Abstract
The aim is to examine whether the changes in pleural fluid interleukin (IL)-1β, IL-2, IL-6, and IL-8 levels were significant in differential diagnosis of childhood pleural effusions. IL-1β, IL-2, IL-6, and IL-8 levels in pleural fluids of all 36 patients were measured. The levels of IL-1β, IL-2, IL-6, and IL-8 in pleural fluids were statistically significantly higher in the transudate group compared with those of the exudate group. The levels of IL-1β, IL-6, and IL-8 were also found to be statistically significantly higher in the empyema group compared with both the parapneumonic and the tuberculous pleural effusion groups. The levels of IL-2 and IL-6 were detected to be statistically significantly higher in the tuberculous pleural effusion group in comparison with those of the parapneumonic effusion group. The results showed that pleural fluids IL-1β, IL-2, IL-6, and IL-8 could be used in pleural fluids exudate and transudate distinction.
INTRODUCTION
Pleura is divided into a parietal layer which lines the inner aspect of the chest wall and a visceral layer which covers the interlobar fissures. These two layers are separated with a cavity containing 5–15 mL fluid. Fluid collection within the pleural cavity can be assessed with clinical and radiological means. When pleural effusion is detected, the characteristics of the fluid (exudate or transudate) must be revealed using thoracocentesis. Pneumonias and congestive heart failure are the most frequently encountered causes of transudative and exudative effusions, respectively [1].
Inflammation develops on the ground of the complex mechanism of cytokine discharges. Accumulating knowledge about cytokines demonstrated their important roles in the pathogenesis of most of the infectious diseases [2]. Interleukins (ILs) mediate the reactions of the our organism against foreign antigens and harmful agents. The ILs act through autocrine or paracrine rather than endocrine pathways. In various infectious diseases significant alterations in IL concentrations of blood, as well as of body fluids, occur. Although IL levels in serum and various body fluids have been studied in various diseases, very few researches involving IL levels in pleural effusions of children have been conducted [3, 4]. It is already acknowledged that established criteria used for the differentiation between exudative and transudative pleural effusions in adults are not valid for children and further studies are required to determine diagnostic criteria for children [5]. The aim of this study is to compare IL levels in exudative and transudative pleural fluids by measuring IL levels in pleural effusions developed owing to various etiological factors in childhood. We aimed to examine whether the changes in pleural fluid protein, glucose, and lactate dehydrogenase (LDH) parameters, as well as in IL-1β, IL-2, IL-6, and IL-8 levels, were significant in differential diagnosis of childhood pleural effusions.
MATERIALS AND METHODS
The study group consisted of 26 patients admitted in the Department of Pediatrics, Medical Faculty, Firat University with a diagnosis of pleural effusions. Thoracal ultrasonograms were obtained from patients thought to have pleural effusions in consideration of medical history, physical examination, and chest X-rays. The presence and the amount of pleural fluids were determined and then thoracocentesis was performed. All samples were obtained by thoracentesis at the diagnosis time (Table 1, Figure 1). Pleural fluids were collected in three separate tubes. The collected samples were evaluated as for color and appearance (turbidity). The first samples were centrifuged immediately for the measurement of IL concentration and the supernatant layers were stored at −70°C until testing for cytokines. The second samples were inoculated in aerobic, anaerobic, and Löwenstein-Jensen media and stained with Gram, Giemsa, and Ziehl-Nielsen dyes. The third samples were analysed for protein, glucose, and LDH. As indicators of infection, peripheral leukocyte counts (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) measurements, blood culture, and purified protein derivate (PPD) tests were performed. Serum total protein and LDH levels were measured. Acid fast bacilli (AFB) were cultivated in fasting gastric secretions.
Table 1.
