Abstract
A double-blind placebo-controlled crossover Phase I trial was conducted to assess the safety and tolerability of N-Acetylcysteine (NAC) in healthy, cocaine-dependent humans. Thirteen participants attended a three-day hospitalization in which they received placebo or NAC. Subjects were crossed over to receive the opposite medication condition during a second three-day hospitalization, which occurred the following week. Across placebo and NAC conditions, only mild side effects were noted, and the number of subjects reporting side effects did not differ. There were trends for a greater reduction in withdrawal symptoms and craving within the NAC condition. These preliminary results suggest that NAC is well tolerated in healthy, cocaine-dependent individuals and may reduce cocaine-related withdrawal symptoms and craving. (Am J Addict 2006;15:105-110)
Although overall rates of cocaine use and the number of individuals seeking treatment for cocaine dependence has declined over the last decade, cocaine continues to be the most frequently mentioned illicit drug in drug-related emergency department reports.1 Cocaine use has been associated with numerous adverse health consequences.2-4 Crack cocaine use has also been associated with multiple high-risk sexual practices5 as well as criminal activity.6 At the present time, there is no effective FDA-approved treatment for cocaine dependence, despite over two decades of intense research. A pharmacological treatment that could serve as an effective adjunct to psychosocial treatment for cocaine dependence would constitute a major public health advancement.
Preclinical investigations have suggested that cocaine-seeking behavior is mediated by levels of glutamate within the nucleus accumbens.7 Specifically, studies have shown that cocaine addiction results in a neuroadaptation characterized by lower basal levels of glutamate in the accumbens.8 The lower basal level of glutamatergic tone results in an exaggerated release of glutamate within the nucleus accumbens during acute cocaine challenge, which in turn produces a stronger display of cocaine-seeking behavior.8-11 N-Acetylcysteine, an amino acid and cysteine pro-drug, appears to restore basal levels of glutamate in the accumbens, leading to a marked reduction in drug-seeking behavior after a cocaine challenge.12 These findings implicate N-acetylcysteine as a possible medication for the treatment of cocaine dependence.
N-acetylcysteine (NAC) is the N-acetyl derivative of the naturally occurring amino acid cysteine. NAC is a white, crystalline compound with a molecular formula of C5H9NO3S and weight of 163.2.13 Worldwide, NAC has been available for several years in intravenous, oral, and nebulizer forms. It is used as a mucolytic agent for bronchopulmonary disorders that produce viscous sputum, such as cystic fibrosis and chronic obstruction lung disease.14 Because NAC is a precursor for glutathione synthesis and forms complexes with toxic reactive metabolites of acetaminophen, which prevents hepatic cell necrosis, it has been used for many years as an antidote to treat acetaminophen poisoning.15 In Europe, NAC in intravenous form is preferred for the treatment of acetaminophen overdose. In the United States, NAC has been FDA approved and has been available in liquid form for nebulizer use in cystic fibrosis and in oral form for acetaminophen toxicity.16 In June 2004, the FDA approved the intravenous form to treat acetaminophen poisoning.
In addition to the FDA-approved indications described above, research studies have suggested that NAC may treat a number of other conditions. NAC has been shown to be useful in preventing X-ray contrast nephropathy.17 There is also evidence to suggest that NAC can beneficially effect CNS function, as evidenced by a recent study that showed improved cognitive functioning in dementia patients treated with NAC.18 Finally, NAC is sold as an orally administered, over-the-counter tablet in health food stores, usually promoted as improving overall mental functions.
Orally administered NAC appears to be well tolerated. A meta-analysis of studies evaluating the long-term oral treatment with NAC for chronic bronchopulmonary disease found that NAC use was associated with mild adverse effects, mostly gastrointestinal (nausea, vomiting, diarrhea) and self-limiting.14 In the context of acetaminophen overdose, oral NAC has been reported to produce increased blood pressure, chest pain, hypertension, rectal bleeding, respiratory distress, headache, lethargy, fever, and skin rash.19 It should be noted, however, that because these adverse events occur in the context of acetaminophen toxicity, it is difficult to determine the extent that they occur as a result of NAC alone.
