Abstract
Thyroid hormone (TH) action is mediated principally through binding of the hormone ligand, 3,3,5-triiodothyronine (T3), to TH receptors (TRs). This hormone-receptor interaction recruits other proteins to form complexes that regulate gene expression by binding to DNA sequences in the promoter of target genes. We recently described an extranuclear mechanism of TH action that consists of the association of TH-liganded TRβ with p85α [regulatory subunit of phosphatidylinositol 3-kinase (PI3K)] in the cytosol and subsequent activation of the PI3K, generating phosphatidylinositol 3,4,5-triphosphate [PtdIns(3,4,5)P3]. This initiates the activation of a signaling cascade by phosphorylation of Akt, mammalian target of rapamycin (mTOR) and its substrate p70S6K, leading to the stimulation of ZAKI-4α synthesis, a calcineurin inhibitor. Furthermore, we found that this same mechanism leads to induction of the transcription factor hypoxia-inducible factor (HIF-1α), and its target genes, glucose transporter (GLUT)1, platelet-type phosphofructokinase (PFKP), and monocarboxylate transporter (MCT) 4. These genes are of special interest, because their products have important roles in cellular glucose metabolism, from glucose uptake (GLUT1) to glycolysis (PFKP) and lactate export (MCT4). These results demonstrate that the TH-TRβ complex can exert a non-genomic action in the cytosol leading to changes in gene expression by direct (HIF-1α) and indirect (ZAKI-4α, GLUT1, PFKP) means.
Classical, genomic, thyroid hormone action
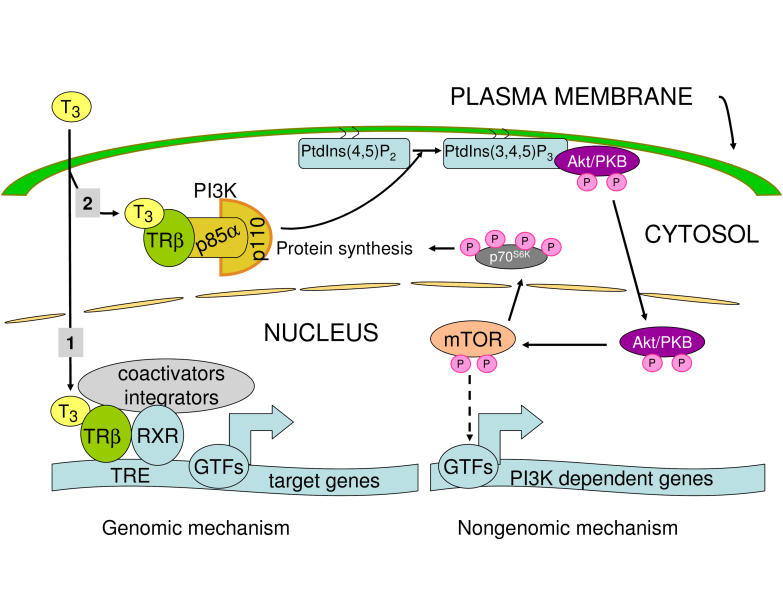
Thyroid hormone (TH) is essential for normal development, growth and metabolism. Its effects are mediated principally through triiodothyronine (T3), which acts as a ligand for the TH receptors (TRs) β1, β2 and α1 [Harvey and Williams, 2002; Yen, 2001]. In the classical model of genes positively regulated by TH, the TR first binds as a heterodimer or homodimer on TH response elements (TRE) located in the promoter regions of target genes, where it interacts with corepressors. Upon ligand binding, the TR homodimers are dissociated in favor of heterodimer formation with the retinoid-X receptor (RXR), resulting in release of the corepressors and recruitment of coactivators. This new complex attracts a large number of proteins which engage the RNA polymerase II in the transcription of the targeted gene (Figure 1, part 1). This classical mechanism can also lead to increased expression of genes devoid of TREs, if they are target genes for transcription factors that are induced by this mechanism.
Figure 1. Genomic and non-genomic action of TH.