The demographic features of all groups.
| Groups | Study group (n(%)) | Age (year, mean ± SE) | Gender (F/M) |
| Transudates | 9 (25) | 7.1 ± 0.9 | 5/4 |
| Exudates | 27 (75) | 5.1 ± 0.6 | 8/19 |
| Parapneumonic | 12 (44) | 5.4 ± 0.8 | 4/8 |
| Empyema | 12 (44) | 4.2 ± 0.8 | 3/9 |
| Tuberculous | 3 (12) | 8.5 ± 3.5 | 1/2 |
| Total | 36 | 5.6 ± 0.5 | 13/23 |
Figure 1.
The source and the type of pleural effusion samples.
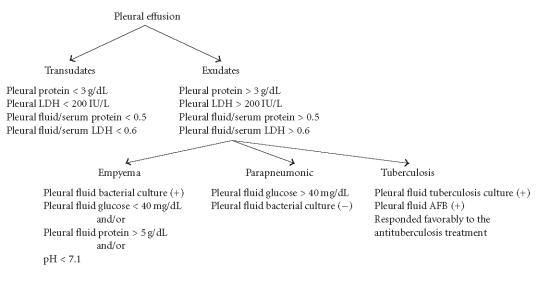
Pleural effusions were categorized as exudates and transudates according to the clinical and laboratory findings. Light's criteria were used to differentiate between transudates and exudates [5]. The cutoff values for the differentiation between the pleural transudates and exudates were determined as follows: protein, 3 g/dL; LDH, 200 IU/L; pleural fluid/serum protein ratio, 0.5; and pleural fluid/serum LDH ratio, 0.6. Pleural effusions with values above these cutoffs were classified as exudates and those below as transudates. Exudates were also grouped as empyematous, parapneumonic, or tuberculous effusions. Empyematous effusions were characterised by the presence of Gram-positive bacteria in pleural fluid smears, attendant pneumonia with or without bacterial growth in the cultures of pleural fluid samples, and typical glucose (< 40 mg/dL) and/or protein (> 5 g/dL) and/or LDH (> 1000 IU/L), and pH (< 7.1) values. The samples of pleural fluids with higher glucose concentrations (> 40 mg/dL) whose Gram-stained smears did not reveal any specific characteristics or bacterial growth were considered as parapneumonic effusions. The patients whose pleural fluid smears revealed AFB which could be cultured in Löwenstein-Jensen media and also responded favorably to the antituberculostatic treatment were acknowledged as cases with tuberculous pleurisy [5, 6, 7].
Pleural fluids preserved at −70°C were thawed and IL-1β, IL-2, IL-6, and IL-8 levels in the samples were measured using enzyme-linked immunosorbent assay (ELISA) and IL-1β, IL-2, IL-6, and IL-8 Bender Medsystems commercial kits.
The clinical and laboratory characteristics of the patients were given as means (± standard deviation (SD)). Statistical evaluations between different groups were done with Kruskal-Wallis variance analysis and post-hoc Tukey-Scheffe tests using SPSS 10.0 software programs.
RESULTS
In our study group pleural effusions were of exudative (n = 27, 75%) and transudative (n = 9, 25%) type. The ages of the study subjects ranged between 9 months and 14 years. Twelve parapneumonic (44%), 12 empyematous (44%), and 3 (12%) tuberculous pleural effusions were detected in the exudate group (Table 1).
A statistically significant difference was found between CRP values of the exudate (140.2 ± 92.4 mg/dL) and the transudate (9.6 ± 3.2 mg/dL) groups (P < .001) (Figure 2). LDH concentrations in pleural fluids were 1828.9 ± 1835.6 U/L in the exudate and 89.8 ± 20.3 U/L in the transudate groups (P < .001). Protein levels in pleural fluids were measured as 5.1 ± 1.2 g/dL and 1.6 ± 0.5 g/dL in the exudate and the transudate groups, respectively (P < .001). Glucose levels in pleural fluids were measured as 53.3 ± 36.6 mg/dL in the exudate group and 83.8 ± 14.7 mg/dL in the transudate group (P < .001) (Figure 3).
Figure 2.
