Abstract
Regulatory T (Treg) cells are the major arbiter of immune responses, mediating actions through the suppression of inflammatory and destructive immune reactions. Inappropriate Treg cell frequency or functionality potentiates the pathogenesis of myriad diseases with ranging magnitudes of severity. Lack of suppressive capability hinders restraint on immune responses involved in autoimmunity and alloreactivity, while excessive suppressive capacity effectively blocks processes necessary for tumor destruction. Although the etiology of dysfunctional Treg cell populations is under debate, the ramifications, and their mechanisms, are increasingly brought to light in the medical community. Methods that compensate for aberrant immune regulation may not address the underlying complications; however, they hold promise for the alleviation of debilitating immune system-related disorders. The dominant immunoregulatory nature of Treg cells, coupled with recent mechanistic knowledge of natural immunomodulatory compounds, highlights the importance of Treg cells to practitioners and researchers of complementary and alternative medicine (CAM).
Keywords: alloreactive immunity, autoimmunity, CAM, Cancer, regulatory T Cells, Treg
Behind the Line of Defense: Treg Cell Relations to Self and Allogeneic Bodies
The intricacies of immune system constituents and interrelationships have been recognized, along with a descriptive appraisal of regulatory T (Treg) cell function in relation to allergy and infection (1,2). The objective of this article is (i) to explain Treg cell function in cancer, autoimmunity and alloresponses and (ii) to examine the pathological costs of irregular Treg cell activity. Due to the high frequency and established knowledge of cancer, autoimmunity and allogeneic immunity, these three afflictions will be utilized as models to communicate the significance and relevance of Treg cells to complementary and alternative medicine (CAM).
Enhanced cell- and humoral-mediated inflammatory responses, resulting from autoimmune and allogeneic diseases, destroy tissues, while depressed immune responses to tumor tissue allow for tumor immunity. Recent evidence has served to elucidate the mechanism of action and substantiate the usage of a wide array of traditional herbs, folk medicines, plant-derived polyphenols and other compounds found in nature, that are employed to attenuate complications related to aberrant functioning of immune responses in these diseases (3–9). Of interest to practitioners, researchers and patients of CAM modalities are those compounds that maintain powerful immunoregulatory capacity via direct or indirect action on Treg cells (see Table 1).
Table 1.
Biologically active CAM products with immunomodulatory capacity
| Natural product | Plant source | Medicinal system | Utility | Mechanism | Reference |
|---|---|---|---|---|---|
| Triptolide | Tripterygium wilfordii Hook F. | Chinese | Immunosuppression in graft acceptance | Inhibition of NF-κB activation | (10,11) |
| Inhibit maturation and trafficking of DCs and effector cells | |||||
| Berbamine | Berberis julianae, Berberis poiretii | Chinese | Immunosuppression in graft acceptance | Decrease ratio of CD4+ to CD8+ cells | (12) |
| Piperine | Piper longum Linn. | Asian and the Pacific Island | Immunostimulation in cancer, especially melanoma | Increase white blood cell count | (13,14) |
| Increase bone marrow cellularity and α-esterase cell population | |||||
| Increase circulating antibodies and antibody cells | |||||
| Inhibit NF-κB | |||||
| Andrographolide | Andrographis paniculata | Indian Ayurveda | Immunostimulation in cancer | Increase lymphocyte proliferation | (15,16) |
| Increase production of IL-2 and TNF-α | |||||
| Herbkines | Eight species of Oriental herbs | Oriental Medicine | Immunostimulation in cancer. | Enhance TH1 and TH2 cytokine production of IFN-γ, TNF-α, IL-2, IL-4 and IL-12 | (17) |
| Sairei-to | Twelve species of medical herbs | Japanese Kampo | Immunosuppression in autoimmune diseases, especially rheumatoid arthritis systemic lupus ethythematosus | Decrease TH1 cell-mediated inflammation | (18) |
| Enhance TH2 cell functionality | |||||
| Decrease IgG1 levels |
Cancer manifestation and severity depends on a number of factors including the location and character of the malignancy as well as occurrence of metastasis. It is mainly a disease of the later years and one of the leading causes of death in developed nations. Traditional therapies for cancer including surgery, chemotherapy and radiotherapy are losing popularity due to gradual development of tumor resistance to therapy and non-specific toxicity toward normal cells (19). New therapeutic options and possibilities with higher specificity, efficacy and safety are desirable.
