Abstract
American ginseng root displays the ability to achieve glucose homeostasis both experimentally and clinically but the unknown mechanism used by ginseng to achieve its therapeutic effects on diabetes limits its application. Disruption in the insulin secretion of pancreatic β cells is considered the major cause of diabetes. A mitochondrial protein, uncoupling protein-2 (UCP-2) has been found to play a critical role in insulin synthesis and β cell survival. Our preliminary studies found that the extracts of American ginseng inhibit UCP-2 expression which may contribute to the ability of ginseng protecting β cell death and improving insulin synthesis. Therefore, we hypothesized that ginseng extracts suppress UCP-2 in the mitochondria of pancreatic β cells, promoting insulin synthesis and anti-apoptosis (a programmed cell-death mechanism). To test the hypothesis, the serum-deprived quiescent β cells were cultured with or without interleukin-1β (IL-1β), (200 pg ml−1, a cytokine to induce β cell apoptosis) and water extracts of American ginseng (25 μg per 5 μl administered to wells of 0.5 ml culture) for 24 h. We evaluated effects of ginseng on UCP-2 expression, insulin production, anti-/pro-apoptotic factors Bcl-2/caspase-9 expression and cellular ATP levels. We found that ginseng suppresses UCP-2, down-regulates caspase-9 while increasing ATP and insulin production/secretion and up-regulates Bcl-2, reducing apoptosis. These findings suggest that stimulation of insulin production and prevention of β cell loss by American ginseng extracts can occur via the inhibition of mitochondrial UCP-2, resulting in increase in the ATP level and the anti-apoptotic factor Bcl-2, while down-regulation of pro-apoptotic factor caspase-9 occurs, lowering the occurrence of apoptosis, which support the hypothesis.
Keywords: American ginseng, apoptosis, ATP, β cell, Bcl-2, caspase, insulin, mitochondria, UCP-2
Introduction
As a devastating illness with significant morbidity and mortality, diabetes mellitus has increased steadily worldwide (1,2). Type I juvenile diabetes is known as an auto-immune disease possibly caused by an over-immune reaction resulting in extensive destruction of the insulin-producing β cells in the islets of Langerhans in the pancreas (3). While the cause of type II adult-onset diabetes remains poorly understood, all patients with type I diabetes and part of type II diabetes require daily insulin shots, replacement of the destroyed β cell mass via islets or transplant of pancreas in order to survive (4).
The use of herbal remedies has been on the rise in the United States (380% over the last 7 years) (3,5–7). Historical treatment of diabetes utilizes traditional medicine (8,9) and integrates of ginseng root extracts in tonic form into the diet. Ginseng root has been used for millennia in ancient Asian tonics for its restorative and curative properties. American (Panax quinquefolius L.) and Asian ginsengs (Panax ginseng CA Meyer) stand as the two widely used species of ginseng (5). However, the species display contrasting effects. Asian ginseng exhibits an effect on blood flow and decreases fatigue, while American ginseng leans towards decreasing aging and regulating digestion (5). The role of American ginseng has been shown to facilitate anti-hyperglycemia but whether American ginseng improves on pancreatic β cell function is still unknown (10–13).
β cell dysfunction and death are attributed to the inability to produce sufficient amounts of insulin, which can be caused by many factors. One possibility contributing to the loss of insulin would be the gradual loss of β cells, possibly through programmed cell death (apoptosis) in which cells appear to shrink, chromatin condenses and DNA is cleaved into pieces at internucleosomal regions (14). Membrane ‘blebbing’ eventually leads to the breakdown of the cell into small bodies that are quickly taken up by neighboring cells without inflammation. The Bcl-2 proto-oncogene is the most fully characterized protein substance which plays a key role in regulating programmed cell death (15–18). In addition, initiation of the Caspase cascade involves translocation of cytochrome c from mitochondria to the cytoplasm to activate caspase-9 then cleave pro-caspase-3, initiating the proteolytic cascade essential for apoptosis (19). Lack of insulin production results from a shortage of ATP produced within the mitochondria, indicating that mitochondrial ATP plays a critical role in insulin production (20,21).
A mitochondrial protein, uncoupling protein-2 (UCP-2), uncouples electron transport from ATP production by transporting protons across the inner membrane, suppressing ATP production. Studies have shown that high levels of UCP-2 negatively regulate insulin secretion in vivo and in vitro by yielding ATP generation (21–25). Some degree of uncoupling exists in many if not all cells and tissues and evidence suggests that uncoupling might be useful in limiting chronic exposure to harmful reactive oxygen species generated in the mitochondria but the inefficiency inherent with uncoupling may provide a significant disadvantage in cellular survival (26,27). Accordingly, studies have shown that even modestly increased levels of UCP-2 protein reduce cell survival (28).
The purpose of the study is to explore the effects of American ginseng root extracts on the pancreatic β cell's function and survival with a cytokine, interleukin-1β (IL-1β) induced β cell dysfunction model (29,30). Mechanisms involved in achieving up-regulated insulin production and lowered apoptosis levels were determined by evaluating the activation of ATP, UCP-2, Bcl-2 and caspase-9.