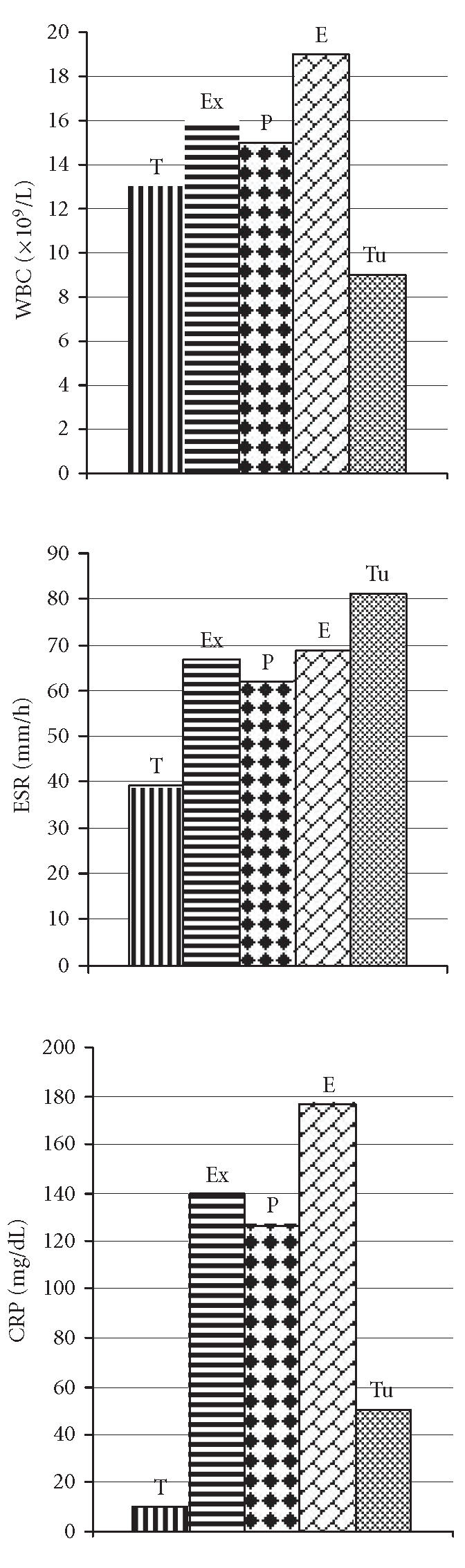
The levels of blood acute phase reactants in the study groups.
Figure 3.
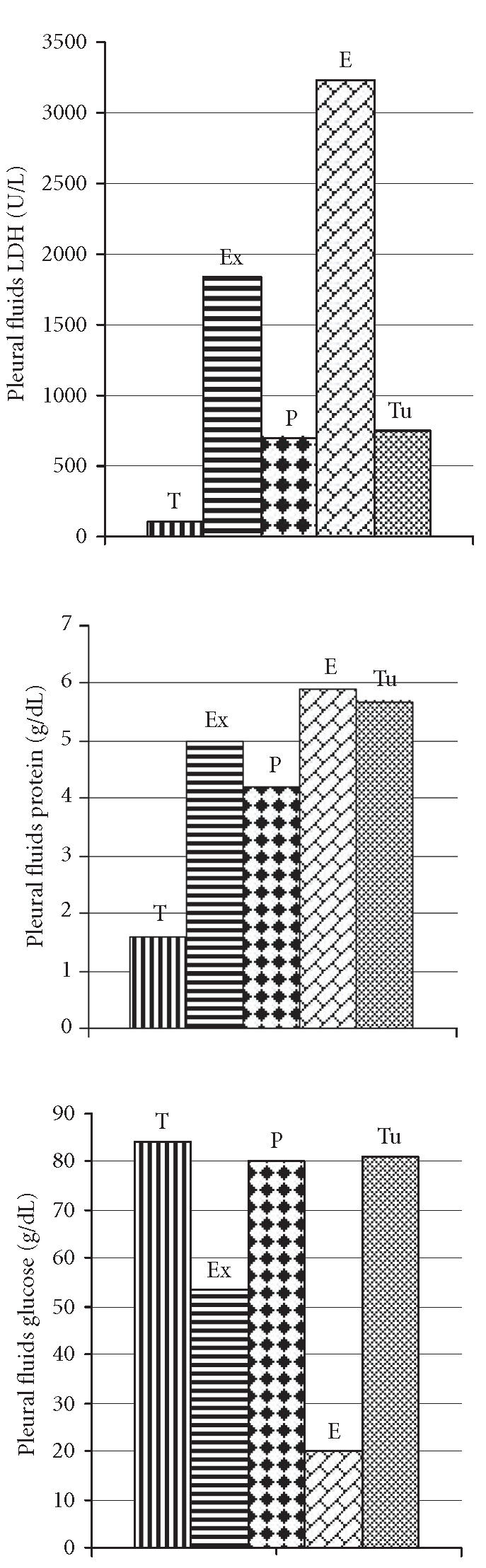
The levels of pleural fluids biochemical characteristics in the study groups.