Andrographis paniculata is a medicinal plant of Ayurveda, known as ‘kalmegh’, which grows abundantly in India and is cultivated in China and Thailand. The phytochemical extracts from the leaves and stems include diterpenes, flavonoids and stigmasterols, granting it a variety of pharmacological activities and potential for usage in traditional systems (16). Andrographolide, a biologically active constituent of A. paniculata, is a potential anticancer agent, mediating these effects through the inhibition of cancer cell proliferation and the destruction of cancer cells (16). The proposed mechanisms by which Andrographolide exerts its anticancer effects include direct cell cycle arrest and indirect stimulation of immune system cells. Immunostimulatory activity of andrographolide is evidenced by increased IL-2 and TNF-α production and enhancement of lymphocyte proliferation, resulting in strengthened response and cytotoxic activity of lymphocytes against cancer cells (15,16). The pharmacological activity suggests that andrographolide is good candidate for development as a therapeutic agent or a lead compound in anticancer and immunomodulatory therapeutics (16).
Therapeutics designated for immune suppression in autoimmunity and alloresponses in Graft-versus-Host-Disease (GvHD) and Host-versus-Graft-Disease (HvGD) include total body irradiation, chemotherapy and immunosuppression, via corticosteroids. Each of these treatments is weighted down with a variety of deleterious side effects, primarily increased incidence and severity of infection and abnormal tissue growth (2,20).
Triptolide (TPT) is a biologically active compound that is isolated from the Chinese medicinal plant, Tripterygium wilfordii Hook F. (10,11). TPT demonstrates potent anti-inflammatory and immunosuppressive actions inhibiting autoimmunity, allograft rejection and GvHD (11). These effects were previously attributed to the suppression of T cells; however, recent studies of its functions on dendritic cells (DCs), in T cell-mediated immunity, has been explored. Usage of TPT in a model for skin graft rejection in mice, demonstrated that TPT impairs allostimulatory functions, i.e. inhibition of maturation and trafficking of DCs, resulted in the prevention of graft rejection (10). Chemoattraction of neutrophils and T cells by DCs may favor their interactions and the initiation of immune response; therefore, the attenuation of DC frequency and activity by TPT significantly impairs chemoattraction of effector T cells (11).
Treg cells have been demonstrated to suppress antigen-specific T cell responses against tumors and allografts, and implicated in the control of autoimmune diseases (21–24).
CAM benefits from the research done to establish a scientific basis for various CAM treatment modalities, as it lends credibility and, most importantly, offers efficacious treatment options to a large segment of the population afflicted with cancer, autoimmune disease and alloreactive responses. Knowledge of the dynamic relationship between Treg cells and immune system responses to self and allogeneic antigens is essential in order to approach Treg cells as a clinical target for the alleviation of severe complications arising from immune system dysregulation.
Too Much of a Good Thing: Excessive Treg Cell Suppression in Tumor Immunity
Cancer is a category of diseases characterized by the uncontrolled division of cells and the ability of these cells to invade other tissues through implantation or metastasis. Excessive and uncontrollable cell division is due to mutations in the gene's encoding for protein regulators of cell cycle and mitosis, e.g. proto-oncogenes and tumor suppressor genes (TSG), such that signals for cell growth overwhelm regulatory signals. Mutations may be passed down through genetic inheritance or can be caused by carcinogens, radioactive materials and viral genome insertions. The rate of mutations increases with age, leading to an accumulating reservoir of damaged DNA sufficient to transform a normal cell into a malignant one.