Side effects for intravenous NAC include vomiting and diarrhea.16,20 In one series of 529 consecutive patients treated for acetaminophen poisoning using intravenously administered NAC, 45 patients (8%) developed an allergic reaction to NAC.21 Most of these were cutaneous reactions consisting of rash, pruritus, and flushing. Eighteen patients appeared to have systemic allergic reactions to NAC, consisting of bronchospasm, angioedema, and nausea. These were managed with the use of antihistamines, corticosteroids, and/or epinephrine. All patients made a full recovery. The investigators noted that patients with a history of asthma were three times more likely to develop systemic allergic reactions than patients with no history of asthma. A past history of medication allergy in general did not increase the risk of allergic reactions to NAC. There has been one reported case of seizure activity in a two-year old girl who developed status epilepticus during intravenous NAC therapy for acetaminophen overdose.22
Taken together, the bulk of the data suggest that NAC in both oral and IV form is generally well tolerated and safe across the treatment of several conditions. Nevertheless, the few existing documented instances of severe reaction to NAC, particularly in IV use,23,24 underscore the need for caution when NAC is used in medical interventions. At present, there are no safety or tolerability data with respect to NAC use in cocaine-dependent humans. This fact, combined with the promising results observed for NAC in preclinical studies, has led to the initiation of this Phase I trial for NAC in cocaine-dependent humans. The present study consisted of a double-blind, placebo-controlled crossover trial. It involved the administration of NAC and placebo to non-treatment-seeking, cocaine-dependent volunteers who were free of cocaine at the time of the trial. It was predicted that NAC would be safe and well tolerated in healthy, cocaine-dependent individuals. Secondarily, it was predicted that the use of NAC would lower ratings of cocaine craving and reduce symptoms associated with cocaine withdrawal.
METHOD
Subjects
Subjects were six males and seven females with a mean age of 37.1 (SD = 7.6, range were = 23 to 45), of whom nine were African-American and four were Caucasian, who met DSM-IV criteria for cocaine dependence but were non-treatment seeking. Of those subjects, eight were primarily crack smokers, two primarily used nasal powder, while the remaining subjects used a mixture of crack, powder, and freebase cocaine. Self-report accounts of cocaine use for the ninety days prior to study participation indicated that subjects used forty out of the ninety days on average (SD = 22.3, range = 10 to 90), or about 44% of the time, spending a daily average of $30 (SD $12, range = $10 to $45). Subjects were recruited flyers, newspaper ads, and word-of-mouth. Subjects received monetary compensation for their participation ($100 per each day in the hospital plus $300 for completion of follow-up visits, for a total of $900).
Inclusion Criteria
Subjects were included if they satisfied DSM-IV criteria for current cocaine dependence, produced a positive urine drug screen (UDS), and had a stable residence as well as a significant other who could be contacted in the event of an emergency. Included subjects were in good physical health; a particular emphasis was placed on identifying any physical conditions previously linked with adverse events related to N-Acetylcysteine (eg, history of asthma or seizure activity). Subjects who smoked had to agree to use a nicotine patch while hospitalized.
Exclusion Criteria
Females who were pregnant or refused to use birth control during the trial were excluded. Subjects dependent on any substance other than cocaine, nicotine, marijuana, and alcohol were also excluded. Subjects had to be free of concurrent psychiatric disorder (eg, major depression, schizophrenia, or PTSD). Subjects who had any history of delusions or hallucinations, including during acute use of cocaine, were excluded from the study as well. Subjects taking any psychotropic medications such as SSRIs or benzodiazepines were required to be free of these medications for two weeks prior to participating in the study. Subjects with any serious medical illness or history of asthma or seizures were excluded. Treatment-seeking subjects were not enrolled in the present study; instead, they were referred to treatment as appropriate.
Procedures
Screening Phase
Subjects were screened anonymously by phone to provide an initial assessment of eligibility. Those who met initial eligibility criteria were invited to a 45-minute informed consent session (conducted by the first author) in which the details of the study were carefully described. All subjects then signed written informed consent approved by the Medical University of South Carolina Institutional Review Board. Upon completion of consent, subjects underwent a series of outpatient visits in which they received a physical and medical history, the Mini International Neuropsychiatric Inventory,25 urine drug screens, an EKG, and a TB test. Subjects also completed a ninety-day Time Line Follow-Back26 account of their cocaine usage. Once it was determined that subjects satisfied inclusion/exclusion criteria, they were enrolled in the study and scheduled for two three-day inpatient hospital stays over two consecutive weeks.
