Abstract
Estrogen receptors (ERs) and the aryl hydrocarbon receptor (AhR) are ligand activated transcription factors and members of the nuclear receptor and bHLH-PAS superfamilies, respectively. AhR is involved in xenobiotic metabolism and in mediating the toxic effects of dioxin-like compounds. Crosstalk has been observed among AhR and nuclear receptors, but has been most well studied with respect to ER signaling. Activated AhR inhibits ER activity through a number of different mechanisms, whereas ERα has been reported to have a positive role in AhR signaling. Here we will discuss recent data revealing that dioxin bound AhR recruits ERα to AhR regulated genes. We will also consider the implications of ER recruitment to AhR target genes on ER and AhR signaling.
Introduction
Estrogen receptor α (ERα) and ERβ, and related steroid hormone receptors mediate the signals of small hydrophobic molecules by acting as ligand-dependent transcription factors. Ligand binding induces receptor homodimerization, DNA binding to estrogen response elements in the promoter regions of target genes, recruitment of coregulators and changes in transcription. Although ligand binding is an important mechanism of nuclear receptor activation, many receptors, including ERα and ERβ, are activated by growth hormones, kinases, and other hormone-bound steroid receptors and transcription factors [Kato et al., 1995; Kousteni et al., 2001]. The aryl hydrocarbon receptor (AhR) is a member of the basic-helix-loop-helix Per (Period)–ARNT (aryl hydrocarbon nuclear translocator)–SIM (single minded) (bHLH-PAS) family [Gu et al., 2000]. Upon ligand binding, the AhR translocates from the cytoplasm to the nucleus where it binds its dimerization partner ARNT. The activated AhR/ARNT heterodimer complex binds to its cognate DNA sequences, termed xenobiotic response elements (XREs), and activates the expression of AhR target genes, such as cytochrome P4501A1 (CYP1A1) and CYP1B1 [Hankinson, 1995]. AhR null animals reveal that the AhR mediates most, if not all, of the toxic effects of 2,3,7,8-tetrachlorodibenzo-[p]-dioxin (TCDD) [Lahvis and Bradfield, 1998]. Although its physiological role is unknown, the AhR has been shown to play an important role in liver development and female reproduction [Baba et al., 2005; Schmidt et al., 1996].
Inhibitory crosstalk between AhR and ER signaling
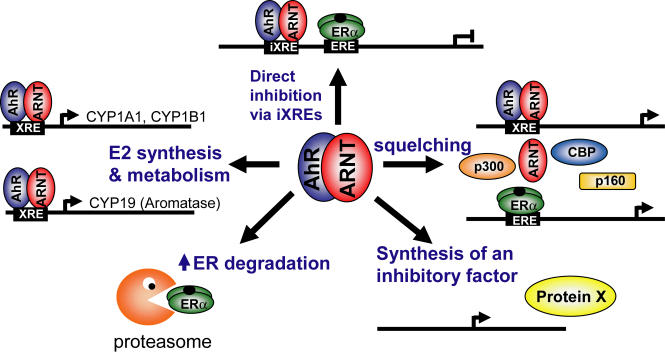
Inhibitory crosstalk between the AhR and ER signaling was suggested by early experiments examining the long-term effects of TCDD treatment in Sprague Dawley rats [Kociba et al., 1978]. Among the findings, were the observations that the incidences of both mammary and uterine tumors were decreased in female rats [Kociba et al., 1978], which were supported by other reports demonstrating that TCDD inhibits the formation of 7,2-dimethylbenz[a]anthracene (DMBA) induced mammary tumors [Holcomb and Safe, 1994]. Several studies have since reported that activated AhR inhibits the expression of E2 induced genes [Safe and Wormke, 2003]. The precise molecular mechanisms for this crosstalk are unclear, and may be a combination of several different mechanisms (Figure 1).
Figure 1. Proposed mechanisms of crosstalk between AhR and ER signaling pathways.
AhR has been reported to inhibit ER activity through a combination of several different mechanisms: direct inhibition by the activated AhR/ARNT heterodimer through binding to inhibitory XRE (iXRE) present in ER target genes; squelching of shared coactivators, including ARNT; synthesis of an unknown inhibitory protein; increased proteasomal degradation of ER; and altered estrogen synthesis/metabolism through increase in aromatase, cytochromeP450 1A1 and 1B1 expression. Modified from Safe and Wormke, 2003.
