INTRODUCTION
A plant lateral organ, defined here as either a leaf or a leaf-like organ of the shoot or flower, arises from a group of initial cells within the flanks of the shoot apical meristem (SAM) or floral meristem. For example, the tobacco leaf is formed from a group of ∼100 initial cells in all three histogenic layers of the SAM (Poethig and Sussex, 1985). Lateral organ initial cells follow fates that are very different from the more central SAM or neighboring cells that will form tissues of the shoot or floral axis. They change their patterns of expansion and division and elaborate new axes of growth—away from the SAM and laterally. As a lateral organ matures, cell division ceases and cells differentiate. The mature leaf typically consists of several million cells and ∼20 different cell types. However, it shows a characteristic shape, size and pattern of tissues. For example, the Antirrhinum leaf, which is typical of many species, shows a patterned arrangement of tissues that is particularly apparent along the dorsiventral (adaxial–abaxial) organ axis and in the distribution of veins. Patterning is also seen in the nonrandom distribution of epidermal cell types, including trichomes and stomatal guard cells (Figure 1).
Figure 1.
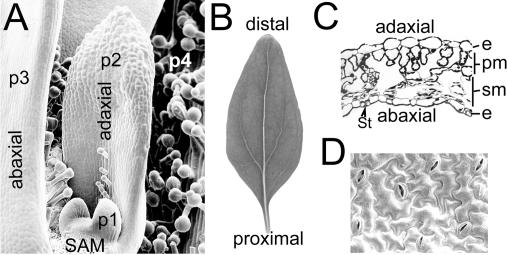
Growth and Patterning of a Dicot Leaf.
(A) Leaves are initiated in opposite pairs from the periphery of the Antirrhinum shoot apical meristem (SAM). Progressively older primordia are denoted (P1–P4). The leaves begin to flatten by lateral growth soon after primordium initiation. Trichomes (leaf hairs) are formed from the adaxial leaf surface in a nonrandom arrangement.
(B) The mature Antirrhinum leaf has undergone considerable growth along it proximal–distal axis and shows a characteristic pattern of veins.
(C) A transverse section through its blade shows an asymmetric arrangement of cell types along the adaxial–abaxial axis. e, epidermis; pm, palisade mesophyll; sm, spongy mesophyll; st, stoma.
(D) In the abaxial leaf epidermis, stomata show a nonrandom arrangement and are separated by at least one jigsaw piece–shaped pavement cell.
Two contrasting mechanisms are commonly invoked to explain such patterned growth and cell identity: either that it is an intrinsic function of initial cells, in which case daughter cells must inherit information about their identities from their parents; or that cells sense their position and respond to it, which implies that fate specification involves cell–cell signaling.
The fate of a cell in a developing lateral organ involves a characteristic pattern of growth and division and finally, differentiation of its progeny into specific cell types. If inherited cell identity has a role in this process, we would expect cells that share a common ancestry to follow similar fates. Such a relationship between cell lineage and fate is inherent in some aspects of lateral organ development. For example, the majority of cell divisions in a developing leaf lamina involve the formation of new anticlinal cell walls (i.e., parallel to the dorsiventral axis). Therefore, daughter cells are likely to remain within the same cell layer and to differentiate as cells of the same type (e.g., as palisade mesophyll; Figure 1). Restricted orientation of division could therefore provide the basis for heritable cell identity within a layer. It also raises the possibility that cell identity might itself affect cell division patterns, maintaining the clonal distinction of layers and effectively creating developmental compartments within which cells inherit identity as they proliferate. Similar lineage restrictions may create different developmental compartments earlier in lateral organ development. They occur, for example, between the initials of Antirrhinum floral organs at about the same time that each begins to express the unique combination of homeotic genes that specifies its identity (Vincent et al., 1995). Therefore, cells that have been specified as initials of one floral organ type (e.g., petal) are likely to remain within the developing primordium of that organ.
Lineage, however, is clearly incapable of explaining all aspects of lateral organ development. Early experiments with periclinal chimeras, in which the clonally distinct layers of the SAM were marked genetically, allowed the origins of cells in the mature leaf to be identified (Stewart and Derman, 1975). This revealed that a single tissue layer within the leaf is derived from multiple cells of the early leaf primordium. In the case of the leaf mesophyll layers, these initials usually involve two distinct cell lineages (the L2 and L3 layers of the SAM; Figure 2), suggesting that cell–cell interactions are involved in specifying their fate as mesophyll initials. Similarly, rare periclinal division within the L1 layer of a leaf consisting of a wild-type outer layer (L1) overlying an L2 carrying a chlorophyll mutation (Figure 2) displaces a wild-type daughter cell into the underlying mutant L2. The displaced cell shows the growth and division appropriate to its new internal position, ultimately giving rise to a clone of green cells with mesophyll identity rather than the epidermal identity expected from its ancestry (e.g., Stewart and Derman, 1975; Figure 2B). This implies that interactions between cells can override lineage in controlling cell fate.
Figure 2.
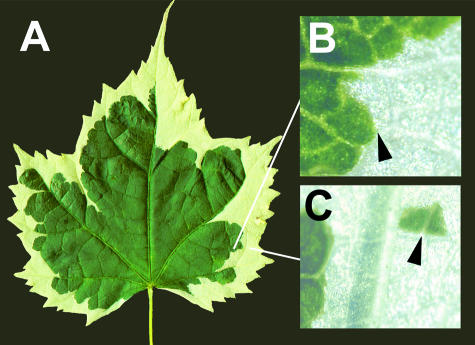
Cell Lineage in a Periclinal Chimera.
This shoot apical meristem of this Abutilon x hybridum cultivar has a mutant sub-epidermal layer (L2) that is unable to produce chlorophyll.
In the mature leaf (A), the contribution of cells derived from the L2 (yellow) and from the (L3) is variable. Compare, for example, the outline of the yellow-green boundary in the two halves of the leaf. Although many boundaries between L2- and L3-derived cells correspond to veins, others pass through groups of cells that have assumed the same mesophyll fate, regardless of their ancestry (arrowhead in [B]). The wild-type L1 layer mostly gives rise to the epidermis, which does not synthesize chlorophyll, except in guard cells. Rare periclinal division, however, can displace an L1 cell internally, where it gives rise to mesophyll cells appropriate to this internal position (arrowhead in [C]).
While these and many other observations reveal the importance of cell–cell signals in re-specifying the fate of displaced cells, they do not preclude the involvement of inherited cell identity in cells that are not displaced. For example, lineage might have a significant role in maintaining cell identity but can be over-ridden to prevent occasionally displaced cells retaining inappropriate identities. In contrast, a purely signaling-based mechanism would involve each daughter cell defaulting to a developmental ground state and having to reinterpret its identity de novo after each normal cell division. Re-specification of displaced cells also has a major experimental consequence in largely preventing the use of cell displacement to test effects of lineage-dependent fate. More telling, though experimentally more difficult tests would involve following the fates of cells isolated from sources of signals or defective in signal sensing.
One exception to the general observation that displaced cells assume fates appropriate to their new positions has been provided by the tangled1 (tan1) mutant of maize. tan1 mutants are affected in the polarity and timing of cell divisions (Smith et al., 1996). Aberrant divisions in the single layer of bundle-sheath (bs) cells surrounding each leaf vein can displace cells into the surrounding mesophyll cells, and the displaced cells retain bs identity rather than assuming mesophyll fate (Jankovsky et al., 2001). These divisions occur after expression of C4 photosynthetic enzymes characteristic of bs cells and after normal bs cells have ceased division. It is therefore possible that bs cells are displaced after the re-specification mechanism is able to operate. Normal bs cells are more closely related to each other and to other vein cells than they are to mesophyll (Langdale et al., 1989), raising the possibility that lineage has a role in their specification.
