog consisting of only the N-terminal 14 amino acids competes with systemin for binding to Lycopersicon peruvianum cell membranes, while the peptide MQTD does not (Meindl et al., 1998).
A cDNA encoding a 200–amino acid systemin precursor called prosystemin contains one copy of the systemin sequence near the C terminus (McGurl et al., 1992), indicating that systemin, like many animal and yeast polypeptide hormones, is proteolytically processed from a larger protein. Prosystemin does not exhibit a signal sequence at its N terminus, nor is it glycosylated (Delano-Frier et al., 1999). The lack of a signal sequence and the absence of any post-translational modification suggest that prosystemin is not synthesized through the secretory pathway but probably on free ribosomes in the cytosol.
The transgenic antisense plants with an antisense prosystemin gene, driven by the constitutive 35S promoter (McGurl et al., 1992), exhibit a severely reduced systemic induction of proteinase inhibitors in response to wounding that results in a reduced defense against Manduca sexta larvae attacks (Orozco-Cardenas et al., 1993). On the other hand, plants transformed with a prosystemin cDNA in its sense orientation, driven by the 35S promoter, synthesize defense proteins constitutively, as if they are in a permanently wounded state (McGurl et al., 1994). This phenotype is apparently caused by the expression of the gene in cells throughout the plant in which it is not normally expressed. Grafting wild-type tomato plants to root stocks overexpressing the prosystemin gene causes the wild-type scions to accumulate defense proteins as if they are wounded, suggesting either that systemin is transported from the root stalks to the scions or that it is producing another systemic signal involved in signaling the amplification of systemin or in directly signaling defense gene activation. Wild-type plants grafted onto wild-type rootstocks do not exhibit this response unless the rootstalks are wounded (McGurl et al., 1994). These experiments provide further evidence that systemin is a key signal for the systemic wound response to occur in the scions.
Systemin interacts with a membrane-bound protein with a Kd of ∼10−10 M (Meindl et al., 1998; Scheer and Ryan, 1999). The binding of an 125I-systemin analog is found in both isolated membranes and on the surface of suspension-cultured cells of L. peruvianum (Meindl et al., 1998; Scheer and Ryan, 1999). The binding is rapid and reversible, and both systemin and the inactive analog Ala-17–systemin, described earlier as an antagonist of systemin, are powerful competitors of 125I-systemin binding. A single 160-kD membrane-bound protein, identified by photoaffinity labeling, is present in the surface of L. peruvianum cells (Scheer and Ryan, 1999), which exhibits the same electrophoretic mobility under reducing and nonreducing conditions. The receptor has been purified 8200-fold to homogeneity from L. peruvianum cell membranes and identified as a leucine-rich repeat receptor kinase (Scheer and Ryan, 2002).
Prosystemin, synthesized with an Escherichia coli expression system (Delano-Frier et al., 1999) and with a baculovirus/insect cell expression system (Vetsch et al., 2000), is as active as systemin when supplied to young excised tomato plants through their cut stems. Whether the intact prosystemin protein is active without being processed or whether it is processed after being supplied to the plants has not been determined. A prosystemin lacking the systemin sequence is totally inactive when supplied to excised tomato plants (Dombrowski et al., 1999), indicating that the systemin sequence must be present for signaling to occur.
Systemins from Other Solanaceous Species
Homologs of the tomato prosystemin gene are found in potato, black nightshade, and bell pepper (Constabel et al., 1998). The encoded prosystemins exhibit high amino acid sequence identities with tomato prosystemin that range from 73 to 88%. When the systemin sequences are compared with the tomato systemin sequence, they exhibit only two to three amino acid replacements, with no replacements found within the seven C-terminal amino acids. All four prolines are conserved among all of the systemins. Each of the systemins is an active inducer of defense genes in leaves of young tomato plants when supplied to the plants through their cut stems, although nightshade systemin was ∼10-fold less active than the others.
A homolog of the tomato prosystemin gene could not be found in tobacco, another solanaceous species, and tomato systemin did not induce defense gene expression in leaves of tobacco plants (Constabel et al., 1998; Pearce et al., 2001a). Tobacco does, however, exhibit a wound response that systemically activates the synthesis of a family of tobacco trypsin inhibitors that are homologous to tomato Inh II (Pearce et al., 1993b). A five-year effort to isolate the systemic signal from tobacco leaves resulted in the isolation of only minute quantities of polypeptide signals that yielded limited structural data. A sensitive assay was recently developed (Pearce et al., 2001a), based on the observation that systemin caused the alkalinization of the medium of suspension-cultured tomato cells (Felix and Boller, 1995). Challenging 1-mL aliquots of L. peruvianum suspension-cultured cells with a few microliters from fractions from chromatography columns causes the pH of the cell medium to increase when signaling polypeptides are present. This assay facilitated the isolation of two 18–amino acid polypeptides from tobacco leaves that possess powerful tobacco trypsin inhibitor–inducing activities (Pearce et al., 2001a). They are called tobacco systemin I (Tob Sys I) and tobacco systemin II (Tob Sys II), in accordance with the definition of systemins as polypeptides that are released from plants at wound sites and that act locally or distally to activate signaling pathways for defense (Ryan and Pearce, 2001).
Tob Sys I and Tob Sys II are not homologous to each other or to tomato systemin. Both tobacco systemins contain hydroxyproline residues and are glycosylated (Pearce et al., 2001a). Each of the tobacco polypeptides induces the synthesis of tobacco trypsin inhibitors when supplied to young excised tobacco leaves through their cut stems, and both activate a MAP kinase when supplied to tobacco suspension-cultured cells, similar to the response caused by tomato systemin when it is supplied to tomato plants (Stratmann and Ryan, 1997). A third polypeptide is found in much lower concentrations than Tob Sys I and II, and is identical to Tob Sys II except for the presence of an additional Leu at the C terminus.
The amino acid sequences of Tob Sys I and II are compared with the sequence of tomato systemin in Figure 1. Both tobacco polypeptides contain pentose units, suggesting that, in contrast to tomato systemin, the tobacco systemins are synthesized through the secretory pathway. However, a common feature of all three systemins is the presence of proline and/or hydroxyproline residues.
Figure 1.

Amino Acid Sequences of Tobacco and Tomato Systemins.
Pearce et al. (2001a) isolated a cDNA from a tobacco leaf cDNA library by reverse transcriptase–mediated PCR, which encoded a 165–amino acid precursor of both polypeptides: Tob Sys I resides in the N-terminal region and Tob Sys II in the C-terminal region. This demonstrates that plant multiple polypeptide signals can arise from a single precursor, a scenario commonly found in animals and yeast (Bourbonnais et al., 1991; Steiner et al., 1992).
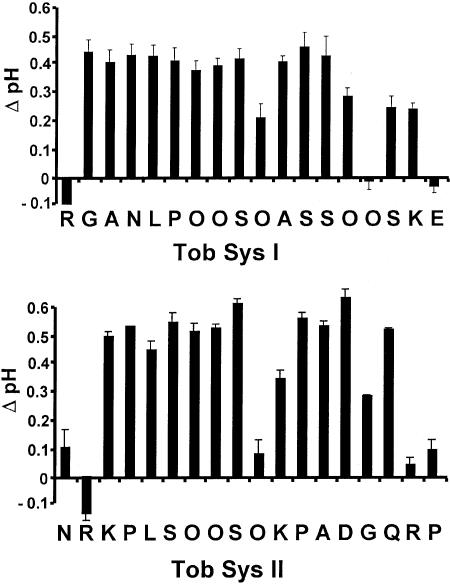
The two chemically synthesized tobacco systemins, lacking carbohydrates, are weakly active in inducing the alkalinization response in tobacco suspension cultures and in inducing synthesis of tobacco trypsin inhibitors in tobacco leaves (Pearce et al., 2001a), indicating that the carbohydrates are essential for full activity. Ala substitutions in Tob Sys I for the N-terminal Arg and for the C-terminal Glu totally eliminated inducing activity (Figure 2). Substitution of Ala for Hyp15 also eliminated activity, and substitution at Hyp10 and Hyp14 severely reduced activity. Ala substitutions in Tob Sys II at the N-terminal Asn or Arg, at the C-terminal Arg and Pro, and Hyp10, all severely reduced inducing activity. Therefore, in both tobacco systemins, the N- and C-terminal amino acids, together with the hydroxyprolines, appear to be important for activity.
Figure 2.
Activities of Synthetic Tob Sys I and II.
Synthetic Tob Sys I and II lack carbohydrates, so Ala substitutions have been introduced at each position in the primary structure. The analogs were assayed by monitoring the change in pH in the medium at 15 min after supplying 25 nmol of the systemins to 1 mL of L. peruvianum cultured cells. Error bars indicate standard deviation from three experiments.