Hospitalization Phase
Upon arrival for admission to the hospital, all female participants were screened for pregnancy. Participants were required to be free of cocaine at the time of admission, as verified by UDS. Along with the initial assessment of vital signs, blood and urine samples were collected in order to complete a complete blood count (CBC), complete metabolic panel (CMP), and urinalysis (UA). Cocaine withdrawal was assessed using the Cocaine Selective Severity Assessment (CSSA).27 Baseline ratings of craving were collected as well. Craving ratings included five items that asked participants to rate desire to use, level of craving, and other similar constructs on a ten-point likert scale. At approximately two hours after admission, subjects received their first dose of either N-Acetylcysteine 600 mg or an identically appearing placebo capsule containing lactose powder. Because previous studies have suggested that peak plasma concentration of NAC occurs approximately 1-2 hours after ingestion,28 subjects were assessed two hours after receiving medication, at which time vitals were taken, ratings of craving were collected, and subjects were assessed for side effects. This was repeated three times at twelve-hour intervals for a total of four doses of NAC (2400 mg over the study period). Additional assessments for craving and side effects occurred 14 and 26 hours after the last dose of medication. On the second full day of hospitalization, an EKG was performed. In addition, three of the thirteen subjects completed a diagnostic EEG to assess for any signs of brain activity that might be indicative of seizures (these same three subjects completed this under both placebo and NAC conditions). By the time participants were discharged, 26 hours had elapsed from the last dosage. This helped to ensure that subjects had little or no serum levels of NAC remaining at discharge. At time of discharge, cocaine withdrawal symptoms were assessed using the CSSA. In the days immediately following discharge, subjects were contacted by phone to assess for any further side effects. Four days later, subjects were admitted to the hospital for a second stay. All procedures were identical except that subjects who received NAC during the first week were crossed over to receive placebo during the second, and those who received placebo during the first week were crossed over to receive NAC during the second. In contrast to the first hospitalization, when labs were collected immediately after admission, labs (CBC, CMP, and UA) were collected immediately prior to discharge. Thus, the first set of lab values provides a baseline pre-study estimate of lab levels, while the second set of lab values provides a post-study estimate of these same values.
Follow-up Phase
Subjects returned for follow-up visits one week and two weeks post-discharge and were assessed for any further side effects. Self-reported use of cocaine was also assessed using the Time Line Follow Back procedure.
RESULTS
Side Effects
Across both hospitalizations, twenty instances of side effects were reported within the NAC condition, thirteen within placebo. The side effects reported in the NAC vs. placebo conditions included pruritus (1 vs. 5), headache (3 vs. 1), flatulence/diarrhea (2 vs. 2), abdominal cramps (2 vs. 0), local rash (1 vs. 1), fatigue (1 vs. 0), instances of increased blood pressure (4 vs. 2), sweat (1 vs. 1), ears “popping” (1 vs. 0), increased appetite (1 vs. 0), canker sore (1 vs. 0), chest pain (1 vs. 0), crying (1 vs. 0), and dizziness (0 vs. 1). None of these side effects was serious or unexpected. McNemar’s test for dependent proportions revealed that the proportion of subjects that reported side effects in the NAC condition (eight of thirteen) was not different (p < .71) from the proportion of subjects that reported side effects in the placebo condition.
Results of Clinical Diagnostic Laboratory Tests
Lab values were inspected for changes from baseline to post-study. Creatinine, bilirubin, SGOT, SGPT, white blood cell count (WBC), and hemoglobin levels were inspected for statistically significant as well as clinically significant changes (see Table 1). Statistical analyses revealed no significant changes. There was a trend toward a decrease in WBC levels (p < .07), but this decrease was not clinically significant as mean values remained well within the normal range. EKGs indicated no clinically significant problems or changes during either session. For the three participants who underwent the standard diagnostic EEGs, no abnormalities were noted for participants regardless of medication condition.