Genomic (1) and non-genomic (2) actions of TH are illustrated. Genomic action requires thyroid hormone responsive elements (TREs) for the recognition of genes for direct transcriptional regulation. Non-genomic action is initiated by the TH-dependent activation of PI3K as illustrated in Figure 2. Activation of PI3K leads to sequential activation of Akt/PKB-mTOR-p70S6K. Although not well defined, this cascade leads to transcriptional upregulation of some genes such as ZAKI-4α and HIF-1α. GTF: general transcription factors. For details see text.
Nongenomic action of thyroid hormone
In addition to the classical, “nuclear” mode of TH action, a number of rapid effects taking place in the cytosol and at the plasma membrane have been subsequently identified. TH can control Ca2+ entry, intracellular protein trafficking and regulation of protein kinase C [Davis and Davis, 2002; Davis et al., 2002]. The MAPK pathway can be activated by TH binding to the integrin αVβ3, located in the cell membrane, without entering the cell. This mechanism leads to phosphorylation of nuclear receptors and can induce angiogenesis and promote cell growth [Bergh et al., 2005; Tang et al., 2004]. A derivative of TH, 3-iodothyronamine (T1AM), can induce bradycardia and hypothermia within minutes through a mechanism that remains unknown [Scanlan et al., 2004]. These nongenomic actions of TH are mostly extranuclear, appear to be independent of TRs and have rapid effects on proteins rather than modulate gene expression.
Cytosolic activation of the PI3K pathway by TR
As all proteins, TRs are synthesized in the cytoplasm from where they are translocated into the nucleus to exert their genomic effect summarized above. A dynamic nucleo-cytoplasmic shuttling has been described [Baumann et al., 2001]. We recently identified a new mechanism of TH action in which the liganded TRβ interacts with the regulatory subunit of PI3K (p85α ) in the cytosol [Cao et al., 2005] (Figure 2). This leads to activation of PI3K (Figure 2) and its downstream signaling cascade (Figure 1 part 2), sequential phosphorylation and activation of the serine/threonine kinase Akt, mammalian target of rapamycin (mTOR) and its substrate p70S6K. mTOR activation is rapid, with detectable phosphorylation as early as 10 minutes after T3 treatment, and not sensitive to cycloheximide (CHX) treatment, indicating that this effect of TH uses preexisting proteins. TH acts through the TRβ, because in human fibroblasts that express the WT TRβ, introduction of a dominant negative mutant TRβ abrogated the effect of TH. Furthermore, a direct interaction between TRβ and PI3K could be demonstrated by coimmunoprecipitation of TRβ1 with the p85α subunit of PI3K. However, activation of PI3K requires the presence of T3. The interaction between TRβ and PI3K most likely takes place in the cytosol. Within minutes after activation by T3, phosphorylated Akt, as part of the PI3K pathway, is translocated from the cytosol into the nucleus (Figure 1 part 2). This TH action is very rapid and independent of protein synthesis, which is typical of nongenomic action. Yet, two aspects distinguish this mechanism of TH action from most other nongenomic effects of the hormone: 1) it requires TR binding and 2) its ultimate effect is ‘genomic’ with specific genes induced by this mechanism. For example, the calcineurin inhibitor ZAKI-4α had been previously described as a TH responsive gene [Cao et al., 2002; Cao and Seo, 2003; Miyazaki et al., 1996]. This effect was blocked by inhibitors of PI3K and by a dominant negative p85α subunit of PI3K [Cao et al., 2005]. Rapamycin, an inhibitor of mTOR also abrogated the TH-dependent induction of ZAKI-4α, suggesting the requirement of sequential activation of kinases initiated by the activation of PI3K. ZAKI-4α expression is also CHX sensitive, indicating that protein synthesis is necessary for the induction of ZAKI-4α. These results show that ZAKI-4α represents an example of an indirectly induced gene downstream of nongenomic action of TH. The transcription factor linking the PI3K pathway to ZAKI-4α expression has not yet been identified. A similar activation of PI3K has been observed for other steroid hormone nuclear receptors [Simoncini et al., 2000].