Activation of the aryl hydrocarbon receptor (AhR) on Treg cells induces proliferation and subsequent Treg cell-mediated immune suppression. Carcinogenic hydrocarbons found in cigarette smoke, broiled meats and elsewhere in the environment have the capacity to act as potent activating ligands for AhR. The induction of profound immune suppression via AhR activation can result in tumor development (25,26). Although, these compounds are of great toxicological concern, they offer researchers the ability to elucidate Treg cell manipulation through AhRs.
T cells are essential for the destruction of cancer cells; therefore, inefficient immune responses to cancer cells allow for their preservation (27). Many tumor-associated antigens are normal self-constituents; therefore, they are presumably under the control of Treg cells (28). Since Treg cells are involved in the suppression of TH1 cell-mediated immune system function, it follows that Treg cells protect tumors from attack. Elevated levels of tumor-specific Treg cells have been found in tumor sites as well as tumor-infiltrating lymphocyte populations, in lung, breast and ovarian tumors and implicated in Hodgkin's lymphoma (27,29). The mechanism for sequestration of Treg cells to tumor tissue, which yields the increased suppression of immune system attack, is apparently due to chemokine ligand 22 (CCL22). CCL22 is secreted by tumor cells and attracts Treg cells from their normal residence, in the lymph nodes, to the tumor tissue area (30–34). The result is significant suppression of CD8+ cells, allowing for tumor immunity and progression (35). Measuring the ratio of Treg cells to total T cells present, in tumor tissue, showed that the higher the ratio, the farther the cancer had progressed and the more dire the prognosis (28). Interestingly, TGF-β secreted by most melanomas could play a critical role, as it is one of the suppressive mechanisms of Treg cells (29). Also naive Th0 cells may develop into Treg cells when exposed to TGF-β (29).
Misidentification of Self as Foe: Dysfunctional Immunoregulation in Autoimmune Responses
Autoimmune disease occurs when there is a breech in the normal processes producing tolerance to self, i.e. the failure to respond to specific autoantigens (28). Antigen presenting cells (APC), either DCs or macrophages, process specific autoantigens that migrate to the draining lymph nodes. In the lymph node region, APCs can present these autoantigens to autoreactive T cells, which have escaped negative selection by the thymus and have evaded peripheral tolerance. This activation of autoreactive T cells leads to their clonal expansion and migration to the specific tissues, where they induce inflammation and tissue destruction (36).
Central tolerance and peripheral tolerance comprise the two mechanisms by which the immune system hinders the autoreactive T cells from inducing their deleterious functions. Central tolerance is mediated through negative selection. This process entails the elimination, through clonal deletion of autoreactive T cells, during ontogenic development in the thymus (28).
Peripheral tolerance is the backup available if central tolerance fails and autoreactive cells escape the thymus. This mechanism of tolerance occurs continuously throughout life, keeping autoreactive cells in check through immune ignorance, peripheral deletion and active suppression. Treg cells maintain peripheral tolerance and regulate autoimmunity (28,37). Thymically derived Treg cells (nTreg) are the main regulatory cells involved, utilizing an array of TCRs targeted towards autoantigen recognition in order to maintain immune homeostasis in the periphery, and regulate autoimmunity and pathogenic immune responses (22–24). The pathological response of autoreactive effector cells can be suppressed by actions of nTreg cells and the peripherally induced Tr1 and TH3 cells, each using different mechanisms (28,38,39).
Treg cells mediate the prevention of autoimmunity in two ways. The first involves the prevention of autoreactive T cell priming and differentiation in the draining lymph nodes. Located around DCs in the lymph nodes, Treg cells can prevent the early stages of T cell activation (40). If this early stage of T cell activation is not suppressed, autoreactive T cells migrate to target tissue inducing inflammation and tissue destruction (36,41). The second of Treg cell suppression involves the activation, proliferation and trafficking of Treg cells to the affected tissue to suppress effector cell functions locally. Treg cell activity is confined to the microenvironment where they are activated, due to their antigen-specific nature (23).