IL-1β levels of pleural fluids in the exudate and the transudate groups were detected as 304.1 ± 127.7 pg/mL and 70.1 ± 23.4 pg/mL, respectively (P < .001). IL-1β levels were demonstrated as 210 ± 43.5 pg/mL in the parapneumonic effusion group, 430.3 ± 69.9 pg/mL in the empyematous effusion group, and 175.3 ± 36.3 pg/mL in the tuberculous effusion groups. Statistically significantly higher values were found in the empyematous group when compared with the parapneumonic and tuberculous pleural effusion groups (P < .001).
IL-2 concentrations of pleural fluids were ascertained to be at undetectable levels in 12 (44.4%) and 176.6 ± 173.6 pg/mL in 15 (55.6%) patients in the exudate group. These concentrations were wholly at undetectable levels in the transudate group. Statistically significant difference was uncovered between the exudate and the transudate groups (P < .001). Statistically significant differences were not revealed between the empyematous group and the other two groups (P > .05). Statistically significantly higher values were recognized in the tuberculous pleural effusion group rather than the parapneumonic effusion group (P < .01).
IL-6 levels were detected to be 18589.5 ± 3631.8 pg/mL in the exudate group and 65.6 ± 16.0 pg/mL in the transudate group (P < .001). The corresponding values were 15295.0 ± 22678.1 pg/mL in the parapneumonic effusion group, 20518.0 ± 1410.9 pg/mL in the empyematous effusion group and 24050.0 ± 223.3 pg/mL in the tuberculous pleural effusion group. In the empyematous group, statistically significantly higher values were obtained when compared with those of the parapneumonic effusion group, while the corresponding values detected in the tuberculous pleural effusion group were also significantly higher than those found in the parapneumonic effusion group (P < .001).
IL-8 levels of pleural fluids were assessed to be 3068.5 ± 1762.7 pg/mL in the exudate group and 108.8 ± 92.0 pg/mL in the transudate group (P < .001). The concentrations of IL-8 were 1884.5 ± 366.7 in the parapneumonic effusion group, 4789.8 ± 1013.2 pg/mL in the empyematous effusion group and 919.0 ± 9454.5 pg/mL in the tuberculous pleural effusion group. The values were statistically significantly higher in the empyematous group when compared with the other two groups (P < .001) (Figure 4).
Figure 4.