A further and more specialized case of lineage-determined fate involves daughter cells that assume two different fates in response to the intrinsic asymmetry of their parental cell or of the process of DNA replication or mitosis that gives rise to them. Asymmetric cell fate is found in several aspects of lateral organ development, including the divisions that give rise to stomatal initials and less-specialized epidermal cells, discussed below.
A number of mechanisms might account for inheritance of the same or different cell identities. DNA methylation status or chromatin modification have been proposed to represent the inherited factor in transmission of stable states of gene expression or of asymmetric daughter cell fates in fission yeast (e.g., Dalgaard and Klar, 2001). Preliminary analyses of genes required for these processes in plants, including the Chromatin Assembly Factor–like proteins encoded by Arabidopsis FASCIATA genes (Kaya et al., 2001), hint at roles in maintaining stable states of regulatory gene expression (reviewed by Finnegan and Kovac, 2000; Habu et al., 2001). An alternate explanation for lineage-dependent identity is that it involves inheritance of intracellular molecules or extracellular factors associated with the cell wall. Such inheritance of cell wall components is implicated in the maintainence of fate during early development of the brown alga Fucus (Berger et al., 1994), but has yet to be demonstrated for higher plants. Inheritance of intracellular molecules is implicated in asymmetric cell fate in the maize leaf epidermis. Here, a subsidiary cell adjacent to a stomatal guard cell and a larger epidermal cell are formed by asymmetric division of a precursor cell. The location of an internal patch of actin in the precursor cell correlates with subsidiary cell fate, even in mutants with defects in orientation of cell divisions. Similarly, mutations that disrupt formation of the patch also disrupt asymmetric division and fate (Gallagher and Smith, 2000).
While ability of position to override effects of cell lineage largely obscures any role of inherited cell identity, there is overwhelming evidence for cell–cell interactions in re-specifying displaced cell fate. Cell–cell interactions also appear likely to operate routinely in other aspects of lateral organ development, including organ initial specification within the SAM. The development of plant lateral organs, particularly leaves, is also highly plastic and responds to environmental cues. For example, the frequency of stomata is influenced by the availability of water, light, and CO2 (e.g., Woodward and Kelly, 1995), and therefore environmental signals must be integrated with intrinsic developmental processes.
Cell–cell interactions in animal development can be broadly classified according to the distances over which the signals act. Morphogens, which are involved in specifying major axes of asymmetry, such as the body and appendage axes of Drosophila, lie at one extreme (Vincent and Briscoe, 2001). These are secreted proteins or transcription factors that form concentration gradients extending over many cells (or nuclei in the embryo). Cells respond according to the concentration of morphogen they experience, effectively converting a gradient into discontinuous domains of gene expression and cell identity. At the other extreme, signaling may involve interactions only between adjacent cells. In Drosophila neurogenesis, for example, the membrane-bound ligand Delta binds its receptor, Notch, at the surface of adjacent cells to prevent their assuming the same identity—a case of lateral inhibition (Artavanis-Tsakonas et al., 1995).
Here we compare three aspects of plant lateral organ development in which cell–cell interactions occur over different distances: (1) specification of lateral organ fate at the shoot apex, (2) asymmetry within lateral organs, and (3) stomatal cell fate in the leaf epidermis. We do not consider several equally important and well-studied aspects of lateral organ development that are also likely to involve signaling, notably leaf venation (which has been reviewed recently by Dengler and Kang, 2001) and control of trichome fate in the lateral organ epidermis, which in Arabidopsis appears partly homologous to specification of hair cells and non-hair cells in the root epidermis (reviewed by Schiefelbein, 2000). Despite the need to invoke cell–cell interactions in all these processes, the mechanisms of interaction remain poorly understood, perhaps because redundancy, lethality, or sheer complexity has hindered identification of the relevant genes by mutation. An alternate explanation is that plants employ less-conventional mechanisms than the ligand-receptor signaling prevalent in animals, which makes them harder to identify genetically. We consider one potential alternative, physical force, in the context of lateral organ fate specification.
Specification of Lateral Organ Fate at the SAM
Lateral organ initiation occurs at regular positions in the periphery of the meristem in a pattern termed phyllotaxy. When each node has one organ, and those at adjacent nodes are opposite, as in maize, the phyllotaxy is termed distichous or alternate. Spiral phyllotaxy also involves a single organ at each node, but with organs at adjacent nodes typically offset by ∼137°. Organs therefore occur in one or more spirals along the shoot axis, as in Arabidopsis vegetative and inflorescence shoots, and the spiral can be either left- or right-handed. Two opposite organs at each node that are offset by 90° from those at adjacent nodes is usually termed decussate, and the term whorled is often used for more than two organs at each node, as in the flowers of many species.
One way to address the question of how lateral organ and non–lateral organ fates are specified at the shoot apex is through analysis of genes involved early in lateral organ development. Lateral organ initials within the Arabidopsis SAM are marked by expression of a number of genes that control early stages of lateral organ development including AINTEGUMENTA (ANT; Elliott et al., 1996; Klucher et al., 1996), LEAFY (LFY; Blazquez et al., 1997) and ASYMMETRIC LEAVES1 (AS1; Byrne et al., 2000), and members of the YABBY family (Sawa et al., 1999; Siegfried et al., 1999). They are also characterized by lack of expression of the knotted1-like homeobox (knox) gene, SHOOT MERISTEMLESS (STM), which is found throughout the remainder of the SAM (Long et al., 1996). Patterned expression of these genes therefore predicts lateral organ fate from at least one plastochron before morphological signs of primordium initiation. (Plastochron is used here to denote the time between initiation of successive primordia.) In the case of the Arabidopsis PINHEAD/ZWILLE gene, expression in basal vascular initials is proposed to reflect the positions at which the next five primordia will initiate (Lynn et al., 1999), suggesting that leaf primordium identity may respond to a genetic prepattern acting several plastochrons before primordium formation, when leaf initials become clonally distinct (Poethig and Sussex, 1985).
Although analysis of genes acting early in lateral organs has suggested when leaf fate might first be specified, it has revealed little of the underlying mechanisms of specification. STM, for example, is needed to repress AS1 expression in the SAM and, although AS1 is not required for lateral organ formation, its ectopic expression leads to SAM cells of stm mutants assuming lateral organ–like fates (Byrne et al., 2000). Repression of AS1 by STM can therefore be considered part of the mechanism responsible for elaborating lateral organ and non–lateral organ fates in shoots, but does not identify the underlying prepattern to which STM expression responds.
An additional category of genes overlaps in expression with STM in cells that will form non–lateral organ cells of the shoot axis. These include the Arabidopsis CUP-SHAPED COTYLEDON1 and 2 (CUC1 and 2) that encode members of the NAC family of potential transcriptional regulators (Aida et al., 1997; Takada et al., 2001). CUC1 and CUC2 are proposed to limit growth of non–lateral organ regions because cuc1 cuc2 double mutants can form united organs. Similarly, loss of activity of a related Petunia gene, NO APICAL MERISTEM, leads to initiation of additional, ectopic organs in flowers (Souer et al., 1996). Limited epistasis experiments suggest that CUC activity promotes STM expression (Aida et al., 1999; Takada et al., 2001). Because stm mutants have partially united cotyledons similar to those of cuc1 cuc2 mutants, CUC genes might act partly via STM to repress lateral organ fate and growth. Analysis of CUC gene function has therefore revealed a potentially earlier step in elaboration of lateral organ and non–lateral organ fates, although it has not revealed the prepattern regulating CUC gene expression.