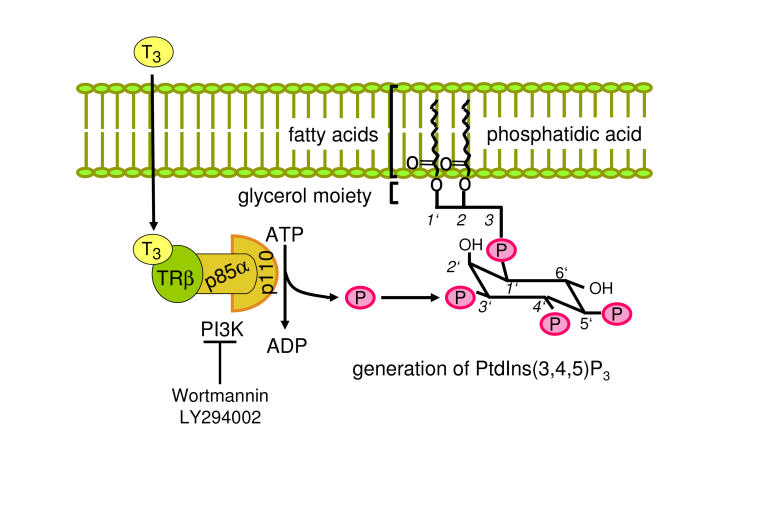
Figure 2. TH-dependent activation of PI3K.
TR present in the cytosol forms a complex with p85α subunit (regulatory subunit of PI3K) in a ligand independent manner. Ligand binding activates PI3K, generating phosphatidyl inositol-3,4,5-triphosphate (PtdIns(3,4,5)P3 from PtdIns(4,5)P2. PI3K activity is blocked by specific inhibitors such as wortmannin or LY294002.
TH target genes
To determine the broad scale effect of TH on gene expression in normal human cells, we measured the expression of more than 15,000 genes in fibroblasts of normal individuals by quantitative fluorescent cDNA microarray [Moeller et al., 2005b]. Fibroblasts from two subjects with resistance to thyroid hormone (RTH) due to mutations in the TRβ gene were used to confirm the specificity of the hormonal effect by the ability to discriminate between normal cells and cells with a defect in TH action. Microarray analysis identified 91 up-regulated and 5 down-regulated genes and confirmation by real-time PCR was obtained in 8 of 10 induced and 2 of 3 repressed genes that were tested. Several new TH responsive genes were identified, both positively (AKR1C1-3, PFKP, RAB3B, HIF-1α, COLVIA3) and negatively regulated (FGF7, ADH1B). Genes that had been found to respond to TH in other species were also found in these human cells (BTEB1, GLUT1, MCT4). Further evidence for T3-specific induction was provided by a graded dose response for induced genes that was absent in fibroblasts from the patients with RTH. Of special interest among the upregulated genes were the transcription factor subunit hypoxia-inducible factor (HIF)-1α, its target genes, glucose transporter (GLUT)1 and platelet-type phosphofructokinase (PFKP), and the monocarboxylate transporter (MCT)4. These genes are functionally related as they have important roles in cellular glucose metabolism, from glucose uptake (GLUT1) to glycolysis (PFKP) and lactate export (MCT4). The response of these genes to TH was reduced or absent in the TH resistant fibroblasts, which confirms the role of the TRβ.