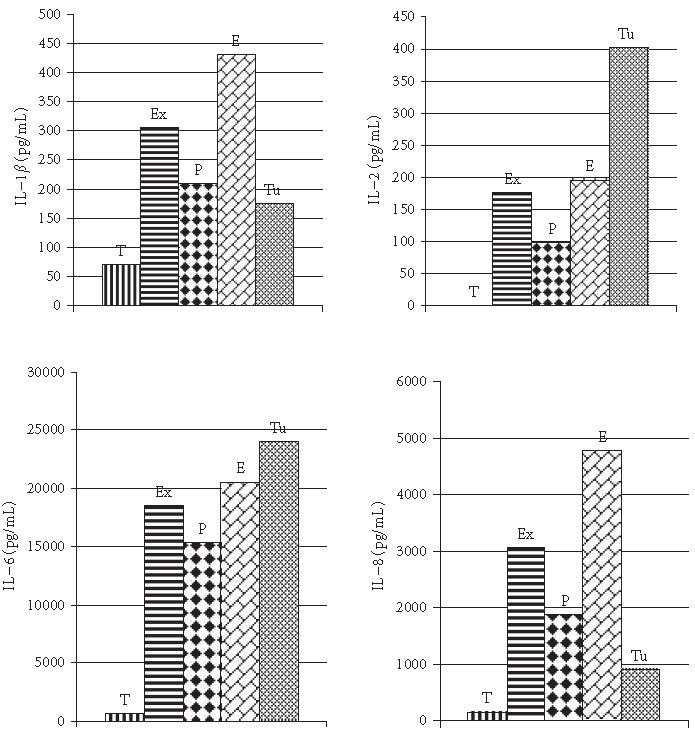
The levels of pleural fluids IL-1β, IL-2, IL-6, and IL-8 in patients with different etiologic diagnosis in the exudate and transudate groups.
DISCUSSION
Pleural effusion is most frequently seen during the course of pneumonias. Pleural effusion can be seen due to the intrusion of an infectious agent or an irritating foreign substance inside the pleural cavity or due a direct access of harmful materials or neoplastic cells into the pleural cavity via hematogenous route [8]. It can be observed following pleural trauma or in association with asbestosis related pleural diseases. Pleural effusion or empyema in association with pneumonia is relatively frequent [8]. Pleural effusions are known to develop secondary to trauma, cardiac, renal, collagenous diseases and malignancies besides pneumonia [9, 10, 11]. From 61% to 80% of the cases with pleural effusions are of infectious origin. Despite the usage of broad spectrum antibiotics, empyema continues to be an important health care problem during childhood. Empyema is also an important cause of mortality in the developing countries. In the remaining 20%–39% of the cases, empyema develops secondary to trauma, malignancies, and renal diseases [5, 12, 13, 14, 15]. In our study group an infectious agent was responsible for 75% of the cases. Many studies have been conducted concerning the levels of IL-1β, IL-2, IL-6, and IL-8 in body fluids in various infections. Although many studies investigating the alterations in levels of IL according to the exudative or transudative nature of pleural effusions in adults have been performed, similar studies pertaining to children are relatively few [16, 17, 18].
IL-1β which is a proinflammatory cytokine plays a key role in various infections. For that reason many studies have analysed IL-1β levels in patients with meningitis, sepsis, urinary tract infections, and empyema. Variable results have been obtained dependent on the presence of different etiological agents [17, 18]. In patients with bacterial and tuberculous meningitis, IL-1β levels of cerebrospinal fluids were found to be significantly higher than those with aseptic meningitis and in the control group. Umblical plasma levels of IL-1β of infected newborns with early-onset sepsis were found to be higher than those of the control group. IL-1β levels in synovial fluids were detected to be higher than those in the pediatric cases with suppurative arthritis due to other causes. Silva-Mejias et al [18] have found comparatively higher IL-1β levels in the empyema group. Similar to our findings, Alexandrakis et al [19] revealed that IL-1β levels in pleural effusions were higher in the exudate group compared with those of the transudate group. Naito et al [20] ascertained that IL-1β levels in pleural effusions were higher than those found in bacterial infections due to other causes. In accordance with the literature in our study, IL-1β levels were nearly 4.5 times higher in the exudate group.
IL-2 levels in pleural effusions due to malignancies were reported to be lower than those with tuberculous pleural effusions [21]. Shimokata et al [22] stated that IL-2 levels were at undetectable levels in 25% of tuberculous pleural effusions and 75% of cancerous pleural effusions. In our study IL-2 levels in pleural fluids were at undetectably lower levels in 44% of the patients in the exudate group while the IL-2 levels of the remaining 56% were assessed as 176.6 ± 33.4 pg/mL. However all the patients in the transudate group demonstrated undetectable IL-2 levels. In our study a statistically significant difference was not present between the empyema group and the other two groups. However a statistically significant difference was detected between the parapneumonic and the parapneumonic pleural effusion groups.