The nature of the prepattern that determines lateral organ and non–lateral organ fates has long fascinated biologists and mathematicians. Early observations of phyllotactic patterns recognized that primordia tend to initiate in the greatest space available in the periphery of the SAM at positions most distant from existing primordia and the SAM apex. Further, spontaneous or induced changes in the position of one primordium could be maintained in subsequent development, for example as a shift in the handedness of spiral phyllotaxy. Such observations suggested that existing primordia are able to determine the position at which lateral organs subsequently form. Surgical and pharmacological experiments conducted early in the last century further supported this view and led to the field theory, which proposed that the central zone (cz) of the meristem and pre-existing primordia produce a diffusible inhibitory signal (Schoute, 1913; Wardlaw, 1949). In this early example of a lateral inhibition model, new primordia arise in the greatest space because this corresponds to the position of least inhibition. An alteration in meristem size without a corresponding change in primordium size is therefore predicted to alter the pattern of leaf initiation, as is seen in a number of mutants that have enlarged meristems, including the Arabidopsis clavata and fasciata mutants (Leyser and Furner, 1992; Clarke et al., 1993) and the maize abphyl1 (abph1) mutant (Greyson et al., 1978). In abph1 plants, an enlarged meristem produces a decussate pattern of leaves, but subsequent reversion to normal meristem size results in a distichous phyllotaxy being re-established (Greyson et al., 1978; Jackson and Hake, 1999). Similarly, reducing the size of lateral organ primordia allows more organs to form from a meristem of the same size, as seen in the whorled arrangement of reduced, needle-like leaves in Antirrhinum phan mutants (Waites and Hudson, 1995).
The inhibitory field model can be most conveniently explained in terms of a single hypothetical inhibitor, originating from existing lateral organs and the cz of the meristem. More recent evidence, however, suggests that lateral organ fate involves at least two specification steps, and therefore, involvement of a single inhibitor is likely to be an oversimplification. Historically, the cz was recognized as a histologically distinct region with lower cell division rates than the surrounding peripheral zone, from which lateral organs initiate (Lyndon, 1998). More recently, the cz has been equated with a population of naive stem cells that, in Arabidopsis, express the signaling ligand CLAVATA3 (CLV3). While the equivalence of these different definitions of cz identity awaits testing, one obvious feature of the cz is that no lateral organs are formed from it. In Arabidopsis, the size of the cz is regulated by the CLV signaling pathway (Nakajima and Benfey, 2002). CLV signaling, involving perception of the CLV3 ligand from the cz by the more widely expressed CLV1–CLV2 receptor, limits expression of the stem cell–promoting transcription factor WUSCHEL (WUS) to more basal and internal cells of the SAM (Brand et al., 2000). WUS, in turn, acts to promote more apical CLV3 expression via an additional, unknown signaling pathway (Schoof et al., 2000). These interactions are therefore sufficient to explain the control of the cz size. If the cz is considered to be the region of the SAM from which lateral organs are not produced, then these interactions can also account for the signaling proposed to inhibit organ formation at the apex of the SAM. How this signaling might regulate genes involved more directly in lateral organ fate is, however, currently unknown.
One candidate for a second signal involved in phyllotaxy is the phytohormone, auxin. It is synthesized at the shoot apex and actively transported between cells. When polar auxin transport (PAT) is inhibited chemically, phyllotactic patterns are altered or lateral organs and floral meristems fail to initiate, resulting in a pin-like inflorescence axis (Okada et al., 1991; Reinhardt et al., 2000). Several Arabidopsis mutants mimic these effects, including pin-formed1 (pin1; Okada et al., 1991), pinoid (pid; Bennett et al., 1995), and monopterous (mp; Berleth and Jürgens, 1993; Przemeck et al., 1996), which are all implicated in auxin transport or responses to auxin signaling.
PIN1 encodes an auxin efflux carrier that is expressed in developing primordia and vascular tissue (Gälweiler et al., 1998; Vernoux et al., 2000). The failure of pin1 mutants to form primordia can therefore be interpreted as a need to either remove auxin from lateral organ initials or to accumulate auxin, depending on whether the hormone inhibits or promotes primordium formation. Alternatively, differences in auxin concentrations between lateral organ and non–lateral organ initials, rather than absolute auxin levels, might affect organ formation. Several lines of evidence support the role of auxin as a lateral organ promoter. First, the pin1 mutant phenotype can be rescued by exogenous auxin (Reinhardt et al., 2000). Local application of auxin can also induce primordium formation at the point of application in wild-type tomato and in wild-type or pin1 mutant Arabidopsis. The size of primordia was further found to be proportional to the amount of auxin applied. Locally applied auxin is, however, unable to induce primordia from the cz, suggesting that auxin signaling acts in lateral organ formation independently of the CLV–WUS interaction. Loss of PID protein kinase activity results in an inflorescence phenotype similar to pin (Bennett et al., 1995). PID has been interpreted as a negative regulator of auxin responses (e.g., its overexpression renders plants less sensitive to auxin and reduces expression of auxin-induced genes; Christensen et al., 2000). Because PID is expressed in lateral organ primordia, loss of primordia in pid mutants would be inconsistent with a role for auxin in promoting lateral organ formation. Other experiments, however, have suggested that PID promotes auxin responses, more consistent with PID expression in lateral organs mediating their formation in response to auxin (Benjamins et al., 2001).
Current evidence therefore favors a model in which existing primordia accumulate auxin and prevent neighboring cells from forming lateral organ primordia by depleting their auxin concentration, as originally proposed by Sachs (see Sachs, 1991). Higher auxin concentrations at more distant positions should therefore allow primordium formation (and perhaps promote increased auxin accumulation).
Recent investigations have also addressed the relationship between auxin signaling and the expression of genes involved early in lateral organ development. Although both pin and pid mutants fail to initiate lateral organ primordia and floral meristems in the inflorescence, they show patterned expression of UNUSUAL FLORAL ORGANS, LFY, and ANT that marks the differences between lateral organs or floral meristems and the apical inflorescence meristem (Christensen et al., 2000; Vernoux et al., 2000). These observations suggest that auxin signaling is required for lateral organ outgrowth but not for the earlier step of organ fate specification. Other studies have shown that lateral organ fate is necessary for the promotion of the activity of the apical meristem (e.g., Waites et al., 1998). This is consistent with pin1 and pid mutants, which retain a functional SAM, presumably as a result of continued specification of lateral organ fate.
Analysis of the PAT mutant pin1 has suggested a more fundamental role for auxin signaling in lateral organ fate specification. It showed that LFY promoter activity, characteristic of lateral organs, occurred in concentric rings around the periphery of the pin1 meristem (Vernoux et al., 2000). This observation is consistent with a model for phyllotaxy involving two signaling processes. One, which does not involve PAT, defines evenly spaced rings of cells that are competent to assume lateral organ fate. And a second, auxin-dependent mechanism partitions each ring into lateral organ and non–lateral organ initials. Such multistep models for phyllotaxy have been proposed previously (e.g., Loiseau, 1969) and are supported by the observation of unpartitioned primordia in an Antirrhinum mutant (Carpenter et al., 1995).
