The classical paradigm of steroid hormone action is that intracellular receptors bind to specific steroids to modulate gene expression within the nuclei of target cells. However, increasing evidence now suggests that many important steroid-induced signaling events are triggered independent of transcription. Examples of “nongenomic” biological responses to steroids include estrogen-induced proliferation of breast cell lines (1), estrogen-mediated dilation of blood vessels (2, 3), and progesterone-induced activation of the acrosomal reaction in sperm (4). The signaling mechanisms responsible for these biological responses are diverse, such as activation of signaling molecules Src, extracellular signal-regulated kinase (ERK), endothelial nitric-oxide synthase (eNOS), and Akt, as well as rapid alterations in intracellular calcium and cAMP levels (5). Experiments designed to identify steroid receptors that may modulate these nongenomic processes have produced several candidates, including classical steroid receptors located in the membrane (1, 3, 6), traditional G protein-coupled receptors (GPCRs) (7, 8), and novel membrane-associated steroid binding proteins (9). In most cases, however, the true physiologic importance of these receptors has yet to be proven. Two articles by Zhu and colleagues (10, 11) in this issue of PNAS provide new and provocative insight toward identifying potentially physiologically relevant steroid receptors capable of mediating nongenomic signaling, describing a novel family of high-affinity membrane steroid receptors with structure and signaling similar to GPCRs.
The authors isolated and characterized these receptors by using one of the best-studied, biologically relevant, nongenomic steroid-mediated processes: steroid-induced maturation of oocytes (12). The maturation of an oocyte refers to its meiotic stage. “Immature” oocytes are arrested in prophase of meiosis I. Just before ovulation, oocytes are induced to reenter the cell cycle, finally resting in metaphase II. “Mature” oocytes are then competent for ovulation and subsequent fertilization, after which meiosis is completed. Steroid-mediated maturation has been best studied in amphibian and fish oocytes, where progesterone is a potent promoter of oocyte maturation in vitro. In vivo studies designed to directly examine ovarian steroid metabolism and production have demonstrated that, although progesterone promotes maturation in vitro, progesterone metabolites may be the true physiologic mediators of oocyte maturation in vivo. Examples of such biologically produced progesterone metabolites are androstenedione and testosterone in Xenopus laevis (13, 14), 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P) in salmon (15), and 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) in the Atlantic croaker and spotted sea trout (16).
Many important steroid-induced signaling events are triggered independent of transcription.
Evidence supporting the nongenomic nature of steroid-induced maturation comes from observations that transcriptional inhibitors such as actinomycin D do not affect steroid-mediated oocyte maturation (17), and that steroids induce decreases in intracellular cAMP that are too rapid (5–10 min) to involve transcriptional regulation (18). Furthermore, several studies have suggested that nongenomic steroid-induced maturation may be mediated by receptors located in the plasma membrane. First, steroids covalently bound to either polymers or BSA still induce maturation (19, 20). Second, direct injection of steroids into oocytes does not appear to promote maturation (21). Third, specific high-affinity steroid binding sites (Kd values in the 10–100 nM range) have been described in oocyte membrane preparations (16, 22–24). Unfortunately, these studies all have significant flaws: (i) BSA-conjugated steroid preparations have been shown to be “leaky,” with significant dissociation of steroid from the protein during the time required for oocyte maturation (25). (ii) Because of steroid insolubility and limits in injection volume, concentrations of injected steroids may not have been sufficient to induce maturation. Overexpression of the enzyme 3β-hydroxysteroid dehydrogenase within Xenopus oocytes results in intracellular conversion of exogenously applied inactive dehydroepiandrosterone (DHEA) to active androstenedione, which in turn leads to oocyte maturation. This result suggests that steroid coming from inside an oocyte is indeed capable of mediating maturation when enough is present (13). (iii) Binding assays using steroids and plasma membranes are often difficult to interpret, given the hydrophobic nature of the ligands and problems with contamination of membrane preparations.
In an effort to unequivocally identify progesterone receptors in the plasma membrane of spotted sea trout oocytes, Zhu and colleagues screened an oocyte expression library by using monoclonal antibodies directed against progestin-binding oocyte membrane proteins. They isolated a novel 352-aa protein termed mPR. The sea trout mPR bears little sequence homology to GPCRs; however, it contains seven putative hydrophobic transmembrane domains and therefore could be considered a novel member of the heptahelical receptor family. Based on the sequence of mPR, the authors then identified a family of mPR proteins from a number of different species, including frog, human, and mouse, some of which bound progesterone.
