Abstract
Brain-derived neurotrophic factor (BDNF) is neuroprotective in the ischemic hippocampus if the neurotrophin is injected directly into the brain. However, the efficacy of BDNF via peripheral (i.v.) administration is limited by the lack of transport of the neurotrophin through the brain capillary wall, which makes up the blood-brain barrier (BBB) in vivo. The present studies describe a molecular reformulation of BDNF that incorporates polyethylene glycol (PEG) moieties at surface carboxyl residues, to optimize plasma pharmacokinetics, and links pegylated BDNF to the OX26 mAb, which undergoes receptor-mediated transport through the BBB via the brain capillary endothelial transferrin receptor. The BDNF-PEG 2000-biotin conjugated to OX26/streptavidin was administered i.v. daily to rats for 1 week after a 12-min period of transient forebrain ischemia. The neuronal density in the CA1 sector of the hippocampus was decreased 68 ± 10% at 1 week after the ischemia. There was no neuroprotective effect of the unconjugated BDNF or unconjugated OX26 mAb. However, the hippocampal CA1 neuronal density was normalized by i.v. administration of the BDNF-PEG 2000-biotin conjugated to OX26/streptavidin. These studies demonstrate that peripherally administered BDNF may have neuroprotective effects in brain, if the neurotrophin is reformulated to (i) optimize plasma pharmacokinetics with carboxyl-directed pegylation, and (ii) enable transport through the BBB by coupling to brain transport vectors.
Keywords: polyethylene glycol conjugation, blood-brain barrier, drug targeting, brain-derived neurotrophic factor
Neurotrophic factors may have therapeutic effects for brain disorders, and more than 30 such neurotrophic factors are known (1). The potential of these peptides is limited by the absence of transport of neurotrophic factors through the brain capillary wall, which makes up the blood-brain barrier (BBB) in vivo. Brain-derived neurotrophic factor (BDNF) normalizes the number of pyramidal neurons in the CA1 sector of the hippocampus after transient forebrain ischemia in the rat, providing the BDNF is administered by continuous intra-cerebral-ventricular (ICV) infusion for a 7-day period after the ischemic insult (2). The therapeutic efficacy of ICV infusion of BDNF is somewhat surprising, because BDNF distributes only to the ependymal surface after ICV administration (3). However, the CA1 sector of the hippocampus is immediately contiguous with the ventricular compartment, and the short diffusion distances in the rat brain may underlie the therapeutic efficacy of ICV infusion of BDNF in the rat.
For neurotrophic factors such as BDNF to have therapeutic value in humans after peripheral (i.v., s.c.) administration, two obstacles must be overcome. First, the neurotrophic factor must be conjugated to a BBB drug delivery system that enables transport across the BBB after peripheral administration (4). Second, the plasma pharmacokinetics of the neurotrophic factor must be optimized to prevent the very rapid rate of removal of neurotrophic factors such as BDNF from the circulation after peripheral administration. Owing to the cationic charge of neurotrophic factors such as BDNF, these molecules are rapidly taken up by liver (5). Recent studies have demonstrated that BDNF may be reformulated with the combined use of pegylation technology, chimeric peptide technology, and avidin-biotin technology (6). Pegylation technology involves the conjugation of polyethylene glycol (PEG) moieties to the surface of the protein to inhibit rapid uptake by peripheral tissues (7). Chimeric peptide technology involves linking drugs such as BDNF to BBB transport vectors that undergo receptor-mediated transcytosis through the BBB in vivo (4). The OX26 murine mAb to the rat transferrin receptor (TfR) undergoes transport through the BBB owing to the high concentrations of TfR on the brain capillary endothelium (8). Approximately 60% of the OX26 mAb that is bound to the BBB TfR is transcytosed through the BBB (9), and the BBB TfR is widely distributed throughout the brain microvasculature (8). Conjugation of pegylated neurotrophic factors to BBB transport vectors, such as the OX26 mAb, is facilitated with the use of avidin/biotin technology. In this approach, the pegylated neurotrophic factor is monobiotinylated and then bound to a conjugate of the OX26 mAb and streptavidin, designated OX26/SA (6). In the present studies, BDNF is pegylated on carboxyl moieties to preserve biologic activity (6) and conjugated to OX26/SA and administered by daily i.v. injection to rats initially subjected to 12 min of transient forebrain ischemia and isoelectric electroencephalogram (EEG). These studies show normalization of the number of pyramidal neurons in the CA1 sector of the hippocampus after transient forebrain ischemia and i.v. treatment with BDNF conjugate.