Various studies have been performed concerning the levels of IL-6 which is the mediator and the regulator of inflammatory responses in miscellaneous inflammatory processes. Blood IL-6 levels were found to be higher in children with Mycoplasma pneumoniae infections who stayed febrile for more than 3 days compared with those having shorter febrile episodes [16]. They were also statistically significantly higher in pediatric cases with urinary tract infections when compared with the control groups. IL-6 levels of cerebrospinal fluid in the patients with bacterial meningitis were reported to be significantly higher than the aseptic meningitis and in the control groups. Blood IL-6 levels above 100 pg/mL were measured in pediatric cases with viral (14%) but especially with bacterial meningitis (53%). Xirouchaki et al [23] and Yokoyama et al [24] reported the levels of IL-6 in pleural fluids to be significantly higher in the exudate group rather than the transudate group. They also found IL-6 concentrations significantly higher in the tuberculous group rather than the parapneumonic effusion group. In our study not only the levels of IL-6 were different between the groups with parapneumonic and empyematous effusions, but at the same time statistically significant differences were found between the empyematous and the parapneumonic or tuberculous pleural effusion groups. Alexandrakis et al [25] measured IL-6 levels in serum and pleural fluids and ascertained that serum IL-6 levels did not differ significantly between the exudate and the transudate groups. However pleural fluid IL-6 levels were detected to be meaningfully higher in the exudate rather than the transudate group. A definite proportion between the serum and pleural fluid IL-6 concentrations could not be assessed. However we found IL-6 levels in the pleural fluid to be 285 times higher than those detected in the exudate group.
IL-8 is the mediator and the regulator of chemotaxis of leukocytes in inflammatory processes. In adults miscellaneous studies have analysed IL-8 levels in pleural fluids. IL-8 levels were found to be higher in cases with infectious pleural effusions compared with the patients with noninfectious effusions [3, 26, 27, 28, 29, 30, 31, 32]. Ceyhan et al [26] reported higher levels in empyematous/parapneumonic effusion groups rather than the tuberculous group. However Antony et al [28] detected higher levels in cases with parapneumonic effusions unlike those found in the group with tuberculous effusions. IL-8 concentrations in the empyematous group were reported to be higher than those measured in the parapneumonic group. In accordance with this study, in our study the highest levels of IL-8 were detected in the empyematous, parapneumonic, and tuberculous pleural effusions in decreasing order. Dlugovitzky et al [29] discovered statistically significantly higher IL-8 values in the tuberculous pleural effusion group in contrast with findings in the parapneumonic pleural effusion group. Ashitani et al [30] and Broaddus et al [32] found higher levels in the empyema group compared with the other groups. Miller and Idell [31] have detected significantly higher levels in the exudate group rather than the transudate group. In this study, similar to the findings of other investigations, IL-8 levels in the exudate group were reported to be 28 times higher than those found in the transudate group.
This study have shown that for the differentiation between the exudative and transudative pleural effusions, in addition to the parametres such as protein, glucose and LDH, pleural fluid IL-1β, IL-2, IL-6, and IL-8 levels could be used. Etiological factors can be differentiated by determining pleural fluid IL levels. Taking these levels into consideration, the exudative or transudative nature of pleural fluids can be ascertained. Accordingly, a definitive diagnosis, a successful treatment and reduction in mortality can be achieved.