Tomato systemin is inactive when supplied to tobacco plants, indicating that the receptor or other recognition factors are missing in tobacco. However, Tob Sys I and II are both active in inducing proteinase inhibitor synthesis in tomato plants and in causing alkalinization of the medium of tomato suspension-cultured cells (G. Pearce and C.A. Ryan, unpublished data). This indicates that receptors present in tomato leaf cells may recognize the tobacco systemins to activate the same signaling pathway that is activated by tomato systemin. Two Hyp-containing glycosylated polypeptides of 18 and 15 amino acids in length were recently isolated from tomato leaves that have strong alkalinating activity when added to either tomato or tobacco suspension-cultured cells (G. Pearce and C.A. Ryan, unpublished data). Whether the glycosylated systemins are systemic signals has not been established, nor have their tissue or cellular specificities been ascertained. Understanding the structures and functions of the various systemins found throughout the Solanaceae family should provide a basis for seeking systemins in other plant species.
RAPID ALKALINIZATION FACTOR
While searching for polypeptide wound signals in young tobacco leaves, using the alkalinization assay described previously (Pearce et al., 2001a), a 49–amino acid polypeptide was identified and isolated (Pearce et al., 2001b). The polypeptide exhibits a stronger and more rapid alkalinization of the medium of tobacco suspension-cultured cells than do the tobacco systemins, and it is therefore called RALF, the rapid alkalinization factor. Like tobacco systemins (Pearce et al., 2001a), RALF induces a MAP kinase activity in the cultured cells. The MAP kinase activity of RALF is also induced much more rapidly than the activities induced by systemins. Despite the similarities between RALF and the various systemin polypeptides in causing the alkalinization and MAP kinase responses, RALF is much larger than the systemins and does not induce the synthesis of tobacco trypsin inhibitors in leaves when supplied to young tobacco plants, suggesting a signaling role other than defense. RALF polypeptides with a high amino acid identity with tobacco RALF were subsequently isolated from tomato leaves and alfalfa leaves. N-terminal sequences of ∼20 amino acids of each of the isolated polypeptides were determined, and a search of databases revealed the existence of RALF homologs in >15 plant species from nine families (Pearce et al., 2001b).
Probing a tobacco cDNA library with a tomato EST probe, a cDNA coding for RALF was isolated that encoded a RALF precursor protein of 115 amino acids in length. The encoded protein contains a putative 25–amino acid signal peptide at its N terminus and a 49–amino acid RALF sequence at its C terminus. The molecular mass of the RALF polypeptide deduced from the precursor matches the molecular mass of the purified tobacco polypeptide, determined by MALDI-MS, indicating that RALF is proteolytically processed from a larger preproprotein. Nine genes that contain highly conserved RALF sequences are present in Arabidopsis (Pearce et al., 2001b). The N-terminal amino acid sequences of each RALF precursor are much less conserved than are the C-terminal sequences encoding the RALF polypeptide (Pearce et al., 2001b). A structural feature of all Arabidopsis RALF precursor proteins is the presence of two Arg residues just upstream from the putative N-termini. In animals, dibasic residues are part of the recognition site for processing polypeptide hormones from their precursors (Harris, 1989). The region just upstream of the dibasic site is rich in polar residues that are highly conserved among RALF precursors from different species and, as in animals, may be part of a recognition site for the RALF-processing proteinase.
Tomato RALF was chemically synthesized, and conditions were established for folding that correctly cross-linked the two disulfide bonds that are present in the native polypeptide. The synthetic RALF is as active as the native RALF in the alkalinization assay, whereas a synthetic, reduced and carboxymethylated RALF is inactive. RALF coupled with an azido-label selectively identified a 120-kD binding protein and a 25-kD binding protein (J. Scheer, G. Pearce, and C.A. Ryan, submitted). The characterization of these proteins will provide further insights into structural characteristics of the receptor(s) and of the signaling cascade that RALF initiates.
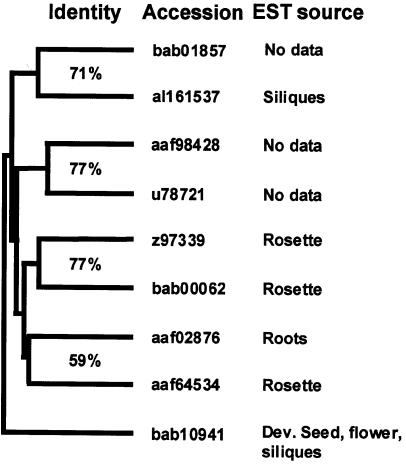
The ubiquity of the RALF polypeptides suggests that they have a fundamental physiological role(s) in different organs and tissues of the plants. On the basis of amino acid sequence identities of the nine Arabidopsis RALF preproproteins, they can be divided into five clusters (Figure 3). The EST for al161537 is found in a library made of green siliques, and aaf02876 is found in a root library. The bab10941 gene is expressed among several tissues, whereas z97339, bab00062, and aaf64534 are all from libraries made of rosette leaves. The remaining genes, bab01857, aaf98428, and u78721, are not found in the current EST databases.
Figure 3.
Tree Plot of the RALF Genes Present in Arabidopsis.
Created by the program Pileup (Genetics Computer Group), this tree plot shows the clustering relationships among the different RALF-like genes found in the Arabidopsis genome. The identity (%) between the clones in each cluster, the clone accession number, and the tissue from which each EST is derived are shown.
An insight into the role of RALF in plants was gained when seedlings of tomato and Arabidopsis were germinated and transferred to medium containing micromolar levels of tomato RALF (Pearce et al., 2001b). Root growth is immediately arrested, but the response is reversible and the roots resume growth when the seedlings are transferred to water. Reduced and alkylated RALF, which was inactive in the alkalinization assay, is inactive in inhibiting root growth. The cause of the inhibition of root growth by RALF is not known, but median longitudinal sections of root tips from RALF-treated seedlings indicate that RALF is inhibiting cell division (D.S. Moura, G. Pearce, and C.A. Ryan, unpublished data). The tomato RALF used in these experiments exhibited a high identity to the RALF encoded by Arabidopsis aaf02876 (Figure 3) that was identified in a root EST library. Because nine RALF genes are present in Arabidopsis, and the many highly conserved ESTs so far identified are found in different organs and cells of different species, RALF may have a more general role in plants. The expression of GFP or GUS under control of the promoters of the various Arabidopsis genes should provide some insight into their temporal and tissue-specific expression.
PHYTOSULFOKINES
Two small polypeptide signals were reported by Matsubayashi and Sakagami (1996) to regulate plant cell proliferation in dispersed asparagus suspension-cultured cells. The rates of cell division of suspension-cultured cells had been known for years to be strictly dependent on the initial cell density (Stuart and Street, 1969). At low density, the mitotic activity is very low, but if the cultures are supplemented with media taken from high-density cell cultures (conditioned medium [CM]), the low-density cells rapidly proliferate. None of the known plant hormones can substitute for CM, and the nature of the active factor remained obscure until two mitogenic factors were isolated from the CM of Asparagus officinalis mesophyll cell cultures (Matsubayashi and Sakagami, 1996). Structural analyses of the two components revealed that they were both small polypeptides modified with sulfate groups (Matsubayashi and Sakagami, 1996), and were named phytosulfokines (PSK-α and -β). The structure of PSK-α is −Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln−, and of PSK-β is −Tyr(SO3H)-Ile-Tyr(SO3H)-Thr−. Sulfated tyrosines are structures found in peptides in animals (Hanai et al., 2000a), but to date the PSKs are the only example of post-translational sulfated tyrosine residues in plants.
PSKs with identical structures are present in the CM of rice (Oryzae sativa) (Matsubayashi et al., 1997), zinnia (Matsubayashi et al., 1999a), and carrot (Hanai et al., 2000b). Both PSK-α and PSK-β were chemically synthesized and each is as active as the native PSKs (Matsubayashi and Sakagami, 1996). Synthetic PSK-αs lacking either one or both of the two C-terminal amino acids are weakly active, compared with native PSK-α. The N-terminal truncated analogs and unsulfated analogs are inactive, suggesting that the N-terminal sulfated tripeptide is the active core. Substitution of Ile with Val, and of Thr with Ser, results in an ∼20- fold decrease in activity. PSK-β is thought to be a degradation product of PSK-α, having lost the C-terminal Gln likely by carboxypeptidase cleavage.
The O. sativa PSK gene (OsPSK) is a single-copy gene (Yang et al., 2000) consisting of one large intron and two exons. The gene codes for a PSK precursor of 725 basepairs encoding an 89–amino acid polypeptide (9.8 kD) with a 22–amino acid leader peptide (Yang et al., 1999b). The PSK-α pentapeptide sequence occurs only once, near the C terminus (Figure 4). Flanking the PSK-α polypeptide are aspartic acid residues that are suggested to be involved in the processing. Because the PSK precursor contains a signal peptide, sulfated tyrosines, and is secreted into the medium of cell cultures, it is most likely synthesized through the secretory pathway. A tyrosyl-protein sulfotransferase activity was shown to be present in microsomal membranes of carrot cells. The specificity of the enzyme is consistent with its role in sulfating the PSK precursor before secretion (Hanai et al., 2000a).