Although phyllotaxy can be explained by the action of signals (e.g., auxin, CLV3, and at least one additional molecule that promotes CLV3 expression) an alternative, but not necessarily exclusive, mechanism has been proposed to involve physical forces. Green et al. (1996) described the meristem as a growing surface (the L1 and L2 layers, or tunica) that is constrained at its margins and supported by an elastic foundation (the L3, or corpus). If the surface has an inherent wavelength, it will tend to deform with this periodicity into a regular series of bumps and hollows, which Green et al. (1996) proposed would represent lateral organ primordia and the creases between them. Zones of inhibition in this model can be viewed as the reluctance of the tunica to bend sharply close to where it has already bent to form an existing primordium. Deformation would therefore represent the prepattern to which expression of lateral organ– and meristem-specific genes respond. Modelling has shown that such a process is theoretically capable of accounting for many aspects of phyllotaxy, including its propagation and self-adjustment after perturbation (Green, 1996). Although such a biophysical mechanism is unlikely to require the action of specific genes, and is difficult to test genetically, several investigations have provided supporting evidence. The sunflower inflorescence (capitulum) is formed from a large, flat meristem that initiates primordia in multiple spirals. Each primordium then gives rise to a bract subtending a flower. Experimental compression of the developing capitulum caused the formation of ridges running along phyllotactic spirals, rather than distinct primordia, and these developed into extended (united) bracts that lacked flowers (Hernandez and Green, 1993). Therefore, physical force could both alter phyllotaxy in a predictable way and change primordium fate.
The protein expansin promotes cell expansion by loosening cell walls. In some species, isoforms of expansin appear to be upregulated in lateral organ primordia, consistent with roles in primordium initiation and growth (Fleming et al., 1997; Reinhardt et al., 1998). When expansin gene expression was induced in the tobacco apex at a position representing initials of the next-but-one primordium, the primordium initiated prematurely and caused reversal of the phyllotactic spiral. Because the resulting leaf developed normally, loosening of the cell wall was proposed to be sufficient to activate all subsequent steps in leaf development (Pien et al., 2001). Earlier experiments, in which outgrowth was induced by application of expansin protein to the SAM, led to outgrowths with only limited leaf-like characteristics (Fleming et al., 1997), presumably because the protein was unable to affect more internal cells. The effects of altered expansin activity provide support for Green's model, in which distortion of the meristem is causal in lateral organ fate. However, expansin was unable to induce primordium formation in positions at which organs would not usually form, suggesting that lateral organ initials are specified before primordium outgrowth, and that expansin merely causes precocious outgrowth of existing initials. Comparing the effects of expansin with expression of genes that act as early markers for lateral organ and non–lateral organ fates, as well as inducing expansin activity in auxin mutants, should help to resolve this question.
A further level of organization within the apex that might be relevant to fate specification is the arrangement of plasmodesmatal connections that potentially allow communication between cells. Plasmodesmata interconnect symplastic fields that may include cells from the same or different lineages. They are also able to regulate the passage of potential signaling molecules, including developmentally important transcription factors or their RNA precursors (e.g., KNOTTED1; Lucas et al., 1995; Haywood et al., 2002), and may be involved in determining the polarity of movement. For example, the Antirrhinum MADS-domain transcription factor DEFICIENS can move outwards to the L1 but not inwards from it (Perbal et al., 1996). Studies of the birch and Arabidopsis meristems reveal that symplastic fields predict some aspects of meristem fate and are highly dynamic (Rinne and van der Schoot, 1998; Gisel et al., 1999). Whether symplastic signaling is important in maintaining fate within a field or for specifying different fates remains to be determined.
SPECIFICATION OF POLARITY IN LATERAL ORGANS
Elaboration of Dorsiventral Asymmetry
Most lateral organs are considered dorsiventral because they are flattened perpendicular to their adaxial–abaxial (or dorsiventral) axis, along which they also show an asymmetric distribution of cell types. The flattened shape of leaves or petals has obvious functional significance, providing a large area and arrangement of cell types specialized for photosynthesis or attraction of pollinators. Because lateral organs form in the periphery of apical meristems, their dorsiventral axis corresponds to a radial axis of the meristem (i.e., their adaxial surface is formed toward the center of the meristem). A connection between dorsiventrality of lateral organs and polarity of the meristem was suggested by early surgical experiments (Sussex, 1955; Snow and Snow, 1959; Hanawa, 1961). When an incision or impermeable barrier was inserted adaxial to a group of leaf initials, the resulting primordium was needle-like and consisted only of abaxial cells. This suggested (i) that signals originating from the center of the SAM are required for adaxial fate, (ii) that abaxial fate occurs in the absence of adaxial, and (iii) that adaxial fate is required for lateral growth of the leaf. Primordia isolated at a later stage of development were able to develop normally, implying that they no longer needed signals from outside the leaf to maintain their dorsiventral asymmetry. Subsequent analysis of the Antirrhinum phantastica (phan) gene, which is required for adaxial cell identity, suggested that interaction between organ cells with adaxial and abaxial identities was responsible for the lateral growth that flattened the organ (Waites and Hudson, 1995). Because phan functions redundantly with cold-sensitive factors, its role in promoting adaxial organ fate could be separated by temperature treatment from an additional function in promoting primordium formation. Temperature shift experiments were unable to separate these two roles in time, suggesting that dorsiventral organ asymmetry is specified before primordium initiation, consistent with the involvement of a signaling mechanism in the SAM (Waites et al., 1998).
Recent analysis in Arabidopsis has provided support for the involvement of an adaxial morphogen within the SAM leading directly to asymmetric gene expression within lateral organ primordia. This involved the identification of a small gene family, encoding likely transcription factors with homeodomain, bZIP, and START motifs that promote adaxial cell types in Arabidopsis lateral organs. These show similar expression patterns in the SAM and lateral organ initials and become restricted to an adaxial domain about the time of primordium initiation. Gain-of-function mutations in two members, PHABULOSA (PHB) and PHAVOLUTA (PHV), cause dose-dependent adaxialisation of organs, which at their most extreme develop only adaxial cell types and fail to grow laterally (McConnell and Barton, 1998; McConnell et al., 2001). Independent gain-of-function mutations involve mis-sense mutations that alter the START domain, a motif that is implicated in binding sterol lipid ligands in a number of functionally diverse proteins (McConnell et al., 2001). This suggests that PHB and PHV proteins are activated by a lipid ligand that is present only in adaxial cells. (An alternative interpretation is that they are repressed by an abaxial ligand.) The gain-of-function mutations are proposed to cause ligand-independent activity in cells positioned more abaxially, resulting in ectopic adaxial fates.
Because the domains of PHB and PHV RNA expression extend abaxially in gain-of-function mutants, the activated proteins are likely to promote accumulation of their own RNA (McConnell et al., 2001). PHB and PHV show graded RNA accumulation in lateral organ primordia, with the highest levels adaxially. This therefore suggests a dorsiventral gradient of protein activity resulting from a gradient of the activating sterol ligand. PHB and PHV expression also extends from lateral organ primordia and initials to the center of the SAM, suggesting that the ligand is also present in the SAM. It is attractive to speculate that this ligand corresponds to the signaling molecule predicted from the surgical experiments, which is produced centrally in the apex, forms a concentration gradient toward the periphery, and specifies adaxial organ fate in a concentration-dependent manner (i.e., is a morphogen). This raises a number of exciting questions about the identity of the ligand or ligands of PHB and PHV and whether they can move between cells. The ability of PHB and PHV to promote their own expression might also provide the basis for the leaf primordium to maintain cell identity without further signals from the SAM, consistent with the ability of isolated primordia to complete normal development.
