Abstract
Our hypothesis is that oxytocin (OT) causes natriuresis by activation of renal NO synthase that releases NO followed by cGMP that mediates the natriuresis. To test this hypothesis, an inhibitor of NO synthase, l-nitroarginine methyl ester (NAME), was injected into male rats. Blockade of NO release by NAME had no effect on natriuresis induced by atrial natriuretic peptide (ANP). This natriuresis presumably is caused by cGMP because ANP also activates guanylyl cyclase, which synthesizes cGMP from GTP. The 18-fold increase in sodium (Na+) excretion induced by OT (1 μg) was accompanied by an increase in urinary cGMP and preceded by 20 min a 20-fold increase in NO3− excretion. NAME almost completely inhibited OT-induced natriuresis and increased NO3− excretion; however, when the dose of OT was increased 10-fold, a dose that markedly increases plasma ANP concentrations, NAME only partly inhibited the natriuresis. We conclude that the natriuretic action of OT is caused by a dual action: generation of NO leading to increased cGMP and at higher doses release of ANP that also releases cGMP. OT-induced natriuresis is caused mainly by decreased tubular Na+ reabsorption mediated by cGMP. In contrast to ANP that releases cGMP in the renal vessels and the tubules, OT acts on its receptors on NOergic cells demonstrated in the macula densa and proximal tubules to release cGMP that closes Na+ channels. Both ANP- and OT-induced kaliuresis also appear to be mediated by cGMP. We conclude that cGMP mediates natriuresis and kaliuresis induced by both ANP and OT.
Keywords: vasopressin, l-nitroarginine methyl ester, nitric oxide, NO synthase, NO synthase immunocytochemistry
Atrial natriuretic peptide (ANP) and oxytocin (OT) are natriuretic hormones that play a fundamental role in the regulation of extracellular fluid volume. The natriuretic action of ANP has been explained by its combination with ANPA receptors on kidney cells that convert GTP into cGMP by activating particulate guanylate cyclase (GC). This form of GC (GCA) is the cell surface receptor for ANP (1). In contrast, OT is a potent natriuretic peptide and OT receptors occur in the kidney, but the mechanism of OT-induced natriuresis is not clearly understood (2–7).
The release of ANP that follows blood volume expansion is partly mediated by renal and arterial baroreceptor input to the brain stem that stimulates OT release from the neurohypophysis. Circulating OT binds to its receptors in the right atrium and stimulates ANP release from atrial myocytes (8, 9). Because the injection of OT evoked concomitant release of ANP and natriuresis (10), the natriuretic action of OT might be mediated by the release of ANP that activates renal GCA receptors localized in glomeruli, their afferent and efferent arterioles, and the tubules (11). ANP selectively dilates preglomerular vessels and constricts efferent arterioles, thereby increasing the filtration fraction (FF). If the glomerular filtration rate (GFR) and tubular reabsorption of sodium (Na+) remain constant, this increase in FF would provide an increased filtered load (FL) of Na+, resulting in natriuresis (12).
In addition to particulate GC, which is a target for ANP, the kidney also contains soluble GC, which is a target for NO. NO is generated from l-arginine by NO synthase (NOS). NO activates soluble GC that synthesizes cGMP, which mediates many of the physiologic actions of NO (13). Soluble GC is expressed largely in the glomerulus, proximal convoluted and straight tubules, and cortical collecting ducts (14). OT receptors have been demonstrated in the macula densa and thin loop of Henle of the rat kidney (15), suggesting a role for OT in the regulation of tubuloglomerular feedback and water and solute transport. This finding led us to the hypothesis that OT also might induce natriuresis by activation of NOS that already has been shown to be localized to macula densa cells (16, 17). To test this hypothesis, we studied the effect of an inhibitor of NOS, l-nitroarginine methyl ester (NAME) (13), on the natriuresis evoked by ANP and OT. Our results, which include the immunolocalization of NOS isoforms in the kidney, support the hypothesis that cGMP mediates the natriuresis induced by both peptides.
METHODS
Animals.
Male Wistar rats (200–250 g) were housed at controlled temperature (23 ± 2°C) and exposed to a daily 12-h light-dark cycle (lights on 07:00 to 19:00 h) with free access to tap water and a pellet diet. They were handled and given a sham gastric gavage daily for 7 days before experiments to decrease emotional stress during the experiments.
Studies in Conscious Rats.
Twenty-four hours before the experiments, animals were anesthetized with 2,2,2-tribromoethanol (Aldrich), and a polyethylene catheter was implanted into the femoral artery for direct blood pressure (BP) recording (19) or into the external jugular vein and advanced to the atrium for blood sample collection or i.v. drug administration (18). After surgery, animals were injected s.c. with 100,000 units of benzyl-penicillin.
The mean arterial BP of unanesthetized freely moving rats was recorded by using a Narco polygraph (Narcotrace 80, Houston) connected to a pressure transducer (Narco model P-1000B, Houston). A paper speed of 0.5 mm/s was used to minimize measurable BP fluctuations. Heart rate was measured at a paper recording speed of 5–10 mm/s.
A water load [5% of body weight (bw), 37°C] was administered by gavage followed by a second identical water load 1 h later. Urine samples were collected at 20-min intervals as described (10). After collection of the first urine sample, test solutions, ANP or OT [1 or 5 μg/rat, respectively, in 200 μl of 150 mM NaCl (saline)] or saline were injected i.v. Thereafter, six urine samples were collected at 20-min intervals. An inhibitor of NOS (NAME, 10 mg/kg, i.v., 200 μl) was injected 30 min before injection of ANP, OT, or saline.