References
- 1.Gotsman I, Fridlender Z, Meirovitz A, Dratva D, Muszkat M. The evaluation of pleural effusions in patients with heart failure. Am J Med. 2001;111(5):375–378. doi: 10.1016/s0002-9343(01)00881-6. [DOI] [PubMed] [Google Scholar]
- 2.Winterbauer RH. Nonmalignant pleural effusions. In: Fishman AP, editor. Fishman's Pulmonary Diseases and Disorders. New York, NY: McGraw-Hill; 1998. pp. 1411–1427. [Google Scholar]
- 3.Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10(4):742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KF, Olak J. Anatomy and physiology of the pleural space. Chest Surg Clin N Am. 1994;4(3):391–403. [PubMed] [Google Scholar]
- 5.Alkrinawi S, Chernick V. Pleural fluid in hospitalized pediatric patients. Clin Pediatr (Phila) 1996;35(1):5–9. doi: 10.1177/000992289603500102. [DOI] [PubMed] [Google Scholar]
- 6.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 7.Metintaş M, Alataş Ö, Alataş F, Çolak Ö, Özdemir N, Erginel S. Comparative analysis of biochemical parameters for differentiation of pleural exudates from transudates Light's criteria, cholesterol, bilirubin, albumin gradient, alkaline phosphatase, creatine kinase, and uric acid. Clin Chim Acta. 1997;264(2):149–162. doi: 10.1016/s0009-8981(97)00091-0. [DOI] [PubMed] [Google Scholar]
- 8.Snider GL. Pleural disorders; pneumothorax. In: Berkow R, Fletcher AJ, editors. Merck Manual. 15th ed. Rahway, NJ: Merck; 1987. pp. 698–705. [Google Scholar]
- 9.Chartrand SA, McCracken GH., Jr Staphylococcal pneumonia in infants and children. Pediatr Infect Dis. 1982;1(1):19–23. doi: 10.1097/00006454-198201000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Chonmaitree T, Powell KR. Parapneumonic pleural effusion and empyema in children. Review of a 19-year experience, 1962–1980. Clin Pediatr (Phila) 1983;22(6):414–419. doi: 10.1177/000992288302200603. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein DM. Diseases of the pleura. In: Behrman RE, Kliegman RM, Arvin AM, editors. Nelson Textbook of Pediatrics. 15th ed. Philadelphia, Pa: WB Saunders; 1996. pp. 1252–1254. [Google Scholar]
- 12.Azevedo I, Sarbeji M, Le Bourgeois M, et al. Diagnostic approach of pleural effusion in children. Report of 59 cases. Pediatrie. 1990;45(11):807–812. [PubMed] [Google Scholar]
- 13.McLaughlin FJ, Goldmann DA, Rosenbaum DM, Harris GB, Schuster SR, Strieder DJ. Empyema in children: clinical course and long-term follow-up. Pediatrics. 1984;73(5):587–593. [PubMed] [Google Scholar]
- 14.Mangete ED, Kombo BB, Legg-Jack TE. Thoracic empyema: a study of 56 patients. Arch Dis Child. 1993;69(5):587–588. doi: 10.1136/adc.69.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdes L, Pose A, San Jose E, Martinez Vazquez JM. Tuberculous pleural effusions. Eur J Intern Med. 2003;14(2):77–88. doi: 10.1016/S0953-6205(03)00018-9. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh CC, Tang RB, Tsai CH, Chen W. Serum interleukin-6 and tumor necrosis factor-alpha concentrations in children with Mycoplasma pneumonia . J Microbiol Immunol Infect. 2001;34(2):109–112. [PubMed] [Google Scholar]
- 17.Freij BJ, Kusmiesz H, Nelson JD, McCracken GH., Jr Parapneumonic effusions and empyema in hospitalized children: a retrospective review of 227 cases. Pediatr Infect Dis. 1984;3(6):578–591. doi: 10.1097/00006454-198411000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Silva-Mejias C, Gamboa-Antinolo F, Lopez-Cortes LF, Cruz-Ruiz M, Pachon J. Interleukin-1β in pleural fluids of different etiologies. Its role as inflammatory mediator in empyema. Chest. 1995;108(4):942–945. doi: 10.1378/chest.108.4.942. [DOI] [PubMed] [Google Scholar]