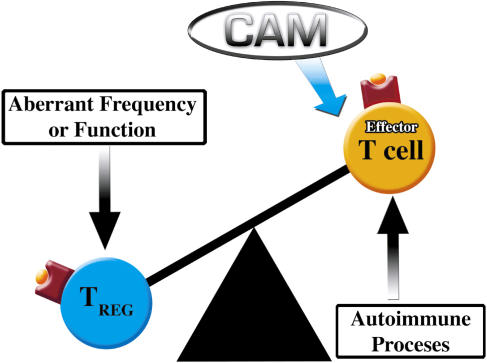
Dysregulation in Treg cell frequency or functioning may lead to a number of debilitating autoimmune diseases including, multiple sclerosis (MS), rheumatoid arthritis (RA), myasthenia gravis (MG), autoimmune polyglandular syndrome type II (APS-II), Hashimoto's thyroiditis (HT), type-1 diabetes (T1D), systemic lupus erythematosus (SLE) and autoimmune lymphoproliferative syndrome (ALS) (37,38,42–46) (Fig. 1).
Figure 1.
Decrease in Treg cell frequency and/or function leaves autoimmune development and progression unregulated. A number of CAM therapeutics may restore balance through suppression of the autoreactive processes directly or indirectly through Treg cell manipulation.
Multiple Sclerosis
Multiple sclerosis is a chronic inflammatory disease characterized by lymphocyte infiltration and inflammation of the central nervous system white matter. Effector T cells specific for myelin protein peptides are involved. Decreased numbers and dysfunction, e.g. low cloning potential in the presence of IL-2, in Treg cells may allow for the over stimulation of CD4+ effector cells upon antigenic challenge, resulting in the production of proinflammatory cytokines and neuronal damage (24,37,42,47).
Experimental autoimmune encephalomyelitis (EAE) is a TH1 cell-mediated inflammatory disease of the central nervous system, and provides a model of human MS from which exploration of Treg cell functionality in autoimmune detriment is possible. Activation of Treg cells with cognate antigen, proteolipid protein-1 (PLP1), carried on a proteolipid protein produced an antigen-specific Treg cell capable of suppressing PLP1 peptide induced EAE in a mouse model (48–50). Thus, there is the suggestion that the induction and activation of peptide-specific Treg cells, by a cognate autoantigen, is essential for the broad immune suppressive functions integral to the attenuation of autoimmunity. Demonstration of the potency of Treg cells, expanded with a single epitope, against autoimmunity is encouraging for CAM researchers to apply this knowledge to patients with MS and other autoimmune diseases.
Rheumatoid Arthritis
Rheumatoid arthritis is a chronic inflammatory disorder leading to the destruction of joint architecture. The pathogenic events leading to the development of RA is not well understood; however, the presence of proinflammatory cytokines plays a key role in the development and maintenance of RA. RA patients' nTreg cells are able to suppress effector T cell proliferation, yet incapable of suppressing TNF-α and IFN-γ production. Suggesting that dysfunction of nTreg cells ability to suppress cytokine production contributes to etiology of RA (42). However, in a number of cases peripheral Treg cells suppress the proliferation of effector T cells, but do not effectively limit proinflammatory cytokine secretion, e.g. anti-TNF-α or anti-IL-17 therapy suppresses inflammation in afflicted patients (43,51).
An increased frequency of Treg cells in directly related to reduced severity of RA. Thus, Treg cell proliferation and activity in the periphery and joints is essential for prevention of rheumatic disease and their dysfunction is implicated in pathogenesis (43).
