Abstract
There is a critical need for alternates to native vein or artery for vascular surgery. The clinical efficacy of synthetic or allogeneic/xenogeneic vessels has been limited by thrombosis, rejection, chronic inflammation, or poor mechanical properties. Using adult human fibroblasts extracted from skin biopsies harvested from patients with advanced cardiovascular disease, we have constructed Tissue Engineered Blood Vessels (TEBVs) that served as arterial bypass grafts in long-term animal models. These TEBVs demonstrated mechanical properties similar to human blood vessels without relying upon synthetic or exogenous scaffolding. The TEBVs were antithrombogenic and mechanically stable through 8 months in vivo. Histology demonstrated complete tissue integration and vasa vasorum formation. The endothelium was confluent and von Willebrand factor positive. A smooth muscle specific α-actin positive cell population developed within the TEBV, suggesting regeneration of a vascular media. Electron microscopy revealed an endothelial basement membrane, elastogenesis and a complex collagen network. These results demonstrate that a completely biological and clinically relevant TEBV can be assembled exclusively from a patient’s own cells.
Cardiovascular Tissue Engineering introduced the prospect of a completely biological, living, and autologous blood vessel produced in vitro to fill the critical need for functional small diameter conduits. Since Weinberg and Bell’s landmark report1, the fundamental challenge in the field has been to produce a vessel with sufficient mechanical strength without relying upon permanent synthetic scaffolds. Using animal cells, two of the most promising tissue engineering models have addressed this challenge2,3, for other models see review 4. However, attempts to transition to human cells underscored the difficulty of producing a clinically relevant conduit. Specifically, these human TEBVs failed to provide requisite mechanical strength or required neonatal/juvenile cells5–8. While the use of human cells in low pressure applications (< 20 mmHg) has shown inspiring clinical success in pediatrics9, the promise of a tissue engineered graft for adult arterial revascularization remains unrealized10.
In this report, we present the first TEBV suitable for autologous small diameter arterial revascularization in adult patients. This TEBV was assembled using a novel method termed Sheet-Based Tissue Engineering. In this approach, fibroblasts are cultured in conditions that promote extracellular matrix deposition to produce a cohesive sheet that can be detached from the culture flask. These sheets, comprised of living cells and a well organized endogenous matrix, demonstrate probe burst loads of 883±42 gf and can be layered into three-dimensional tissues or organs with physiological mechanical strength6,11,12. This method realizes the goal of completely avoiding the use exogenous biomaterials. Sheet-Based Tissue Engineering also marks a departure from the dogma of Cardiovascular Tissue Engineering by eliminating the need for smooth muscle cells (SMCs), whose early senescence is associated with decreased burst pressures in human models7.
Human age- and risk-matched TEBVs were built exclusively with cells isolated from bypass patients (n=6, age 65±8 years, range 54–79). Fibroblast sheets were produced in as little as six weeks (43±10μm thick). Sheet thickness increased at a rate of 5 μm/week through 15 weeks. The TEBVs consisted of three components: a living adventitia, a decellularized internal membrane (IM) and an endothelium. The IM was assembled by wrapping an eight week old fibroblast sheet around a temporary Teflon®-coated stainless steel support tube (3 revolutions). After a maturation period of at least 10 weeks, the individual plies fused together to form a homogenous cylindrical tissue. This tissue was then dehydrated to form an acellular substrate for endothelial cell seeding. The IM was also included to provide a barrier against cell migration towards the lumen. The adventitia was formed in a similar fashion by wrapping a living sheet around the IM. After a second maturation phase, the steel tube was removed, and endothelial cells were seeded in the lumen of the living TEBV. The vessel was then subjected to pulsatile flow that increased from 3 ml/min to 150 ml/min over a three day pre-conditioning period.
TEBVs with an internal diameter (ID) of 4.2 mm and a wall thickness of 407±49 μm had mechanical properties that compared favorably with saphenous veins (Table 1) and surpassed values reported for any other completely biological TEBV produced in vitro2,7,11,13,14. Human TEBVs were xenografted in an immunosuppressed canine model for short-term evaluation (Fig. 1) to confirm that these benchtop mechanical qualities would translate to the surgical setting. The vessels demonstrated positive handling characteristics and suturability. At 14 days, histology revealed a massive immune response (results not shown), confirming that only short-term time-points can be studied in this xenogeneic model.