Expression studies were consistent with mPR playing a possible role in progestin-induced oocyte maturation. First, Northern blots demonstrated that mPR mRNA was expressed primarily in neuroendocrine tissues, including ovary, brain, testes, and pituitary. Second, fractionation and immunohistochemistry studies confirmed that the mPR protein was expressed in the oocyte plasma membrane. Third, mPR expression increased with the developmental stage of the oocyte, consistent with the higher sensitivity of later-stage oocytes to steroid-induced maturation. Fourth, binding studies using bacterially expressed mPR revealed specific, high-affinity binding to progesterone and several progesterone metabolites, with Kd values around 30 nM.
Functional studies also supported a role for mPR in steroid-mediated oocyte maturation. A breast cell line that stably expressed mPR exhibited pertussis toxin-sensitive decreases in cAMP levels in response to progesterone and 20β-S. This result is consistent with decreases in teleost oocyte cAMP levels on stimulation with progestins (16) and suggests that Gαi might be important in mediating maturation in fish. Additionally, progestins induced rapid phosphorylation of ERK1/2 in the same stably transfected cell line, confirming that mPR can mediate more than one nongenomic signal in somatic cells. Finally, although performed in a different species of fish, injection of mPR antisense oligonucleotides into zebrafish oocytes significantly attenuated progestin-induced oocyte maturation, suggesting that endogenous mPR is necessary for normal steroid-induced oocyte maturation.
The discovery of a novel family of membrane steroid receptors that appear to mediate dramatic physiologic responses to steroids both in a somatic cell line and in oocytes provides an exciting and major contribution to the quickly growing field of nongenomic signaling by steroids. The mPRs offer a relatively compelling mechanism that would tie together many of the described nongenomic steroid-induced signals with known GPCR-mediated signaling pathways. These results must be put into perspective, however, because they bring forth an entirely new set of issues that will now need to be resolved.
The first issue involves the nature of the receptors themselves. Nongenomic steroid-mediated signaling has frequently been linked to GPCRs. Examples include progesterone effects on oxytocin (7) and GABA receptor signaling (8), as well as estrogen signaling through GPR30 in breast cells (26). Are these mPRs true classical GPCRs? Although some of them contain seven putative transmembrane regions, they bear little sequence homology to known GPCRs, nor do most of them contain obvious amino-terminal signal peptide sequences found in many classical GPCRs and other membrane proteins with external amino termini. Although mPR is clearly expressed in the plasma membranes of oocytes and the transfected breast cell line, the location of its amino terminus has yet to be confirmed. These disparities will be interesting to examine, because they suggest that the mPRs might be signaling through G proteins in their own novel fashion.
Another important issue concerns the specificity and nature of steroid binding to the mPR family members. Nongenomic steroid-mediated signaling has been reported for nearly every steroid, including progestins, androgens, and estrogens (5). Even within the field of oocyte maturation, both progestins and androgens can promote maturation, depending on the animal (13, 16). Could different members of the mPR family preferentially bind different steroids? Does steroid binding depend on other factors within an individual target cell? Finally, how are these hydrophobic steroids specifically interacting with a receptor that contains no known steroid-binding domain? Interestingly, in contrast to the mPRs, most GPCRs do not retain sufficient structure to bind ligand when solubilized or expressed in bacteria, again emphasizing the unusual nature of the mPR family.
A third issue concerns the generalizability of mPR-mediated oocyte maturation. At this point, it is unclear whether steroids promote oocyte maturation in mammals. In fact, evidence suggests that they may even inhibit maturation under certain conditions (17). Furthermore, in amphibians, the steroid-induced drop in oocyte cAMP levels is not pertussis toxin-sensitive (27), and maturation does not appear to involve direct signaling by Gαi. Instead, Gαs and/or Gβγ may be promoting constitutive inhibitory signals that are overcome on addition of steroids (24, 28, 29). Perhaps different mPRs are capable of signaling through different G proteins, depending on the specific isoform of mPR and/or the cell in which they are expressed.
Finally, although the mPRs might be critical for oocyte physiology, they are unlikely to explain all nongenomic phenomena. For example, compelling evidence suggests that classical estrogen and androgen receptors (ER and AR) are playing important roles in mediating nongenomic effects in bone, endothelium, and breast (1, 3, 6). Even in the frog oocyte, modulation of classical PR levels has been shown to modestly alter progesterone-induced maturation (30–32), classical PRs have been shown to be associated with PI3K in membranes (33), and AR antagonists block androgen-induced maturation (13), suggesting at least a partial role for classical steroid receptors in amphibian oocyte maturation.