AhR agonist dependent recruitment of ERα to AhR regulated promoters
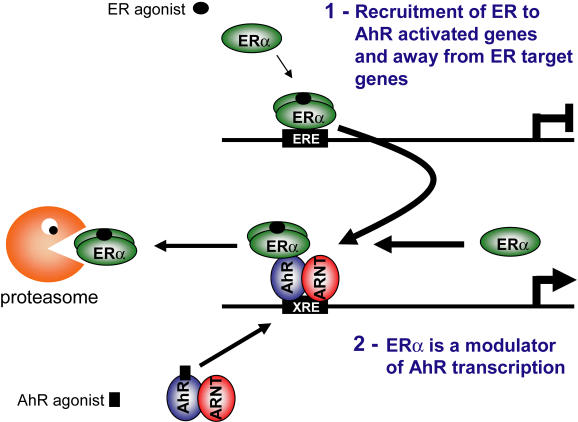
Recent data from our group reveals that in the absence of 17β-estradiol (E2) TCDD bound AhR recruits ERα to AhR regulated genes, CYP1A1 and CYP1B1 [Matthews et al., 2005]. This recruitment most likely occurs through direct protein/protein interactions between ERα and AhR [Beischlag and Perdew, 2005; Ohtake et al., 2003]. Promoter occupancy by ERα at CYP1A1 was increased by cotreatment with TCDD and E2; however, E2 treatment only enhanced TCDD-induced CYP1A1 regulated reporter gene activity and not endogenous CYP1A1 mRNA levels. The molecular mechanisms governing the increase in recruitment of ERα by cotreatment of TCDD and E2 are unclear, but may be a combination of E2-induced homodimerization resulting in recruitment of ERα homodimer rather than the monomer and that the conformational change of ERα induced by E2 better exposes the epitope interaction surfaces allowing improved antibody interaction. Our data support the novel scenario that active AhR can redirect ER from ER target genes to AhR target genes, suggesting that AhR can regulate ERα protein levels and consequently estrogenic responses [Matthews et al., 2005]. For example, TCDD treatment is known to reduce the protein levels of ERα, which are dependent on proteasome activity [Wormke et al., 2003]; the recruitment of ERα to the AhR complex, away from ER regulated genes may serve as a mechanism to regulate ERα protein levels (Figure 2). The signals that recruit ERα to the activated AhR complex are unknown, and may include the activation of other signaling pathways, such as kinases, since the ERα AF1 is needed for recruitment to and interaction with the AhR complex [Ohtake et al., 2003] (Wihlén et al., in preparation).
Figure 2. Modulation of ER and AhR signaling via dioxin dependent interaction of ER with AhR.
The association of ER with activated AhR can be viewed as two separate pathways. 1. Activated AhR recruits unliganded or liganded ERα away from ER regulated genes to AhR regulated genes, inhibiting estrogen signaling. The shuttling via the AhR may serve as a mechanism to regulate ER protein levels. 2. ERα has been shown to modulate AhR-dependent transcription, suggesting that ERα is an important regulator of AhR activity.
Is AhR a coregulator of ER-dependent transcription?
3-Methylcholanthrene, a polycyclic aromatic hydrocarbon (PAH) AhR agonist has been reported to activate AhR such that it interacts with unliganded ERα and the entire AhR/ERα complex is recruited to estrogen responsive genes, suggesting that ER signaling is modulated by co-regulatory-like functions of AhR [Ohtake et al., 2003]. However, PAHs can be metabolized to relatively potent estrogenic compounds and independent studies have reported estrogenic effects of 3MC and other AhR agonists in the absence of AhR [Pearce et al., 2004; Shipley and Waxman, 2005]. AhR agonists, such as 3MC, have recently been shown to directly activate ERα, and are suggested to represent a new class of mixed AhR/ER agonists [Abdelrahim et al., 2006]. Moreover, ARNT directly interacts with ERs and has been shown by transient transfection to coactivate ER-dependent gene expression [Brunnberg et al., 2003].