Table 1.
Mechanical properties of age- and risk-matched human TEBVs.
| Vessel Type | Burst Pressure (mmHg) | Compliance (%)† | Suture Retention (gf) | Wall Thickness (μm) |
|---|---|---|---|---|
| TEBV (4.5 mm ID) | 3,468 ± 500 (n=5) | 1.5 ± 0.3 (n=3) | 162 ± 15 (n=9) | 407 ± 49 (n=5) |
| TEBV (1.5 mm ID) | 3,688 ± 1865 (n=9) | ND | ND | 200 ± 41 (n=3) |
| Human Saphenous Vein | 1,680–2,273 6,18 | 0.7–1.5 19,20 | 196 ± 2 (n=7) | ≈250 21,22 |
| Human Artery | 2,031–4,225 (n=13) | 4.5–6.2 19,23,24 | 200 ± 119 (n=9) | 350–710 25,26 |
Calculated for a pressure change from 80 to 120 mmHg. ND: not determined.
Figure 1. Short-term evaluation of TEBV in canine model.
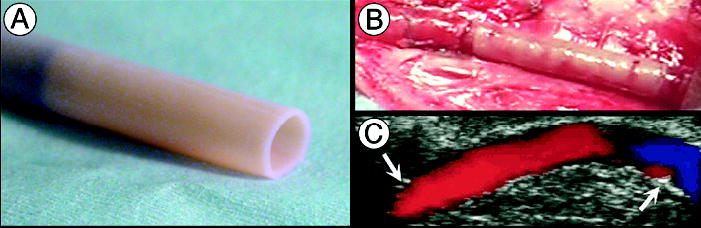
(a) Age- and risk-matched TEBV before implantation (4.2 mm ID) being removed from its temporary tubular support. (b) TEBV, anastomosed as end-to-end interpositional femoral graft, immediately after removal of the cross-clamps. (c) Doppler-ultrasound imaging at 2 weeks shows a patent vessel with uniform lumen and normal flow (arrows indicate anastomoses). TEBVs were endothelialized with autologous canine endothelial cells.
Two approaches were considered for longer-term in vivo evaluation of the TEBV: 1) to develop an autologous animal model or 2) to implant the human model in an immunodeficient animal. Since we (unpublished data), and others, have demonstrated that TEBV mechanical properties are highly species dependent2,7, we concluded that the outcome of an autologous animal model would not likely be representative of the performance of a human TEBV. Therefore, age- and risk-matched human TEBVs (1.5 mm ID) were implanted in nude rats as abdominal interpositional grafts. A pilot group (n=13) received single-layer vessels (without an IM). Overall patency was 85% with time-points up to 225 days. Graft diameter, estimated using Doppler-ultrasound imaging, increased over time (results not shown) suggesting the need for increased mechanical strength. A second group (n=14) received two-layer TEBVs (with IM) to determine if thicker and stronger vessels would maintain diameter. Twelve patent vessels were explanted between 90 and 225 days (86% patency) demonstrating impressive graft incorporation into the surrounding tissue, no blood infiltration in the wall, a smooth lumen, intact anastomoses and no signs of lumen narrowing or aneurysm formation (Figs. 2 and 3). Doppler-ultrasound evaluation suggested a stable diameter and no mechanical failures were observed. Overall patency for the two studies was 85% which, considering the small diameter of the graft and the lack of post-operative antiplatelet therapy, clearly demonstrates the anti-thrombogenicity of the TEBV.
Figure 2. Early remodeling of age- and risk-matched human TEBV after implantation in athymic rats.