The cytosolic action of TH induces HIF-1α and glycolytic genes
The transcription factor HIF-1 consists of two subunits, α and β. While the β subunit is constitutively expressed, the α subunit is tightly regulated. Cellular hypoxia leads to increase of HIF-1α protein levels by decreased ubiquitination [Semenza, 2001] and in normoxia, growth factors and hormones (e.g. EGF, IGF-1, insulin, androgens) lead to increased HIF-1α by posttranscriptional mechanisms [Bardos and Ashcroft, 2004; Kasuno et al., 2004]. The latter is mediated by cellular signal transduction pathways, especially the PI3K-Akt/PKB-mTOR pathway and the MAPK pathways [Fukuda et al., 2002; Kasuno et al., 2004]. We examined the involvement of the PI3K pathway by treating human skin fibroblasts with T3. In contrast to the response to growth factors, TH increased HIF-1α mRNA levels. mRNA of HIF-1α was increased after 3 hours and the effect lasted for the 24 hours of the experiments. mRNA induction led to significant increase in the protein, demonstrated by western blotting for HIF-1α as well as PFKP. Addition of the PI3K inhibitor LY294002 abrogated TH-dependent induction of mRNAs for HIF-1α as well as GLUT1, PFKP and MCT4 [Moeller et al., 2005a]. HIF-1α induction was direct and translation-independent, as CHX pretreatment did not inhibit HIF-1α mRNA increase after T3 treatment. As expected, expression of HIF-1’s target genes, GLUT1 and PFKP, was CHX-sensitive. The MAPK pathway was examined by pretreatment with PD98059, but inhibition of T3 effect was not observed. The MAPK pathway seems not to be involved expression of these genes in human fibroblasts. These results further demonstrate that activation of the PI3K signaling pathway by TH and TRβ in the cytosol can lead to direct (HIF-1α) and indirect (GLUT1, PFKP, MCT4) gene expression.
Conclusion and perspective
Until recently, TH-mediated changes in gene expression were thought to be primarily, if not solely, initiated by direct nuclear TR binding to a TRE in the promoter of a target gene. We found that the same TRβ can also exert extranuclear actions. It is capable of activating cellular signaling pathways in the cytosol, as demonstrated for the PI3K pathway, which leads to induction of ZAKI4α, HIF-1α and its target genes. This cytosolic mechanism of TH-mediated TRβ-dependent action contributes to the overall effect of TH on gene expression. Surprisingly, some of the most highly induced genes in our experiments were later found to be downstream of the PI3K pathway (e.g. ZAKI-4α, PFKP). A possible explanation is that kinase cascades have the potential to amplify the signal from kinase to kinase, whereas on a TRE amplification steps are limited. The definition of the TRs therefore has broadened beyond being merely ligand-dependent nuclear transcription factors. We conclude that TH responsive genes can be directly induced by nuclear (e.g. BTEB1) and cytosolic action of TH (e.g. HIF-1α) without requirement of prior protein synthesis. Indirectly induced genes, following expression of a transcription factor, exist for both modes of TH action. We expect that more TH-responsive genes will be described as ‘nongenomically’ induced and that more signaling pathways will be found to be activated by the TRs.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (DK 15070 and DK20595) and the US public Health Service (RR 00055) (to S.R.); Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (Monbukagakusho) of Japan (nos. 13470217 and 16390269) (to HS); by the 21st Century COE program “Integrated Molecular Medicine for Neuronal and Neoplastic Disorders” of Monbukagakusho (to HS and XC), the Howard Hughes Medical Institute (Predoctoral Fellowship to AMD) and the Deutsche Forschungsgemeinschaft DFG (Mo 1018/1-1) (to LCM).