Autoimmune Myasthenia Gravis
Autoimmune myasthenia gravis is a well characterized autoimmune disease affecting neuromuscular transmission. MG is CD4+ T cell dependent, mediated by anti-acetylcholine receptor (anti-AChR) autoantibodies. MG patients show no difference in frequency and proliferation from normal controls, but exhibit a markedly attenuated ability to suppress effector T cell proliferation. Therefore, the pathogenesis and progression of MG may be dependent upon aberrant Treg cell functioning due to abnormally low levels of FoXP3 production, and subsequent decrease in Treg cell regulatory capacity (43).
Autoimmune Polyglandular Syndrome Type II
Autoimmune polyglandular syndrome type II is a multiple endocrine disease initiated by an autoimmune process. The hallmarks of APS-II include the occurrence of two or more of the following diseases: Addison's disease, T1D or autoimmune thyroid disease. No difference is seen in frequency, surface markers, death rates or FoXP3 expression of Treg cells in APS-II patients; however, there is a significant decrease in suppressor function, resulting in failure to suppress proliferation of effector T cells (42).
Autoimmune Thyroiditis
Hashimoto thyroiditis (HT) is an organ-specific autoimmune disease characterized by lymphocyte infiltration of the thyroid that leads to follicular destruction. Thyroglobulin (Tg)-specific T cells are generated and migrate to the thyroid where they produce IFN-γ, facilitating apoptosis of thyrocytes through caspase activation. The magnitude of the attack on the thyroid increases by means of further expansion and accumulation of activated Tg-specific T cells (52). IL-10 produced by Treg cells, induced by DCs, is essential for suppression of Tg-specific T cell responses, targeted lymphocyte infiltration and follicular destruction (53,54).
Type-1 Diabetes
Type-1 diabetes (T1D) is a chronic TH1 cell-mediated autoimmune disease that destroys the insulin-producing β cells in the islets of Langerhans within the pancreas, in genetically prone individuals (36,42). A decrease in several immunoregulatory lineages, including natural killer T cells and Treg cells is found in T1D, leaving little suppression of the effector antigen-specific CD4+ and CD8+ T cells involved in the pathogenesis of T1D (36). The deficit of Treg cells is observed in both newly diagnosed individuals and those with long-term conditions. Functional capacity of Treg cells is also needed (42).
Two methodologies have proven effective in the pursuit of utilizing Treg cells to prevent or reverse T1D. Boosting the regulation of T cells via healthy Treg cells has been accomplished through the activation of Treg cells that respond to the antigen of islet cells and the adoptive transfer of Treg cells from a non-T1D mouse into a diabetes-prone mouse (55,56).
Systemic Lupus Erythematosus and Autoimmune Lymphoproliferative Syndrome
Systemic lupus erythematosus (SLE) and autoimmune lymphoproliferative syndrome are chronic, systemic, autoimmune diseases demonstrated to involve a decreased frequency of nTreg cells. SLE patients also display B cell hyperreactivity, defects in lymphoid activation processes, aberrant cytokine production and a lower percent of Treg cells in population of peripheral blood mononuclear cells (PBMCs). It is unclear whether Treg cell function is involved in SLE or ALS etiology and pathogenesis (42).
Psoriasis
Psoriasis is an inflammatory skin disease which has many characteristics of a TH1 cell-mediated autoimmune disease. Activation of autoreactive T cells, and their cytokine secretions, trigger keratinocytes to proliferate and produce psoriasis (57–61). Dysfunctional Treg cells, with decreased capacity for CD4+ suppression, may be the culprit for the unrestrained T cell proliferation observed (62–65). These findings represent a critical component of organ-specific autoimmune disease and their implications for possible therapeutic manipulation of Treg cells (see Fig. 1).