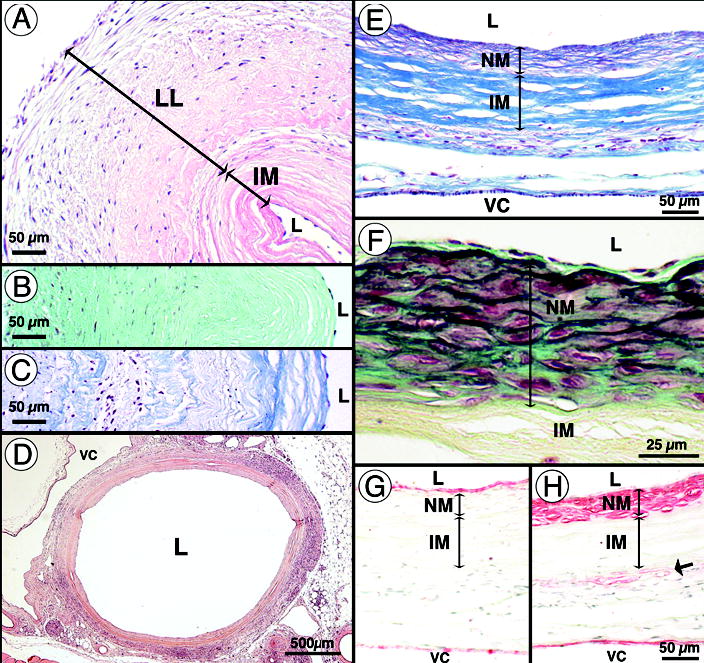
(a–c) Pre-implantation histology of TEBV (1.5 mm ID). (a) H&E staining reveals a decellularized internal membrane (IM), the living layer (LL) and the lumen (L) of TEBV. Lumens of vessel were seeded with syngeneic rat endothelial cells. Note that the wall is scalloped because this vessel was an unused surgical sample that contracted prior to fixation. (b) Movat staining reveals the large proteoglycan (aqua green) content of the TEBV at the time of implantation. (c) Verhoff-Masson staining indicates the high collagen (blue) content of the TEBV. (d) 90 days after implantation, H&E staining of the perfusion-fixed graft shows complete tissue integration with minimal inflammatory/immune response and a modest neomedia formation. VC: vena cava. (e) Verhoff-Masson staining clearly indicates that the IM was largely 16 intact and still acellular. The neomedia was rich in collagen and cells and appeared to have elastic fibers (black). (f) At higher magnification, a Movat staining reveals the forming elastic fibers and a developing internal elastic lamella-like structure forming under a confluent endothelium. Note that proteoglycans were present in the neomedia and subendothelial space but not no longer in the IM (yellowish staining indicates collagen). (g–h) Immunohistochemical staining for von Willebrand factor and smooth muscle α-actin reveals the confluent endothelium and the SMC layers (respectively) of the TEBV and adjacent vena cava. Note the presence of SMC-specific α-actin positive cells at the IM/adventitia interface.
Figure 3. Late remodeling of age- and risk-matched human TEBV after implantation in athymic rats.
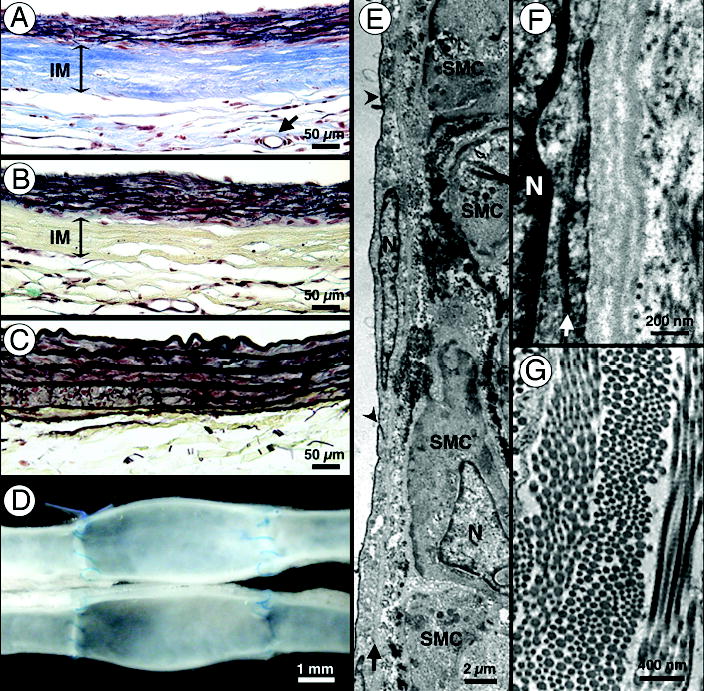
(a–b) After 180 days, Verhoff-Masson and Movat stainings of the perfusion-fixed TEBV wall demonstrate a largely intact and acellular IM as well as abundant elastic fiber formation in the neomedia. Vasa vasorum formation was common (arrow in a). (c) Movat staining of native rat aorta. Note the resemblance with the remodeled wall of the TEBV shown in (b). (d) At the latest time point (225 days), grafts appear fully integrated in the surrounding tissue and show no signs of thrombosis, stenosis or mechanical failure. (e–g) Transmission electron micrographs (N=nucleus). (e) Flat endothelial cells with accompanying basement membrane (arrow) line the lumen of the TEBV 17 (arrow head: cell-cell junction). Underlying SMCs are rich in microfilaments and dense bodies suggesting a contractile phenotype. The dark staining material along the surface of the SMCs, as revealed by ruthenium red, resembles forming elastic fibers. (f) Higher magnification of the endothelium reveals tight endothelial cell-cell junctions (arrow) and close apposition to a continuous basement membrane. (g) Multi-directional bundles of collagen fibers in the IM. Note the periodic banding pattern typical of collagen fibers.