Methods
Ginseng Root Preparation
Sliced American ginseng (Panax quinquefolium L.) root was purchased from H.S.U Corp. (WI, USA). The ginseng root was soaked in 10 ml of ddH2O for 24 h at 4°C on a rotator with gentleshaking. The supernatant appeared yellow. Following centrifugation, the supernatant was lyophilized to yield the solid product which was weighed and diluted to yield a final concentration of 5 μg μl−1. One lot of ginseng was used to prepare this solution over the course of the study and it was stored at − 4°C for less than 1 week. There was no evidence of degradation during the 1 week storage period since ginsenoside is stable in pH 7.0 water solution but unstable in acid solution. The concentration is determined by weight per volume. Components of American ginseng present in the solution include the most water- soluble parts (Ginsenoside Ro, Rb1, Re, Rg1, Rg2, Rg3, Rf, quinquenoside R1, gypenosede X1 and water soluble material, Vitamins and metals as well as undefined elements) (31,32).
Cell Culture
Rat pancreatic β cell derived cell line, INS-1, was a gift from Dr Claes Wollheim (Centre Médical Universitaire, Geneva, Switzerland). INS-1 was cultured in 24-well cell culture plates (Corning®, St Louis, MO) for insulin assay and 4-well culture slides (Lab-Tek™ Chamber slide™ system, Rochester, NY) for immunohistochemistry. Cells were cultured in RPMI-1640 with 10% fetal bovine serum and 50 μM 2-mercaptoethanol to maintain differentiation. Semi-confluent (80–90%) monolayer cells in 100 mm polystyrene dishes (Becton Dickinson, Lincoln Park, New Jersey) and 15–40 passage cells were used for the experiments. IL-1β (200 pg ml−1) was added to cell cultures to induce cell apoptosis.
Fluorescence Immunohistochemistry
Cells were washed with phosphate-buffered saline (PBS) twice and fixed with fixation solution (acetone:methanol:dH2O) for 20 min at room temperature (RT). Slides were then blocked with 1% normal goat serum for 10 min at RT and incubated with primary insulin antibody (mouse monoclonal anti-insulin 1:30 in PBS-T) at RT for 2 h. The slides were washed with PBS-T three times and anti-mouse second antibody (Texas Red Anti-Mouse 1:200) was then applied for 20 min. After the second antibody, the slides were washed with PBS-T five times and DAPI was added (for nuclei staining; Vector Labs, Burlingame, CA). Finally, the slides were covered with cover glass.
Tunel Staining (Apoptosis Test)
The detection of apoptotic nuclei using the TdT-mediated dUTP nick-end labeling (TUNEL) method was performed with ApoAlert™ DNA Fragmentation Assay Kit (Clontech, Palo Alto, CA). Briefly, cells were fixed in 3% paraformaldehyde and incubated in 0.3% H2O2 in methanol for 5 min to block endogenous peroxidase. The slides were then incubated with fluorescent-conjugated avidin in PBS for 2 h at RT. After three washes, slides were coverslipped with glycerol-phosphate buffer. Apoptotic cells were counted in at least 10 random fields and expressed as a percentage of the total cell number (apoptotic index).
Measurement of ATP Content (Bio-Luminescence)
The Enliten™ ATP Assay system with a bioluminescence detection kit (Promega Madison, WI) was used for ATP measurement. The principle of the assay is that luciferase from Photinus pyralis catalyses d-luciferin in the presence of ATP and oxygen to oxyluciferin, Pi, AMP, carbon dioxide and light. As described above, cells after removal or removal/incubation were transferred to 1.5 ml Eppendorf tubes with 50 μl TCA (5%) and immediately homogenated with a plastic homogenizer. The suspension was spun at 2000 r.p.m. and the supernatant stored at −80°C until assay. ATP was measured according to the kit protocol. Briefly, samples were neutralized to pH 7.4 with 10 μl 4 M Tris and 10 μl added to a new tube with 90 μl ATP-free water. The luciferase reagent was added 1 s before a 5 s measurement in the luminometer, as suggested by the supplier. Light photons were measured by a luminometer and were compared with an ATP standard curve to calculate ATP concentration (33). Protein content in the supernatant was determined with a spectrophotometric Protein Assay Kit (Pierce, Rockford, IL). The ATP level was standardized by divided protein content. ATP content is expressed in micromolar.