Studies in Anesthetized Rats.
GFR and renal plasma flow were measured by inulin (20) and para-aminohippurate clearances (21), respectively in rats anesthetized with pentobarbital sodium (50 mg/kg bw, i.p.) as described (22). The drugs were infused in saline at a rate of 2.8 ml/h. After a period of stabilization (30 min), urine was collected for the control period of at least 1 hr, and OT (5 μg), ANP (5 μg), or NAME (10 mg/kg) diluted in 500 μl of saline then were administered i.p. Urine and blood were sampled at 30-min intervals.
Na+ and potassium (K+) in plasma and urine were measured by flame photometry (Micronal, model B 262, São Paulo, Brazil), and urine osmolality was determined by freezing point depression (Fiske OS Osmometer, Norwood, MS). Inulin and para-aminohippurate in plasma and urine samples were measured by the methods of Fuehr et al. (20) and Smith et al. (21), respectively.
Urinary nitrate excretion (NO3−, a metabolite of NO) was determined by colorimetric assay with a nitrate/nitrite assay kit (no. 780001, Cayman Chemicals, Ann Arbor, MI). Urinary cGMP excretion was determined by the method of J.G. (unpublished work).
To determine the effect of an arginine vasopressin V2-receptor blocker on OT-induced antidiuresis and natriuresis, the rats were injected i.v. with [adamantaneacetyl1, O-Et-d-Tyr2, Val4, Abu6, Arg8,9]vasopressin, a selective V2-receptor blocker (5 μmg/kg bw), 30 min before 1 μg of OT or saline injection (23).
NOS Immunohistochemistry.
The expression of neuronal NOS (nNOS) and endothelial NOS (eNOS) was detected by using affinity purified antibodies: a polyclonal rabbit anti-nNOS, and a monoclonal anti-eNOS (clone 3), diluted 50- and 100-fold, respectively, both from Transduction Laboratories (Lexington, KY). Antibodies were diluted in blocking buffer (25). Tissue for light microscopy and immunoperoxidase staining was fixed in methyl Carnoy’s solution (24). Fixed tissues were paraffin-embedded, and 5-μm sections were cut and mounted on gelatin-coated microscope slides.
Enhancement of NOS immunoreactivity was obtained by a modification of the procedure of Zanardo et al. (24). Sections were boiled in 50 mM Tris⋅HCl buffer, pH 9.5, in a microwave oven (Sharp) for 15 min at 900 W. The nNOS antibody was detected with a biotinylated swine anti-rabbit IgG (Dako) diluted 400-fold. The eNOS antibody was detected with a biotinylated rabbit anti-mouse IgG (Dako) diluted 500-fold. Biotinylated antibodies were detected with the ABC technique (Elite ABC kit, Vector Laboratories), using diaminobenzidine (Pierce) as chromogen. Blocking buffer substituted for primary antibodies in the negative controls. In control experiments, kidney sections adjacent to those microwave-treated were not boiled, but were otherwise identically processed for immunohistochemistry.
Statistical Analysis.
The significance of differences among multiple groups was determined by ANOVA with repeated measures and significance between groups determined by the Newman–Keuls multiple comparison test. Student’s t test was used to determine significance of differences between two groups.
RESULTS
Effect of ANP on Urine Flow, Electrolyte Excretion, and Urine Osmolality in Conscious Rats.
Diuresis. In the saline-injected rats, the rate of urine flow increased from a basal value (35.7 ± 2.3 μl/min per 100 g bw) to a peak (52.4 ± 6.6 μl/min per 100 g bw) at 20 min, then declined to a minimum (24 ± 5.0 μl/min per 100 g bw) at 100 min. This pattern was not altered significantly by NAME pretreatment. In the ANP-treated rats the urine flow was significantly increased (P < 0.05) only at 20 min (87.0 ± 10.0 μl/min per 100 g bw), and this increase was slightly, but significantly, inhibited by NAME pretreatment (66.0 ± 5.0 μl/min per 100 g bw).
Natriuresis.
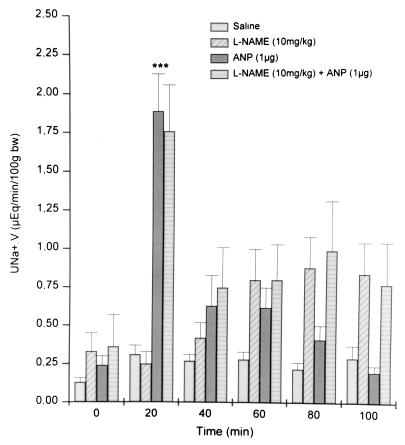
Na+ excretion in the saline-injected rats increased slightly from a basal value to 0.31 ± 0.06 μEq/min per 100 g bw at 20 min and remained on this plateau until 100 min (Fig. 1). Thirty minutes after injection of NAME (time 0) Na+ excretion was minimal but significantly greater than that in control rats. After the 20-min period, Na+ excretion rose linearly to the highest value at 80 min, nearly 2.5 times the initial value. ANP evoked a rapid and significant 14.8-fold increase (P < 0.01) in natriuresis at 20 min that then returned to a value not statistically different from that observed in saline-injected rats. ANP produced no significant natriuresis for the remainder of the experimental period. This dramatic, transient natriuresis induced by ANP, observed only at 20 min after ANP injection, was not affected by pretreatment with NAME. Indeed, there was no difference between values of rats treated with ANP alone and those injected with NAME + ANP except at 80–100 min at which time natriuresis in rats injected only with NAME peaked and was 2- to 4-fold greater than that of rats injected with NAME + ANP. This effect probably was caused by the prior natriuresis in ANP-injected rats.