MATERIALS AND METHODS
Materials.
Human recombinant BDNF is produced in Escherichia coli and was obtained from Amgen Biologicals under Material Transfer Agreement. [125I] was obtained from Amersham. Methoxy-PEG-hydrazide (Hz) of molecular weight 2,000 Da (designated PEG 2000-Hz), and biotin-PEG 2000-Hz, were synthesized by Shearwater Polymers (Huntsville, AL). High-trap copper affinity columns and Superose 12HR(10/30) columns were obtained from Pharmacia. N-methyl-N′-3(dimethylaminopropyl)carbodiimide hydrochloride, recombinant SA, and all other reagents were obtained from Sigma. Adult male Sprague–Dawley rats (300–400 g) were purchased from Harlan Breeders (Indianapolis).
Preparation of BDNF-PEG 2000-Biotin/OX26-SA Complex.
125I-labeled BDNF was prepared with lactoperoxidase as reported (10) and used within 24 hr of labeling. [125I]-BDNF (30 μCi, 3 μg of protein) and 6 mg of unlabeled BDNF (0.48 μmol BDNF monomer) were mixed in 200 μl of 0.3 M pyridine-HCl (pH 4.8); 240 mg of PEG 2000-Hz and 18 mg of biotin-PEG 2000-Hz were added. The reaction was initiated by the addition of 26 mg of N-methyl-N′-3(dimethylaminopropyl)carbodiimide hydrochloride and allowed to proceed in the dark at room temperature overnight. The solution was brought to 1 ml by the addition of buffer A (0.05 M Na2HPO4/0.5 M NaCl, pH 7.0), and subsequently applied to a 5-ml Hi-Trap copper affinity column, to remove unreacted PEG 2000. The column was preactivated with 0.1 M CuSO4. After washing with 20 ml of buffer A, the BDNF-PEG 2000-biotin was eluted with 15 ml of 50 mM EDTA in buffer A, and 1-ml fractions were collected. The blue-colored fractions of copper and pegylated BDNF were pooled and concentrated in a Centriprep-10 microconcentrator (Amicon) as described (6). After bicinchoninic acid protein assay (Pierce) and measurement of [125I] radioactivity, the pegylated BDNF was stored at −20°C until use. Twenty milligrams (100 nmol) of OX26/SA was added to 2.8 mg of the BDNF-PEG 2000-biotin (100 nmol) containing 12 μCi of [125I]-BDNF-PEG 2000-biotin. OX26/SA was prepared as described (6). The mixture was incubated at room temperature for 30 min, and the sample was applied to a 2.6 × 96 cm column of Sephacryl S300HR (Pharmacia) followed by elution in 0.01 M Na2HPO4/0.5 M NaCl, pH 7.4/0.05% Tween-20 at 30 ml/hr; and 3-ml fractions were collected. The conjugate peak (fractions 65–90), as determined by measurement of absorption at 280 nm and [125I] counting, was selected, pooled, and concentrated to 2.0 ml by using a Centriprep-10 microconcentrator. The final yield of BDNF-PEG 2000-biotin conjugated to OX26/SA was 65%. The molar ratio of BDNF-PEG 2000-biotin to OX26-SA was 1:1. The proportion of the BDNF-PEG 2000-biotin/SA-OX26 that was BDNF homodimer was calculated from the molecular weight (25,000 Da) and the specific activity (μCi/μg) of the BDNF, which was computed from the small amount of [125I]-BDNF added initially to the unlabeled BDNF. The free [125I]-BDNF-PEG 2000 peak (fractions 91–105) also was collected from the Sephacryl S300 column and concentrated by using a Centriprep-10 microconcentrator.
Gel Filtration Fast Protein Liquid Chromatography (FPLC).
To study the in vivo metabolic stability of [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA, adult male Sprague–Dawley rats received 50 μCi of [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA by i.v. injection under anesthesia of ketamine (100 mg/kg, i.p.) and xylazine (2 mg/kg, i.p.). One hour after administration, an arterial blood sample was collected, and the animals were decapitated for the removal of brain. The plasma was separated, and 25 μl of the plasma from each of three rats was pooled, mixed with 200 μl of buffer A, and injected onto a Superose 12HR (1 × 30 cm) column that was eluted with buffer A at the rate of 0.5 ml/min for FPLC. The rat forebrain was homogenized in 2 vol of buffer A and centrifuged at 10,000 g for 30 min. The supernatant (100 μl) was injected on the Superose 12HR FPLC column with elution as described for the plasma sample. Either [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA (0.1 μ Ci) or unconjugated [125I]-BDNF-PEG 2000 (0.1 μ Ci) also was injected onto the Superose 12HR column. Fractions (0.5 ml) were collected, and [125I] radioactivity was counted by using a Beckman gamma counter.