Enzyme-Linked Immunosorbent Assay for Insulin Measurement
Insulin concentrations in the specimen (culture medium and cell extracts) were measured using Ultra Sensitive Rat Insulin ELISA Kit (Crystal Chem Inc., Downer Grove, IL) according to the vendor's instruction. The collected culture medium was used to perform insulin assay. The cell extracts were extracted using 5% TCA then neutralized with 4 M Tris buffer. Briefly, insulin standards and appropriately diluted (1:50) samples were added to an insulin antibody-coated 96-well microplate and incubated for 2 h at 4°C. After washing five times, anti-rat insulin enzyme conjugate was added to the well and incubated for 30 min at RT. After washing seven times, an enzyme substrate solution was added and then incubated up to 45 min at RT in the dark. The reaction was halted by adding 1 N sulfuric acid. Absorbance at 450 nm was read with a μQuant microplate reader (Bio-Tek Instruments, Inc., Winooski, VT) and concentrations were calculated by KC Junior® microplate reader software (Bio-Tek Instruments, Inc.).
Western Blotting Analysis
Pancreatic β cells (INS-1) were plated in a monolayer at 106 cells ml−1 on 35 × 10 mm plates. The protein was extracted with lysis buffer (0.5% NP-40, 10 mM Tris, 10 mM NaCl, 1 mM EDTA and protease inhibitor 2 mM AEBSF, 0.13 mM Bestatin, 1 μM leupeptin, 0.3 μM Aprotinin) and equal amounts of protein were loaded on to 15–20% SDS–PAGE. After electrophoresis (200 V for 45 min), the proteins were transferred onto a nitrocellulose membrane for 2 h in a transfer buffer (25 mM Tris, pH 8.3, 192 mM glycine and 20% methanol). The nitrocellulose membrane was incubated with 1:1000 rabbit anti-caspase-9 antibody (Santa Cruz, CA), Bcl-2 antibody or primary UCP-2 antibody (Anti-Rabbit monoclone antibody, Alpha-Diagnostic Intl, San Antonio, TX) in PBS-T (PBS–0.5% Tween 20) containing 5% non-fat dry milk at 4°C for 24 h. The membrane was then washed and incubated with a second antibody coupled with horseradish peroxidase diluted to 1:2000 in PBS-T at RT for at least 2 h. The primary antibody immunoreaction was detected by incubating the membrane with an enhanced chemiluminescence (ECL) kit (Amersham, Piscataway, NJ) for 5 min. The membrane was then exposed to Kodak film that captured the signal. The nitrocellulose membranes were stripped with anti-actin antibody (Sigma-Aldrich, St Louis, MO). Analysis of the immunoreactions was carried out using specialized imaging software (NIH image analysis system) and computer equipment from the Central Research Facility at Rhode Island Hospital. The results were normalized by actin signal.
Statistical Analysis
All the data are represented as means ± SEM and analyzed by analysis of variance (ANOVA) followed by the Tukey–Kramer test unless otherwise indicated.
Results
American Ginseng Increases Pancreatic B Cell Insulin Secretion/Production and Cell Survival
The effect of American ginseng was observed to be dosage-dependent on both insulin levels and apoptosis. In situ imaging results show that American ginseng extracts in β cell culture increased insulin positive cells as well as prevented IL-1β (200 pg ml−1) induced apoptosis (Fig. 1A). An apoptotic detection kit (TUNEL ASSAY) was used to detect IL-1β induced apoptosis in β cells, which clearly showed the pro-apoptotic effects of IL-1β in cultures and American ginseng extract was able to prevent IL-1β induced β cell apoptosis (Fig. 1B).
Figure 1.
Insulin cell staining and apoptosis: the results of immunohistochemistry and TUNEL assays showed that American ginseng extracts (5 μg μl−1) increases insulin producing cells (A) and also protected against IL-1β induced apoptosis (B). Although American ginseng extracts had a positive effect on the cells, an over dosage (250 μg per 50 μl) showed little effect on apoptosis protection (A) while better effects were found to prevent IL-1β induced apoptosis (B). (n = 12, *P < 0.01 versus control, **P < 0.01 versus IL-1β 200 pg ml−1.)
Quantifying Insulin Production/Secretion
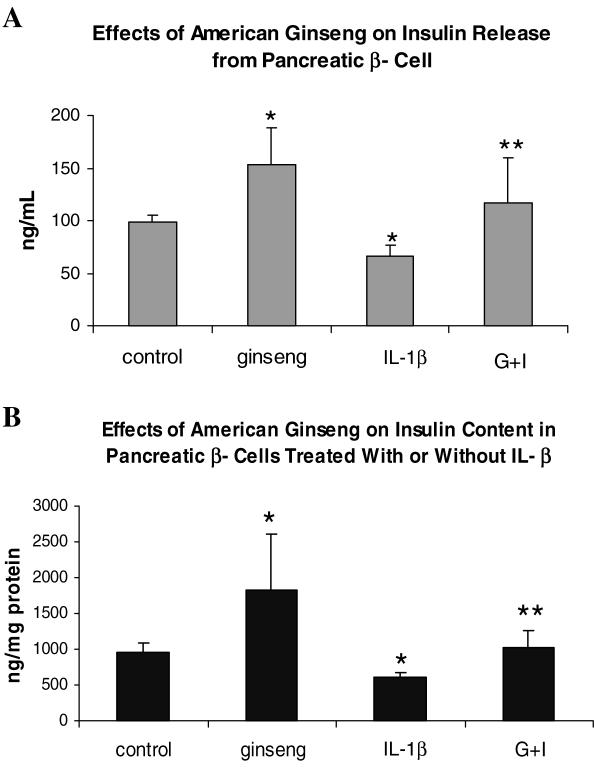
American ginseng was able to increase the amount of insulin-producing cells as indicated by Fig. 1A. Using enzyme-linked immunosorbent assay (ELISA) to quantify the amount of insulin in cultures [secretion/medium (2a) and production/cell content (2b)] with or without American ginseng extracts administered, it was found that cultures with American ginseng extracts displayed significant increases in insulin levels while cells administered with IL-1β exhibited significant decreases in insulin, while American ginseng was able to reverse IL-1β induced insulin reduction (Fig. 2).