TABLE 1.
Laboratory values for all hospitalized subjects at baseline and post-study (n = 13)
| Baseline |
Post-study |
|||
|---|---|---|---|---|
| M | SE | M | SE | |
| Creatinine | 0.84 | 0.05 | 0.82 | 0.05 |
| Bilirubin | 0.64 | 0.05 | 0.55 | 0.04 |
| SGOT | 36.69 | 7.61 | 36.31 | 5.66 |
| SGPT | 41.38 | 11.29 | 46.46 | 11.44 |
| White blood cell count* | 8.22 | 0.82 | 7.37 | 0.79 |
| Hemoglobin | 13.81 | 0.44 | 13.80 | 0.41 |
Change from pre- to post-study showed a trend towards significance, p < .07.
Craving Measures
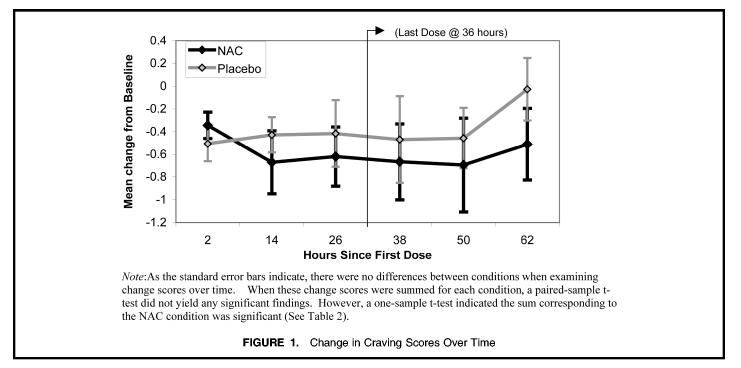
Because the distribution of craving scores tended to deviate from normality, all values were log transformed. Craving scores were then calculated as change from baseline (time immediately before medication) for all six craving assessments, and the change scores were summed for Weeks 1 and 2. A preliminary 2× 2 (week × medication condition) ANOVA was performed to assess for the effects of week (first week vs. second) and medication condition (NAC at Week 1 vs. placebo at Week 1). The effect for week was non-significant, p < .12, as was the main effect for medication condition (p < .63) and the week × medication condition interaction, p < .67. In a post-hoc analysis, craving data for the NAC condition and placebo condition were pooled irrespective of week. Craving scores were again calculated as change from baseline for all six craving assessments (see Fig. 1).
FIGURE 1.
Change in Craving Scores Over Time
Change scores were summed and compared using a paired-samples t-test. While the paired-samples t-test did not detect a significant difference between the mean change score for the NAC vs. placebo condition, a one-sample t-test revealed that the change score for the NAC condition was significantly different from zero, while the change score for the placebo condition was not (see Table 2). These results suggested a statistically significant drop in craving scores within the NAC condition but not in the placebo condition.
TABLE 2.
Secondary outcome variables: Craving, withdrawal (CSSA Scores), and cocaine use data
| Change in craving and CSSA totals scores by condition (n = 13) | NAC condition |
Placebo condition |
||
|---|---|---|---|---|
| M | SE | M | SE | |
| Sum of craving change scores | -3.50* | 1.57 | -2.30 | 1.36 |
| Change for CSSA total scores† | -7.38‡ | 2.69 | -2.62 | 2.10 |
| Pre- and post-study self-reported average daily dollar value of cocaine used and proportion of days used (n = 13) | Pre-study |
Post-study |
||
| M | SE | M | SE | |
| Daily $ use of cocaine§ | $30.31 | 3.44 | $8.77 | 2.52 |
| Mean proportion days used∥ | .41 | .07 | .27 | .07 |
One-sample t-test, t12 = -2.24, p < .05.
Change from intake to discharge.
One-sample t-test, t12 = -2.75, p < .05.
Daily $ use was lower during post-study follow-up, Wilcoxon signed rank, Z = -1.99, p < .05.
Proportion of days used was lower during post-study follow-up, Wilcoxon signed rank, Z = -3.18, p < .01.