ERα is a ligand activated modulator of AhR-dependent transcription
Our studies confirm a proposed “hijacking” of ERα by activated AhR [Brosens and Parker, 2003], but we and others observe ERα to be present at AhR target genes [Abdelrahim et al., 2006; Beischlag and Perdew, 2005; Matthews et al., 2005]. As we have suggested above this recruitment could have important consequences on ER signaling, but also reveals an important role for ERα in AhR-dependent transcription. There are conflicting reports on the effect of E2 on TCDD-induced expression of AhR target genes. Some studies report an inhibition [Beischlag and Perdew, 2005; Kharat and Saatcioglu, 1996], whereas we and others observe a slight activation or no effect [Hoivik et al., 1997; Matthews et al., 2005]. These differences may be due to different cell culture conditions, cell passage, serum lots and treatment regimes. The observation that transfection of ERα into ER negative breast cancer cell lines restores AhR responsiveness as determined by CYP1A1 induction, suggests a positive role for ER in AhR responses [Thomsen et al., 1994]. Consistent with ERα positively influencing AhR activity are our findings that short hairpin RNA (shRNA) mediated knockdown of ERα expression significantly reduced TCDD-induced CYP1A1 expression, whereas cotreatment with E2 had no further effect [Matthews et al., 2005]. Stable knockdown of ERα in HC11 mouse mammary cells confirmed an important role for ERα in AhR agonist-induced CYP1A1 expression, although no reduction was observed in induced CYP1B1 expression (Matthews et al. submitted). These data suggest that ERα is a promoter-specific modulator of AhR-dependent transcription, and demonstrate the complexity of crosstalk between these two receptor pathways (Figure 2). Whether ERα acts as a coregulator or recruits additional cofactors that modulate AhR transcription is currently under investigation and an important area for future research. The ERα modulation of AhR activity represents a new mechanism of ERα signaling. The recruitment of ERα by AhR agonist to CYP1A1 and CYP1B1 and its enhancement by E2, also leads to the hypothesis that this process may represent a feedback regulation in estrogen signaling, since both CYP1B1 and CYP1A1 are involved in E2 metabolism [Lee et al., 2003].
Conclusions and Future Prospects
Dioxins and compounds that mimic the activities of estrogen are found throughout our environment, and exposure to these compounds has been suggested to increase the risk of many hormone related diseases (“endocrine disruption”). Despite many studies our understanding of the molecular mechanisms of AhR and ER crosstalk is far from complete. We believe that the AhR agonist dependent association of ERα with AhR represents an important mechanism mediating inhibitory crosstalk between AhR and ER signaling pathways, but also a new mechanism of ER action. Some of the major future challenges are to identify the regulatory signals that cause ER to interact with AhR and to confirm that ERs are present at AhR target promoters in vivo. Current data would suggest that AhR influences ERβ signaling in a similar manner to ERα signaling; however, little is known about how AhR influences ERβ activity and vice versa. It will also be important to determine if ERα and/or ERβ are recruited to all AhR target genes or restricted to the CYP1A1 and 1B1 promoters. Studies of cell-based and genetically modified animal models will be important in assessing the physiological importance of crosstalk between these receptor systems and in uncovering potential ER subtype specific regulation.
Acknowledgments
The authors would like to thank all members of the Receptor Biology Unit for their assistance and helpful discussions. This work was supported by a postdoctoral fellowship from the David and Astrid Hageléns Foundation (to J.M.) and by grants from the Swedish Cancer Fund and KaroBio AB.
Abbreviations
- AF1
activation function 1
- AhR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- bHLH-PAS
basic-helix-loop-helix Per (Period)–ARNT–SIM (single minded)
- CYP1A1
cytochrome P4501A1
- DMBA
7,2-dimethylbenz[a]anthracene
- E2
17β-estradiol
- ER
estrogen receptor
- shRNA
short hairpin RNA
- TCDD
2,3,7,8-tetrachlorodibenzo-[p]-dioxin.