Histological analysis of the explanted TEBV at 90 days (Fig. 2) revealed tissue integration, vasa vasorum formation and cell accumulation on the luminal side of the IM. Surprisingly, the IM was largely intact and still acellular. This strongly suggests that the cell-synthesized extracellular matrix does not create an inflammatory response, which has been linked to stenosis15. The limited thickness of the “neomedia” observed in the lumen of the TEBV is consistent with this hypothesis. The neomedia was highly cellular, composed of SMC-specific α-actin positive cells intertwined with collagen fibers and developing elastic fibers, and was covered by a confluent endothelium (Fig. 2e–g). At 6 months, the thicknesses of the neomedia and IM were essentially unchanged (Fig. 3a–c). Graft diameter also appeared unchanged as evidenced by the fact that the initial diameter mismatch to the <1 mm ID abdominal aorta was still visible in the middle of the graft (Fig. 3d). Due to the exclusion of the SMC layer and the lack of immediate contractility, the remodeling to match the diameter of the native artery appears to be a slow process. The increased thickness and number of elastic fibers made the TEBV more closely resemble the native aorta (Fig. 3c). Likewise, ultrastructural examination demonstrated cell and extracellular matrix organization that were reminiscent of natural tissues (Fig. 3e–f). Although circulating progenitor cells may have participated in the formation of the neomedia, SMCs migrating from the anastomoses were the likely source. While this remodeling process is unlikely in grafts of clinically relevant length, SMC-specific α- actin positive cells could be seen at the IM/adventitia interface (Fig. 2h). This suggest that SMC-like cells can be recruited from the local environment, as is the case in vasculogenesis, or that implanted fibroblasts have differentiated into myofibroblasts16. This recruitment, which increased with time (data not shown), may be a load-induced response as the IM gradually remodels.
Proteoglycan expression, which is often observed in pathological or developing tissues17, was seen throughout the TEBV prior to implantation (Fig. 2b). Interestingly, 90 days after grafting, the TEBV was essentially devoid of proteoglycans, suggesting remodeling into a more physiological tissue (Fig. 2f). Similarly, proteoglycans that were present in the newly formed neomedia at 90 days (Fig. 2f) were absent three months later, again suggesting a positive maturation process (Fig. 3b).