Figure 4.
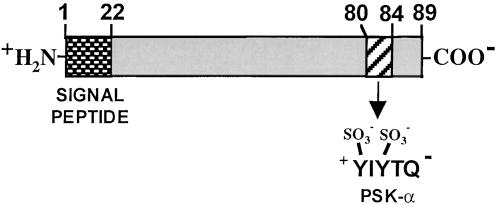
Proteolytic Processing of the PSK-α.
PSK-α is proteolyically processed from the C terminus of a preproprotein precursor. The precursor is synthesized through the secretory pathway where the signal sequence is removed, and where the sulfate groups are attached to tyrosine residues.
A 1.9-kb 5′ region of the gene is sufficient to drive the expression of the GUS gene in transformed cultured rice cells. Transforming rice cells with sense and antisense OsPSK genes driven by the actin promoter results in sense transformants with cell division rates two times faster than those of wild-type cells, and antisense transformants that divide much more slowly than the wild type. This indicates that the OsPSK sense construct is expressed and that the endogenous OsPSK gene expression is reduced by the OsPSK antisense construct. The expression of the OsPSK gene is detected throughout the rice seedling but in higher abundance in root and shoot apices, where it is likely to have a physiological role. Although PSK mRNA has been identified in rice and Arabidopsis cell suspension cultures (Yang et al., 1999b, 2001) and PSK polypeptides have been identified in cell cultures of asparagus (Matsubayashi and Sakagami, 1996), rice (Matsubayashi et al., 1997), zinnia (Matsubayashi et al., 1999a), and carrot (Hanai et al., 2000b), immunologically detectable PSK polypeptides have not been reported in whole-plant extracts.
GUS expression regulated by the rice PSK gene promoter in rice cells is dependent on the presence of both 6-benzyladenine and auxin in the medium. The two hormones together enhance the production of GUS activity. The presence of PSK-α in suspension-cultured asparagus mesophyll cells is first detected immunologically 48 hr after initiating the cultures and 48 hr before the observation of the first cell division (Matsubayashi et al., 1999b).
The in vivo functional role of PSK-α in plants has not been established, but the polypeptide enhances the differentiation of cell cultures of zinnia into tracheary elements (Matsubayashi et al., 1999b) and causes somatic embryogenesis in carrot (Kobayashi et al., 1999; Hanai et al., 2000b). The polypeptide also induces adventitious root and bud formation in Antirrhinum callus (Yang et al., 1999a) and induces the formation of adventitious roots on cucumber hypocotyls (Yamakawa et al., 1998). Tracheary differentiation caused by PSK-α has an absolute requirement for 1-naphthaleneacetic acid and/or 6-benzyladenine, but adventitious bud formation from Antirrhinum callus cultures occurs in the absence of phytohormones.
[3H]PSK-α exhibits high affinity binding to rice membranes, with a Kd of 1.4 nM (Matsubayashi and Sakagami, 1999). The binding is specific, competitive, and reversible, and only active analogs can compete with the labeled PSK-α. An estimated 104 PSK-α binding sites per cell are present, based on the assumption that a 40% recovery of plasma membranes had been achieved. Specific binding is also detected in plasma membrane fractions derived from cultured cells of asparagus, rice, maize, tobacco, tomato, and carrot (Matsubayashi and Sakagami, 1999).
An 125I-labeled photoactive 4-azidosalicylic acid ligand linked to the α-amino group of PSK-α labels membrane proteins of 120- and 160-kD (Matsubayashi and Sakagami, 2000) that appear to be components of a receptor complex. Treatment of the photoaffinity-labeled membranes with glycosidase enzymes to remove carbohydrate moieties, followed by electrophoresis and identification of labeled proteins, causes a decrease of ∼10-kD for each of the 160- and 120-kD proteins, which suggests that they are glycosylated.
The cumulative data indicate that PSK-α has a role related to cell division and development, but its mechanism of action, its signaling pathway, its relationship to other hormones, and its role in regulating developmental processes remain to be determined.
CLAVATA3
CLAVATA3 (CLV3) is an extracellular signaling polypeptide of 79 amino acids in length that is involved in the determination of cell fate in the shoot apical meristem (SAM) of Arabidopsis. The origin of above-ground organ position and identity in plants is initiated and established in SAM (Clark, 1997; Meyerowitz, 1997), where a balance between the division of stem cells (central zone) and the differentiating cells (peripheral zone) must be maintained as the plant grows. The stem cells divide slowly, while the surrounding cells divide more actively. Two genes, CLV1 and CLV3 (Clark et al., 1993, 1995; Nakajima and Benfey, 2002), present in Arabidopsis SAM, are fundamental to maintaining the balance of cells in each zone. Mutations in either CLV1 or CLV3 produce identical phenotypes in which many more cells are found in both apical meristems and floral meristems than are found in meristems of wild-type plants (Clark, 1997; Meyerowitz, 1997). Double clv1-clv3 mutants exhibit the same phenotypes as plants with single mutations (Clark et al., 1995), providing strong evidence that both CLV1 and CLV3 are components of a common signaling pathway.
CLV3 was cloned using two mutant alleles, clv3-3, identified by a T-DNA insertion, and clv3-7, which resulted from the integration of the maize transposable element En-1 (Fletcher et al., 1999). The gene contains three exons and two small introns that encode a protein of 96 amino acids having an 18–amino acid N-terminal leader peptide. The processed polypeptide is therefore composed of 78 amino acids. There is no evidence that any further processing of CLV3 occurs after removal of the signal peptide, but a 13–amino acid conserved domain exists in the C terminus of five maize endosperm-specific polypeptides (ZmEsrs) (Opsahl-Ferstad et al., 1997; Cock and McCormick, 2001) and in the C terminus of 21 Arabidopsis CLV3-like polypeptides (DeYoung and Clark, 2001), which begin with either a dibasic (20 polypeptides) or a single basic residue (6 polypeptides). The possibility that these residues are candidates for processing sites to produce a family of small highly conserved polypeptides that may have a role in signaling requires further investigation.
CLV3 mRNAs are found in cells of the SAM and in the central regions of floral meristems (Fletcher et al., 1999). The gene is always expressed in the most central, nondifferentiated cells (stem cells), especially near the apical surface. The polypeptide synthesized from CLV3 is hypothesized to be secreted from superficial cell layers in the central region of the SAM, interacting with CLV1, a 105-kD receptor-like kinase (Clark et al., 1997) in underlying cells, coordinating the enlargement of the CLV1 domain with a restriction of the stem cell population (Fletcher et al., 1999). The amino acid sequence of CLV1 suggests that it is a transmembrane protein consisting of a leader sequence, a putative extracellular domain of 21 leucine-rich repeats, and 15 putative N-linked glycosylation sites.
CLV1 is thought to be associated with CLV2, a 720–amino acid LRR receptor-like protein lacking a kinase domain to form a heterodimer (Jeong et al., 1999). The association is thought to be through disulfide bonds (Trotochaud et al., 1999). CLV1 and CLV2 are expressed in the central layer of the SAM and are part of a larger membrane-bound protein complex that includes KAPP, a protein phosphatase (Stone et al., 1998) expressed in the same regions as CLV1, and a Rho-GTPase–related protein called Rop (Trotochaud et al., 1999). Using cauliflower, which has multiple meristems and is closely related to Arabidopsis, a complex containing both CLV1 and KAPP was immunoprecipitated with CLV1-specific antibody, demonstrating that those two proteins interact in vivo (Stone et al., 1998). CLV1 protein is present in two complexes, one of 185 kD and one of 450 kD (Trotochaud et al., 1999). The two complexes are present in Arabidopsis meristem extracts, and reducing agents dissociate the complexes, supporting the involvement of disulfide bridges in their structural integrity. CLV1 is present in both complexes, while KAPP is only found in the 450-kD complex. Using antibodies raised against pea Rop protein, cross-reacting proteins were found only in the 450-kD complex. Both the 185- and 450-kD complexes are therefore considered significant components of the signaling pathway. The 185-kD complex is thought to be a heterodimer composed of CLV1 and CLV2, and the 450-kD complex composed of the heterodimer plus KAPP and Rop, and possibly additional proteins (Trotochaud et al., 1999). KAPP has been shown to bind to various receptor-like kinases (Braun et al., 1997) and clearly regulates negatively CLV signaling (Williams et al., 1997; Stone et al., 1998) by binding to phosphorylated CLV1.