Figure 2.
ELISA was used to measure insulin from β cells cultured with IL-1β only or IL-1β and American ginseng together. A β cell cultured without treatment served as control. The data indicate American ginseng promoted insulin secretion (medium) and production (cell extract), thus preventing IL-1β induced reduction of insulin synthesis. (G = American ginseng 25 μg per 5 μl, I = IL-1 β 200 ng ml−1, n = 12, *P < 0.01 versus control, **P < 0.01 versus IL-1β.)
Bcl-2 and caspase-9 Involved in American Ginseng on Apoptosis
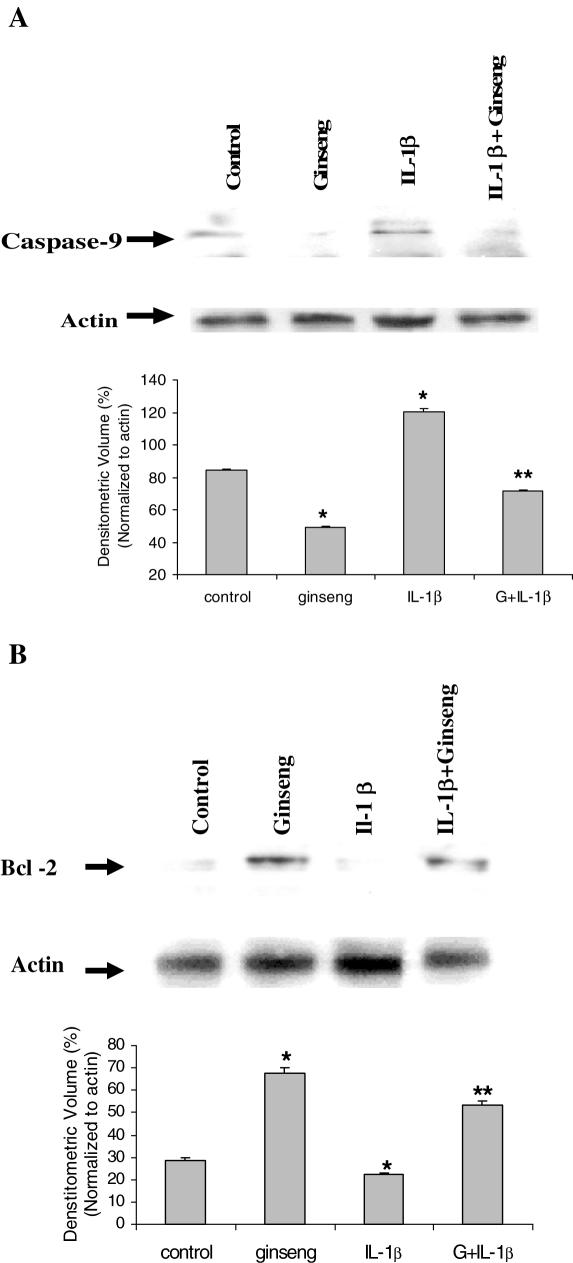
Pro-apoptotic protein caspase-9 and anti-apoptotic protein Bcl-2 have been known to be major factors in the regulation of the cellular apoptotic cascade. American ginseng was proposed to be able to regulate pancreatic β cell apoptosis through mediation of caspase-9 and Bcl-2 activation. Measurement of the response of Bcl-2 and caspase-9 to American ginseng extracts in cultures showed American ginseng inducing a higher expression of Bcl-2 and preventing activation of caspase-9 by western blotting. The determining levels of caspase-9 (Fig. 3A) and Bcl-2 (Fig. 3A) in cultures was quantified by densitometry analysis and normalized by actin.
Figure 3.
(A) Western blotting results indicate that the level of caspase-9 activation in cells induced by IL-1β was significantly reduced by American ginseng extracts. (B) Western blotting results indicate that the level of Bcl-2 activation in cells induced by IL-1β was significantly increased by American ginseng extracts. (G = American ginseng 25 μg per 5 μl, n = 3, *P < 0.05 versus control, **P < 0.01 versus IL-1 β.)