While the small diameter of the rat model created a challenging environment (microsurgery and potential thrombosis), the circumferential wall stress, which is proportional to the diameter, was significantly lower than in clinically relevant TEBVs (3–6 mm ID). To evaluate the TEBV in a more representative biomechanical environment, age- and risk-matched human TEBVs (4.2 mm ID) were implanted as interpositional arterial grafts in three immunosuppressed cynomolgus primates. This model was also chosen for its well-defined immunosuppression protocol utilized for xenogeneic transplantation. All vessels were patent at explantation (n=1 at 6wks and n=2 at 8wks). Doppler-ultrasound, CT-angiography (Fig. 4a) and necropsy (Fig. 4b) confirmed that the vessels were patent with smooth lumens, intact anastomoses and no sign of lumen narrowing or aneurysm formation. Histological observation revealed a moderate immune response to the human tissue, limited focal neomedia formation, vasa vasorum formation and re-endothelialization of an acellular and intact IM (Fig. 4c–f). Proteoglycan expression was consistent with that observed in rat model (Fig. 4e) suggesting positive remodeling. Also similar to the rat model, SMC-specific α-actin positive cells were seen at the IM/adventitia interface (Fig. 4g). Moreover, abundant α- actin staining was evident in the perivascular tissue. These observations suggest a possible mechanism for the long-term regeneration of a contractile media.
Figure 4. Implantation of age- and risk-matched human TEBV in non-human primates.
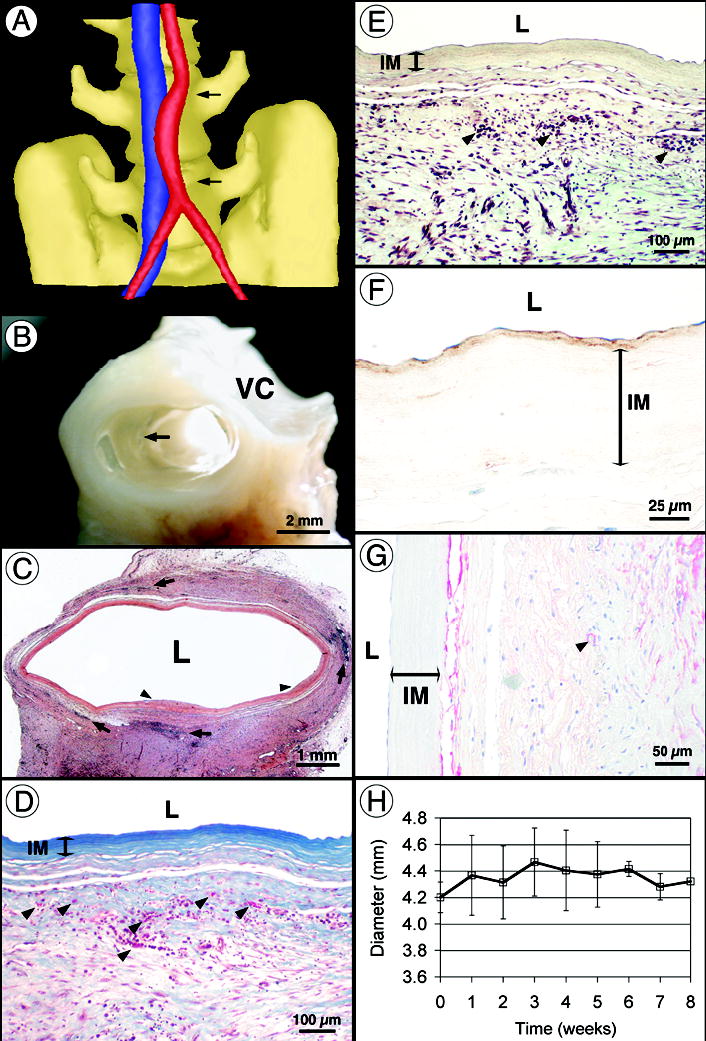
(a) Computed tomography angiogram after 8 weeks of implantation showing a patent TEBV (red) and the adjacent vena cava (blue). Note that the initial diameter mismatch is still visible in the middle of the graft and that the curved shape is typical of an interpositional graft. (b) Perfusion-fixed TEBV at 8 weeks shows complete tissue integration and a smooth lumen with no signs of thrombosis, stenosis or mechanical failure. Arrow indicates suture line. VC: vena cava. (c) Histology (H&E) of the graft at 8 weeks shows multiple leukocyte infiltrations (arrows) and rare sites of limited luminal growth (arrow heads). (d–e) At higher magnification, Verhoff-Masson and Movat stainings show an intact and still acellular IM. Note that proteoglycans are no longer present in the TEBV. Vasa vasorum formation was noted (arrows in D). The significant immune response observed (arrows in e) may be responsible for 18 incomplete tissue integration. (f) Immunolabeling for von Willebrand factor reveals the confluent endothelium and subendothelial matrix (nuclei counterstained in blue). (g) Immunolabeling for SMC-specific α-actin stains a cell population at the IM/adventitia interface (blue counterstain). Arrow indicates small blood vessel. (h) TEBV internal diameter was measured at the center of the graft by weekly ultrasound-Doppler examination of all animals. After an increase of about 5% associated with initial pressurization, the diameter remained constant for the duration of the study.