- 19.Alexandrakis MG, Kyriakou D, Alexandraki R, Pappa KA, Antonakis N, Bouros D. Pleural interleukin-1 beta in differentiating transudates and exudates: comparative analysis with other biochemical parameters. Respiration. 2002;69(3):201–206. doi: 10.1159/000063620. [DOI] [PubMed] [Google Scholar]
- 20.Naito T, Ohtsuka M, Ishikawa H, Satoh H, Hasegawa S. Clinical significance of cytokine measurement in pleural effusion. Kekkaku. 1997;72(10):565–572. [PubMed] [Google Scholar]
- 21.Okubo Y, Namushi NR, Nakata M, Kuroiwa Y, Ota T, Kusama S. Purified protein derivative induced cytotoxicity in carcinomatous and tuberculous pleurisy. Jpn J Med. 1998;27(3):255–260. doi: 10.2169/internalmedicine1962.27.255. [DOI] [PubMed] [Google Scholar]
- 22.Shimokata K, Saka H, Murate T, Hasegawa Y, Hasegawa T. Cytokine content in pleural effusion. Comparison between tuberculous and carcinomatous pleurisy. Chest. 1991;99(5):1103–1107. doi: 10.1378/chest.99.5.1103. [DOI] [PubMed] [Google Scholar]
- 23.Xirouchaki N, Tzanakis N, Bouros D, et al. Diagnostic value of interleukin-1alpha, interleukin-6, and tumor necrosis factor in pleural effusions. Chest. 2002;121(3):815–820. doi: 10.1378/chest.121.3.815. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama A, Maruyama M, Ito M, Kohno N, Hiwada K, Yano S. Interleukin 6 activity in pleural effusion. Its diagnostic value and thrombopoietic activity. Chest. 1992;102(4):1055–1059. doi: 10.1378/chest.102.4.1055. [DOI] [PubMed] [Google Scholar]
- 25.Alexandrakis MG, Coulocheri SA, Bouros D, Eliopoulos GD. Evaluation of ferritin, interleukin-6, interleukin-8 and tumor necrosis factor alpha in the differentiation of exudates and transudates in pleural effusions. Anticancer Res. 1999;19(4C):3607–3612. [PubMed] [Google Scholar]
- 26.Ceyhan BB, Özgün S, Çelikel T, YalÇin M, Koç M. IL-8 in pleural effusion. Respir Med. 1996;90(4):215–221. doi: 10.1016/s0954-6111(96)90290-5. [DOI] [PubMed] [Google Scholar]
- 27.Segura RM, Alegre J, Varela E, et al. Interleukin-8 and markers of neutrophil degranulation in pleural effusions. Am J Respir Crit Care Med. 1998;157(5 pt 1):1565–1572. doi: 10.1164/ajrccm.157.5.9711116. [DOI] [PubMed] [Google Scholar]
- 28.Antony VB, Godbey SW, Kunkel SL, et al. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol. 1993;151(12):7216–7223. [PubMed] [Google Scholar]
- 29.Dlugovitzky D, Rateni L, Torres-Morales A, et al. Levels of interleukin-8 in tuberculous pleurisy and the profile of immunocompetent cells in pleural and peripheral compartments. Immunol Lett. 1997;55(1):35–39. doi: 10.1016/s0165-2478(96)02649-1. [DOI] [PubMed] [Google Scholar]
- 30.Ashitani J, Mukae H, Nakazato M, et al. Elevated pleural fluid levels of defensins in patients with empyema. Chest. 1998;113(3):788–794. doi: 10.1378/chest.113.3.788. [DOI] [PubMed] [Google Scholar]
- 31.Miller EJ, Idell S. Interleukin-8: an important neutrophil chemotaxin in some cases of exudative pleural effusions. Exp Lung Res. 1993;19(5):589–601. doi: 10.3109/01902149309031730. [DOI] [PubMed] [Google Scholar]
- 32.Broaddus VC, Hebert CA, Vitangcol RV, Hoeffel JM, Bernstein MS, Boylan AM. Interleukin-8 is a major neutrophil chemotactic factor in pleural liquid of patients with empyema. Am Rev Respir Dis. 1992;146(4):825–830. doi: 10.1164/ajrccm/146.4.825. [DOI] [PubMed] [Google Scholar]