Decrease UCP-2 Levels
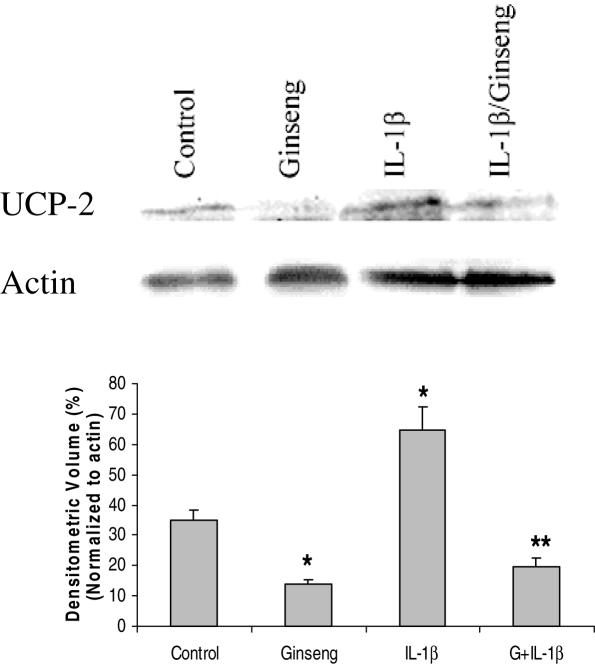
UCP-2 negatively mediates ATP production and causes cell death.The effects of ginseng on UCP-2 levels were investigated in order to see which factors American ginseng affects to achieve cell longevity. The UCP-2 levels in cultures with or without American ginseng extracts were measured by western blotting. Cultures with American ginseng extracts displayed a low expression of UCP-2 while cells with IL-1β exhibited enhanced levels. American ginseng was able to reverse the effects of IL-1β induced UCP-2 over-expression (Fig. 4).
Figure 4.
Western blotting images (top) display the reverse regulatory effects of American ginseng on IL-1β induced pro-activation of UCP-2. Densitometry analysis indicates there is significance between control and variable groups (see graph). (G = American ginseng 25 μg per 5 μl, n = 3, *P < 0.05 versus control, **P < 0.01 versus IL-1β 200 pg ml−1.)
American Ginseng Enhances ATP Production
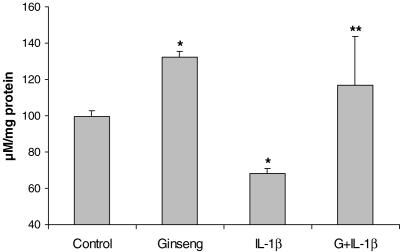
Cellular ATP levels are closely linked to insulin production and mitochondrial function, which is regulated perhaps by UCP-2. American ginseng was able to regulate UCP-2 levels, possibly linked to mitochondrial ATP production. ATP levels showed an inverse correlation between UCP-2 and ATP in response to ginseng treatment. Data showed American ginseng to be an enhancer of ATP production under IL-1β induced anti-ATP circumstance. ATP levels were drastically lowered by IL-1β but American ginseng stimulated an increased output of ATP (Fig. 5).
Figure 5.
Bio-luminescence ATP data displays ginseng's ability to counteract IL-1β caused ATP effects. Administration of American ginseng increased ATP generation and also increased insulin synthesis (see Fig. 2) and inhibited UCP-2 levels in the mitochondria (see Fig. 4). (G = American ginseng 25 μg per 5 μl, n = 3, *P < 0.01 versus control, **P < 0.01 versus IL-1β 200 pg ml−1.)
Discussion
Throughout history, the ginseng root has been used in its water-extract form. Evaluation of the water soluble material from ginseng in extracts could be beneficial in the attempt to find ginseng's biological activation. The main active component in ginseng, which is responsible for ginseng's medical value, has been identified as glycoside. Other than the glycosides, minor constituents include volatile oils (member of vitamin B complex), manganese, vanadium, copper, cobalt, fatty acids, amino acids, simple sugars and other carbohydrates [Dixon, P. Ginseng. Gerald Duckworth & Co. Ltd (1976)]. Glycoside is a naturally occurring substance consisting of a sugar and non-sugar moiety. A glycoside which produces froth under agitation by reducing water surface tension is called a saponin. A group of saponins in ginseng were named ginsenosides, and were classified into subclasses Ro, Ra, Rb, Rc, Rd, Re, Rf, Rg, and Rh. These ginsenosides were differentiated based on their retention factor (Rf) values in thin layer chromatography (TLC), which is the distance that the ginsenosides travel up the TLC column (34). A range of chemical components with biological activity including ginsenosides, alkaloids, polypeptides and polysaccharides have been isolated. By 1992, Liu and Xiao reported that 28 ginsenosides had been found in different parts of the ginseng plant (35). The report summarized the range of effects that ginsenoside had, which included cardio-protective, immunomodulatory, anti-fatigue and hepato-protective effects (36). Oriental ginseng can be distinguished from American ginseng using ginsenoside profiles as the former contains ginsenoside Rf while the latter does not (37). The anti-hyperglycemic effects of the total ginsenosides extracted from Chinese ginseng (TGCG) were evaluated in diabetic C57BL/6J ob/ob mice. The results indicated that in a diabetic ob/ob mouse model TGCG was endowed with significant anti-hyperglycemic and anti-obesity properties (38). One ginsenoside component, Re was evaluated in the same animal model and the anti-hyperglycemic effect had been found to be caused by improving the muscle's metabolism (39). No evaluation of pancreatic function was reported in these studies. However, ginsenoside components could potentially be substances which improve the pancreatic islet function in creating the anti-hyperglycemic effect.