Transient Forebrain Ischemia.
Forebrain ischemia in the rat was induced according to the method of Smith et al. (11). One day before the ischemia, the rat was anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (2 mg/kg, i.p.) and was placed into a small animal stereotaxis device. Two metal screws (3.2 mm, 0–80 × 1/8) were anchored to the skull bone (3 mm posterior from bregma, and 2 mm lateral to the sagittal suture) after a midline skin incision. After fasting overnight, the animal was lightly anesthetized with inhalation of halothane, and endotracheal intubation was performed by transillumination (12). The endotracheal catheter was PE100 tubing (7 cm long) and was connected to a model 680 Harvard small animal ventilator. The animal was artificially ventilated with a mixture of 70% N2O, 30% O2, and 0.5% halothane at a rate of 90 strokes/min and a volume of 5 ml/stroke. The body temperature was maintained by using a Harvard thermoblanket with a rectal probe. The systolic blood pressure was measured by using a model 29 rat tail amplifier (IITC Inc./Life Science Instruments, Woodland Hills, CA). The left femoral artery and vein were cannulated with PE50 tubing, and both common carotid arteries were surgically isolated. The halothane component of the anesthesia then was discontinued. Blood was collected via the femoral artery catheter, and arterial blood pH, pCO2 and pO2, were measured with a model 238 pH/blood gas analyzer (Chiron). After muscle paralysis was achieved by an i.v. dose (0.5 mg/kg) of suxamethonium (Nycomed, Arzneimittel, Germany), the EEG was recorded via the two anchored metal screws by using a Student Oscillograph and an Isolated Preamplifier (Harvard Apparatus). During the ensuing 30 min, three arterial blood samples were measured for pH, pCO2, and pO2. After the blood gas values were stabilized, forebrain ischemia, as indicated by the isoelectric EEG, was induced by clamping both common carotid arteries and by lowering the blood pressure to a level below 50 mmHg. The hypotension was achieved by a single i.v. dose (5 mg/kg) of trimethaphan (Hoffmann–La Roche), and by bleeding via the femoral artery catheter. After 12 min of the ischemic insult, the carotid clamps were released, and the shed blood was slowly reinfused (2 ml/min). The animal was allowed to recover under a heating lamp for 4 hr. If the blood pH was below 7.30, then sodium bicarbonate (2 ml/kg of 4.2% NaHCO3) was i.v. injected to neutralize the systemic acidosis.
Drug Treatment Schedule.
The rats with the ischemic insult were randomly assigned into four groups. The first group received buffer A by i.v. injection; the second group received 1.7 mg/kg per day of the OX26 mAb by i.v. injection; the third group received 0.25 mg/kg per day of unconjugated BDNF by i.v. injection; and the fourth group received BDNF-PEG 2000-biotin conjugated to OX26/SA, which was equivalent to 0.25 mg/kg per day of BDNF, and 1.7 mg/kg per day of OX26 mAb. The first dose was given immediately after the recovery from ischemia via the femoral vein catheter, whereas the remaining six doses were given daily in the morning over the next 6 days by tail vein injection. The animals were sacrificed 24 hr after the final i.v. injection. Tail vein injections in lightly immobilized, conscious rats was performed by dilation of the tail vein in warm (40–45°C) water.
Nissl Stain and Hippocampal Neuron Quantitation.
After removal of brains, three coronal sections (2 mm thick) were prepared after discarding 3 mm of frontal cortex. The slabs were immersion fixed in 10% formalin/90% PBS, pH 7.0, and paraffin embedded; 6-μm sections were cut with a microtome, deparaffinized, and Nissl-stained. The pyramidal neurons in the hippocampal CA1, CA3, and CA4 regions were counted with a measuring grid under light microscopy at ×100 magnification (13). Only neurons with a visible nucleolus were scored.
Data were presented as the mean ± SEM of each group. Student’s t test was performed for the different groups, and P < 0.05 was considered statistically significant.