The mechanical requirements for completely biological tissue engineered grafts are not limited to demonstrating physiological burst pressure and compliance. It is also important that the graft be resistant to rapid degradation and fatigue-induced aneurysm formation in vivo. Hence, we followed graft diameter via ultrasound imaging through the course of the primate study (Fig. 4h). The constant diameter of the TEBV through 8 weeks indicates that the graft is mechanically stable during the phase of incorporation into the surrounding tissue. This data, coupled with positive histological observations and the absence of aneurysm formation in the long-term rat model, suggests that this TEBV has promise as a clinical graft. Clearly, the key to clinical efficacy will be the long-term remodeling responses in the completely autologous environment. Further xenogeneic or even autologous animal models would offer little insight into this remodeling process in humans. Therefore autologous TEBVs are currently being evaluated clinically for peripheral arterial revascularization. While the time to produce these TEBVs (≈28 weeks) clearly precludes urgent clinical use, the vast majority of patients lacking autologous vessels for revascularization can be identified months prior to surgical intervention. For example, patients with hemodialysis-dependent end-stage renal disease, with coronary disease and chronic stable angina, or with peripheral vascular disease and claudication, follow a well-defined disease progression and can have months-long pre-operative waiting periods. Should initial clinical studies prove successful, future efforts will focus on reducing vessel production time and exploring the use of allogeneic TEBVs produced by Sheet-Based Tissue Engineering.
METHODS
Tissue culture
Human skin fibroblasts were extracted as previously described6. For sheet production, cells at passage 3–5 were seeded at 104cells/cm2 on gelatin coated T-75 flasks and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with Ham’s F12 (20%), FetalClone® bovine serum (20%), glutamine (2mM), penicillin (100U), streptomycin (100μg/ml), sodium ascorbate (500μM). For maturation, vessel were cultured in 45 ml of the same medium, but the sodium ascorbate was decreased to 250μM. Endothelial cells were extracted from saphenous veins and cultured using endothelial cell growth factor and heparin as previously described6. Endothelial cells were seeded at 105cells/cm2 in the TEBV and allowed to adhere for 3 hours. All media were changed three times weekly.