Figure 1.
Sodium excretion (UNa+.V) before and after i.v. injection of saline, ANP, NAME, and NAME + ANP (n = 10–15 for each group). Values are mean ± SEM; ∗∗∗, P < 0.001 versus saline.
Kaliuresis.
Kaliuresis was unchanged in saline-injected rats, but NAME more than doubled kaliuresis throughout the experiment. ANP (1 μg) evoked a significant (P < 0.01) 2-fold increase in K+ excretion, but only in the initial 20-min collection period. Beginning at 40 min and continuing until the end of the experiment, kaliuresis in NAME + ANP-injected rats was similar to that in rats injected with NAME alone, and significantly greater than that in ANP-injected animals, so that over the course of the experiment NAME- or NAME + ANP-injected rats excreted significantly more K+ than those injected with either saline or ANP (data not shown).
Effect of OT on Urine Flow and Electrolyte Excretion.
Diuresis. OT (1 μg) induced a significant decrease in urine flow at 20 min that reached a minimum (P < 0.01) at 40 min, 40% of the flow at 0 min, and then returned toward the initial level (Fig. 2). The antidiuretic effect of OT at 40 min still occurred in the NAME-pretreated rats (data not shown).
Figure 2.
Diuresis before and after i.v. injection of saline, AVP V2-receptor blocker (5 μg/kg bw), OT (1 μg/rat), and AVP V2-receptor blocker + OT (n = 10). Values are mean ± SEM; ∗∗, P < 0.01 versus saline; +++, P < 0.01 versus other treatment group.
Natriuresis.
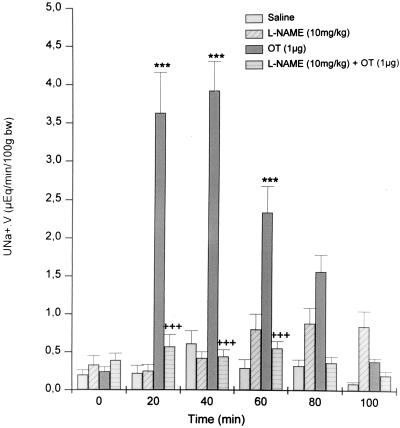
Except for a transient, significant increase at 40 min, Na+ excretion in the saline-injected rats did not change during the entire experimental period (Fig. 3). Pretreatment with NAME induced a similar increase in Na+ excretion at 40 min as occurred in the saline-injected rats, but Na+ excretion increased significantly further at 60 min and remained at this level, significantly greater than that of the controls for the rest of the experiment. OT (1 μg) evoked a rapid, significant 15-to 18-fold increase in natriuresis at 20–40 min, respectively, that rapidly declined but remained significantly elevated in comparison with values observed in saline-injected rats for the remainder of the experiment (P < 0.001). The OT-induced natriuresis was almost completely inhibited (P < 0.001) by NAME pretreatment.
Figure 3.
Sodium excretion before and after i.v. injection of saline, OT, NAME, and NAME + OT (n = 10–15). Values are mean ± SEM; ∗∗∗, P < 0.001 versus saline; +++, P < 0.01 versus other treatment group.
Kaliuresis.
As with injection of ANP, kaliuresis increased slightly 20 min after injection of saline and then declined linearly to reach values below the starting excretion at the end of the experiment (data not shown). As before NAME increased kaliuresis significantly throughout the experiment. OT evoked significant kaliuresis that was indistinguishable from that induced by NAME but was no longer significant by 100 min. Kaliuresis in rats injected with NAME + OT was indistinguishable from that induced by either NAME or OT alone except at 100 min at which time OT induced significantly less K+ excretion than either NAME or NAME + OT but still significantly more than saline-injected rats.
Urinary cGMP and nitrate excretion.
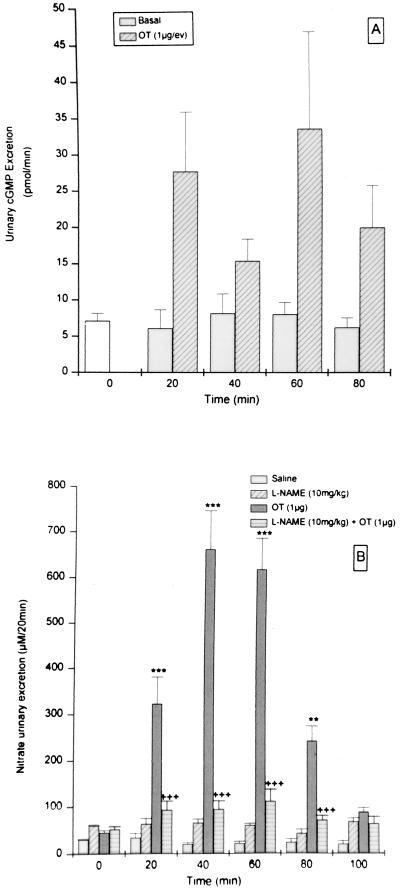
After injection of OT, urinary cGMP excretion was significantly increased (Fig. 4A) with the same time course as that of Na+ excretion (Fig. 2). Urinary excretion rates of NO3− were low in control urine samples and did not change during the experiment (Fig. 4B). NO3− excretion was increased significantly by pretreatment with NAME on comparison to control rats at time zero, 30 min after NAME injection, and remained elevated throughout the experiment. OT induced a dramatic 10-fold increase (P < 0.001) in NO3− excretion at 20 min that peaked at 40–60 min with a 22-fold increase. This increase in urinary NO3− excretion, induced by OT, observed at 20–80 min, was almost completely blocked by pretreatment with NAME (P < 0.001) (Fig. 4B). A small, but significant, elevation remained. In this same group of rats, the increasing OT-induced natriuresis preceded by 20 min the increasing NO3− excretion (Fig. 3).