Wild-type Arabidopsis plants constitutively overexpressing CLV3 cease formation of organs after the emergence of the first leaves. Floral primordia fail to initiate whorls with stamens and carpels in plants in which CLV3 is expressed weakly, confirming that CLV3 expression contributes to cell fate determination. Increasing the copy number of the CLV3 of Arabidopsis plants does not cause alterations in shoot or floral meristem development. Another consequence of CLV signaling is a reduction of the expression of WUSCHEL (WUS), which promotes cell formation and maintenance of cells in a narrow domain deeper in the meristems and acts antagonistically to the CLV genes (Laux et al., 1996). The positive and negative feedback interactions between CLV1/CLV2/CLV3 genes and WUS maintain the balance required for stem cell proliferation, both temporally and spatially (Schoof et al., 2000). CLV1 has not been found to affect root meristem development.
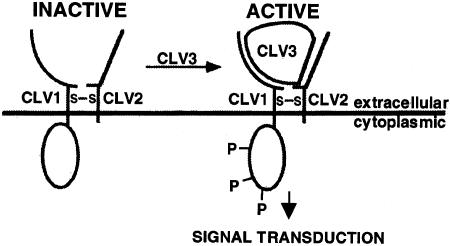
A model for the interaction of CLV3 with CLV1 and CLV2 is shown in Figure 5. CLV3 exists as a multimer of ∼25 kD (Trotochaud et al., 2000), and its interaction with the receptor results in phosphorylation of the intracellular domains of CLV1 and the activation of signaling. Subsequent dephosphorylation of CLV1 terminates signaling.
Figure 5.
Model for the Receptor Complex and Its Interaction with the Polypeptide CLV3.
A model for the receptor complex and its interaction with the polypeptide CLV3, which regulates the balance of cell proliferation and organ formation in the shoot and flower meristems of Arabidopsis. Upon binding of CLV3 to the CLV1-CLV2 receptor complex, the protein kinase domain becomes activated, leading to the autophosphorylation of CLV1 and subsequent signaling pathway events. (Adapted from Trotochaud et al., 1999.) s-s, disulfide bonds; P, phosphorylation.
The CLV signaling pathway has provided an exceptional insight into signaling for meristem development. That such fundamental developmental processes are governed in part by a polypeptide signal and its receptor complex may be a prototype for many aspects of meristem and floral development, and of other key steps in growth and differentiation in plants.
S-LOCUS SIGNALING
A family of intercellular signaling polypeptides of 47 to 60 amino acids in length, collectively called S-locus cysteine-rich proteins (SCR) or S-locus protein 11 (SP11) (Schopfer et al., 1999; Takayama et al., 2000), play a central role in pollen self-incompatibility in the Brassicaceae. During pollination, the pollen tubes invade the female stigmatic and pistil tissues before fertilization. Self-recognition between pollen and stigma in crucifers is controlled by multi-allelic, dominant self-incompatibility (SI) genes at the S-locus (Bateman, 1955). During SI, when the pollen comes in contact with the papilli of the stigma, a self-recognition occurs that arrests the pollination process (Kachroo et al., 2002). The SI response leads to dehydration of the papilli, with aquaporin playing a major role in regulating water loss (Ikeda et al., 1997; Franklin-Tong and Franklin, 2000).
SCR/SP11 is composed of a family of small cysteine-rich proteins of 74 to 83 amino acids, including their signal peptides (Brugière et al., 2000; Nasrallah, 2000). Each mature polypeptide contains eight cysteine residues. The amino acid sequences of the signal peptides are highly conserved, but the mature, processed polypeptide signals, while conserved at the cysteine residues, exhibit weak identities at other residues. The high identity among the leader peptides and not among the mature polypeptides is in contrast to most secreted proteins in which the amino acids in signal peptides among homologs are less conserved than are the mature proteins. The 100% conservation of the cysteines suggests that the polypeptides may share conformational similarities that are not readily apparent from sequences alone (Schopfer and Nasrallah, 2000). The properties of the SCR polypeptides are consistent with their roles as signals that determine pollen SI specificity when recognized by the appropriate receptor. The polypeptides are secreted from developing microspores in the tapetum and transported to the pollen coat exine layer (Stephenson et al., 1997; Schopfer and Nasrallah, 2000; Takayama et al., 2000). SCR translocates into cell walls of the stigma epidermal cells at the pollen–stigma contacts, activating a receptor-mediated cascade leading to SI (Schopfer et al., 1999; Brugière et al., 2000; Nasrallah, 2000).
SCR/SP11s interact with an S-locus receptor kinase (SRK) on the cell walls of stigma papillae. The SRK has a functional serine/threonine kinase at its C terminus (Goring and Rothstein, 1992; Stein and Nasrallah, 1993), with a transmembrane region in the middle and an extracellular cysteine-rich domain at its N-terminal, extracellular region (Stein et al., 1991). An abundant soluble S-locus glycoprotein (SLG) appears to be associated with SRK, with SLG often present in a 100-fold excess, and both SLG and SRK display genotype-specific sequence polymorphisms that are involved in haplotype specificity (McCubbin and Kao, 2000). SRK is inherently unstable, and SLG may interact with SRK to facilitate the maintenance of levels that can be functional (Dixit et al., 2000), perhaps by contributing to pollen–stigma adhesion (Luu et al., 1999). However, SLG is not sufficient to cause S-haplotype specificity (Nasrallah and Nasrallah, 1993; Takasaki et al., 2000; Takayama et al., 2000), and in one self-incompatible line of Brassica oleraceae the SLG gene is missing (Okazaki et al., 1999).
At least three gene products have been identified from yeast two-hybrid libraries that interact with the kinase domain of SRK, and they all appear to have roles in the intracellular signaling cascade initiated by the SCR/SRK/SLG complex. This complex is composed of ARC1, an arm-repeat protein associated with protein–protein interactions in yeast (Gu et al., 1998), and two thioredoxin h-like proteins that have been implicated in the modulation of protein–protein interactions by reducing disulfide bridges (Bower et al., 1996). However, recent evidence indicates that thioredoxin may not directly participate in the activation of SRK by SCR/SP11 (Takayama et al., 2001) but may prevent spontaneous autophosphorylation of SRK in the absence of SCR/SP11 (Cabrillac et al., 2001).
Synthetic SP11, with its four cysteine bridges correctly oxidized, did not interact with SLG but did bind to whole cells of the stigma and to microsomal membranes prepared from the corresponding haplotype. Cross-linking experiments using 125I-labeled SP11 and microsomal membranes indicated that SRK and SLG may form a high-affinity receptor complex on the stigma membrane surface (Takayama et al., 2001). Using recombinant SCR and the extracellular domain of SRK, high-affinity allele-specific binding between the two signaling components was demonstrated (Kachroo et al., 2001; Takayama et al., 2001), providing direct evidence for the ligand-receptor interaction that regulates SI. Arabidopsis, a self-compatible Brassica relative, does not have in its genome the S-locus genes SLG, SRK, and SCR (Takada et al., 2001). However, the Arabidopsis genome has been shown to contain a large family of genes coding for polypeptides with homology to SCR and pollen coat proteins (Vanoosthuyse et al., 2001). More complete descriptions of this system are presented in recent review articles (Brugière et al., 2000; McCubbin and Kao, 2000; Nasrallah, 2000) and in a companion article in this issue of The Plant Cell (Kachroo et al., 2002).
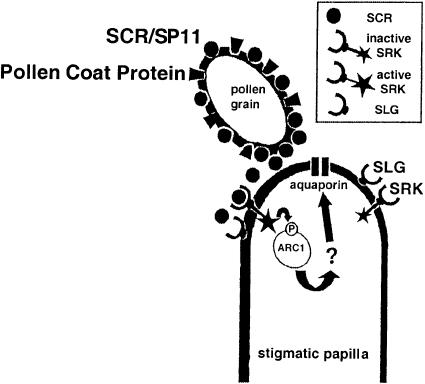
Shown in Figure 6 is a recently proposed model for the important features that are relevant to the SI signaling cascade (Franklin-Tong and Franklin, 2000). This includes the polypeptide signal SCR/SP11 and the receptor complex, consisting of SRK and associated proteins. This pathway, as in other signaling pathways in plants, is still in its early stages of understanding. However, the identification of SCR/SP11, SRK, and the intracellular components associated with this complex has provided a major advance for understanding the molecular basis of SI in the Brassicaceae.
Figure 6.
SI Signaling in Brassica.
Shown is a model for the initial steps of SI signaling in Brassica (Franklin-Tong and Franklin, 2000). When pollen grains come in contact with the papilla surface of the stigma, the polypeptide signal, SCR, interacts with the SLG-SRK receptor kinase complex to activate a signaling cascade that leads to the incompatible SI response. The complex results in the activation of the receptor kinase and the phosphorylation of ARCI, which may lead to the activation of aquaporins to limit the access of water to the incompatible pollen. P, phosphorylation.