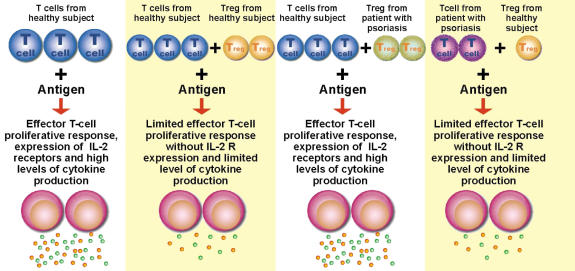
The role of Treg cells in the active suppression of psoriasis, seen in Fig. 2, may be applied to other autoimmune diseases discussed previously. The great potentiality of adoptive antigen-specific Treg cell transfer impels the research of CAM modalities and remedies to facilitate such processes with efficiency and safety.
Figure 2.
The importance of regulatory T cells in controlling lymphoproliferative response of CD4+ effector cells, expression of IL-2 receptor and level of cytokine production in healthy subjects and patients with psoriasis. These criss-cross experiments were set for testing psoriatic Treg cells in their ability to inhibit normal T cell response, and vice versa.
Rejecting Valuable Donations: Insufficient Treg Cell Suppression of Allogeneic Reactions
Bone marrow (BM) transplants are utilized to correct myriad afflictions ranging from primary immunodeficiencies to hematologic malignancies. In order for a BMT to be successful it must overcome two alloreactive obstacles: GvHD and graft rejection, also known as HvGD.
GvHD is common to allogeneic BM transplantations. It involves the immunologic attack on the cells and tissues of the recipient by the T cell contaminants contained in the donor BM. GvHD primarily affects the skin, liver and gastrointestinal tract through T cell infiltration of epithelia of these areas. T cells present in the graft recognize the host tissues as antigenically foreign and begin an offensive proinflammatory reaction utilizing TNF-α and IL-1, the conditioning of T cell activation with cytokine production, adhesion molecule expression and maturation and trafficking of effector T cells to the area (66). A variety of host antigens presented to donor T cell major histocompatibility complexes (MHC), in particular human lymphocyte antigens (HLAs), are responsible for the initiation of GvHD. Donor T cells are undesirable as effector cells; however, they are beneficial for graft acceptance by preventing the recipients immune system from rejecting the graft. The dual quality of donor T cells implores research in order to maximize graft acceptance through downplaying GvHD aspects of donor T cells.
Graft rejection is mediated by the host immune system in response to the foreign graft cells (20). Dominant transplantation tolerance to BM and tissue grafts has been induced in mice. The presence or addition of Treg cells can induce antigen-specific tolerance to BM grafts, while reduction of Treg cells may accelerate GvHD and graft rejection. The proposed mechanisms of Treg cell functionality in graft acceptance include IL-10 secretion and IL-2 receptor-mediated suppression of donor T cell expansion (66). Co-injection of donor BM and Treg cells, stimulated with donor-type APC and high levels of IL-2 ex vivo, into mice, resulted in long-term alloantigen-specific protection of the BM graft (20).
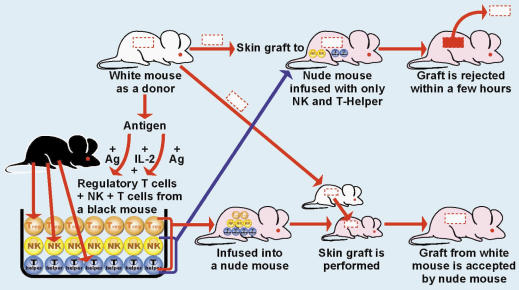
In a similar fashion, Treg cells promote conditions conducive to long-term acceptance of allografts (67). Activation of Treg cells could result in the acceptance of donor organs as seen in Fig. 3. Culturing of Treg cells with IL-2 and antigen from a donor mouse produced antigen-specific Treg cells. These cells were infused into skin graft recipient mice, preventing skin graft rejection even though the mice were also infused with NK and T cells (68,69). Graft recipient mice, without introduction of antigen-specific Treg cells, rejected the skin graft. The observed induction of specific tolerance occurred with just one injection of antigen-specific Treg cells. This shows the possibility the generation of antigen-specific Treg cells, followed by their infusion into a patient, prior to organ transplantation, may encourage success of organ or tissue transplants (70).