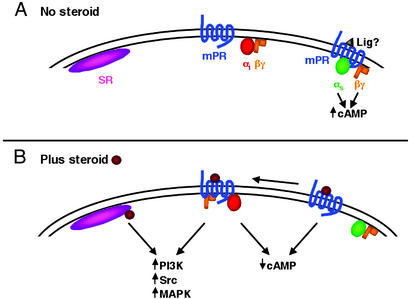
To reconcile the existing data, the following model for early nongenomic signaling in steroid-induced oocyte maturation is proposed (Fig. 1). Perhaps both the classical and newly described membrane steroid receptors are used, depending on the target cell and steroid involved. This would be similar to nongenomic signaling by estrogen, where both the ER and the GPCR GPR30 appear to be important for mediating nongenomic estrogen-induced events in some breast cells, whereas the ER alone seems to mediate up-regulation of NOS in endothelial cells. Oocytes may contain both membrane (mPR) and classical (SR) steroid receptors at or near the plasma membrane. In fish, stimulation of the mPR with steroid might lead to rapid activation of Gαi, resulting in a rapid decrease in cAMP (Fig. 1, center). Other signals, such as activation of the PI3K or mitogen-activated protein kinase (MAPK) pathways, might also be rapidly altered, although very early activation of either pathway by steroids has not been reported in oocytes. Alternatively, some mPRs might be constitutively signaling through Gαs and/or Gβγ, either autonomously (34) or through stimulation by a yet unknown ligand, to block maturation (Fig. 1, right). Steroids might then act as antagonists to block these signals, resulting in a decrease in intracellular cAMP. This “release of inhibition” concept for oocyte maturation would be consistent with mammalian systems, where unidentified factors that inhibit oocyte maturation, possibly by keeping intracellular cAMP levels high, have been found in the ovaries of pigs and humans (35, 36). Interestingly, activated β2-adrenergic receptors have been shown to “switch classes” from Gαs to Gαi signaling (37), which would result in even greater reductions in intracellular cAMP. If mPRs were functioning similarly, then yet another level of complexity could be attributed to mPR signaling. Finally, steroid binding to membrane-associated classical receptors might be signaling independently or in concert with the mPR to promote signaling through Src, PI3K, and MAPK (Fig. 1, left).
Figure 1.
Model for early nongenomic signaling in steroid-induced oocyte maturation. (A) In the absence of steroid, both the classical (SR) and membrane steroid (mPR) receptors are expressed at or near the oocyte plasma membrane (left and center). In some species, mPR might also be constitutively signaling through Gαs and/or Gβγ, either autonomously or through stimulation by an unknown ligand (Lig?), to block maturation (right). (B) Addition of steroid could lead to any combination of the following: (i) Steroid-bound mPR might rapidly activate Gαi, resulting in a decrease in intracellular cAMP (center). mPR-mediated activation of the PI3K or MAPK pathways might also occur. (ii) Steroid binding to the constitutively activated mPR could shut off signaling, which would also lower intracellular cAMP (right). These inactivated receptors could even “switch classes” from Gαs to Gαi signaling, resulting in even greater reductions in intracellular cAMP. (iii) Steroid binding to membrane-associated classical receptors might be signaling independently or in concert with the mPR to promote signaling through Src, PI3K, and MAPK (left).
In summary, the studies presented by Zhu and colleagues reemphasize the complexity of both steroid and GPCR-mediated signaling and may redefine the way that we think about both of these processes. Perhaps just as classical steroid receptors dramatically alter genomic signaling when in the nucleus, steroid receptors within the membrane may modulate multiple nongenomic membrane signaling cascades. Defining the interplay between these genomic and nongenomic signals will likely be critical for understanding the diverse biological responses to steroids.