PHB and PHV act to restrict expression of two families of regulatory genes involved in abaxial cell fate specification, the KANADIs and YABBYs, which are initially expressed throughout lateral organ initials and become restricted to the abaxial domain about the time that PHB and PHV are localized to adaxial cells (Eshed et al., 1999, 2001; Sawa et al., 1999; Siegfried et al., 1999; Kerstetter et al., 2001). Ectopic adaxial expression of KANADI (KAN) genes causes adaxial cells to assume abaxial fates, suggesting that much of the role of PHB and PHV in promoting adaxial fate might result from repression of KAN. Expression of all three gene families, however, overlaps in lateral organ initials within the SAM, suggesting that other factors, specific to organ primordia, are required for the activation of PHB/PHV or repression of KAN.
Interaction between primordium cells with adaxial and abaxial identities is proposed to be necessary for lateral growth of the leaf (Waites and Hudson, 1995). The time at which genes promoting adaxial or abaxial fate become restricted to their respective domains broadly corresponds with the changes in patterns of cell division that accompany the start of leaf blade growth (Sawa et al., 1999; Siegfried et al., 1999; Eshed et al., 2001; Kerstetter et al., 2001; McConnell et al., 2001). Early divisions are highest in two lateral domains of the primordium, termed blastozones, that probably act as lamina initials. Expression of organ polarity genes persists in the growing organ, suggesting that adaxial–abaxial interaction might be required for oriented cell divisions until relatively late in development. While there is increasing evidence to support the role of adaxial–abaxial interaction in controlling lateral organ growth, the signaling mechanisms remain unknown. The leaf lamina, however, is formed from as few as four layers of cells along its dorsiventral axis, and therefore the proposed interactions between dorsal and ventral cells need control cell division over only two cell diameters.
Elaboration of Proximal–Distal Asymmetry in Lateral Organs
The dorsiventral organ axis typically shows an asymmetric distribution of tissues but undergoes little growth. In leaves, more growth typically occurs along the proximal–distal (P–D) axis (representing the base to tip of the mature leaf). However, limited P–D asymmetry of tissues in dicot leaves hinders testing of the developmental relevance of this axis and the mechanisms that might be involved in its specification and elaboration. Monocot leaves, in contrast, often show obvious P–D patterning. The maize leaf consists of a proximal sheath and distal blade with ligule and auricle structures between. Disruptions to this pattern result from ectopic expression of Class I knox genes that are normally restricted to cells of the SAM and internode initials. Although such gain-of-function phenotypes are inherently difficult to interpret, one explanation for the effect of ectopic knox gene expression is that it alters the developmental age of cells, allowing them to respond to a P–D prepattern in a way that is inappropriate for their position (Freeling, 1992). The basis for the prepattern, however, remains elusive.
The dicot gynoecium, in contrast to the dicot leaf, shows marked asymmetry of tissues along its P–D axis, which often also represents its predominant axis of growth. The Arabidopsis gynoecium is a complex organ—it has a pollen-receptive stigma distally, followed by the style, ovary, and a short internode (gynophore) separating it from the pedicel at its proximal end. The ovary consists of two lateral valves and is divided into two locules by a septum that is formed by postgenital fusion of outgrowths from the ovary walls. This distinct arrangement of cell types has allowed identification of mutations that disrupt P–D patterning of the gynoecium.
Disruptions to the arrangement of gynoecium tissues occur in mutants defective in auxin transport and perception (e.g., pin1, pid, and mp) or following application of inhibitors of PAT (Bennett et al., 1995; Przemeck et al., 1996; Nemhauser et al., 2000). This has lead to the proposal that auxin acts as a morphogen in carpel development. In this model, auxin is produced distally and is actively transported proximally forming a concentration gradient to which cells respond; stigma and style, valve, and gynophore identities are promoted by high, medium, and low auxin concentrations, respectively (Figure 3). The apical boundaries between style and valve initials and the basal boundary between valve and internode therefore represent the points at which two different threshold concentrations are reached within the gradient. PAT inhibitors shift both of these boundaries distally (i.e., cells adopt more basal fates than normally appropriate for their positions) but also reduce the spacing between boundaries, so that the gynoecium shows an increase in stigma/style and gynophore identities at the expense of ovary (Nemhauser et al., 2000). This is consistent with PAT inhibitors causing a steeper auxin concentration gradient.
Figure 3.
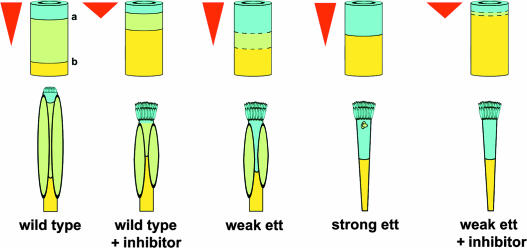
Development of the Arabidopsis Gynoecium.
The gynoecium primordium is depicted as a cylinder in which the apical boundary (a) separates the stigma/style (blue) from the ovary valve (green), and the basal boundary (b) separates valve from gynophore (yellow). Auxin is produced apically and transported basally, forming a concentration gradient within the developing gynoecium (red arrow). The predicted effects of reduced polar auxin transport and ETT activity on the concentration gradient and positioning of boundaries are shown above the resulting gynoecium phenotypes. Dashed lines in ett mutant primordia indicate boundaries that are disrupted through a loss of ETT activity. When polar auxin transport (PAT) is inhibited, the apical and basal boundaries are shifted apically and the distance separating them reduced. The effects on gynoecium development are proposed to reflect a steeper morphogen gradient. Gynoecia of weak ett mutants display similar characteristics, which are suggested to involve both boundaries moving toward each other because sensitivity to auxin has been altered. Application of PAT inhibitor to the weak ett mutant causes a further reduction in valve region. This, together with the disrupted boundaries, results in a loss of valve identity and a phenotype that is similar to strong ett mutants. Strong ett mutants are not affected by PAT inhibitors. (Figure adapted from Nemhauser et al., 2000).
The effect of PAT inhibitors is similar in several respects to that of Arabidopsis ettin (ett) mutations. Decreasing activity in a series of ett mutants leads to progressive replacement of proximal valve tissue with tissue resembling both abaxial style and gynophore and tissues between the valves of the ovary with adaxial style tissue (Sessions and Zambryski, 1995; Sessions, 1997). The most severe ett mutants lack all valve tissue and form only a style-like structure. Application of PAT inhibitors to weak ett mutants causes a more severe mutant phenotype, suggesting that ETT perceives the auxin concentration gradient (Nemhauser et al., 2000). Changes to ETT activity (mutations) alter the threshold levels that give rise to boundaries (Figure 4).
Figure 4.
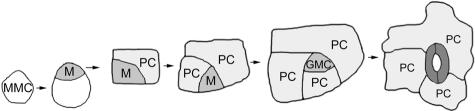
The Stereotypic Division Model for Stomatal Spacing.
A meristemoid mother cell (MMC) divides asymmetrically to form a smaller meristemoid (M). The meristemoid then undergoes three rounds of asymmetric cell divisions, in which the larger daughters assume fates as less-specialized pavement cells (PC). The meristemoid then becomes a guard mother cell (GMC) that divides symmetrically to form two guard cells surrounding a central pore. This pattern of division would result in each stoma being surrounded by at least one pavement cell derived from the same meristemoid. (Redrawn from Geisler et al., 2000).
Consistent with this view is the finding that ETT encodes a member of the auxin response factor family of transcriptional regulators that show auxin-dependent binding to auxin response promoter elements (Sessions et al., 1997; Kepinsky and Leyser, 2002). Therefore, the ETT protein could potentially act to transduce an auxin concentration gradient into patterned gene expression (Sessions, 1997; Nemhauser et al., 2000). ETT activity, however, appears unlikely to set the apical and basal boundaries in response to auxin, because its domain of RNA expression corresponds to the region between the boundaries and is unaffected by auxin treatment (Nemhauser et al., 2000).