Figure 3.
The importance of regulatory T cells in the prevention of organ rejection.
The aforementioned problems necessitate the discovery of new methods for immune tolerance, in order to maintain the life saving benefits of BM grafts without the potential complications (20).
Mechanisms of Self Preservation: Integral Functions of Treg Cells for Maintenance of Immune Harmony
Innovative research of Treg cell involvement in various pathologies related to autoimmune and alloimmune responses elucidates the mechanisms involved in disease pathogenesis and, subsequently, identifies plausible means for ameliorative therapies. It is apparent that the Treg cell quantity and activation state are integral and equally important factors in the development and progression of autoimmunity, alloimmunity and cancer (71). Increased research is necessary in order to determine Treg cell functioning in relation to individual disease states. This will afford CAM researchers insight into the appropriate means of approaching a variety of human disorders with respect to Treg cells.
Treg cell's essential role in the management of immune response to specific auto, allo and tumor antigens has been detailed. Harmony between regulatory and effector arms of the immune system is a necessity for good health (1,2). Treg cell intricacy and specificity to individual antigens impels further research and highlights Treg cell's overall importance to human health and CAM. The conceptual framework laid down provides a solid basis from which to explore the diversity of therapeutic methodologies possible for the direct manipulation of Treg cells to attenuate hypersensitivity, cancer, infection, autoimmunity and alloimmunity.
Conflict of Interest
Aristo Vodjani is co-owner of Immunosciences Lab, Inc. and Jonathan Erde is an employee of Immunosciences Lab Inc. They declare no conflict of interest.
Acknowledgments
We greatly appreciate the contributions of Mr Joel Bautista for the design and illustration of figures 2 and 3 in this article.
References
- 1.Vojdani A, Erde J. Regulatory T Cells, a potent immunoregulatory target for CAM researchers: the ultimate antagonist (I) Evid Based Complement Alternat Med. 2006;3:25–30. doi: 10.1093/ecam/nek022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: modulating allergic and infectious disease pathology (II) Evid Based Complement Alternat Med. 2006;3:209–15. doi: 10.1093/ecam/nel020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellavite P, Conforti A, Piasere V, Ortolani R. Immunology and homeopathy. 1. Historical background. Evid Based Complement Alternat Med. 2005;2:441–52. doi: 10.1093/ecam/neh141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naser B, Bodinet C, Tegtmeier M, Lindequist U. Thuja occidentalis (Arbor vitae): a review of its pharmaceutical, pharmacological and clinical properties. Evid Based Complement Alternat Med. 2005;2:69–78. doi: 10.1093/ecam/neh065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padmavathi B, Rath PC, Rao AR, Singh RP. Roots of Withania somnifera inhibit forestomach and skin carcinogenesis in mice. Evid Based Complement Alternat Med. 2005;2:99–105. doi: 10.1093/ecam/neh064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminogawa S, Nanno M. Modulation of immune functions by foods. Evid Based Complement Alternat Med. 2004;1:241–50. doi: 10.1093/ecam/neh042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian Green Propolis against in vitro and in vivo ischemic neuronal damage. Evid Based Complement Alternat Med. 2005;2:201–7. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindequist U, Niedermeyer TH, Julich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–99. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki N, Shibata T, Itoh T, Suzuki T, Tanaka H, Nakamura T, et al. Inhibition of IgE-dependent mouse triphasic cutaneous reaction by a boiling water fraction separated from mycelium of Phellinus linteus. Evid Based Complement Alternat Med. 2005;2:369–74. doi: 10.1093/ecam/neh105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Murakami T, Oppenheim JJ, Howard OM. Triptolide, a constituent of immunosuppressive Chinese herbal medicine, is a potent suppressor of dendritic-cell maturation and trafficking. Blood. 2005;106:2409–16. doi: 10.1182/blood-2005-03-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Chen T, Chen G, Li N, Wang J, Ma P, et al. Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-kappaB activation. Biochem Biophys Res Commun. 2006;345:1122–30. doi: 10.1016/j.bbrc.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Luo CN, Lin X, Li WK, Pu F, Wang LW, Xie SS, et al. Effect of berbamine on T-cell mediated immunity and the prevention of rejection on skin transplants in mice. J Ethnopharmacol. 1998;59:211–5. doi: 10.1016/s0378-8741(97)00117-7. [DOI] [PubMed] [Google Scholar]