Figure 4.
cGMP (A) and nitrate (B) excretion before and after i.v. injection of saline, OT (1 μg), NAME (10 mg/kg), and NAME + OT (B) (n = 8). Values are mean ± SEM; ∗∗, P < 0.01 and ∗∗∗, P < 0.001 versus saline; +++, P < 0.01 versus other treatment group.
Effects of AVP-V2 blocker on OT-induced antidiuresis and natriuresis.
The OT-induced antidiuretic effect was completely reversed by pretreatment with the V2-receptor blocker (5 μg/kg bw) (Fig. 2), but OT-induced natriuresis was only slightly decreased (data not shown).
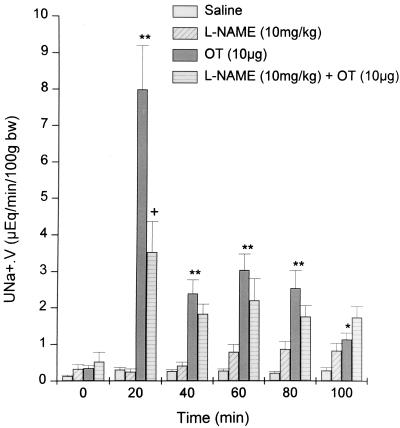
The effect of a 10-fold higher dose of OT (10 μg) on natriuresis.
This higher dose of OT produced a sharper, higher peak of Na+ excretion (30-fold) (Fig. 5) than that obtained with 1 μg (Fig. 3). In contrast to the nearly complete blockade of the natriuresis evoked by the lower dose of OT, NAME only partially blocked the natriuresis induced by this higher dose. As in the previous experiment, NAME alone induced natriuresis.
Figure 5.
Sodium excretion (UNa+.V) before and after i.v. injection of saline, OT (10 μg), NAME (10 mg/kg), and NAME + OT (n = 11–15). Values are mean ± SEM; ∗, P < 0.05 and ∗∗, P < 0.01 versus saline; +, P < 0.05 versus other treatment group.
Arterial BP.
Injection of saline, OT (5 μg), or ANP (5 μg) had no effect on mean arterial BP. NAME (10 mg/kg) increased BP significantly at 5 min, and it remained high for 60 min after injection. Significant bradycardia was observed concurrent to the BP increase induced by NAME (data not shown).
Studies in Anesthetized Rats.
Effects of ANP, OT, and NAME. OT and NAME had no significant effect on GFR (Table 1). However, there was a small, but significant, increase in GFR induced by ANP and NAME + ANP. The largest GFR occurred in rats injected with NAME + OT that was greater (P < 0.05) than in rats receiving NAME only (Table 1). None of the treatments altered renal plasma flow (data not shown). The percentage of FF was more than doubled in rats pretreated with NAME (P < 0.001). After injection of either ANP or OT in NAME-injected rats, there was a greater, highly significant increase in the %FF (P < 0.001).
Table 1.
Effect of ANP (5 μg), OT (5 μg), l-NAME (10 mg/kg), l-NAME + ANP, l-NAME + OT on GFR, plasma Na+, Na+ FL, urinary Na+ excretion (UNa+.V), Na+ reabsortion, and Na+ fractional reabsortion (%)
| Treatment | GFR, ml/min | PNa+, μEq/ml | Na+ F.load, μEq/min | UNa+.V, μEq/min | Na+ Reabs., μEq/min | Na+ F.Reabs., % |
|---|---|---|---|---|---|---|
| Saline (28) | 0.84 ± 0.05 | 142.7 ± 1.24 | 123.8 ± 7.8 | 1.2 ± 0.2 | 122.6 ± 7.5 | 0.99 ± 0.001 |
| l-NAME (15) | 0.96 ± 0.06 | 145.9 ± 1.09 | 139.9 ± 9.0 | 3.0 ± 0.7∗∗ | 136.9 ± 9.3 | 0.98 ± 0.01∗∗ |
| ANP (8) | 1.10 ± 0.14∗ | 142.5 ± 2.99 | 163.5 ± 27.8 | 6.7 ± 1.8∗∗∗ | 156.8 ± 27.2 | 0.95 ± 0.02∗∗∗ |
| OT (6) | 0.87 ± 0.08 | 142.2 ± 3.64 | 123.8 ± 12.1 | 11.3 ± 1.2∗∗∗ | 112.5 ± 11.5 | 0.91 ± 0.01∗∗∗ |
| l-NAME + ANP (8) | 1.08 ± 0.09∗ | 145.3 ± 2.18 | 157.1 ± 13.4 | 10.7 ± 1.7∗∗∗+ | 145.9 ± 12.9 | 0.93 ± 0.01∗∗∗ |
| l-NAME + OT (8) | 1.23 ± 0.10∗∗∗+ | 146.9 ± 1.16 | 169.5 ± 18.7∗ | 3.9 ± 0.4∗∗∗+++ | 165.3 ± 19.2∗+ | 0.97 ± 0.01∗∗∗+++ |
Values are mean ± SEM for the final 30 min of each period. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 versus saline. +, P < 0.05; +++, P < 0.001 versus ANP or OT by Student’s t test.