Further doubt about the proposed role of ETT has been raised by the finding that it represses the basic helix-loop-helix transcription factor gene, SPATULA (SPT; Heisler et al., 2001). SPT is expressed distally, outside the ETT domain, and promotes development of distal gynoecium structure (spt mutant gynoecia have reduced stigmatic tissues and lack internal distal cell types; Alvarez and Smyth, 1998, 1999). This repression might involve direct binding of ETT protein to auxin response promoter elements in the SPT promoter. Mis-expression of SPT can account for most of the carpel defects of ett mutants, including the aberrant positions of the apical and basal boundaries, because these are restored in spt ett double mutants (Alvarez and Smyth, 1998; Heisler et al., 2001). Thus, it appears that the effects of ett mutants on the carpel boundaries result indirectly from SPT expression and not from an inability to respond to an auxin gradient. This could be tested further by examining the effects of PAT inhibitors on boundary placement in ett spt mutant gynoecia.
CONTROL OF STOMATAL CELL FATE
Stomata are pores formed between a pair of specialized epidermal guard cells that allow gas exchange to be regulated. Arabidopsis is typical of many dicots in which the guard cells of one stoma are separated from others by at least one less-specialized epidermal pavement cell (e.g., Figure 1D; Kagan et al., 1992; Korn, 1993). The pair of guard cells is formed by symmetric division of a guard mother cell (GMC). The GMC results from asymmetric divisions of a meristemoid or meristemoid mother cell (MMC; Figure 4). A number of mechanisms have been proposed to explain the pattern of stomatal and non-stomatal cells in the epidermis. At one extreme, an invariant pattern of cell divisions is proposed to result in each GMC being completely surrounded by non-GMCs derived from the same lineage (Figure 4; Larkin et al., 1996). Such a pattern could be an intrinsic property of the MMC and need not involve communication between cells. This view has received support from the observation that the boundaries of clones marked early in development rarely pass between guard cells and their neighboring pavement cells, consistent with the surrounding cells and GMC arising from the same lineage (Larkin et al., 1997; Serna and Fenoll, 2000). Alternatively, spacing might involve cell–cell interactions that prevent cells close to MMCs, meristemoids, or GMCs from assuming similar fates (a lateral inhibition model) or that influence cell division polarity.
Such questions were addressed by carefully analyzing the origins of epidermal cells through daily observations of Arabidopsis leaves or cotyledons (Geisler et al., 2000). This study revealed that the cell divisions giving rise to a GMC were far from stereotypic (as in Figure 4) and involved a variable number of meristemoid divisions. The majority of guard cell pairs therefore had at least one adjacent pavement cell that was derived from a different MMC, so purely lineage-based mechanisms were unable to explain stomatal spacing. MMCs could form adjacent to existing guard cells or their precursors (MMCs, meristemoids, and GMCs), although they did so less frequently and never formed from a cell adjacent to two existing guard cell precursors. This suggested that cell–cell interactions were involved in specification of MMC fate but that these interactions were insufficient to account for spacing of all stomata. While MMCs located next to each other showed random orientation of cell division, those adjacent to existing meristemoids, GMCs, or guard cells always divided to form a new satellite meristemoid opposite the existing guard cell precursor. This asymmetric division did not appear to reflect an inherited cellular polarity, because it could occur in cells derived from a lineage different from that of the guard cell precursor. This again suggested the involvement of signaling, rather than cell intrinsic polarity. Because three quarters of stomata arose from satellite meristemoids, such oriented cell division constituted a major spacing mechanism. When adjacent meristemoids were formed, they very rarely gave rise to adjacent stomata, either because they were separated by an oriented division or because one of them assumed pavement cell fate. Adjacent meristemoids are derived from different lineages, implying the involvement of cell–cell interaction in these processes.
Several important aspects of stomatal patterning can therefore be explained by signaling that acts within one cell diameter of guard cell precursors to inhibit cell fate and orient cell divisions (although it does not rule out a role for inherited asymmetry in some cells). This mechanism is also compatible with the effects of environmental and developmental cues that alter the frequency of stomata (Gray et al., 2000; Berardini et al., 2001; Brownlee, 2001), and it will be interesting to determine which spacing mechanisms these cues affect (e.g., MMC frequency, the number of meristemoid divisions, etc). Because MMC fate involves lateral inhibition, it can also explain the relative insensitivity of stomatal frequency to differences in organ size.
All these aspects of stomatal spacing are affected by loss-of-function mutations in the Arabidopsis TOO MANY MOUTHS (TMM) gene (Yang and Sack, 1995). tmm mutants show several defects consistent with compromised signaling; for example, they form MMCs adjacent to existing stomatal lineages, and the divisions that form satellite meristemoids are oriented randomly (Geisler et al., 2000). This suggests that TMM is involved in signaling and that a common TMM-dependent signaling process may be involved in all the mechanisms contributing to stomatal spacing. The role of TMM and other genes with related phenotypes (Yang and Sack, 1995), should cast more light on this process.
CONCLUSIONS
We have considered several aspects of plant lateral organ development that appear to be dependent on cell–cell signaling, although the signaling mechanisms involved are largely obscure. Auxin, sterol lipids, and physical forces have been invoked as signals acting over distances of several cells and even acting as morphogens in specifying different fates according to concentration. The involvement of auxin (which can have pleiotropic effects) and of physical force (which is unlikely to depend directly on gene activity) is difficult to test genetically. Other signaling mechanisms, however, lend themselves to genetic characterisation. Identification of PHB-like transcription factors provides a potential route to identifying their activating ligands and so testing the roles of the ligands in cell–cell signaling. In the case of stomatal cell placement, much of the observed patterning can be explained by signals acting between adjacent cells, for example, a mechanism analogous to Delta-Notch signaling in Drosophila. Further insights into the identity of these signals may come when the genes altered in the stomatal spacing mutants have been identified. Perhaps the strongest evidence for the nature of a cell–cell signaling mechanism is provided by the CLV pathway, in which the secreted CLV3 peptide probably acts as a ligand for the CLV1 receptor kinase. The Arabidopsis genome contains numerous CLV3-like genes and potential CLV1-like receptors (Cock and McCormick, 2001), raising the possibility that homologous signaling mechanisms operate in many aspects of plant development.
NOTE ADDED IN PROOF
A recently published paper by Kessler et al. (2002) shows that cell identity in a maize leaf may be specified through lineage, as cells displaced from the L1 to the L2 layer in leaves of the xcl1 mutant retain their L1 identity.
Kessler, S., Seiki, S., and Sinha, N. (2002). Xcl1 causes delayed oblique periclinal cell divisions in developing maize leaves, leading to cellular differentiation by lineage instead of position. Development 129, 1859–1869.
Acknowledgments
We would like to thank Jill Harrison and Pete Newton for their helpful comments on this manuscript. JFG is supported by a fellowship from the European Molecular Biology Organization.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.000828.