Previous studies have revealed that American ginseng lowers blood glucose in diabetic patients (5) and benefits pancreatic β cell insulin production, secretion and prevents β cell apoptosis (40). Data presented in the current project suggest that the effect of American ginseng in stimulating insulin production/secretion and anti-apoptosis is dosage dependent, which indicates biological specificity of ginseng extracts on β cells. A high dosage of ginseng reversed IL-1β induced apoptosis (Fig. 1B), which suggests that high dosage of American ginseng is required for healing/repairing damaged β cells but not for normal cells (lesser effect of high-dosage American ginseng on β cell apoptosis; see Fig. 1A).
Regulation of UCP-2 activation in the mitochondria could be a major mechanism for the effects of American ginseng on β cell function. Elevations of UCP-2 levels were observed under an IL-1β cytokine induced pancreatic β cell dysfunction model (Fig. 4). UCP-2, a protein belonging to the large family of the anion mitochondrial transporters, is believed to negatively regulate metabolism. The uncoupling protein family consists of three main uncoupling proteins. UCP-1 is well understood and characterized as an uncoupler in brown adipocytes and controls heat production. UCP-2 and 3 are not well known but are proposed as ancestors of UCP-1. The role of UCP ancestral functions was to facilitate the adaptation of cells to oxygen through uncoupling of respiration. It is believed that UCP-2 is linked to anti-hyperinsulinemia after the presence of the UCP-2 gene was detected in pancreatic β cells (26). Potassium channels are uncoupled by UCP-2 upon the inner membrane of the mitochondria, relieving the osmotic proton pressure via alternative channels (25). It is known that the ATP/ADP ratio controls insulin secretion, which could be through the inhibition of mitochondrial UCP-2 expression. UCP-2 levels in relation to ATP and insulin production were observed by the over expression of UCP-2 inhibited insulin secretion and increased ATP levels. An inhibition of UCP-2 production reversed results (26). In this study, we found that IL-1β significantly increased UCP-2 expression, which was reversed by American ginseng root extracts accompanied by an increase in ATP and insulin levels in cultured β cells. This is consistent with the ability of ATP in playing a critical metabolic role that controls insulin secretion, and is mediated primarily by UCP-2 (23). Thus, stimulation of insulin synthesis and ATP production by American ginseng can be achieved through the regulation of the mitochondria and mitochondrial proteins, UCP-2, by affecting the ADP/ATP ratio response.
Though little information and research shows the specific role of UCP-2 on cell viability and longevity, cells with an abnormally high UCP-2 level tend to exhibit a decrease in cell livelihood (28). A major cause of shortened cell livelihood would be the process of programmed cell death (apoptosis). A model utilizing cytokine IL-1β to induce apoptosis provided a platform for observing the effects of American ginseng on apoptotic factors and overall apoptosis. Several protease families are implicated in apoptosis, the most prominent being caspases (18,19,41). Apoptosis is inhibited by the mitochondrial integral membrane protein Bcl-2, suggesting that the mitochondria are a crucial mediator in the apoptosis process. Bcl-2 inhibits the activation of the Caspase cascade by suppressing the release of mitochondrial proteins (18). At the commencement of the mitochondria-regulated apoptosis, cytochrome c, a factor in electron transport is released from the mitochondria, which leads to the activation of pro-caspase-9, followed by activation of the entire cascade and nuclear defragmentation (19).
In our studies, American ginseng extracts reversed IL-1β induced increase of UCP-2 and pro-apoptotic protein caspase-9 and reduced anti-apoptotic protein Bcl-2 and ATP levels, indicating that the anti-apoptotic effects of American ginseng could be achieved through regulating mitochondrial UCP-2, ATP levels (Fig. 5) and apoptotic factors (caspase-9/Bcl-2 Fig. 3), thus influencing insulin synthesis and apoptotic cascades in β cells. It is the first study to show that American ginseng root extracts can directly regulate the β cell biochemical processes, which contribute to improving β cell function with regard to insulin production and cell survival, all of which may be directly connected to American ginseng's anti-hyperglycemic effects (Fig. 6). Further research is required to fully understand the effects of American ginseng extracts on pancreatic β cells and factors utilized by American ginseng to achieve cell regulation and function. American ginseng may enhance pancreatic β cell viability through various alternative factors.