The rates of urine flow and sodium (Na+) excretion in rats infused with saline remained stable during the entire experimental period (Table 1). NAME produced a significant increase in urinary volume and Na+ excretion. Intravenous injection of ANP (5 μg) or OT (5 μg) caused a significant increase in urine flow (P < 0.01) and also a significant large increase in Na+ excretion (P < 0.001). The natriuretic and diuretic effect of ANP was additive with that of NAME, whereas the OT-induced natriuresis was drastically inhibited (P < 0.001).
Tubular reabsorption of Na+ was calculated by subtracting Na+ excretion from the FL of Na+. The FL was increased significantly above that of saline-infused animals only in the case of animals injected with NAME, NAME + ANP, or OT. This increase was accounted for by the increase in GFR in those animals.
Na+ excretion was increased by all treatments with the smallest increase occurring with l-NAME followed by a larger increase with ANP and the largest increase obtained with OT. The increase obtained with ANP could be added to the increase obtained with NAME to produce the excretion obtained by combined action of NAME and ANP, whereas the Na+ excretion in animals injected with NAME + OT returned to the excretion in animals injected with NAME alone. The total tubular reabsorption of Na+ was unaffected except with the combination of NAME + ANP or OT. This increase in tubular reabsorption was accounted for by the dramatic increase in FL. Thus, none of the treatments actually reduced the absolute reabsorption of Na+. However, when the fractional reabsorption was calculated by dividing the reabsorption by the GFR, it was clear that all of the treatments significantly decreased the fractional reabsorption of Na+. NAME alone produced a highly significant decrease; much larger, significant decreases occurred with ANP, OT, and NAME + ANP that did not modify the reduction in fractional reabsorption obtained with ANP. In contrast, the fractional reabsorption in NAME + OT-injected rats was drastically and highly significantly increased to a level obtained with NAME alone.
To determine the effect of the various treatments on K+ reabsorption, we calculated the FL of K+ and determined that l-NAME itself increased the FL above that of saline-injected animals (P < 0.05) (Table 2). ANP had no effect on the FL of K+ except when injected together with NAME. In this case, as in the case of NAME alone, the FL was increased; however, it was not significantly greater than that obtained with NAME alone. Because of a significant decrease in plasma [K+], the FL of K+ in OT-injected animals was significantly reduced below that of saline-injected controls. This reduction in FL was eliminated in animals treated with both NAME and OT, because of the increased GFR and the significantly increased plasma [K+]. Therefore, it returned to the level obtained in animals injected with NAME alone. Injection of NAME had no significant kaliuretic effect. However, kaliuresis of approximately equal magnitude, roughly 20% above saline controls, was induced by ANP, OT, and the combination of NAME with ANP or OT. The kaliuresis obtained with both ANP and OT was not significantly altered by NAME. K+ reabsorption was not affected by either NAME or ANP, but was dramatically reduced by OT, resulting in a net secretion of K+. The reabsorption obtained with ANP was not modified by NAME; however, the secretion obtained with OT was reversed and the reabsorption was now equivalent to that obtained in the other groups.
Table 2.
Effect of ANP (5 μg), OT (5 μg), l-NAME (10 mg/kg), l-NAME + ANP, l-NAME + OT, or saline on GFR, plasma K+, K+ FL, urinary K+ excretion, K+ reabsortion, and K+ fractional reabsortion (%)
| Treatment | GFR, ml/min | PK+, μEq/ml | F.load K+, μEq/min | UK+.V, μEq/min | K+ Reabs., μEq/min | K+ F.reabs., % |
|---|---|---|---|---|---|---|
| Saline (28) | 0.84 ± 0.05 | 4.17 ± 0.22 | 3.49 ± 0.25 | 1.74 ± 0.13 | 1.75 ± 0.21 | 0.50 ± 0.03 |
| l-NAME (15) | 0.96 ± 0.06 | 4.73 ± 0.33 | 4.56 ± 0.46∗ | 1.94 ± 0.16 | 2.61 ± 0.48 | 0.54 ± 0.05 |
| ANP (8) | 1.10 ± 0.14∗ | 5.03 ± 0.63 | 4.50 ± 0.58 | 2.32 ± 0.21∗ | 2.09 ± 0.56 | 0.42 ± 0.08 |
| OT (6) | 0.87 ± 0.08 | 2.93 ± 0.23∗ | 2.54 ± 0.31 | 2.74 ± 0.38∗∗ | −0.20 ± 0.27∗∗∗ | −0.09 ± 0.11∗∗∗++ |
| l-NAME + ANP (8) | 1.08 ± 0.09∗ | 4.88 ± 0.57 | 5.27 ± 0.78∗∗ | 2.49 ± 0.53∗ | 2.61 ± 0.47 | 0.49 ± 0.06 |
| l-NAME + OT (8) | 1.23 ± 0.10∗∗∗+ | 4.56 ± 0.47+ | 5.29 ± 0.67∗∗++ | 2.36 ± 0.33∗ | 2.74 ± 0.74++ | 0.48 ± 0.09 |
Values are mean ± SEM for the final 30 min of each period. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 versus saline. +, P < 0.05; ++, P < 0.01; versus ANP or OT by Student’s t test.