Mechanical testing
The mechanical strength of fibroblast sheets (n=6, 8 weeks of culture) was determined using an indentation test. The center of a circular sheet, mounted on a force transducer, was loaded with a spherical tip (9mm) of a probe at a rate of 20mm/min until perforation. Force was digitally recorded. Other mechanical testing followed ANSI/AAMI test standard VP20 with minor modifications for biological material. Burst pressure was achieved by increasing hydrostatic pressure within the vessel at a rate of 80 mmHg/minute until failure. Pressure was digitally recorded. Clean longitudinal slits were the noted failure mechanism in TEBVs. Human mammary arteries were unused distal segments harvested during bypass surgery or vessels obtained at necropsy. Vessels were carefully dissected to expose collaterals for ligation. Three right internal mammary artery segments and 10 left internal mammary artery segments from 8 patients were tested (age: 70 ± 10 y, range 61–87). Results were separated in two groups: failures that appeared associated with the presence of a collaterals or wall defects (2,031 ± 872, n=8), and high pressure failures that may reflect maximum material properties (4,225 ± 1368, n=5). To measure suture retention strength, a single throw of 5-0 prolene® suture (2.2 ± 0.3mm bite) was pulled (2mm/sec) through the TEBV segment which was secured to a force transducer. Force was digitally recorded. Samples were obtained from the saphenous veins of 2 patients, arteries from 3 patients (2 radial and 3 internal mammary). Compliance (c) was calculated by measuring the change in internal diameter (d) as the pressure (P) was varied at 0.2 Hz between 80 and 120 mmHg. High resolution digital images were used to determine TEBV outer diameter. The TEBV wall was assumed to be incompressible. Compliance was calculated using the equation:
| (1) |
Canine studies
Human TEBVs (4.2mm ID, ≈ 6 cm long) were implanted following standard surgical techniques (n=5 grafts in 4 animals) using 8-0 prolene. Immunosuppressive and antiplatelet therapies were administered starting 48 hours before surgery: cyclosporine (7.5 mg/kg), mycophenolate mofetil (20 mg/kg), methyl prednisolone (1 mg/kg qd), acetylsalicylic acid (325 mg qd) and clopidogrel (300 mg initial dose and 75 mg qd after surgery). This protocol was approved by the ethics committee of the Palo Alto Veteran’s Administration Hospital.
Nude Rats studies
Human TEBV (1.5mm ID, ≈ 7 mm long) were anastomosed using 10-0 prolene. In the pilot study (no IM), animals received a single dose of antiplatelet agent (clopidogrel, 30mg/kg, PO) 30 minutes prior to surgery. Patent vessels were harvested at days 14 (n=2), 30 (n=1), 60 (n=1), 90 (n=5), 180 (n=1) and 225 (n=2). Thrombosed vessels were explanted at days 3 (n=1) and 166 (n=1). In the second study (with IM), no antiplateletdrug was given. Patent vessels were explanted at days 90 (n=3), 120 (n=2), 180 (n=5) and 225 (n=2). Thrombosed vessels were explanted at days 90 (n=1) and 180 (n=1). This protocol was approved by the ethics committee of the Stanford University.
Primate studies
Human TEBVs (4.2mm ID, ≈ 3 cm long) were implanted using 8-0 prolene as end-to end iliac (n=1) or abdominal aortic (n=2) interpositional grafts. Immunosuppressive and antiplatelet therapies were administered starting 2 days before surgery: cyclosporine A (target plasma level of 250–300ng/ml until end), mycophenolate mofetil (target plasma level of 1.5–2.0μg/ml until end), rabbit antithymocyte globulin (5mg/kg for 5 days), acetylsalicylic acid (10mg/kg until day 14) and clopidogrel (0.5mg/kg, until day 4 then wean over 10 days). Patent grafts were explanted at 6 weeks (iliac) and 8 weeks (abdominal aorta). This protocol was approved by the ethics committee of the University of California, Davis and the Regional Primate Center.
Tissue fixation and histology
Grafts and control vessels were fixed in situ with cold phosphate buffered parafolmaldehyde 4% following a rinse with cold phosphate saline solution containing 5 mM Ca++. Animals received heparin prior to sacrifice. In rats, whole body fixation was performed while only the target vasculature was fixed in dogs and primates. Antibodies against alpha-SMC actin and vonWillebrand factors were revealed with streptavidin-alkaline phosphatese LSAB 2 system (DakoCytomation #N1584, A0082) and a substrate kit (Vector labs # SK-5100). Transmission electronic microscopy samples were prepared as previously described6 and further stained with ruthenium red.
Acknowledgments
We thank Genzyme Transplant for graciously providing ATG, Roche for graciously providing Mycophenolate mofetil (CellSept®), and LifeNet for tissue procurement assistance. We thank Dr. Mark Haidekker for his help with the image processing of the CT angiogram. This work was supported in part by a grant from the NIH/SBIR (2R44HL64462).