ENOD40 Short ORFs
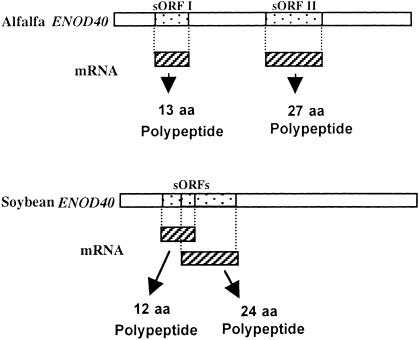
ENOD40 is a nodule-associated gene present in a number of legume species (Yang et al., 1993; Mylona et al., 1995) and also found in tobacco and rice (van de Sande et al., 1996; Kouchi et al., 1999). The expression of ENOD40 gene has been associated with nodule formation in response to nodulation factors and cytokinin (Fang and Hirsch, 1998) and with a change in the response to auxins (van de Sande et al., 1996). The modification of the response to the phytohormone auxin has been re-evaluated, and the previous results could not be reproduced (Schell et al., 1999). The ENOD40 gene contains no long ORF, but it does contain short ORFs (sORFs) in highly conserved regions, called boxes I and II (van de Sande et al., 1996). In alfalfa, box I is found in the 5′ region of the gene and codes for a 13–amino acid polypeptide. Box II is in the central region of the gene, coding for a polypeptide of 27 amino acids (Sousa et al., 2001) (Figure 7). The polypeptides have no signal sequences at their N termini and may be synthesized in the cytosol, similar to tomato systemin. The expression of either region of a Medicago truncatula enod40 in Medicago sativa roots causes a response similar to that evoked by the complete gene, that is, cortical cell division (Charon et al., 1997). The 13–amino acid polypeptide is homologous to sORFs found in soybean, pea, alfalfa, and vetch ENOD40s (van de Sande et al., 1996). Microtargeting of the wild-type and mutated M. truncatula ENOD40 genes into Medicago roots has shown that the translation of the two sORFs as well as the presence of an inter-ORF region are involved in the regulation of ENOD40 activity in Medicago roots (Sousa et al., 2001). Using polyclonal antibodies specific for the 13–amino acid sORF, immunocompetition experiments revealed the presence of small amounts of an antigen in extracts of mature nodules. Northern analyses indicate that in the same tissues, the ENOD40 transcripts constitute ∼0.5% of the total mRNA. When the synthetic 13–amino acid polypeptide is added to nodule extracts, the polypeptide totally disappears in <1 min, indicating that it is being rapidly degraded, or else binds to a component in the extract that masks its immunological reactivity. In vitro translation of the gene does not result in any measurable signals, and when the synthetic 13–amino acid polypeptide is added to the translation mix, it disappears within a minute (Sousa et al., 2001).
Figure 7.
Short ORFs Derived from the ENOD40 Genes from Alfalfa and Soybean.
In soybean, a 12–amino acid polypeptide and a 24–amino acid polypeptide are translated from overlapping ORFs. aa, amino acids.
The search for the role of ENOD40 polypeptides has recently become more interesting with the finding that a second sORF of 24 amino acids overlaps the box I 12–amino acid sORF of the soybean ENOD40 gene (Figure 7) (Röhrig et al., 2002). The 24–amino acid polypeptide but not the 12–amino acid polypeptide was identified in nodule extracts using gel blot techniques. Both polypeptides, however, strongly bind to a 93-kD nodule protein that was identified as a subunit of sucrose synthase. The authors proposed that the two ENOD40 polypeptides may serve a regulatory role in unloading sucrose from the phloem, either by modulating enzyme activity or by directing the enzyme to specific intracellular sites. The sORFs of alfalfa have not yet been investigated for a similar regulatory role.
The data strongly support the hypothesis that translations of sORFs as well as the presence of the inter-ORF regions are important for ENOD40 function and that they likely play an important role in early nodulation events. However, if the sORFs are active only in the cells in which they are synthesized, they would not be signals that fit the classical definition of hormones but rather would serve novel roles as modulators of cellular activities. These findings introduce a new level of investigation of the signaling pathways that regulate cell division early in the nodulation process.
OTHER POLYPEPTIDES WITH SIGNALING POTENTIALS
Several other polypeptides have been suggested to be hormone-like signaling molecules in plants with various roles related to growth, development, and differentiation. A small gene, called POLARIS, is expressed in Arabidopsis seedlings and root tips and is required for correct cytokinin and ethylene signaling during root growth (Lindsey, 2001). The gene encodes a 450-bp mRNA that has an ORF encoding a 36–amino acid polypeptide, but its possible role in signaling is still being investigated. It is of interest that ENOD40, PSK-α, RALF, and POLARIS are all associated with some aspect of root growth and development.
Several laboratories have looked for homologs of animal polypeptide hormones in plants. A 4-kD polypeptide called leginsulin (Watanabe et al., 1994) was isolated that caused the autophosphorylation of a 7S globulin that exhibits some characteristics of an insulin receptor. However, no function has been identified for either the polypeptide or the putative receptor, and the inclusion of this polypeptide in the insulin family is premature until an insulin-like role in plants is associated with the polypeptide and/or the soluble 7S globulin. In a somewhat similar study, a 20-kD protein was isolated from maize tissue using affinity chromatography with an insulin-Sepharose column (Garcia-Flores et al., 2001). The protein accelerated maize seedling growth and enhanced phosphorylation of a ribosomal subunit from maize axes. A polypeptide has been isolated from Jack bean seed coats that has the identical structure as bovine insulin (Oliveira et al., 1999), but no biological activity in plants has been associated with the polypeptide, nor has an insulin gene been reported from plants.
A group of polypeptides from animals, called natriuretic peptides, function in animals as regulators of salt and water balance, and a mixture of 3- to 10-kD polypeptides could be immunoprecipitated from Hedera helix with anti-animal natriuretic peptide antibodies (Gehring, 1999). The crude mixture caused biological effects in plants, including increases in cGMP levels and stomatal opening in maize roots. However, no plant natriuretic peptides have been purified and characterized, nor have the mRNAs or genes that encode such polypeptides been identified.
CONCLUDING REMARKS
Polypeptide signaling has been known for more than fifty years to be of fundamental importance in a wide spectrum of physiological processes in animals, and research over the past decade has revealed their importance in plants. Polypeptide signaling may have had its origins in ancestral organisms that predated plants and animals, providing a foundation for the evolution of polypeptide signaling in all modern eucaryotes. In animals, polypeptide hormones classically fall into two major categories: endocrine hormones (Douglass et al., 1984) and membrane-anchored growth factors (Massague and Pandiella, 1993). The endocrine polypeptide hormones, and some growth factors, are synthesized as preprohormones in the secretory pathway of specialized cells and are processed and sequestered in vesicles until released into extracellular compartments in response to appropriate physiological cues. The hormones are then transported to target cells, either nearby or long distances away, where they are recognized by specific receptors to initiate signaling pathways. Some well-known examples are insulin and endorphins. Polypeptide hormones are usually small, being derived from precursors that are ∼100 to 500 amino acids in length (Douglass et al., 1984). Some of these polypeptides are produced as a single copy from a precursor, such as insulin, but some precursors harbor several copies of the hormone, such as proenkephalin, while still other precursors are processed to produce multiple hormones having different physiological roles, such as pro-opiomelanocortin. The variability of structural conformations is critcial for the high specificity of receptor binding that exists among hundreds of polypeptide hormones.
Processing of polypeptide hormones from larger prohormone precursors in vesicles is mediated by members of the subtilisin family of endopeptidases. These enzymes are membrane-associated and cleave proprotein precursors at the C terminus of the dibasic pair. This family of processing proteinases is found in both animals and yeast, and homologs have been reported in plants, where no known substrates have been identified (Schaller and Ryan, 1994; Berger and Altmann, 2000). Conserved dibasic pairs of amino acids that are candidates for processing sites are found in all tomato-related prosystemins (Constabel et al., 1998), in all nine of the Arabidopsis RALF precursor proteins (Pearce et al., 2001b), in three of four PSK-α precursor proteins (Yang et al., 2001), and in 20 of 26 CLV3 precursor proteins (DeYoung and Clark, 2001). In systemins, the dibasic amino acids may be sites of degradation, and whether the dibasic sites in other polypeptide signals lead to the production of polypeptide signals or to degradation remains to be determined.
A different scenario for polypeptide synthesis and release is found with membrane-anchored growth factor precursors (Massague and Pandiella, 1993). Examples include transforming growth factor TGF-α (50 amino acids derived from a 160–amino acid precursor), epidermal growth factor EGF (53 amino acids derived from a 1207–amino acid precursor), and tumor necrosis factor TNF-α (157 amino acids derived from a 233–amino acid precursor). The precursors of most members of this class of polypeptides are generally between 150 to 650 amino acids in length, although some are larger. They are synthesized through the secretory pathway; the proproteins, however, are not processed in vesicles but are anchored in vesicle membranes. The vesicles fuse with the plasma membrane of the cell, exposing the polypeptide to the extracellular space. Upon appropriate cues, membrane-associated proteases are activated that release the factors, which can be recognized by their receptors.