RESULTS
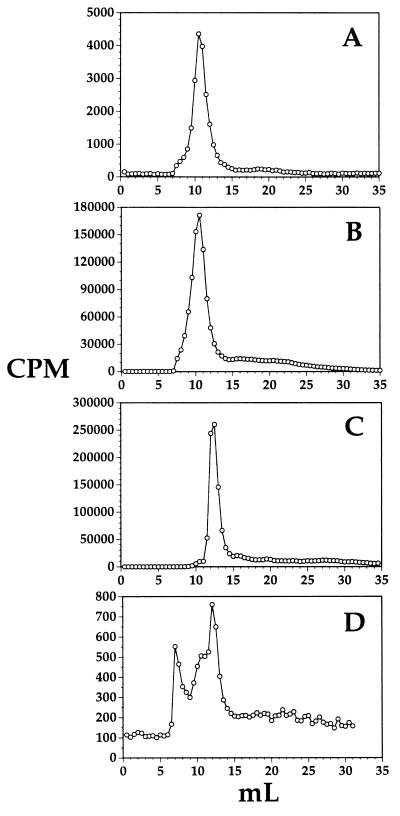
The structure of the pegylated BDNF conjugated to OX26/SA is shown in Fig. 1. The conjugate was radiolabeled by attachment of [125I] iodine to BDNF tyrosine residues with lactoperoxidase, and the metabolic stability of the conjugate in vivo in anesthetized rats was assessed. Superose 12HR gel filtration FPLC analysis was performed on rat plasma taken 60 min after i.v. injection of [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA (Fig. 2A), [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA before injection into rats (Fig. 2B), [125I]-BDNF-PEG 2000 without attachment to OX26/SA and without injection into rats (Fig. 2C), and rat brain extract taken 60 min after i.v. injection of [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA (Fig. 2D). The control conjugate elutes at 10.0 ml (Fig. 2B) and the unconjugated BDNF-PEG 2000 elutes at 12.5 ml (Fig. 2C). The only radioactive species detected in rat plasma 60 min after i.v. injection of the conjugate is the form of BDNF-PEG 2000-biotin that is conjugated to OX26/SA (Fig. 2A). There is no unconjugated BDNF-PEG 2000 detected in rat plasma and there is no low molecular weight metabolite (e.g., 125I-tyrosine) detected in rat plasma 60 min after i.v. injection of the conjugate (Fig. 2A). Samples of rat brain 60 min after injection of the conjugate also were analyzed with Superose 12HR FPLC, and these studies showed the presence of three peaks in brain: a peak that migrated in the void volume of the column, a principal peak that comigrated exactly with the conjugate peak, and another peak that comigrated exactly with [125I]-BDNF-PEG 2000-biotin without attachment to OX26/SA (Fig. 2D).
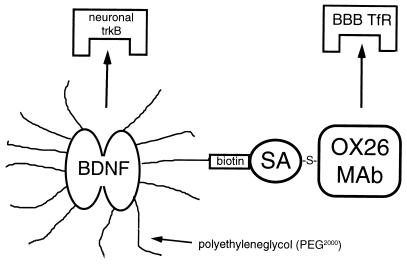
Figure 1.
Structure of conjugate of BDNF attached to the OX26 mAb by using both carboxyl-directed pegylation and avidin-biotin technology. The stable thioether (-S-) linkage between OX26 and SA is prepared in parallel with monobiotinylation of BDNF. BDNF is monobiotinylated by using a bifunctional PEG 2000 derivative that contains a Hz moiety at one end for attachment to BDNF carboxyl groups, and a biotin group at the other end of the PEG strand. The BDNF is pegylated with PEG 2000-Hz and 7% of the PEG 2000-Hz contains the biotin-PEG 2000-Hz. The BDNF-PEG 2000-biotin then is conjugated to OX26/SA. The trifunctionality of the BDNF formulation is illustrated: binding to trkB receptor for pharmacologic effect in brain, binding to the BBB TfR, and pegylation as a pharmacokinetic enhancer to delay clearance of drug from plasma.
Figure 2.
Superose 12HR gel filtration FPLC analysis of (A) rat plasma taken 60 min after i.v. injection of [125I]-BDNF-PEG 2000-biotin-conjugated OX26/SA, (B) [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA before injection into rats, (C) [125I]-BDNF-PEG 2000 without attachment to OX26/SA and without injection into rats, and (D) rat brain taken 60 min after i.v. injection of [125I]-BDNF-PEG 2000-biotin conjugated to OX26/SA.