References
- Abdelrahim M., Ariazi E., Kim K., Khan S., Barhoumi R., Burghardt R., Liu S., Hill D., Finnell R., Wlodarczyk B., Jordan V. C., Safe S. 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor α. Cancer Res. 2006;66:2459–67. doi: 10.1158/0008-5472.CAN-05-3132. [DOI] [PubMed] [Google Scholar]
- Baba T., Mimura J., Nakamura N., Harada N., Yamamoto M., Morohashi K., Fujii-Kuriyama Y. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25:10040–51. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag T. V., Perdew G. H. ER α-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J Biol Chem. 2005;280:21607–11. doi: 10.1074/jbc.C500090200. [DOI] [PubMed] [Google Scholar]
- Brosens J. J., Parker M. G. Gene expression: Oestrogen receptor hijacked. Nature. 2003;423:487–8. doi: 10.1038/423487a. [DOI] [PubMed] [Google Scholar]
- Brunnberg S., Pettersson K., Rydin E., Matthews J., Hanberg A., Pongratz I. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc Natl Acad Sci U S A. 2003;100:6517–22. doi: 10.1073/pnas.1136688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y. Z., Hogenesch J. B., Bradfield C. A. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–61. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–40. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Hoivik D., Willett K., Wilson C., Safe S. Estrogen does not inhibit 2,3,7, 8-tetrachlorodibenzo-p-dioxin-mediated effects in MCF-7 and Hepa 1c1c7 cells. J Biol Chem. 1997;272:30270–4. doi: 10.1074/jbc.272.48.30270. [DOI] [PubMed] [Google Scholar]
- Holcomb M., Safe S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 1994;82:43–7. doi: 10.1016/0304-3835(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H., Metzger D., Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kharat I., Saatcioglu F. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin are mediated by direct transcriptional interference with the liganded estrogen receptor. Cross-talk between aryl hydrocarbon- and estrogen-mediated signaling. J Biol Chem. 1996;271:10533–7. doi: 10.1074/jbc.271.18.10533. [DOI] [PubMed] [Google Scholar]
- Kociba R. J., Keyes D. G., Beyer J. E., Carreon R. M., Wade C. E., Dittenber D. A., Kalnins R. P., Frauson L. E., Park C. N., Barnard S. D., Hummel R. A., Humiston C. G. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Kousteni S., Bellido T., Plotkin L. I., O'Brien C. A., Bodenner D. L., Han L., Han K., DiGregorio G. B., Katzenellenbogen J. A., Katzenellenbogen B. S., Roberson P. K., Weinstein R. S., Jilka R. L., Manolagas S. C. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–30. [PubMed] [Google Scholar]
- Lahvis G. P., Bradfield C. A. Ahr null alleles: distinctive or different? Biochem Pharmacol. 1998;56:781–7. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Lee A. J., Cai M. X., Thomas P. E., Conney A. H., Zhu B. T. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–98. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- Matthews J., Wihlen B., Thomsen J., Gustafsson J. A. Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor α to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol Cell Biol. 2005;25:5317–5328. doi: 10.1128/MCB.25.13.5317-5328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F., Takeyama K., Matsumoto T., Kitagawa H., Yamamoto Y., Nohara K., Tohyama C., Krust A., Mimura J., Chambon P., Yanagisawa J., Fujii-Kuriyama Y., Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–50. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Pearce S. T., Liu H., Radhakrishnan I., Abdelrahim M., Safe S., Jordan V. C. Interaction of the aryl hydrocarbon receptor ligand 6-methyl-1,3,8-trichlorodibenzofuran with estrogen receptor α. Cancer Res. 2004;64:2889–97. doi: 10.1158/0008-5472.can-03-1770. [DOI] [PubMed] [Google Scholar]
- Safe S., Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor α cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–16. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Schmidt J. V., Su G. H., Reddy J. K., Simon M. C., Bradfield C. A. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley J. M., Waxman D. J. Aryl hydrocarbon receptor-independent activation of estrogen receptor-dependent transcription by 3-methycholanthrene. Toxicol Appl Pharmacol. 2005 doi: 10.1016/j.taap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Thomsen J. S., Wang X., Hines R. N., Safe S. Restoration of aryl hydrocarbon (Ah) responsiveness in MDA-MB-231 human breast cancer cells by transient expression of the estrogen receptor. Carcinogenesis. 1994;15:933–7. doi: 10.1093/carcin/15.5.933. [DOI] [PubMed] [Google Scholar]
- Wormke M., Stoner M., Saville B., Walker K., Abdelrahim M., Burghardt R., Safe S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor α through activation of proteasomes. Mol Cell Biol. 2003;23:1843–55. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]