Withdrawal Measures
CSSA total scores were calculated for baseline measures as well as measures collected at discharge. Change scores were calculated as the difference between discharge and baseline values. A preliminary 2 × 2 (week × medication condition) ANOVA was performed to assess for effects of week (Week 1 vs. Week 2) and medication condition (NAC at week 1 vs. placebo at week 1). Neither the effect for week (p < .88), nor the effect for medication condition (p < .84) was significant. The week × medication medication condition interaction was also non-significant, p < .22. In a post-hoc analysis, change scores were calculated for NAC and placebo conditions irrespective of week. While paired samples t-test did not detect a significant difference between change scores, one-sample t-tests indicated that the change score for the NAC condition was significantly different from zero, while the change score for the placebo condition was not (see Table 2).
Self-Reported Use
The proportion of days of use and average daily dollar value was calculated for the ninety days prior to the initial screening. The proportion of days used as well as average daily dollar value were calculated for the two-week follow-up period as well. Subjects provided an average of seven days of follow-up data, ranging from five to fourteen days (M = 10.7, SD = 3.5). Because the data were non-normally distributed, non-parametric Wilcoxon signed rank tests for related samples were used to calculate differences between pre- and post-study use. Results indicated a significant difference between pre- and post-study proportion of days used, Z = -1.99, p < .05, as well as a significant difference between pre- and post-study average daily dollar value, Z = -3.18, p <.01.
DISCUSSION
These results suggest that NAC was safe and well tolerated in non-treatment-seeking-cocaine dependent adults. The number of subjects reporting side effects was not significantly different across NAC and placebo conditions. Importantly, no unexpected side effects were noted, and none of the serious side effects associated with intravenous NAC use were noted (e.g., seizure or severe allergic reactions). No statistically or clinically significant changes in laboratory values were noted upon completion of the procedures. There were also indications that individuals within the NAC condition experienced a greater reduction in cocaine-related withdrawal symptoms and craving. Finally, participants reported less use after the completion of the hospital phase of the present study.
While these results are encouraging, they must be interpreted with a substantial degree of caution. Most notably, this study is limited by a small sample size. Thus, the larger number of side effects in the NAC condition may reach significance with a larger sample. In addition, though no serious side effects were observed, the probability of their occurrence could increase with a larger sample size.
The data pertaining to cocaine withdrawal and craving must also be interpreted with caution. There were no differences between the NAC and placebo conditions when the data from the two conditions were directly compared to one another; indeed, only the one-tailed t-tests for the NAC condition reached significance. It is possible that within a larger sample, one-tailed t-tests for the placebo condition will reach significance as well, even as the comparisons between conditions remain non-significant. The drop in self-reported cocaine use must also be interpreted with caution. While it is encouraging that the self-report data suggested lower use after the completion of the study, the design of the present study makes it impossible to attribute this drop to any specific drug effect at the present time.
Despite the caveats noted above, the present results are cause for cautious optimism and argue for further studies with NAC as a treatment for cocaine dependence. If the safety and efficacy of NAC is borne out in future work, this could prove to be a significant advance in the treatment of cocaine dependence for a number of reasons. On a practical level, NAC is already marketed as a relatively inexpensive dietary supplement, which means it would be a readily available treatment option for those who might not otherwise have the resources to receive treatment for substance abuse. More importantly, while basic science studies have elucidated the role of glutamate in cocaine addiction, this finding has not yet been translated in a way that advances the treatment of human cocaine addiction. The use of NAC could provide important evidence supporting the further development of agents targeting the glutamatergic system in the treatment of addictions.
CONCLUSION
The present results suggested that N-acetylcysteine (NAC) was safe and well tolerated in thirteen non-treatment-seeking cocaine-dependent adults, as the reported side effects were mild. Moreover, the number of subjects reporting side effects did not differ significantly between NAC and placebo conditions. Finally, the data hint that NAC may reduce craving and symptoms associated with cocaine withdrawal. While the present results are preliminary, they are nevertheless encouraging and grounds for further investigation.
Footnotes
This research was made possible by the support of grants DA07288 (Dr. Jacqueline McGinty), DA015369 (Dr. Myrick), RR01070 (MUSC), and DA018501 (Dr. LaRowe) from the National Institute on Drug Abuse, Bethesda, Md., and with the co-operation of the MUSC General Clinical Research Center, Center for Drug and Alcohol Programs, Neurobiology of Addictions Research Center, and Division of linical Neurosciences.