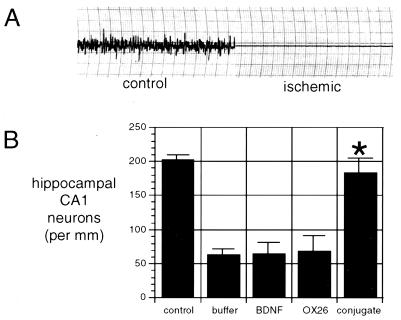
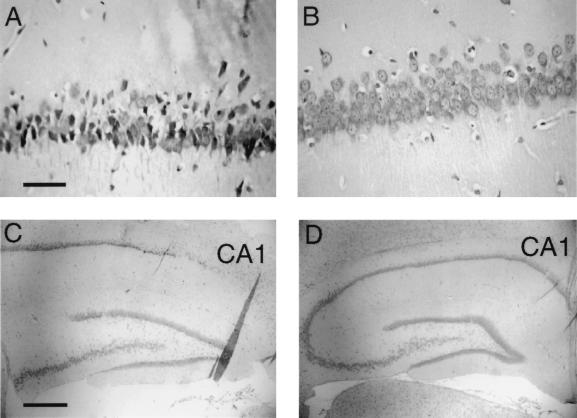
The physiologic parameters of the rats subjected to transient forebrain ischemia are shown in Table 1 for the four treatment groups. During the 10-min ischemic period, the blood pressure ranged from 22 ± 2 to 27 ± 6 mm Hg in all four groups. There were no significant differences in the temperature during the ischemic period of the four groups. There were no significant differences between the arterial pH, the arterial pO2, and the arterial pCO2 in the four groups either before or after the ischemic episode. Ischemia was confirmed in all rats investigated by EEG recordings, and an illustrative EEG both before and during the ischemic period is shown in Fig. 3A. The animals were treated i.v. immediately after recovery from the ischemic period and daily through the next 6 days. Seven days after the ischemic episode, animals were sacrificed for Nissl staining and neuron counting in the hippocampal CA1 sector. These data show a 68 ± 10% decrease in the hippocampal CA1 neurons in the rats subjected to transient forebrain ischemia (Fig. 3B), and there was no therapeutic effect observed with the i.v. administration of BDNF without attachment to the BBB delivery system, nor was any therapeutic effect achieved by the administration of the OX26 mAb without attached BDNF. However, there was a normalization of the CA1 sector density of pyramidal neurons by the i.v. treatment with the BDNF conjugated to OX26/SA (Fig. 3B). Representative Nissl stains are shown in Fig. 4. The neurons were reduced in number and size in the CA1 sector of the hippocampus in the rats subjected to transient forebrain ischemia and treated with unconjugated BDNF (Fig. 4 A and C). However, there is normalization of the CA1 sector neuron density in the animals treated with BDNF-PEG 2000-biotin conjugated to OX26/SA (Fig. 4 B and D). There were no significant changes in the neuronal density in hippocampal CA3 or CA4 sectors in any of the animals (Table 2).
Table 1.
Physiologic parameters
| Saline (4) | BDNF (6) | OX26 (4) | Conjugate (6) | |
|---|---|---|---|---|
| BP, mm Hg | ||||
| pre | 128 ± 20 | 138 ± 10 | 103 ± 6 | 121 ± 10 |
| dur | 26 ± 5 | 22 ± 2 | 23 ± 5 | 27 ± 6 |
| post | 108 ± 22 | 138 ± 13 | 131 ± 13 | 151 ± 7 |
| T, °C | ||||
| pre | 36.7 ± 0.1 | 36.4 ± 0.1 | 36.8 ± 0.2 | 36.5 ± 0.2 |
| dur | 36.3 ± 0.1 | 36.2 ± 0.3 | 36.5 ± 0.1 | 36.4 ± 0.3 |
| post | 37.1 ± 0.1 | 36.8 ± 0.1 | 37.0 ± 0.1 | 36.7 ± 0.1 |
| pH | ||||
| pre | 7.41 ± 0.02 | 7.38 ± 0.02 | 7.37 ± 0.04 | 7.34 ± 0.01 |
| post | 7.36 ± 0.01 | 7.34 ± 0.01 | 7.32 ± 0.03 | 7.33 ± 0.01 |
| pO2, mm Hg | ||||
| pre | 135 ± 22 | 117 ± 14 | 141 ± 17 | 117 ± 10 |
| post | 151 ± 19 | 154 ± 9 | 160 ± 16 | 125 ± 9 |
| pCO2, mm Hg | ||||
| pre | 44 ± 3 | 48 ± 3 | 51 ± 5 | 49 ± 2 |
| post | 41 ± 2 | 42 ± 1 | 50 ± 6 | 45 ± 3 |
BP, mean arterial blood pressure; dur, during ischemia; and post, after recovery. Mean ± S.E. (n = 4–6 rats per group).