References
- Aida, M., Ishida, T., Fukaki, H., Fujisawa, H., and Tasaka, M. (1997). Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida, M., Ishida, T., and Tasaka, M. (1999). Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: Interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 119 823–831. [DOI] [PubMed] [Google Scholar]
- Alvarez, J., and Smyth, D.R. (1998). Genetic pathways controlling carpel development in Arabidopsis thaliana. J. Plant Res. 111 295–298. [Google Scholar]
- Alvarez, J., and Smyth, D.R. (1999). CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development 126 2377–2386. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas, S., Matsuno, K., and Fortini, M. (1995). Notch signalling. Science 268 225–230. [DOI] [PubMed] [Google Scholar]
- Benjamins, R., Quint, A., Weijers, D., Hooykaas, P., and Offringa, R. (2001). The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067. [DOI] [PubMed] [Google Scholar]
- Bennett, S.R.M., Alvarez, J., Bossinger, G., and Smyth, D.R. (1995). Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 8 505–520. [Google Scholar]
- Berardini, T.Z., Bollman, K., Sun, H., and Poethig, R.S. (2001). Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291 2405–2407. [DOI] [PubMed] [Google Scholar]
- Berger, F., Taylor, A., and Brownlee, C. (1994). Cell fate determination by the cell wall in early Fucus development. Science 263 1421–1423. [DOI] [PubMed] [Google Scholar]
- Berleth, T., and Jürgens, G. (1993). The role of the monopterous gene in organising the basal body region of Arabidopsis embryo. Development 118 575–587. [Google Scholar]
- Blazquez, M.A., Soowal, L.N., Lee, I., and Weigel, D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124 3835–3844. [DOI] [PubMed] [Google Scholar]
- Brand, U., Fletcher, J., Hobe, M., Meyerowitz, E., and Simon, R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289 617–619. [DOI] [PubMed] [Google Scholar]
- Brownlee, C. (2001). The long and the short of stomatal density signals. Trend. Plant Sci. 6 441–442. [DOI] [PubMed] [Google Scholar]
- Byrne, M.E., Barley, R., Curtis, M., Arroyo, J.M., Dunham, M., Hudson, A., and Martienssen, R.A. (2000). Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408 967–971. [DOI] [PubMed] [Google Scholar]
- Carpenter, R., Copsey, L., Vincent, C., Doyle, S., Magrath, R., and Coen, E. (1995). Control of flower development and phyllotaxy by meristem identity genes in Antirrhinum. Plant Cell 7 2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.K., Dagenais, N., Chory, J., and Weigel, D. (2000). Regulation of auxin response by the protein kinase PINOID. Cell 100 469–478. [DOI] [PubMed] [Google Scholar]
- Clarke, S.E., Running, M.P., and Meyerowitz, E.M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397–418. [DOI] [PubMed] [Google Scholar]
- Cock, J.M., and McCormick, S. (2001). A large family of genes that share homology with CLAVATA3. Plant Physiol. 126 939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard, J.Z., and Klar, A.J. (2001). Does S. pombe exploit the intrinsic asymmetry of DNA synthesis to imprint daughter cells for mating-type switching? Trend. Genet. 17 153–157. [DOI] [PubMed] [Google Scholar]
- Dengler, N., and Kang, J. (2001). Vascular patterning and leaf shape. Curr. Opin. Plant Biol. 4 50–56. [DOI] [PubMed] [Google Scholar]
- Elliott, R.C., Betzner, A.S., Huttner, E., Oakes, M.P., Tucker, W.Q.J., Gerentes, D., Perez, P., and Smyth, D.R. (1996). AINTEGUMENTA, an APETELA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., and Bowman, J.L. (1999). Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99 199–209. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., Perea, J.V., and Bowman, J.L. (2001). Establishment of polarity in lateral organs of plants. Curr. Biol. 11 1251–1260. [DOI] [PubMed] [Google Scholar]
- Finnegan, E.J., and Kovac, K.A. (2000). Plant DNA methyltransferases. Plant Mol. Biol. 43 189–201. [DOI] [PubMed] [Google Scholar]
- Fleming, A.J., McQueen-Mason, S., Mandel, T., and Kuhlemeier, C. (1997). Induction of leaf primordia by the cell wall protein expansin. Science 276 1415–1418. [Google Scholar]
- Freeling, M. (1992). A conceptual framework for leaf development. Dev. Biol. 153 44–58. [DOI] [PubMed] [Google Scholar]
- Gallagher, K., and Smith, L.G. (2000). Roles for polarity and nuclear determinants in specifying daughter cell fates after an asymmetric cell division in the maize leaf. Curr. Biol. 10 1229–1232. [DOI] [PubMed] [Google Scholar]
- Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230. [DOI] [PubMed] [Google Scholar]
- Geisler, M., Nadeau, J., and Sack, F.D. (2000). Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. Plant Cell 12 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisel, A., Barella, S., Hempel, F.D., and Zambryski, P.C. (1999). Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development 126 1879–1889. [DOI] [PubMed] [Google Scholar]
- Gray, J.E., Holroyd, G.H., vander Lee, F.M., Bahrami, A.R., and Sijmons, P.C. (2000). The HIC signalling pathway links CO2 perception to stomatal development. Nature 408 713–716. [DOI] [PubMed] [Google Scholar]
- Green, P.B., Steele, C.S., and Rennich, S.C. (1996). Phyllotactic patterns: a biophysical mechanism for their origin. Ann. Bot. 77 515–527. [Google Scholar]
- Green, P.B. (1996). Expression of form and pattern in plants–a role for biophysical fields. Semin. Cell Dev. Biol. 7 903–911. [Google Scholar]
- Greyson, R.I., Walden, D.B., Humes, J.A., and Erickson, R.O. (1978). The ABPHYL syndrome in Zea mays. II. Patterns of leaf initiation and the shape of the shoot meristem. Can. J. Bot. 56 1545–1550. [Google Scholar]
- Habu, Y., Kakutani, T., and Paszkowski, J. (2001). Epigenetic developmental mechanisms in plants: Molecules and targets of plant epigenetic regulation. Curr. Opin. Genet. Dev. 11 215–220. [DOI] [PubMed] [Google Scholar]
- Hanawa, J. (1961). Experimental studies of leaf dorsiventrality in Sesamum indicum L. Bot. Mag. Tokyo 74 303–309. [Google Scholar]
- Haywood, V., Kragler, F., and Lucas, W. (2002). Plasmodesmata: Pathways for protein and riboprotein signalling. Plant Cell 14 (suppl.), S303–S325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler, M.G.B., Atkinson, A., Bylstra, Y.H., Walsh, R., and Smyth, D.R. (2001). SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development 128 1089–1098. [DOI] [PubMed] [Google Scholar]
- Hernandez, L.F., and Green, P.B. (1993). Transductions for the expression of structural pattern: Analysis in sunflower. Plant Cell 5 1725–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, D., and Hake, S. (1999). Control of phyllotaxy in maize by the abphyl1 gene. Development 126 315–323. [DOI] [PubMed] [Google Scholar]
- Jankovsky, J.P., Smith, L.G., and Nelson, T. (2001). Specification of bundle sheath cell fates during maize leaf development: Roles of lineage and positional information evaluated through analysis of the tangled1 mutant. Development 128 2747–2753. [DOI] [PubMed] [Google Scholar]
- Kagan, M.L., Novoplansky, N., and Sachs, T. (1992). Variable cell lineages form the pea epidermis. Ann. Bot. 69 303–312. [Google Scholar]
- Kaya, H., Shibahara, K.I., Taoka, K.I., Iwabuchi, M., Stillman, B., and Araki, T. (2001). FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104 131–142. [DOI] [PubMed] [Google Scholar]
- Kepinsky, S., and Leyser, O. (2002). Ubiquitination and auxin signalling: A degrading story. Plant Cell 14 (suppl.), S81–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter, R.A., Bollma, K., Taylor, R.A., Bomblies, K., and Poethig, R.S. (2001). KANADI regulates organ polarity in Arabidopsis. Nature 411 706–709. [DOI] [PubMed] [Google Scholar]
- Klucher, K.M., Chow, H., Reiser, L., and Fischer, R.L. (1996). The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell 8 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn, R.W. (1993). Evidence in dicots for stomatal patterning by inhibition. Int. J. Plant Sci. 154 367–377. [Google Scholar]
- Langdale, J.A., Lane, B., Freeling, M., and Nelson, T. (1989). Cell lineage analysis of maize bundle sheath and mesophyll cells. Dev. Biol. 133 128–139. [DOI] [PubMed] [Google Scholar]
- Larkin, J.C., Marks, M.D., Nadeau, J., and Sack, F. (1997). Epidermal cell fate and patterning in leaves. Plant Cell 9 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J.C., Young, N., Prigge, M., and Marks, M.D. (1996). The control of trichome spacing and number in Arabidopsis. Development 122 997–1005. [DOI] [PubMed] [Google Scholar]
- Leyser, H.M.O., and Furner, I.J. (1992). Characterisation of three shoot apical meristem mutants of Arabidopsis thaliana. Development 116 397–403. [Google Scholar]
- Loiseau, J.E. (1969). La Phyllotaxie. (Paris: Masson et Cie).
- Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the SHOOTMERISTEMLESS gene of Arabidopsis. Nature 379 66–69. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J., Bouché-Pillon, S., Jackson, D.P., Nguyen, L., Baker, L., Ding, B., and Hake, S. (1995). Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270 1980–1983. [DOI] [PubMed] [Google Scholar]
- Lyndon, R.F. (1998). The Shoot Apical Meristem—Its Growth and Development. (Cambridge, UK: Cambridge University Press).
- Lynn, K., Fernandez, A., Aida, M., Sedbrook, J., Tasaka, M., Masson, P., and Barton, M.K. (1999). The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development 126 469–481. [DOI] [PubMed] [Google Scholar]
- McConnell, J.R., and Barton, M.K. (1998). Leaf polarity and meristem formation in Arabidopsis. Development 125 2935–2942. [DOI] [PubMed] [Google Scholar]
- McConnell, J.R., Emery, J.F., Eshed, Y., Bao, N., Bowman, J., and Barton, M.K. (2001). Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411 709–713. [DOI] [PubMed] [Google Scholar]
- Nakajima, K., and Benfey, P.N. (2002). Signalling in and out: Control of cell division and differentiation in the shoot and root. Plant Cell 14 (suppl.), S265–S276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser, J.L., Feldman, L.J., and Zambryski, P.C. (2000). Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127 3877–3888. [DOI] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal, M.C., Haughn, G., Saedler, H., and Schwarz-Sommer, Z. (1996). Non-cell-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development 122 3433–3441. [DOI] [PubMed] [Google Scholar]
- Pien, S., Wyrzykowska, J., McQueen-Mason, S., Smart, C., and Fleming, A. (2001). Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc. Natl. Acad. Sci. USA 98 11812–11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig, S., and Sussex, I.M. (1985). The cellular parameters of leaf development in tobacco: A clonal analysis. Planta 165 170–184. [DOI] [PubMed] [Google Scholar]
- Przemeck, G.K.H., Mattsson, J., Hardtke, C.S., Sung, Z.R., and Berleth, T. (1996). Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200 229–237. [DOI] [PubMed] [Google Scholar]
- Reinhardt, D., Mandel, T., and Kuhlemeier, C. (2000). Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Wittwer, F., Mandel, T., and Kuhlemeier, C. (1998). Localized upregulation of a new expansin gene predicts the site of leaf formation in the tomato meristem. Plant Cell 10 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne, P.L., and van der Schoot, C. (1998). Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125 1477–1485. [DOI] [PubMed] [Google Scholar]
- Sachs, T. (1991). Pattern Formation in Plant Tissues. (Cambridge, UK: Cambridge University Press).
- Sawa, S., Watanabe, K., Goto, K., Kanaya, E., Morita, E.H., and Okada, K. (1999). FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 13 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein, J.W. (2000). Constructing a plant cell. The genetic control of root hair development. Plant Physiol. 124 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof, H., Lenhard, M., Haecker, A., Mayer, K., Jürgens, G., and Laux, T. (2000). The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644. [DOI] [PubMed] [Google Scholar]
- Schoute, J.C. (1913). Beiträge zur Blattstellungslehre. Ré. Trav. Bot. Néerl. 10 153–235. [Google Scholar]
- Serna, L., and Fenoll, C. (2000). Stomatal development and patterning in Arabidopsis leaves. Physiol. Plant 109 351–358. [Google Scholar]
- Sessions, A., Nemhauser, J., McCall, A., Roe, J.L., Feldman, K.A., and Zambryski, P.C. (1997). ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124 4481–4491. [DOI] [PubMed] [Google Scholar]
- Sessions, R.A. (1997). Arabidopsis (Brassicaceae) flower development and gynoecium patterning in wild type and ettin mutants. Am. J. Bot. 84 1179–1191. [PubMed] [Google Scholar]
- Sessions, R.A., and Zambryski, P.C. (1995). Arabidopsis gynoecium structure in the wild type and ettin mutants. Development 121 1519–1532. [DOI] [PubMed] [Google Scholar]
- Siegfried, K.R., Eshed, Y., Baum, S., Otsuga, D., Drews, G.N., and Bowman, J.L. (1999). Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126 4117–4128. [DOI] [PubMed] [Google Scholar]
- Smith, L.G., Hake, S., and Sylvester, A.W. (1996). The tangled-1 mutation alters cell division orientations throughout maize leaf development without altering leaf shape. Development 122 481–489. [DOI] [PubMed] [Google Scholar]
- Snow, M., and Snow, R. (1959). The dorsiventrality of leaf primordia. New Phytol. 58 188–207. [Google Scholar]
- Souer, E., van Houwelingen, A., Kloos, D., Mol, J., and Koes, R. (1996). The No Apical Meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85 159–170. [DOI] [PubMed] [Google Scholar]
- Stewart, R.N., and Derman, H. (1975). Flexibility in ontogeny as shown by the contribution of the shoot apical layers to leaves of periclinal chimeras. Am. J. Bot. 62 935–947. [Google Scholar]
- Sussex, I.M. (1955). Experiments on the cause of dorsiventrality in leaves. Nature 174 351–352. [DOI] [PubMed] [Google Scholar]
- Takada, S., Hibara, K., Ishida, T., and Tasaka, M. (2001). The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 128 1127–1135. [DOI] [PubMed] [Google Scholar]
- Vernoux, T., Kronenberger, J., Grandjean, O., Laufs, P., and Traas, J. (2000). PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127 5157–5165. [DOI] [PubMed] [Google Scholar]
- Vincent, C.A., Carpenter, R., and Coen, E.S. (1995). Cell lineage patterns and homeotic gene activity during Arabidopsis flower development. Curr. Biol. 5 1449–1458. [DOI] [PubMed] [Google Scholar]
- Vincent, J.P., and Briscoe, J. (2001). Morphogens. Curr. Biol. 11 852–854. [DOI] [PubMed] [Google Scholar]
- Waites, R., and Hudson, A. (1995). phantastica: A gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121 2143–2153. [Google Scholar]
- Waites, R., Selvadurai, H., Oliver, I.R., and Hudson, A. (1998). The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93 779–789. [DOI] [PubMed] [Google Scholar]
- Wardlaw, C.W. (1949). Further experimental observations on the shoot apex of Dryopteris aristata Druce. Philos. Trans. R. Soc. Lond. B. 233 415–451. [Google Scholar]
- Woodward, F.I., and Kelly, C.K. (1995). The influence of CO2 concentration on stomatal density. New Phytol. 131 311–327. [Google Scholar]
- Yang, M., and Sack, F.D. (1995). The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell 7 2227–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]