REFERENCES
- 1.Drug Abuse Warning Network . Office of Applied Studies; Washington, DC: 2001. [Google Scholar]
- 2.Brust JC, Richter R. Stroke associated with cocaine abuse. New York State Journal of Medicine. 1977;77:1473–1475. [PubMed] [Google Scholar]
- 3.Schwartz KA, Cohen JA. Subarachnoid hemorrhage precipitated by cocaine snorting. Arch Neurol. 1984;41:705. doi: 10.1001/archneur.1984.04050180027008. [DOI] [PubMed] [Google Scholar]
- 4.Cregler LL, Mark H. Medical complications of cocaine abuse. N Eng J Med. 1986;315:1495–1500. doi: 10.1056/NEJM198612043152327. [DOI] [PubMed] [Google Scholar]
- 5.Booth RE, Watters JK, Chitwood DD. HIV risk-related sex behaviors among injection drug users, crack smokers, and injection drug users who smoke crack. Am J Public Health. 1993;83:1144–1148. doi: 10.2105/ajph.83.8.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conaboy RP. United States Sentencing Commission; Washington, DC: 1995. Cocaine and Federal Sentencing Policy. [Google Scholar]
- 7.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce RC, Bell K, Duffy P, et al. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 10.Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- 11.Reid MS, Hsu KJ, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Baker DA, Khroyan TV, O’Dell LE, et al. Differential effects of intra-accumbens sulpiride on cocaine-induced locomotion and conditioned place preference. J Pharmacol Exp Ther. 1996;279:392–401. [PubMed] [Google Scholar]
- 13.Mosby’s Drug Consult . 14th ed Mosby; St. Louis, Mo.: 2004. pp. II-29–II-31. [Google Scholar]
- 14.Grandjean EM, Berthet P, Ruffman R, et al. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published, double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 15.Smilkstein MJ, Knapp GL, Kulig KW, Rumak BH. Efficacy of oral N-acetylcysteine in treatment of acetaminophen overdose: analysis of the national multicenter study. N Eng J Med. 1998;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 16.Mucomyst [package insert] Bristol Meyers Squibb; Princeton, NJ: 2001. [Google Scholar]
- 17.Birck R, Krzossok S, Markowetz F, Schnulle P, van der Woude FJ, Braun C. Acetylcysteine for the prevention of contrast nephropathy: meta-analysis. Lancet. 2003;362:598–603. doi: 10.1016/S0140-6736(03)14189-X. [DOI] [PubMed] [Google Scholar]
- 18.Adair JC, Knoefel JE, Morgan Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology. 2001;57:1515–1517. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- 19.Miller LF, Rumack BH. Clinical safety of high oral doses of acetylcysteine. Semin Oncol. 1983;10:76–85. [PubMed] [Google Scholar]
- 20.Kozer E, Koren G. Management of paracetamol overdose: current controversies. Drug Saf. 2001;24:503–512. doi: 10.2165/00002018-200124070-00003. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. Br J Clin Pharmacol. 2001;51:87–91. doi: 10.1046/j.1365-2125.2001.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershkovitz E, Shorer Z, Levitas A, Tal A. Status epilepticus following intravenous N-acetylcysteine therapy. Israel Journal of Medical Science. 1996;32:1102–1104. [PubMed] [Google Scholar]
- 23.Mant TGK, Tempowski JH, Volans GN, Talbot JCC. Adverse reactions to acetylcysteine and effects of overdose. BMJ. 1984;289:217–219. doi: 10.1136/bmj.289.6439.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Death after N-acetylcysteine [Notes and News] Lancet. 1984;1:1421. [Google Scholar]
- 25.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 26.Sobell MB, Sobell LC. Behavioral Treatment of Alcohol Problems. Plenum Press; New York, NY: 1978. [Google Scholar]
- 27.Kampman KM, Volpicelli JR, McGinnis DE, et al. Reliability and validity of the cocaine selective severity scale. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 28.Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokineti. 1991;20:123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]