Figure 3.
(A) EEG for rats before ischemia and isoelectric EEG during the 12-min ischemic period. (B) Hippocampal CA1 neuron density in control animals not subjected to transient forebrain ischemia and in four groups of animals subjected to 12 min of transient forebrain ischemia and isoelectric EEG. These four groups of animals were treated i.v. with either buffer, unconjugated BDNF, OX26 without BDNF attached, or BDNF-PEG 2000-biotin conjugated to OX26/SA. Only the latter group resulted in a statistically significant therapeutic effect after i.v. administration. ∗, P < 0.0025 difference between BDNF and BDNF-PEG conjugate.
Figure 4.
Nissl staining of hippocampal CA1 sector 7 days after 12 min of transient forebrain ischemia sufficient to induce isoelectric EEG. Intravenous treatment immediately after the ischemic injury and daily for 7 days consisted of unconjugated BDNF at a dose of 250 μg/kg per day (A and C) or BDNF-PEG 2000-biotin (250 μg/kg per day) conjugated to OX26/SA (B and D). The neuronal cell density is markedly reduced in the animals treated with unconjugated BDNF, but is normalized by the treatment with the BDNF-PEG-OX26 conjugate. [Magnification bars: (A) 21 μm, and (C) 212 μm.]
Table 2.
Neuronal cell density in postischemic hippocampus after drug treatment
| Hippocampal region | Treatment
|
||||
|---|---|---|---|---|---|
| Control (6) | Saline (4) | BDNF (6) | OX26 (4) | Conjugate (6) | |
| CA3 | 129 ± 14 | 156 ± 7 | 135 ± 13 | 159 ± 6 | 123 ± 7 |
| CA4 | 145 ± 5 | 154 ± 2 | 123 ± 22 | 142 ± 2 | 144 ± 4 |
Data are mean ± SE (n = 4–6 animals per treatment group) with units of neurons/mm length of measuring grid.
DISCUSSION
These studies demonstrate the therapeutic efficacy of BDNF in cerebral ischemia after i.v. administration, providing the neurotrophic factor is (i) conjugated to a BBB drug delivery system, and (ii) pegylated to optimize plasma pharmacokinetics (Fig. 1). The molecular formulation of the BDNF conjugate depicted in Fig. 1 incorporates several specific characteristics that enable the successful neuroprotective effects of this conjugate after i.v. administration. First, the BDNF is pegylated to optimize plasma pharmacokinetics. That is, the attachment of PEG moieties to the surface of the neurotrophic factor greatly reduces hepatic uptake in vivo and prolongs the circulation time of the neurotrophic factor in the blood (10). This results in an increase in the plasma area under the concentration curve (AUC). The brain delivery of the BDNF, expressed as percent of injected dose (%ID/g brain), is directly proportional to the BBB permeability-surface area product and the plasma AUC (4). It is difficult to generate pharmacologically active concentrations of BDNF in brain, even if the neurotrophic factor is conjugated to a BBB drug delivery system such as the OX26 mAb, if the plasma AUC is not increased in parallel by using pegylation technology. The metabolic stability of the pegylated neurotrophic factor is demonstrated by the FPLC studies of rat plasma (Fig. 2A), which corroborate previous measurements of plasma stability of BDNF by using trichloroacetic acid (TCA) percipitability (10). In the absence of the pegylation modification, BDNF is rapidly removed from the blood stream with a half time of approximately 5 min and rapidly converted to low molecular weight TCA soluble metabolites (10).
The second important factor in the formulation of BDNF depicted in Fig. 1 is attachment of the PEG molecules to surface carboxyl groups of the neurotrophic factor. Previous studies describing protein pegylation involve the attachment of PEG moieties to surface lysine residues (14–16). However, the lysine residues on BDNF, and other members of the nerve growth factor family of neurotrophic factors, form a “cationic groove” that is critical to binding to the neuronal trkB receptor (17), and conjugation of these surface amino groups results in inactivation of the neurotrophic factor (18). Conversely, previous studies demonstrated complete retention of biologic activity, based on cell survival assays and trkB autophosphorylation assays, when PEG moieties are attached to surface carboxyl groups on the neurotrophin by using Hz linkers (6, 10).