References
- 1.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 2.Niklason LE, et al. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 3.Chue WL, et al. Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular grafts. J Vasc Surg. 2004;39:859–867. doi: 10.1016/j.jvs.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–54. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 5.L’Heureux N, Germain L, Labbe R, Auger FA. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. Journal of Vascular Surgery. 1993;17:499–509. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 6.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 7.McKee JA, et al. Human arteries engineered in vitro. EMBO Rep. 2003;4:633–8. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poh M, et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–4. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 9.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–3. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 10.Niklason LE. Replacement arteries made to order. Science. 1999;286:1493–4. doi: 10.1126/science.286.5444.1493. [DOI] [PubMed] [Google Scholar]
- 11.L’Heureux N, et al. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. Faseb J. 2001;15:515–24. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]
- 12.Michel M, et al. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev Biol Anim. 1999;35:318–26. doi: 10.1007/s11626-999-0081-x. [DOI] [PubMed] [Google Scholar]
- 13.Berglund JD, Mohseni MM, Nerem RM, Sambanis A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials. 2003;24:1241–54. doi: 10.1016/s0142-9612(02)00506-9. [DOI] [PubMed] [Google Scholar]
- 14.Grenier G, et al. Isolation and culture of the three vascular cell types from a small vein biopsy sample. In Vitro Cell Dev Biol Anim. 2003;39:131–139. doi: 10.1007/s11626-003-0007-y. [DOI] [PubMed] [Google Scholar]
- 15.Davis C, Fischer J, Ley K, Sarembock IJ. The role of inflammation in vascular injury and repair. J Thromb Haemost. 2003;1:1699–709. doi: 10.1046/j.1538-7836.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 16.Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–94. doi: 10.1161/01.atv.19.7.1589. [DOI] [PubMed] [Google Scholar]
- 17.Wight, T.N. The Vascular Extracellular Matrix. in Artherosclerosis and Coronary Artery Disease (eds. Fuster, V., Ross, R. & Topol, E.) 421–440 (Raven Press, New York, 1996).
- 18.Lamm P, Juchem G, Milz S, Schuffenhauer M, Reichart B. Autologous endothelialized vein allograft: a solution in the search for small-caliber grafts in coronary artery bypass graft operations. Circulation. 2001;104:I108–14. [PubMed] [Google Scholar]
- 19.Dobrin PB. Mechanical behavior of vascular smooth muscle in cylindrical segments of arteries in vitro. Ann Biomed Eng. 1984;12:497–510. doi: 10.1007/BF02363919. [DOI] [PubMed] [Google Scholar]
- 20.Cambria RP, et al. The evolution of morphologic and biomechanical changes in reversed and in-situ vein grafts. Ann Surg. 1987;205:167–74. doi: 10.1097/00000658-198702000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Lugt A, et al. Femorodistal venous bypass evaluated with intravascular ultrasound. Eur J Vasc Endovasc Surg. 1995;9:394–402. doi: 10.1016/s1078-5884(05)80006-1. [DOI] [PubMed] [Google Scholar]
- 22.Varty K, Porter K, Bell PR, London NJ. Vein morphology and bypass graft stenosis. Br J Surg. 1996;83:1375–9. doi: 10.1002/bjs.1800831015. [DOI] [PubMed] [Google Scholar]
- 23.Chamiot-Clerc P, Copie X, Renaud JF, Safar M, Girerd X. Comparative reactivity and mechanical properties of human isolated internal mammary and radial arteries. Mar. 1998;37:811–9. doi: 10.1016/s0008-6363(97)00267-8. [DOI] [PubMed] [Google Scholar]
- 24.Girerd XJ, et al. Incompressibility of the human arterial wall: an in vitro ultrasound study. J Hypertens Suppl. 1992;10:S111–4. [PubMed] [Google Scholar]
- 25.van Son JA, Smedts F, Vincent JG, van Lier HJ, Kubat K. Comparative anatomic studies of various arterial conduits for myocardial revascularization. J Thorac Cardiovasc Surg. 1990;99:703–7. [PubMed] [Google Scholar]
- 26.van Andel CJ, Pistecky PV, Borst C. Mechanical properties of porcine and human arteries: implications for coronary anastomotic connectors. Ann Thorac Surg. 76:58–64. doi: 10.1016/s0003-4975(03)00263-7. discussion 64–5 (2003). [DOI] [PubMed] [Google Scholar]