When the fractional reabsorption was calculated, there was no effect from any of the treatments except OT and the fractional reabsorption of K+ remained approximately 50%, which dramatically contrasted with the 99% reabsorption of Na+. Only OT produced a drastic reduction and a reversal of the fractional K+ reabsorption, which now became a slight fractional K+ secretion that was not statistically significant. The change in reabsorption was highly significant versus either saline-injected controls or the animals treated with NAME + OT.
nNOS and eNOS immunoreactive-like isoforms.
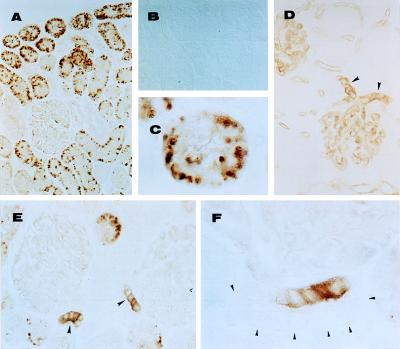
A very intense staining for nNOS was observed in the cytoplasm of cells of the macula densa (Fig. 6 E and F) but not in the remainder of the distal tubules. Staining also was pronounced in the proximal tubules of the renal cortex (Fig. 6 A and C). Glomerular capillaries and blood vessels in the kidney (Fig. 6D) expressed eNOS. NO staining was not detected when primary antibodies were omitted (Fig. 6B). Both types of NOS were detected with or without microwave antigen enhancement; however, the staining was more intense after microwave treatment in alkaline solution. Our data on the immunolocalization of eNOS and nNOS, using paraffin-embedded tissue, are in good agreement with those obtained using frozen tissue sections (16, 17) except for the prominent localization of nNOS in cortical proximal tubules shown here.
Figure 6.
Immunolocalization of nNOS (A–C, E, and F) and eNOS (D). (B) An adjacent section to that shown in A, in which nNOS antibody was omitted (control). (C) Cross section of a cortical tubule stained for nNOS. (D) Arrowheads indicate afferent and efferent glomerular arterioles immunostained for eNOS. (E) Arrowheads indicate macula densa stained for nNOS. (F) Arrowheads indicate nonstained cells in the same tubule that exhibits macula densa cells strongly stained for nNOS. [Magnifications: 33-fold (A and B); 132-fold (D and E), and 333-fold (C and F); Nomarski optics.]
DISCUSSION
Our results indicate that the inhibition of NOS by NAME produced significant pharmacological effects in conscious rats, among them an increase in BP, associated with bradycardia, and significant urinary changes (a small increase in Na+ and K+ excretion). These urinary changes were accompanied by an increase in FF and a decrease in fractional Na+ reabsorption.
NAME administration by decreasing NO formation that relaxes vascular smooth muscle via cGMP leads to a decrease in NO vasodilator tone, with resultant increase in BP that activates baroreceptors that induce compensatory bradycardia by activating the parasympathetic and inhibiting the sympathetic nervous system. The elevated BP also can cause a reflex release of vasopressin that may account for the increased urine osmolality observed (data not shown). The significant increase in NO3− excretion in NAME-treated rats is puzzling. Probably the NAME-induced hypertension reflexively activated parasympathetic axons both in the heart and in the vascular endothelium to release NO that was metabolized to NO3−. This explanation presupposes that the dose of NAME used only incompletely inhibited NOS.
The increased Na+ excretion induced by NAME may be caused in part by inhibition of eNOS leading to reduced release of NO in the renal vascular system, associated with differential constriction of the efferent and afferent renal arterioles, thereby raising intraglomerular pressure that increases FF. However, the doubling of the FF doubled the FL of Na+, producing a small natriuresis resultant from a decreased fractional tubular Na+ reabsorption in the face of an increase in the total Na+ reabsorbed. The results with OT suggest that NO and consequent cGMP release reduced fractional tubular Na+ reabsorption. Therefore, this effect of NAME to do the same appears to be inconsistent with the other results. However, we found that NAME increased the nitrate excretion into the urine, suggesting that there was a compensatory increase in NO production and consequent release of cGMP that accounted for the decreased fractional reabsorption of Na+ in the presence of NAME.
Our results support the hypothesis that the natriuretic effect of OT is caused by production of NO and consequent release of cGMP that activates protein kinase G. Indeed, the potent natriuretic action of OT almost certainly was caused by activation of its receptors on NOergic cells in the proximal tubules and macula densa resulting in reduced fractional Na+ reabsorption, because blockade of NO production by NAME blocked the natriuresis in the face of a very large increase in FL of Na+. The striking inhibitory effect of NAME on OT-induced NO3− excretion, taken together with OT-induced excretion of cGMP, corroborates the above interpretation. These in vivo studies are in agreement with those in vitro relating NO production and cGMP (26–28).