A third factor involved in the molecular reformulation of BDNF to enable BBB transport is monobiotinylation of the pegylated neurotrophin. Owing to the multivalency of SA binding of biotin (19), the presence of two or more biotin residues on the pegylated BDNF would result in the formation of high molecular weight aggregates that are rapidly cleared by the reticulo-endothelial system in vivo. Previous SDS/PAGE results demonstrated the attachment of approximately 6–12 PEG moieties per BDNF monomer (6, 10). Because there are 12 aspartate and glutamate residues per BDNF monomer (20), 50–100% of these carboxyl moieties have extended PEG molecules attached. However, only one of these PEG molecules must have an extended biotin bridge to enable attachment to OX26/SA. The BDNF-PEG 2000 without attached biotin or the BDNF-PEG 2000-biotin with two or more biotin moieties attached per BDNF homodimer is removed by Sephacryl S300 gel filtration chromatography as described (6).
The fourth critical factor involved in the molecular reformulation of the BDNF conjugate shown in Fig. 1 is the placement of the biotin residue at the tip of the PEG strand rather than at the surface of the neurotrophic factor. Attachment of the biotin to the surface of the neurotrophic factor would cause mutual steric hindrance between the pegylated BDNF and the OX26/SA, which would reduce the bifunctionality of the conjugate and impair binding to either neuronal trkB receptor or the BBB TfR. Conversely, placement of the biotin bridge at the tip of the PEG strand causes a spatial separation of the two components of the conjugate that allows for an uninhibited binding to the two respective receptor systems shown in Fig. 1 (6).
The daily dose of BDNF used in these studies is 250 μg/kg per day. Given a brain uptake of 0.15% injected dose/g of the conjugate (6), this dose generates a brain BDNF concentration of 100 ng/g, which causes a complete autophosphorylation of the trkB receptor (6). Because significant autophosphorylation of trkB receptors is induced by concentrations as low as 10 ng/ml of the BDNF conjugate (6), it is possible that lower systemic doses of the BDNF conjugate also will have therapeutic effects in vivo after i.v. administration. Because more than 90% of the total body trkB receptor is located in the central nervous system and less than 10% in peripheral tissues (21), the i.v. administration of the BDNF conjugate may have minimal peripheral side effects. The effects of long-term delivery of neurotrophic factors through the BBB and into brain are unknown. Also unknown is the maximal duration between the ischemic insult and the initiation of therapy such that therapeutic effects are still observed. The mRNA for caspase-3, an enzyme in the apoptosis cascade, increases markedly in the hippocampal CA1 sector for the first 72 hr after the ischemia, and then normalizes at 96 hr (22). Therefore, the therapeutic “window” may be up to 48–72 hr after ischemia.
The therapeutic efficacy of BDNF conjugates in vivo may be further increased by the use of dual peptide therapy. Although other neurotrophic factors such as ciliary neurotrophic factor do not have therapeutic effects in transient forebrain ischemia after ICV administration (23), neurotrophic factors such as hepatocyte growth factor (HGF) or epidermal growth factor (EGF) may have synergistic effects with BDNF. The intra-striatal injection of HGF or the ICV infusion of EGF protects hippocampal CA1 neurons after transient forebrain ischemia (24, 25).
In summary, these studies demonstrate the combined effects of carboxyl-directed pegylation of BDNF, and attachment to a BBB drug delivery system, such as the OX26 mAb, results in neuroprotection after transient forebrain ischemia, and i.v. administration of the neurotrophic factor. There are more than 30 known neurotrophic factors (1), and these molecules may prove to be powerful neuropharmaceuticals should they be enabled to undergo transport through the BBB with optimized plasma pharmacokinetic properties. The results of the present investigation indicate that if the neurotrophic factor undergoes a defined molecular reformulation, such as that depicted in Fig. 1, both to enable BBB transport and to optimize plasma pharmacokinetics, then these molecules may have therapeutic effects in the brain after peripheral administration.