Several plant polypeptide hormones described in this review are synthesized as preproproteins, apparently through the secretory pathway. These include PSK-α, CLV3, SCR, and the tobacco systemins. Although PSK-α, CLV3, and tobacco systemins appear to be secreted to interact directly with their receptors, SCR appears to be unique in that it is synthesized in the tapetum, transported to the pollen, and then to the stigma receptors. The precursors of tomato prosystemin and of ENOD40s do not have signal sequences and are apparently synthesized in the cytosol on free ribosomes, a scenario not found with animal polypeptide hormone precursors. However, systemin must somehow be transported to the extracellular spaces upon wounding, where it can interact with its receptor on cell surfaces. ENOD40 does not appear to fit the classical definition of polypeptide hormones, acting as an intracellular modulator.
To date, the receptors for polypeptide hormones appear to be either leucine-rich repeat receptors (CLV1 and the systemin receptor) or cysteine-rich receptor kinases (SRK). These, and others currently under investigation, such as the PSK-α receptor and the RALF receptor, will provide excellent systems for structural analyses to study ligand-receptor interactions and to determine how the complexes transmit information across plant plasma membranes to facilitate intracellular signaling.
Although only a small number of plant polypeptide signals and their receptors have been identified, polypeptide signaling in plants has become a new subject for scientific inquiry. Major research efforts in this area are necessary to gain a full understanding of the importance of polypeptide signaling in plants and of the fundamental biochemistry of polypeptide-receptor interactions and the mechanisms that underlie their signaling of plant growth and development, plant defense, and plant responses to the environment. This research area holds much promise for developing new fundamental knowledge about plant biology as well as providing novel biotechnological approaches for the benefit of agriculture and human health.
Acknowledgments
Financial support from the Washington State University College of Agriculture and Home Economics and the National Science Foundation Grant No. IBN 0090766 is gratefully acknowledged.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010484.
References
- Banting, F.G., and Best, C.H. (1922). The internal secretions of the pancreas. J. Lab. Clin. Med. 5 251–266. [Google Scholar]
- Bateman, A.J. (1955). Self-incompatibility systems in angiosperms, III. Crucifera. Heredity 9 52–68. [Google Scholar]
- Berger, D., and Altmann, T. (2000). A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 14 1119–1131. [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais, Y., Germain, D., Lathcinian-Sadek, L., Boileau, G., and Thomas, D.Y. (1991). Prohormone processing by yeast proteases. Enzyme 45 244–256. [DOI] [PubMed] [Google Scholar]
- Bower, M.S., Matias, D.D., Fernandes-Carvalho, E., Mazzurco, M., Tiesheng, G., Rothstein, S.J., and Goring, D.R. (1996). Two members of the thioredoxin-h family interact with the kinase domain of a Brassica S locus receptor kinase. Plant Cell 8 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, D.M., Stone, J.M., and Walker, J.C. (1997). Interaction of the maize and Arabidopsis kinase interaction domains with a subset of receptor-like protein kinases: Implications for transmembrane signaling in plants. Plant J. 12 83–95. [DOI] [PubMed] [Google Scholar]
- Brugière, N., Cui, Y., and Rothstein, S.J. (2000). Molecular mechanisms of self-recognition in Brassica self-incompatibility. Trends Plant Sci. 5 432–438. [DOI] [PubMed] [Google Scholar]
- Cabrillac, D., Cock, J.M., Dumas, C., and Gaude, T. (2001). The S-locus receptor kinases are inhibited by thioredoxins and activated by pollen coat proteins. Nature 410 220–223. [DOI] [PubMed] [Google Scholar]
- Charon, C., Johansson, C., Kondorosi, E., Kondorosi, A., and Crespi, M. (1997). enod40 induces dedifferentiation and division of root cortical cells in legumes. Proc. Natl. Acad. Sci. USA 94 8901–8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E. (1997). Organ formation at the vegetative shoot meristem. Plant Cell 9 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397–418. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1995). CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121 2057–2067. [Google Scholar]
- Clark, S.E., Williams, R.W., and Meyerowitz, E.M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89 575–585. [DOI] [PubMed] [Google Scholar]
- Cock, J.M., and McCormick, S. (2001). A large family of genes that share homology with CLAVATA3. Plant Physiol. 126 939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constabel, C.P., Yip, L., and Ryan, C.A. (1998). Prosystemin from potato, black nightshade, and bell pepper: Primary structure and biological activity of predicted systemin polypeptides. Plant Mol. Biol. 36 55–62. [DOI] [PubMed] [Google Scholar]
- Delano-Frier, J.P., Dombrowski, J.E., and Ryan, C.A. (1999). The expression of tomato prosystemin in E. coli: A structural challenge. Protein Expression Purif. 17 74–82. [DOI] [PubMed] [Google Scholar]
- DeYoung, B.J., and Clark, S.E. (2001). Signaling through the CLAVATA1 receptor complex. Plant Mol. Biol. 46 505–513. [DOI] [PubMed] [Google Scholar]
- Dixit, R., Nasrallah, M.E., and Nasrallah, J.B. (2000). Post-translational maturation of the S receptor kinase of Brassica correlates with co-expression of the S locus glycoprotein in the stigmas of two Brassica strains and in transgenic tobacco plants. Plant Physiol. 124 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski, J.E., Pearce, G., and Ryan, C.A. (1999). Proteinase inhibitor-inducing activity of the prohormone prosystemin resides exclusively in the C-terminal systemin domain. Proc. Natl. Acad. Sci. USA 96 12947–12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass, J., Civelli, O., and Herbert, E. (1984). Polyprotein gene expression: Generation of diversity of neuroendocrine peptides. Annu. Rev. Biochem. 53 665–715. [DOI] [PubMed] [Google Scholar]
- Fang, Y., and Hirsch, A.M. (1998). Studying early nodulin gene ENOD40 expression and induction by nodulation factor and cytokinin in transgenic alfalfa. Plant Physiol. 116 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G., and Boller, T. (1995). Systemin induces rapid ion fluxes and ethylene biosynthesis in Lycopersicon peruvianum cells. Plant J. 7 381–389. [Google Scholar]
- Ferris, P.J., Woessner, J.P., Waffenschmidt, S., Kilz, S., Drees, J., and Goodenough, U.W. (2001). Glycosylated polyproline II rods with kinks as a structural motif in plant hydroxyproline-rich glycoproteins. Biochemistry 40 2978–2987. [DOI] [PubMed] [Google Scholar]
- Fletcher, J.C., Brand, U., Running, M.P., Simon, R., and Meyerowitz, E.M. (1999). Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283 1911–1914. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong, V.E., and Franklin, F.C.H. (2000). Self-incompatibility in Brassica: The elusive pollen S gene is identified. Plant Cell 12 305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Flores, C., Aguilar, R., Reyes De La Cruze, H., Albores, M., and Sánchez de Jiménez, E. (2001). A maize insulin-like growth factor signals to a transduction pathway that regulates protein synthesis in maize. Biochem. J. 358 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring, C.A. (1999). Natriuretic peptides—A new class of plant hormones? Ann. Bot. 83 329–334. [Google Scholar]
- Goring, D.R., and Rothstein, S.J. (1992). The S-locus receptor kinase gene in a self-incompatible Brassica napus line encodes a functional serine/threonine kinase. Plant Cell 4 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, T.R., and Ryan, C.A. (1972). Wound-induced proteinase inhibitor in plant leaves: A possible defense mechanism against insects. Science 175 776–777. [DOI] [PubMed] [Google Scholar]
- Gu, T., Mazzurco, M., Sulaman, W., Matias, D.D., and Goring, D.R. (1998). Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proc. Natl. Acad. Sci. USA 95 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai, H., Nakayama, D., Yang, H., Matsubayashi, Y., Hirota, Y., and Sakagami, Y. (2000. a). Existence of a plant tyrosylprotein sulfotransferase: Novel plant enzyme catalyzing tyrosine O-sulfation of preprophytosulfokine variants in vitro. FEBS Lett. 470 97–101. [DOI] [PubMed] [Google Scholar]
- Hanai, H., Matsuno, T., Yamamoto, M., Matsubayashi, Y., Kobayashi, T., Kamada, H., and Sakagami, Y. (2000. b). A secreted peptide growth factor, phytosulfokine, acting as a stimulatory factor of carrot somatic embryo formation. Plant Cell Physiol. 41 27–32. [DOI] [PubMed] [Google Scholar]