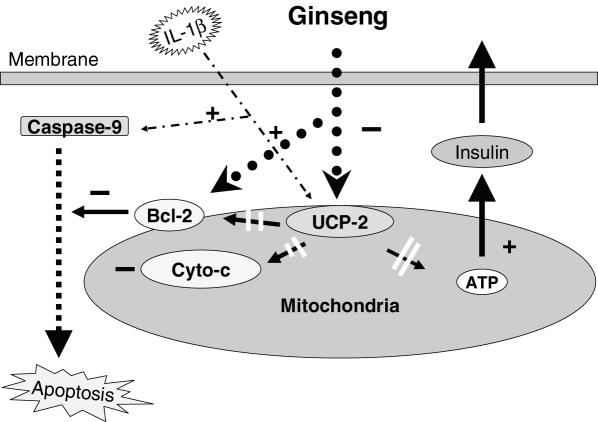
Figure 6.
Our observation showed that American ginseng reversed IL-1β induced apoptosis and increased of insulin synthesis by halting the apoptotic cascade through reversed activation of Bcl-2, consequently, inhibiting caspase-9 and cytochrome c (Cyto-c). American ginseng's inhibition of UCP-2 and increase of ATP production/insulin synthesis suggests that mitochondrial UCP-2 and ATP could be involved in effecting American ginseng on β cell function. With these observations, it is proposed that the mitochondrion is the organelle of target, thus forming the proposal outlined in Fig. 6.
Acknowledgments
This publication was made possible partially by NIH Grant Number P20RR018757 from the National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Honeycutt AA, Boyle JP, Broglio KR, Thompson TJ, Hoerger TJ, Geiss LS, et al. A dynamic Markov model for forecasting diabetes prevalence in the United States through 2050. Health Care Manag Sci. 2003;6:155–64. doi: 10.1023/a:1024467522972. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P. The burden of type 2 diabetes: are we doing enough? Diabetes Metab. 2003;29:6S9–18. doi: 10.1016/S1262-3636(03)72783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuksan V, Sievenpiper JL, Wong J, Xu Z, Bejian-Zdravkovic U, Amason JT, et al. American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr. 2001;73:753–8. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Walberg J, Vethacke A, EI-Ouaghlidi A, Senkal M, Holst JJ, et al. Blood glucose control in healthy subject and patients receiving intravenous glucose infusion or total parenteral nutrition using glucagon-like peptide 1. Regul Pept. 2004;118:89–97. doi: 10.1016/j.regpep.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beijan-Zdravkovic U, Xu Z, et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–13. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 6.Vuksan V, Sievenpiper JL, Wong J, Xu Z, Bejian-Zdravkovic U, Amason JT, et al. American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr. 2001;73:753–8. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- 7.Vuksan V, Stavro MP, Sievenpiper JL, Koo VY, Wong E, Beijan-Zdravkovic U, et al. American ginseng improves glycemia in individuals with normal glucose tolerance: effect of dose and time escalation. J Am Coll Nutr. 2000;19:738–44. doi: 10.1080/07315724.2000.10718073. [DOI] [PubMed] [Google Scholar]
- 8.Punitha IS, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin-nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Gosha-jinki-gan (a herbal complex) corrects abnormal insulin signaling. Evid Based Complement Alternat Med. 2004;1:269–76. doi: 10.1093/ecam/neh028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–8. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 11.Vuksan V, Stavro MP, Sievenpiper JL, Beljian-Zdravkovic U, Leiter LA, Josse RG, et al. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diabetes Care. 2000;23:1221–6. doi: 10.2337/diacare.23.9.1221. [DOI] [PubMed] [Google Scholar]
- 12.Davydov VV, Molokovskii DS, Limarenko A. Efficacy of ginseng drugs in experimental insulin-dependent diabetes and toxic hepatitis. Patol Fiziol Eksp Ter. 1990;5:49–52. [PubMed] [Google Scholar]
- 13.Xie JT, Aung HH, Wu JA, Attel AS, Yuan CS. Effects of American ginseng berry extract on blood glucose levels in ob/ob mice. Am J Chin Med. 2002;30:187–94. doi: 10.1142/S0192415X02000442. [DOI] [PubMed] [Google Scholar]
- 14.Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50(Suppl 1):S44–7. doi: 10.2337/diabetes.50.2007.s44. [DOI] [PubMed] [Google Scholar]
- 15.Allison J, Thomas H, Beck D, Brady JL, Lew AM, Elefanty A, et al. Transgenic overexpression of human Bcl-2 in islet beta cells inhibits apoptosis but does not prevent autoimmune destruction. Int Immunol. 2000;12:9–17. doi: 10.1093/intimm/12.1.9. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovitch A, Suarez-Pinzon W, Strynadka K, Ju Q, Edelstein D, Brownlee M, et al. Transfection of human pancreatic islets with an anti-apoptotic gene (bcl-2) protects beta-cells from cytokine-induced destruction. Diabetes. 1999;48:1223–9. doi: 10.2337/diabetes.48.6.1223. [DOI] [PubMed] [Google Scholar]
- 17.Naito M, Shiina K, Mashima T, Nagashima K, Tsuruo T. Induction of in vitro nuclear apoptosis activity coincides with the production of 50 kDa cytosolic protein. Cell Death Differ. 1997;4:617–22. doi: 10.1038/sj.cdd.4400287. [DOI] [PubMed] [Google Scholar]