Acknowledgments
This work was supported by National Institutes of Health Grant NS-34698.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- BBB
blood-brain barrier
- PEG
polyethylene glycol
- SA
streptavidin
- OX26/SA
conjugate of streptavidin and OX26 mAb
- ICV
intra-cerebro-ventricular
- EEG
electroencephalograph
- Hz
hydrazide
- FPLC
fast protein liquid chromatography
- TfR
transferrin receptor
References
- 1.Hefti F. Annu Rev Pharmacol Toxicol. 1997;37:239–267. doi: 10.1146/annurev.pharmtox.37.1.239. [DOI] [PubMed] [Google Scholar]
- 2.Beck T, Lindholm D, Castren E, Wree A. J Cereb Blood Flow Metab. 1994;14:689–692. doi: 10.1038/jcbfm.1994.86. [DOI] [PubMed] [Google Scholar]
- 3.Yan Q, Matheson C, Sun J, Radeke M J, Feinstein S C, Miller J A. Exp Neurol. 1994;127:23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge W M. J Cereb Blood Flow Metab. 1997;17:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge W M, Kang W-S, Buciak J L. Pharm Res. 1994;11:738–746. doi: 10.1023/a:1018940732550. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge W M, Wu D, Sakane T. Pharm Res. 1998;15:576–582. doi: 10.1023/a:1011981927620. [DOI] [PubMed] [Google Scholar]
- 7.Nucci M L, Shorr R, Abuchowski A. Adv Drug Delivery Rev. 1991;6:133–151. [Google Scholar]
- 8.Bickel U, Kang Y-S, Yoshikawa T, Pardridge W M. J Histochem Cytochem. 1994;42:1493–1497. doi: 10.1177/42.11.7930531. [DOI] [PubMed] [Google Scholar]
- 9.Pardridge W M, Buciak J L, Friden P M. J Pharmacol Exp Ther. 1991;259:66–70. [PubMed] [Google Scholar]
- 10.Sakane T, Pardridge W M. Pharm Res. 1997;14:1087–1093. doi: 10.1023/a:1012117815460. [DOI] [PubMed] [Google Scholar]
- 11.Smith M-L, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo B K. Acta Neurol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 12.Cambron H, Latulippe J-F, Nguyen T, Cartier R. Lab Ani Sci. 1995;45:303–304. [PubMed] [Google Scholar]
- 13.Yokoyama C, Okamura H, Ibata Y. Brain Res. 1995;681:153–159. doi: 10.1016/0006-8993(95)00308-d. [DOI] [PubMed] [Google Scholar]
- 14.Knauf M J, Bell D P, Hirtzer P, Luo Z-P, Young J D, Katre N V. J Biol Chem. 1988;263:15064–15070. [PubMed] [Google Scholar]
- 15.Clark R, Olson K, Fuh G, Marian M, Mortensen D, Teshima G, Chang S, Chu H, Mukku V, Canova-Davis E, et al. J Biol Chem. 1996;271:21969–21977. doi: 10.1074/jbc.271.36.21969. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi Y, Kihira T, Tsunoda S, Kamada H, Nakagawa S, Kaneda Y, Kanamori T, Mayumi T. J Pharmacol Exp Ther. 1996;278:1006–1011. [PubMed] [Google Scholar]
- 17.Ibanez C F, Ebendal T, Barbany G, Murray-Rust J, Blundell T L, Persson H. Cell. 1992;69:329–341. doi: 10.1016/0092-8674(92)90413-7. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg M B, Hawrot E, Breakefield X O. J Neurochem. 1986;46:641–648. doi: 10.1111/j.1471-4159.1986.tb13015.x. [DOI] [PubMed] [Google Scholar]
- 19.Green N M. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- 20.Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde Y-A. Nature (London) 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 21.Katoh-Semba R, Takeuchi I K, Semba R, Kato K. J Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- 22.Ni B, Wu X, Su Y, Stephenson D, Smalstig E B, Clemens J, Paul S M. J Cereb Blood Flow Metab. 1998;18:248–256. doi: 10.1097/00004647-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ogata N, Ogata K, Imhof H G, Yonekawa Y. Acta Neurochir. 1996;138:580–583. doi: 10.1007/BF01411179. [DOI] [PubMed] [Google Scholar]
- 24.Miyazawa T, Matsumoto K, Ohmichi H, Katoh H, Yamashima T, Nakamura T. J Cereb Blood Flow Metab. 1998;18:345–348. doi: 10.1097/00004647-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, Wen T-C, Tanaka J, Maeda N, Matsuda S, Desaki J, Sudo S, Zhang B, Sakanaka M. J Cereb Blood Flow Metab. 1998;18:349–360. doi: 10.1097/00004647-199804000-00002. [DOI] [PubMed] [Google Scholar]