- Harris, R.B. (1989). Processing of pro-hormone precursor proteins. Arch. Biochem. Biophys. 275 315–333. [DOI] [PubMed] [Google Scholar]
- Ikeda, S., Nasrallah, J.B., Dixit, R., Preiss, S., and Nasrallah, M.E. (1997). An aquaporin-like gene required for the Brassica self-incompatibility response. Science 276 1564–1566. [DOI] [PubMed] [Google Scholar]
- Jacinto, T., McGurl, B., Franceschi, V., Delano-Freier, J., and Ryan, C.A. (1997). Tomato prosystemin promoter confers wound-inducible, vascular bundle-specific expression of the β-glucuronidase gene in transgenic tomato plants. Planta 203 406–412. [Google Scholar]
- Jeong, S., Trotochaud, A.E., and Clark, S.E. (1999). The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11 1925–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo, A., Nasrallah, M.E., and Nasrallah, J.B. (2002). Self-incompatibility in the Brassicaceae: Receptor-ligand signaling and cell-to-cell communication. Plant Cell 14 (suppl.), S227–S238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo, A., Schopfer, C.R., Nasrallah, M.E., and Nasrallah, J.B. (2001). Allele-specific receptor-ligand interactions in Brassica self-incompatibility. Science 293 1824–1826. [DOI] [PubMed] [Google Scholar]
- Kende, H., and Zeevaart, J.A.D. (1997). The five “classical” plant hormones. Plant Cell 9 1197–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T., Eun, C.-H., Hanai, H., Matsubayashi, Y., Sakagami, Y., and Kamada, H. (1999). Phytosulfokine-α, a peptidyl plant growth factor, stimulates somatic embryogenesis in carrot. J. Exp. Bot. 50 1123–1128. [Google Scholar]
- Kouchi, H., Takane, K., So, R., Ladha, J., and Reddy, P. (1999). Rice ENOD40: Isolation and expression analysis in rice and transgenic soybean root nodules. Plant J. 18 121–129. [DOI] [PubMed] [Google Scholar]
- Laux, T., Mayer, K.F., Berger, J., and Jürgens, G. (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122 87–96. [DOI] [PubMed] [Google Scholar]
- Lindsey, K. (2001). Plant peptide hormones: The long and the short of it. Curr. Biol. 11 R741–R743. [DOI] [PubMed] [Google Scholar]
- Luu, D.-T., Marty-Mazars, D., Trick, M., Dumas, C., and Heizmann, P. (1999). Pollen–stigma adhesion in Brassica sp. involves SLG and SLR1 glycoproteins. Plant Cell 11 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandava, N.B. (1988). Plant growth–promoting brassinosteroids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39 23–52. [Google Scholar]
- Massague, J., and Pandiella, A. (1993). Membrane-anchored growth factors. Annu. Rev. Biochem. 62 515–541. [DOI] [PubMed] [Google Scholar]
- Matsubayashi, Y., and Sakagami, Y. (1996). Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc. Natl. Acad. Sci. USA 93 7623–7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi, Y., and Sakagami, Y. (1999). Characterization of specific binding sites for a mitogenic sulfated peptide, phytosulfokine-α, in the plasma–membrane fraction derived from Oryza sativa L. Eur. J. Biochem. 262 666–671. [DOI] [PubMed] [Google Scholar]
- Matsubayashi, Y., and Sakagami, Y. (2000). 120- and 160-kDa receptors for endogenous mitogenic peptide, phytosulfokine-α, in rice plasma membranes. J. Biol. Chem. 275 15520–15525. [DOI] [PubMed] [Google Scholar]
- Matsubayashi, Y., Takagi, L., and Sakagami, Y. (1997). Phytosulfokine-α, a sulfated pentapeptide, stimulates the proliferation of rice cells by means of specific high- and low-affinity binding sites. Proc. Natl. Acad. Sci. USA 94 13357–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi, Y., Takagi, L., Omura, N., Morita, A., and Sakagami, Y. (1999. a). The endogenous sulfated pentapeptide, phytosulfokine-α stimulates tracheary element differentiation of isolated mesophyll cells of Zinnia elegans. Plant Physiol. 120 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi, Y., Morita, A., Matsunaga, E., Fruya, A., Hanai, N., and Sakagami, Y. (1999. b). Physiological relationships between auxin, cytokinin, and a peptide growth factor, phytosulfokine-α in stimulation of asparagus cell proliferation. Planta 207 559–565. [Google Scholar]
- McCubbin, A.G., and Kao, T.-h. (2000). Molecular recognition and response in pollen and pistil interactions. Annu. Rev. Cell Dev. Biol. 16 333–364. [DOI] [PubMed] [Google Scholar]
- McGurl, B., Pearce, G., Orozco-Cardenas, M., and Ryan, C.A. (1992). Structure, expression and antisense inhibition of the systemin precursor gene. Science 255 1570–1573. [DOI] [PubMed] [Google Scholar]
- McGurl, B., Orozco-Cardenas, M., Pearce, G., and Ryan, C.A. (1994). Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc. Natl. Acad. Sci. USA 91 9799–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl, T., Boller, T., and Felix, G. (1998). The plant wound hormone systemin binds with the N-terminal part to its receptor but needs the C-terminal part to activate it. Plant Cell 10 1561–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz, E.M. (1997). Genetic control of cell division patterns in developing plants. Cell 88 299–308. [DOI] [PubMed] [Google Scholar]
- Mylona, P., Pawlowski, K., and Bissleing, T. (1995). Symbiotic nitrogen fixation. Plant Cell 7 869–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., and Benfey, P.N. (2002). Signaling in and out: Control of cell division and differentiation in the shoot and root. Plant Cell 14 (suppl.), S265–S276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez-Vasquez, J., Pearce, G., Orozco-Cardenas, M.L., Franceschi, V.R., and Ryan, C.A. (1995). Autoradiographic and biochemical evidence for the systemic translocation of systemin in tomato plants. Planta 19 593–600. [Google Scholar]
- Nasrallah, J.B. (2000). Cell–cell signaling in the self-incompatability response. Curr. Opin. Plant Biol. 3 368–373. [DOI] [PubMed] [Google Scholar]
- Nasrallah, J.B., and Nasrallah, M.E. (1993). Pollen–stigma signaling in the sprophytic self-incompatibility response. Plant Cell 5 1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, C.E., Walker-Simmons, M., Makus, D., Zuroske, G., Graham, J., and Ryan, C.A. (1983). Regulation of synthesis and accumulation of proteinase inhibitors in leaves of wounded tomato plants. In Plant Resistance to Insects, P.A. Hedin, ed (Washington, D.C.: American Chemical Society), pp 103–122.
- Okazaki, K., Kusaba, M., Ockendon, D.J., and Nishio, T. (1999). Characterization of S tester lines in Brassica oleracea: Polymorphism of restriction fragment length of SLG homologues and isoelectric points of S-locus glycoproteins. Theor. Appl. Genet. 98 1329–1334. [Google Scholar]
- Oliveira, A.E.A., Machado, O.L.T., Gomes, V.M., Neto, J.X., Pereira, A.C., Vieira, J.G.H., Fernandez, K.V.S., and Xavier-Filho, J. (1999). Jack bean seed coat contains a protein with complete sequence homology to bovine insulin. Protein Peptide Lett. 6 15–21. [Google Scholar]
- Opsahl-Ferstad, H.G., LeDeunff, E., Dumas, C., and Rogowsky, P.M. (1997). ZmEsr, a novel endosperm-specific gene expressed in a restricted region around the maize embryo. Plant J. 12 235–246. [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas, M., McGurl, B., and Ryan, C.A. (1993). Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc. Natl. Acad. Sci. USA 90 8273–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, G., Strydom, D., Johnson, S., and Ryan, C.A. (1991). A polypeptide from tomato leaves induces wound-inducible inhibitor proteins. Science 253 895–898. [DOI] [PubMed] [Google Scholar]
- Pearce, G., Johnson, S., and Ryan, C.A. (1993. a). Structure–activity of deleted and substituted systemin, an eighteen amino acid polypeptide inducer of plant defensive genes. J. Biol. Chem. 268 212–216. [PubMed] [Google Scholar]
- Pearce, G., Johnson, S., and Ryan, C.A. (1993. b). Purification and characterization from tobacco leaves of six small, wound-inducible, proteinase isoinhibitors of the potato inhibitor II family. Plant Physiol. 102 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, G., Moura, D.S., Stratmann, J., and Ryan, C.A. (2001. a). Production of multiple plant hormones from a single polyprotein precursor. Nature 411 817–820. [DOI] [PubMed] [Google Scholar]
- Pearce, G., Moura, D.S., Stratmann, J., and Ryan, C.A. (2001. b). RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 98 12843–12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrig, H., Schmidt, J., Miklasheyichs, E., Schell, J., and John, M. (2002). Soybean ENOD40 encodes two peptides that bind to sucrose synthase. Proc. Natl. Acad. Sci. USA 99 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, D.J., Pearce, G., Ryan, C.A., and Satterlee, J.D. (1992). Proton NMR assignments of systemin. J. Protein Chem. 11 265–274. [DOI] [PubMed] [Google Scholar]
- Ryan, C.A. (1998). The discovery of systemin. In Discoveries in Plant Biology, Vol. 2, S.-D. Kung and S.-F. Yang, eds (Singapore: World Scientific Press), pp. 175–188.