Abbreviations
- ADH1B
alcohol dehydrogenase 1B
- AKR1
aldo-keto reductase family 1
- BTEB1
basic transcription element binding protein 1
- CHX
cycloheximide
- COLVIA3
collagen type VI α3
- EGF
epidermal growth factor
- FGF7
fibroblast growth factor 7
- GLUT1
glucose transporter 1
- GTF
general transcription factors
- HIF-1
hypoxia-inducible factor 1
- IGF-1
insulin-like growth factor I
- MAPK
mitogen-activated protein kinase
- MCT4
monocarboxylate transporter 4
- mTOR
mammalian target of rapamycin
- p70S6K
p70S6 kinase
- PFKP
platelet phosphofructokinase
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- RAB3B
member RAS oncogene family brain antigen RAB3B
- RTH
resistance to thyroid hormone
- RXR
retinoid X receptor
- T1AM
3-iodothyronamine
- T3
3,3,5-triiodothyronine
- TH
thyroid hormone
- TR
thyroid hormone receptor
- TRE
thyroid hormone response element
References
- Bardos J. I., Ashcroft M. Hypoxia-inducible factor-1 and oncogenic signalling. Bioessays. 2004;26:262–9. doi: 10.1002/bies.20002. [DOI] [PubMed] [Google Scholar]
- Baumann C. T., Maruvada P., Hager G. L., Yen P. M. Nuclear cytoplasmic shuttling by thyroid hormone receptors. multiple protein interactions are required for nuclear retention. J Biol Chem. 2001;276:11237–45. doi: 10.1074/jbc.M011112200. [DOI] [PubMed] [Google Scholar]
- Bergh J. J., Lin H. Y., Lansing L., Mohamed S. N., Davis F. B., Mousa S., Davis P. J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146:2864–71. doi: 10.1210/en.2005-0102. [DOI] [PubMed] [Google Scholar]
- Cao X., Kambe F., Miyazaki T., Sarkar D., Ohmori S., Seo H. Novel human ZAKI-4 isoforms: hormonal and tissue-specific regulation and function as calcineurin inhibitors. Biochem J. 2002;367:459–66. doi: 10.1042/BJ20011797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Kambe F., Moeller L. C., Refetoff S., Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol. 2005;19:102–12. doi: 10.1210/me.2004-0093. [DOI] [PubMed] [Google Scholar]
- Cao X., Seo H. Thyroid hormone-dependent regulation of ZAKI-4[α], an inhibitor of calcineurin, and its implication in brain development and function. Curr Opin Endocrinol and Diabetes. 2003;10:375–363. [Google Scholar]
- Davis P. J., Tillmann H. C., Davis F. B., Wehling M. Comparison of the mechanisms of nongenomic actions of thyroid hormone and steroid hormones. J Endocrinol Invest. 2002b;25:377–88. doi: 10.1007/BF03344022. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Davis F. B. Nongenomic actions of thyroid hormone on the heart. Thyroid. 2002a;12:459–66. doi: 10.1089/105072502760143827. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Hirota K., Fan F., Jung Y. D., Ellis L. M., Semenza G. L. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–11. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- Harvey C. B., Williams G. R. Mechanism of thyroid hormone action. Thyroid. 2002;12:441–6. doi: 10.1089/105072502760143791. [DOI] [PubMed] [Google Scholar]
- Kasuno K., Takabuchi S., Fukuda K., Kizaka-Kondoh S., Yodoi J., Adachi T., Semenza G. L., Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–8. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Kanou Y., Murata Y., Ohmori S., Niwa T., Maeda K., Yamamura H., Seo H. Molecular cloning of a novel thyroid hormone-responsive gene, ZAKI-4, in human skin fibroblasts. J Biol Chem. 1996;271:14567–71. doi: 10.1074/jbc.271.24.14567. [DOI] [PubMed] [Google Scholar]
- Moeller L. C., Dumitrescu A. M., Refetoff S. Cytosolic action of thyroid hormone leads to induction of hypoxia-inducible factor-1alpha and glycolytic genes. Mol Endocrinol. 2005b;19:2955–63. doi: 10.1210/me.2004-0542. [DOI] [PubMed] [Google Scholar]
- Moeller L. C., Dumitrescu A. M., Walker R. L., Meltzer P. S., Refetoff S. Thyroid hormone responsive genes in cultured human fibroblasts. J Clin Endocrinol Metab. 2005a;90:936–43. doi: 10.1210/jc.2004-1768. [DOI] [PubMed] [Google Scholar]
- Scanlan T. S., Suchland K. L., Hart M. E., Chiellini G., Huang Y., Kruzich P. J., Frascarelli S., Crossley D. A., Bunzow J. R., Ronca-Testoni S., Lin E. T., Hatton D., Zucchi R., Grandy D. K. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–42. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- Semenza G. L. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–50. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- Simoncini T., Hafezi-Moghadam A., Brazil D. P., Ley K., Chin W. W., Liao J. K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. Y., Lin H. Y., Zhang S., Davis F. B., Davis P. J. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology. 2004;145:3265–72. doi: 10.1210/en.2004-0308. [DOI] [PubMed] [Google Scholar]
- Yen P. M. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]