Footnotes
References
- 1.Razandi M, Oh P, Pedram A, Schnitzer J, Levin E R. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 2.Simoncini T, Fornari L, Mannella P, Varone G, Caruso A, Liao J K, Genazzani A R. Steroids. 2002;67:935–939. doi: 10.1016/s0039-128x(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 3.Shaul P W. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 4.Luconi M, Bonaccorsi L, Bini L, Liberatori S, Pallini V, Forti G, Baldi E. Steroids. 2002;67:505–509. doi: 10.1016/s0039-128x(01)00173-8. [DOI] [PubMed] [Google Scholar]
- 5.Cato A C, Nestl A, Mink S. Sci STKE. 2002;2002:RE9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- 6.Kousteni S, Bellido T, Plotkin L I, O'Brien C A, Bodenner D L, Han L, Han K, DiGregorio G B, Katzenellenbogen J A, Katzenellenbogen B S, et al. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 7.Grazzini E, Guillon G, Mouillac B, Zingg H H. Nature. 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 8.Falkenstein E, Wehling M. Eur J Clin Invest. 2000;30, Suppl. 3:51–54. doi: 10.1046/j.1365-2362.2000.0300s3051.x. [DOI] [PubMed] [Google Scholar]
- 9.Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. Endocrinology. 1999;140:5999–6002. doi: 10.1210/endo.140.12.7304. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Rice C D, Pang Y, Pace M, Thomas P. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Bond J, Thomas P. Proc Natl Acad Sci USA. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maller J L. Proc Natl Acad Sci USA. 2001;98:8–10. doi: 10.1073/pnas.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz L B, Cole L M, Gupta M K, Kwist K W, Auchus R J, Hammes S R. Proc Natl Acad Sci USA. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang, W. H., Lutz, L. B. & Hammes, S. R. (2003) J. Biol. Chem., in press.
- 15.Nagahama Y, Adachi S. Dev Biol. 1985;109:428–435. doi: 10.1016/0012-1606(85)90469-5. [DOI] [PubMed] [Google Scholar]
- 16.Thomas P, Zhu Y, Pace M. Steroids. 2002;67:511–517. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 17.Smith D M, Tenney D Y. J Reprod Fertil. 1980;60:331–338. doi: 10.1530/jrf.0.0600331. [DOI] [PubMed] [Google Scholar]
- 18.Sadler S E, Maller J L. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:179–194. [PubMed] [Google Scholar]
- 19.Godeau J F, Schorderet-Slatkine S, Hubert P, Baulieu E E. Proc Natl Acad Sci USA. 1978;75:2353–2357. doi: 10.1073/pnas.75.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maller J L, Krebs E G. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- 21.Masui Y, Markert C L. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Patino R. Biol Reprod. 1993;49:980–988. doi: 10.1095/biolreprod49.5.980. [DOI] [PubMed] [Google Scholar]
- 23.Morrill G A, Ma G Y, Kostellow A. Biochem Biophys Res Commun. 1997;232:213–217. doi: 10.1006/bbrc.1997.6190. [DOI] [PubMed] [Google Scholar]
- 24.Lutz L B, Kim B, Jahani D, Hammes S R. J Biol Chem. 2000;275:41512–41520. doi: 10.1074/jbc.M006757200. [DOI] [PubMed] [Google Scholar]
- 25.Stevis P E, Deecher D C, Suhadolnik L, Mallis L M, Frail D E. Endocrinology. 1999;140:5455–5458. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- 26.Filardo E J, Quinn J A, Frackelton A R, Jr, Bland K I. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 27.Sadler S E, Maller J L, Cooper D M. Mol Pharmacol. 1984;26:526–531. [PubMed] [Google Scholar]
- 28.Gallo C J, Hand A R, Jones T L, Jaffe L A. J Cell Biol. 1995;130:275–284. doi: 10.1083/jcb.130.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheng Y, Tiberi M, Booth R A, Ma C, Liu X J. Curr Biol. 2001;11:405–416. doi: 10.1016/s0960-9822(01)00123-3. [DOI] [PubMed] [Google Scholar]
- 30.Bayaa M, Booth R A, Sheng Y, Liu X J. Proc Natl Acad Sci USA. 2000;97:12607–12612. doi: 10.1073/pnas.220302597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian J, Kim S, Heilig E, Ruderman J V. Proc Natl Acad Sci USA. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boonyaratanakornkit V, Scott M P, Ribon V, Sherman L, Anderson S M, Maller J L, Miller W T, Edwards D P. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 33.Bagowski C P, Myers J W, Ferrell J E., Jr J Biol Chem. 2001;276:37708–37714. doi: 10.1074/jbc.M104582200. [DOI] [PubMed] [Google Scholar]
- 34.Seifert R, Wenzel-Seifert K. Naunyn-Schmiedeberg's Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 35.Pomerantz S H, Bilello P A. Gamete Res. 1987;17:267–278. doi: 10.1002/mrd.1120170310. [DOI] [PubMed] [Google Scholar]
- 36.Cameron I L, Lum J B, Nations C, Asch R H, Silverman A Y. Biol Reprod. 1983;28:817–822. doi: 10.1095/biolreprod28.4.817. [DOI] [PubMed] [Google Scholar]
- 37.Lefkowitz R J. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]