- Ryan, C.A. (2000). The systemin signaling pathway: Differential activation of plant defensive genes. Biochem. Biophys. Acta 1477 112–121. [DOI] [PubMed] [Google Scholar]
- Ryan, C.A., and Pearce, G. (1998). Systemin, a polypeptide signal for plant defense. Annu. Rev. Cell Dev. Biol. 14 1–17. [DOI] [PubMed] [Google Scholar]
- Ryan, C.A., and Pearce, G. (2001). Polypeptide hormones. Plant Physiol. 125 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, A. (1999). Oligopeptide signaling and the action of systemin. Plant Mol. Biol. 40 763–769. [DOI] [PubMed] [Google Scholar]
- Schaller, A., and Ryan, C.A. (1994). Identification of a 50-kDa systemin-binding protein in tomato plasma membranes having Kex2p-like properties. Proc. Natl. Acad. Sci. USA 91 11802–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, A., and Ryan, C.A. (1995). Systemin: A polypeptide defense signal in plants. Bioessays 18 27–33. [DOI] [PubMed] [Google Scholar]
- Scheer, J.M., and Ryan, C.A. (1999). A 160-kD systemin receptor on the surface of Lycopersicon peruvanium cultured cells. Plant Cell 11 1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer, J.M., and Ryan, C.A. (2002). The system in receptor SR160 from Lycopersicon esculentum is a member of the LRR receptor kinase family. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- Schell, J., et al. (1999). Re-evaluation of phytohormone-independent division of tobacco protoplast-derived cells. Plant J. 17 461–466. [DOI] [PubMed] [Google Scholar]
- Schoof, H., Lenhard, M., Haecker, A., Mayer, K.F.X., Jürgens, G., and Laux, T. (2000). The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644. [DOI] [PubMed] [Google Scholar]
- Schopfer, C.R., and Nasrallah, J.B. (2000). Self-incompatibility: Prospects for a novel putative peptide-signaling molecule. Plant Physiol. 124 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer, C.R., Nasrallah, M.E., and Nasrallah, J.B. (1999). The male determinant of self-incompatibility in Brassica. Science 286 1697–1700. [DOI] [PubMed] [Google Scholar]
- Sousa, C., Johansson, C., Charon, C., Manyani, H., Sautter, C., Kondorosi, A., and Crespi, M. (2001). Translational and structural requirements of the early nodulin gene ENOD40, a short-open reading frame–containing RNA, for elicitation of a cell-specific growth response in the alfalfa root cortex. Mol. Cell. Biol. 21 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, J.C., and Nasrallah, J.B. (1993). A plant receptor-like gene, the S-locus receptor kinase of Brassica oleracea, encodes a functional serine/threonine kinase. Plant Physiol. 101 1103–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, J.C., Howlett, B.H., Boyes, D.C., Nasrallah, M.E., and Nasrallah, J.B. (1991). Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc. Natl. Acad. Sci. USA 88 8816–8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, D.F., Smeekens, S.P., Ohagi, S., and Chan, S.J. (1992). The new enzymology of precursor processing endoproteases. J. Biol. Chem. 267 23435–23438. [PubMed] [Google Scholar]
- Stephenson, A., Doughty, J., Dixon, S., Elleman, C., Hiscock, S., and Dickinson, H.G. (1997). The male determinant of self-incompatibility in Brassica oleracea is located in the pollen coating. Plant J. 12 1351–1359. [Google Scholar]
- Stone, J.M., Trotochaud, A.E., Walker, J.C., and Clark, S.E. (1998). Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiol. 117 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann, J., and Ryan, C.A. (1997). Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc. Natl. Acad. Sci. USA 94 11085–11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, R., and Street, H.E. (1969). Studies on the growth in culture of plant cells. The initiation of division in suspensions of stationary-phase cells of Acer pseudoplatanus L. J. Exp. Bot. 20 556–571. [Google Scholar]
- Takada, Y., Ito, A., Ninomiya, C., Kakizaki, T., Takahata, Y., Suzuki, G., Hatakeyama, K., Hinata, K., Shiba, H., Takayama, S., Isogai, A., and Watanabe, M. (2001). Characterization of expressed genes in the SLL2 region of self-compatible Arabidopsis thaliana. DNA Res. 8 215–219. [DOI] [PubMed] [Google Scholar]
- Takasaki, T., Hatakeyama, K., Suzuki, G., Watanabe, M., Isogai, A., and Hinata, K. (2000). The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403 913–916. [DOI] [PubMed] [Google Scholar]
- Takayama, S., Shiba, H., Iwano, M., Shimasato, H., Che, F.-S., Kai, N., Watanabe, M., Suzuki, K.G., Hinata, K., and Isogai, A. (2000). The pollen determinant of self-incompatibility in Brassica campestris. Proc. Natl. Acad. Sci. USA 97 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, S., Shimosato, H., Shiba, H., Funato, M., Che, F.-C., Watanabe, M., Iwano, M., and Isogai, A. (2001). Direct ligand–receptor complex interaction controls Brassica self-incompatibility. Nature 413 534–538. [DOI] [PubMed] [Google Scholar]
- Toumadje, A., and Johnson, W.C., Jr. (1995). Systemin has the characteristics of a poly(l-proline) II type helix. J. Am. Chem. Soc. 117 7023–7024. [Google Scholar]
- Trotochaud, A.E., Hao, T., Wu, G., Yang, Z., and Clark, S.E. (1999). The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell 11 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotochaud, A.E., Jeong, S., and Clark, S.E. (2000). CLAVATA3, a multimeric ligand for the CLAVATA1 receptor-kinase. Science 289 613–617. [DOI] [PubMed] [Google Scholar]
- van de Sande, K., Pawlowski, K., Czaja, I., Wieneke, U., Schell, J., Schmidt, J., Walden, R., Matvienko, M., Wellink, J., van Kammen, A., Frannsen, H., and Bisseling, T. (1996). Modification of phytohormone response by a peptide encoded by ENOD40 of legumes and a nonlegume. Science 273 370–373. [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse, V., Miege, C., Dumas, C., and Cock, J.M. (2001). Two large Arabidopsis thaliana gene families are homologous to the Brassica gene superfamily that encodes pollen coat proteins and the male component of the self-incompatibility response. Plant Mol. Biol. 16 17–34. [DOI] [PubMed] [Google Scholar]
- Vetsch, M., Janzik, I., and Schaller, A. (2000). Characterization of prosystemin expressed in the baculovirus/insect cell system reveals biological activity of the systemin precursor. Planta 211 91–97. [DOI] [PubMed] [Google Scholar]
- Watanabe, Y., Barbashov, S.F., Konatsu, S., Hemmings, A.M., Miyagi, M., Tslkunasawa, S., and Hirano, H. (1994). A peptide that stimulates phosphorylation of the plant insulin-binding protein: Isolation, primary structure and cDNA cloning. Eur. J. Biochem. 224 167–172. [DOI] [PubMed] [Google Scholar]
- Williams, R.W., Wilson, J.M., and Meyerowitz, E.M. (1997). A possible role for kinase-associated protein phosphatase in the Arabidoposis CLAVATA1 signaling pathway. Proc. Natl. Acad. Sci. USA 94 10467–10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa, S., Sakuta, C., Matsubayashi, Y., Sakagami, Y., Kamada, H., and Satoh, S. (1998). The promotive effects of a peptidyl plant growth factor, phytosulfokine-α. On the formation of adventitious roots and expression of a gene for a root-specific cystatin in cucumber hypocotyls. J. Plant Res. 111 453–458. [Google Scholar]
- Yang, G., Shen, S., Kobayashi, T., Matsubayashi, Y., Sakagami, Y., and Kamada, H. (1999. a). Stimulatory effects of a novel peptidyl plant growth factor, phytosulfokine-α on the adventitous bud formation from callus of Antirrhinum majus. Plant Biotech. 16 231–234. [Google Scholar]
- Yang, H., Matsubayashi, Y., Nakamura, K., and Sakagami, Y. (1999. b). Oryza sativa PSK gene encodes a precursor of phytosulfokine-α, a sulfated peptide growth factor found in plants. Proc. Natl. Acad. Sci. USA 96 13560–13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., Matsubayashi, Y., Hanai, H., Nakamura, K., and Sakagami, Y. (2000). Molecular cloning and characterization of OsPSK, a gene encoding a precursor for phytosulfokine-α, required for rice cell proliferation. Plant Mol. Biol. 635 635–647. [DOI] [PubMed] [Google Scholar]
- Yang, H., Matsubayashi, Y., Nakamura, K., and Sakagami, Y. (2001). Diversity of Arabidopsis genes encoding precursors for phytosulfokines, a peptide growth factor. Plant Physiol. 127 842–851. [PMC free article] [PubMed] [Google Scholar]
- Yang, W., Katinakis, P., Hendriks, P., Smolders, A., deVries, F., Spee, J., van Kammen, A., Bisseling, T., and Franssen, H. (1993). Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. 3 573–585. [DOI] [PubMed] [Google Scholar]