The drastic increase in urinary NO3− excretion of the same magnitude as the increase in Na+ excretion after OT injection plus the blockade of OT-induced Na+ and NO3− excretion by NAME strongly support the hypothesis that the natriuresis induced by OT is caused by NO. The increased NO3− excretion evoked by OT followed the increased Na+ excretion by 20 min. The delay may be accounted for by two factors: the time required for NO to be metabolized to NO3− and the time required for the NO3− to be secreted into the tubules. If NO was free in the extracellular fluid of the tubular cells, it would nearly instantaneously diffuse into the tubules. Therefore, we hypothesize that it is present as a compound that releases NO. Although cGMP excretion increased in the urine after OT in parallel with Na+, the increase was much less than that of either Na+ or NO3−. We speculate that this result is related to breakdown of cGMP by phosphodiesterase, plus possible delays in secretion of cGMP into the tubules.
Because the natriuresis induced by the 10-fold higher dose of OT (10 μg) was only partially blocked by NAME and this dose produced a much greater increase in plasma ANP than the lower dose (10), we hypothesize that the residual natriuresis in NAME-injected rats was caused by ANP as postulated earlier (10) and discussed in the Introduction.
Our data also show that OT-induced natriuresis was not blocked by the selective V2 receptor antagonist that inhibited OT-induced antidiuresis. The antidiuretic effect of NAME probably was related to reflex activation of vasopressin secretion caused by its elevation of BP, whereas that of OT may have been caused by activation of renal V2 receptors.
Our results also have shown no significant effect of NAME on the natriuretic action of ANP. This finding can be explained by the accepted concept that the ANP-induced natriuresis is caused by its activation of the ANPA-receptor, the particulate GCA, with generation of cGMP.
The natriuretic action of bradykinin also is caused by its release of NO followed by generation of cGMP (29). Indeed, it appears that cGMP is the mediator of natriuresis, whereas overwhelming evidence implicates cAMP in the induction of antidiuresis. Since Stoos et al. (30) have shown that NO inhibited Na+ transport in cultured cortical collecting duct cells associated with increased cGMP content, the mechanism of the natriuretic action of cGMP is thought to be via closure of Na+ channels, whereas the mechanism of the antidiuretic action of cAMP is via opening of CHIP28 water channels (31).
NAME augmented the kaliuretic effect of ANP possibly by increasing the FL of K+, but there was no difference between the kaliuresis in NAME-treated versus NAME + ANP-treated animals, indicating that the kaliuretic effect of ANP also may be caused by cGMP.
Although OT induced a highly significant kaliuretic effect, it was associated with a complete abolition of reabsorption of K+ and indeed a small secretion of the ion. It is established that K+ is largely reabsorbed in the proximal tubules and then is secreted in the distal tubules. Therefore, it would appear that OT partially blocked the proximal tubular reabsorption of K+, and that this blockade was accompanied by a small secretion from the distal tubules. Although the kaliuretic effect of OT was not affected by treatment with NAME, the reabsorption of K+, both in absolute terms and as fractional reabsorption, was drastically increased, such that the total reabsorption was even slightly greater (P, not significant) than in the saline-injected control animals, but was highly significantly greater (P < 0.001) than in the presence of OT alone. Furthermore, the fractional K+ reabsorption returned to levels of the saline-injected controls. Therefore, we conclude that as in the case of Na+, NO via cGMP controls K+ reabsorption and does so by closing K+ channels. A small kaliuretic effect of ANP was not affected by NAME because cGMP is released by ANP’s activation of particulate GC.
A very surprising finding was the significant lowering of plasma [K+] in the animals injected with OT. This decreased plasma [K+] significantly lowered the FL of K+ and may have played a role in the removal of all of the K+ by reabsorption in the proximal tubule. This lowering of [K+] was abolished by NAME, resulting in a highly significant increase in the FL of K+, which complicates the interpretation that the OT inhibition of K+ reabsorption was solely related to NO.
Because the urinary excretion of K+ was not different in OT versus OT + NAME-treated animals, and yet the plasma [K+] was normalized by NAME, it is hard to attribute the decline in plasma [K+] in the animals treated with OT to the renal excretion of K+. Indeed, the results suggest that OT may reduce release of K+ from intracellular stores and that this reduction may be a function of NO and cGMP because the effect was reversed in the NAME + OT-injected rats, even though there was no significant change in K+ output. Further studies are needed to clarify these last points. In any event, the results support a kaliuretic effect of both OT and ANP mediated by cGMP closure of K+ channels analogous to the effect of the cyclic nucleotide on Na+ channels.
Acknowledgments
We thank Mrs. Marina Holanda, Maria Valci, Cleonice G.A da Silva, and Mr. Hildeberto Caldo for the skillful technical assistance and Judy Scott for excellent secretarial expertise. This work was supported by Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo, Programa de Apoio aos Nucleos de Excelência (PRONEX), Federação de Agricultura do Estado do Pará (FAEPA), and Conselho Nacional de Pesquisas (Brazil) to J.A.-R., A.L.V.F., and A.R.M.; and by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK43900 and National Institute of Mental Health Grant MH51853 to S.M.M.