- 18.Iwahashi H, Hanafusa T, Eguchi Y, Nakajima H, Miyagawa J, Itoh N, et al. Cytokine-induced apoptotic cell death in a mouse pancreatic beta-cell line: inhibition by Bcl-2. Diabetologia. 1996;39:530–6. doi: 10.1007/BF00403299. [DOI] [PubMed] [Google Scholar]
- 19.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, et al. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med. 1999;189:381–94. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger K, Sivars U, Winzell MS, Johansson P, Hellman U, Rippe C, et al. Mitochondrial ATP synthase—a possible target protein in the regulation of energy metabolism in vitro and in vivo. Nutr Neurosci. 2002;5:201–10. doi: 10.1080/10284150290008604. [DOI] [PubMed] [Google Scholar]
- 21.Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes. 2002;51:2749–56. doi: 10.2337/diabetes.51.9.2749. [DOI] [PubMed] [Google Scholar]
- 22.Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–6. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- 23.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–55. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 24.Hagen T, Vidal-Puig A. Mitochondrial uncoupling proteins in human physiology and disease. Minerva Med. 2002;93:41–57. [PubMed] [Google Scholar]
- 25.Mattson MP, Liu D. Mitochondrial potassium channels and uncoupling proteins in synaptic plasticity and neuronal cell death. Biochem Biophys Res Commun. 2003;304:539–49. doi: 10.1016/s0006-291x(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 26.Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, et al. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl 1):S130–5. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- 27.Langin D. The role of uncoupling protein 2 in the development of type 2 diabetes. Drugs Today (Barc) 2003;39:287–95. doi: 10.1358/dot.2003.39.4.737960. [DOI] [PubMed] [Google Scholar]
- 28.Mills EM, Xu D, Fergusson MM, Combs CA, Xu Y, Finkel T. Regulation of cellular oncosis by uncoupling protein 2. J Biol Chem. 2002;277:27385–92. doi: 10.1074/jbc.M111860200. [DOI] [PubMed] [Google Scholar]
- 29.Islam Z, Pestka JJ. Role of IL-1(beta) in endotoxin potentiation of deoxynivalenol-induced corticosterone response and leukocyte apoptosis in mice. Toxicol Sci. 2003;74:93–102. doi: 10.1093/toxsci/kfg119. [DOI] [PubMed] [Google Scholar]
- 30.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–60. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagasawa TYT, Nishino Y. Application of HPLC to the isolation of ginsenosides—Rb1, Rb2, Rc, Rd, Re and Rg1 from ginseng saponin. Wakanyaku Shinpojumo (kiroku) Japanese 1980;13:9–15. [Google Scholar]
- 32.Li TSCMG, Conttrell AC. Ginsenosides in roots and leaves of American ginseng. J Agric Food. 1996;44:717–20. [Google Scholar]
- 33.Levy JR, Gyarmati J, Lesko JM, Adler RA, Stevens W. Dual regulation of leptin secretion: intracellular energy and calcium dependence of regulated pathway. Am J Physiol Endocrinol Metab. 2000;278:E892–901. doi: 10.1152/ajpendo.2000.278.5.E892. [DOI] [PubMed] [Google Scholar]
- 34.Otsuka H, Morita Y, Ogihara Y, Shibata S. The evaluation of ginseng and its congeners by droplet counter-current chromatography. Planta Med. 1977;32:9. doi: 10.1055/s-0028-1097551. [DOI] [PubMed] [Google Scholar]
- 35.Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 36.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 37.Proctor JTA. Arlington, VA: ASHS Press; 1996. Ginseng: old crop, new directions. [Google Scholar]
- 38.Xie JT, Wang CZ, Wang AB, Wu J, Basila D, Yuan CS. Antihyperglycemic effects of total ginsenosides form leaves and stem of Panax ginseng. Acta Pharmacol Sin. 2005;26:1104–10. doi: 10.1111/j.1745-7254.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- 39.Xie JT, Mehendale SR, Li X, Quigg R, Wang X, Wang CZ, et al. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim Biophys Acta. 2005;1740:319–25. doi: 10.1016/j.bbadis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Luo JZ, Yano N, Luo L. American ginseng stimulates insulin production and prevents apoptosis induced by IL-1[beta] in pancreatic [beta] cells. Diabetes. 2003;52(Suppl. 1):A354–1534-P. [Google Scholar]
- 41.Sesti G. Apoptosis in the beta cells: cause or consequence of insulin secretion defect in diabetes? Ann Med. 2002;34:444–50. doi: 10.1080/078538902321012397. [DOI] [PubMed] [Google Scholar]