ABBREVIATIONS
- OT
oxytocin
- NOS
NO synthase
- NAME
nitroarginine methyl ester
- ANP
atrial natriuretic peptide
- GC
guanylate cyclase
- FF
filtration fraction
- GFR
glomerular filtration rate
- FL
filtered load
- nNOS
neuronal NOS
- eNOS
endothelial NOS
- bw
body weight
- BP
blood pressure
References
- 1.Ruskoaho H. Pharmacol Rev. 1992;44:479–602. [PubMed] [Google Scholar]
- 2.Buijs R M. Pharmacol Ther. 1983;22:127–141. doi: 10.1016/0163-7258(83)90056-6. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn R E, Samson W K, Fulton R J, Stricker E M, Verbalis J G. Proc Natl Acad Sci USA. 1993;90:10380–10384. doi: 10.1073/pnas.90.21.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn R E, Stricker E M, Verbalis J G. Neuroendocrinology. 1992;56:255–263. doi: 10.1159/000126236. [DOI] [PubMed] [Google Scholar]
- 5.Chan W Y. J Pharmacol Exp Ther. 1988;246:603–609. [PubMed] [Google Scholar]
- 6.Gardner D G, Vlasuk G P, Baxter J D, Fiddes J C, Lewicki J A. Proc Natl Acad Sci USA. 1987;84:2175–2179. doi: 10.1073/pnas.84.8.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue T, Naruse M, Nakayama M, Kurokawa K, Sato T. Am J Physiol. 1993;265:F487–F503. doi: 10.1152/ajprenal.1993.265.4.F487. [DOI] [PubMed] [Google Scholar]
- 8.McCann S M, Franci C R, Gutkowska J, Favaretto A L V, Antunes-Rodrigues J. Proc Soc Exp Biol Med. 1996;213:117–127. doi: 10.3181/00379727-213-44044. [DOI] [PubMed] [Google Scholar]
- 9.Favaretto A L V, Ballejo G O, Albuquerque-Araujo W I C, Gutkowska J, Antunes-Rodrigues J, McCann S M. Peptides. 1997;18:1377–1381. doi: 10.1016/s0196-9781(97)00209-x. [DOI] [PubMed] [Google Scholar]
- 10.Haanwinckel M A, Elias L K, Favaretto A L V, Gutkowska J, McCann S M, Antunes-Rodrigues J. Proc Natl Acad Sci USA. 1995;92:7902–7906. doi: 10.1073/pnas.92.17.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritter D, Dean A D, Gluck S L, Greenwald J E. Kidney Int. 1995;48:1758–1766. doi: 10.1038/ki.1995.474. [DOI] [PubMed] [Google Scholar]
- 12.Endlich K, Steinhausen M. Kidney Int. 1997;52:202–207. doi: 10.1038/ki.1997.320. [DOI] [PubMed] [Google Scholar]
- 13.McDonald L J, Murad F. Adv Pharmacol. 1995;34:263–275. doi: 10.1016/s1054-3589(08)61091-1. [DOI] [PubMed] [Google Scholar]
- 14.Terada Y, Tomita K, Nonoguchi H, Marumo F. J Clin Invest. 1992;90:659–665. doi: 10.1172/JCI115908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arpin-Bott M T, Waltisperger E, Freund-Mercier M J, Stoeckel M E. J Endocrinol. 1997;153:49–59. doi: 10.1677/joe.0.1530049. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox C S, Welch W J, Murad F, Gross S S, Taylor G, Levi R, Schmidt H H. Proc Natl Acad Sci USA. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachmann S, Bosse H M, Mundel P. Am J Physiol. 1995;268:F885–F898. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- 18.Krieger E M. Circ Res. 1964;15:511–521. doi: 10.1161/01.res.15.6.511. [DOI] [PubMed] [Google Scholar]
- 19.Harms P, Ojeda S R. J Appl Physiol. 1974;36:391–393. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- 20.Fuehr Y, Kaczmarczk Y, Kruttgen Y. Klin Wochenschr. 1955;33:729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- 21.Smith H W, Finkelstein N, Aliminosa L, Crawford B, Graber M. J Clin Invest. 1945;24:388–390. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitas A S M, Coimbra T M, Costa R S, Baroni E A. Nephron. 1998;78:302–309. doi: 10.1159/000044940. [DOI] [PubMed] [Google Scholar]
- 23.Manning M B, Lammek B, Kolodziejczyk A M. J Med Chem. 1981;24:701–706. doi: 10.1021/jm00138a012. [DOI] [PubMed] [Google Scholar]
- 24.Zanardo R C O, Costa E, Ferreira H H A, Antunes E, Martins A R, Murad F, De Nucci G. Proc Natl Acad Sci USA. 1997;94:14111–14114. doi: 10.1073/pnas.94.25.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson R J, Garcia R L, Pritzl P, Alpers C E. Am J Pathol. 1990;136:369–374. [PMC free article] [PubMed] [Google Scholar]
- 26.Roczniak A, Burns K D. Am J Physiol. 1996;279:F106–F115. doi: 10.1152/ajprenal.1996.270.1.F106. [DOI] [PubMed] [Google Scholar]
- 27.Ito S, Ren Y-L. J Clin Invest. 1993;92:1093–1098. doi: 10.1172/JCI116615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conrad K P, Gellai M, North W G, Valtin H. Ann NY Acad Sci. 1993;689:346–362. doi: 10.1111/j.1749-6632.1993.tb55559.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimamoto K, Ura N, Iimura O. Brazilian J Med Biol Res. 1994;27:1965–1973. [PubMed] [Google Scholar]
- 30.Stoos B A, Carretero O A, Farhy R D, Scieli G, Garvin J L. J Clin Invest. 1992;89:761–765. doi: 10.1172/JCI115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen S, Smith B L, Christensen E I, Knepper M, Agre P. J Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]