- 13.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol. 2004;90:339–46. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Pradeep CR, Kuttan G. Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int Immunopharmacol. 2004;4:1795–803. doi: 10.1016/j.intimp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol. 2004;92:291–5. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol. 2003;3:147–58. doi: 10.1046/j.1359-4117.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- 17.Hong SH, Jeong HJ, Chung HS, Kim HR, Chae HJ, Shin T, et al. An herbal formula, herbkines, enhances cytokines production from immune cells. J Ethnopharmacol. 2005;98:149–55. doi: 10.1016/j.jep.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Seo N, Yagi H, Ohtani T, Tokura Y, Takigawa M, et al. Unique therapeutic effects of the Japanese–Chinese herbal medicine, Sairei-to, on Th1/Th2 cytokines balance of the autoimmunity of MRL/lpr mice. J Dermatol Sci. 2002;28:198–210. doi: 10.1016/s0923-1811(01)00161-x. [DOI] [PubMed] [Google Scholar]
- 19.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–47. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 20.Joffre O, van Meerwijk JP. CD4(+)CD25(+) regulatory T lymphocytes in bone marrow transplantation. Semin Immunol. 2006;18:128–35. doi: 10.1016/j.smim.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nardelli DT, Cloute JP, Luk KH, Torrealba J, Warner TF, Callister SM, et al. CD4(+) CD25(+) T cells prevent arthritis associated with Borrelia vaccination and infection. Clin Diagn Lab Immunol. 2005;12:786–92. doi: 10.1128/CDLI.12.6.786-792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiappelli F. The molecular immunology of mucositis: implications for evidence-based research in alternative and complementary palliative treatments. Evid Based Complement Alternat Med. 2005;2:489–94. doi: 10.1093/ecam/neh129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–42. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 25.Wojdani A, Alfred LJ. Alterations in cell-mediated immune functions induced in mouse splenic lymphocytes by polycyclic aromatic hydrocarbons. Cancer Res. 1984;44:942–5. [PubMed] [Google Scholar]
- 26.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4148–88. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 27.Wang RF, Peng G, Wang HY. Regulatory T cells and Toll-like receptors in tumor immunity. Semin Immunol. 2006;18:136–42. doi: 10.1016/j.smim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Fehervari Z, Sakaguchi S. Regulatory T cells. In: Lotze MT, Thompson AW, editors. Measuring Immunity. Oxford: Elsevier; 2005. pp. 322–35. [Google Scholar]
- 29.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–8. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90–8. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 31.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 32.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor-a) monoclonal antibody. Cancer Res. 1999;59:3128–33. [PubMed] [Google Scholar]
- 33.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 34.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee R, Wagar D, Stephens TA, Lee-Chan E, Singh B. Identification of CD4+ T cell-specific epitopes of islet-specific glucose-6-phosphatase catalytic subunit-related protein: a novel β cell autoantigen in type 1 diabetes. J Immunol. 2005;174:5306–15. doi: 10.4049/jimmunol.174.9.5306. [DOI] [PubMed] [Google Scholar]
- 37.Viglietta B, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Kuchroo VK, Inobe JI, Hafler DA, Wiener HL. Regulatory T-cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 39.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–95. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 40.Morelli AE, Thompson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 41.Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Alternat Med. 2005;2:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4:351–63. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–59. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 45.Fontenot J, Rudensky AY. A well-adapted regulatory contrivance: regulatory T cell development and the Forkhead family transcription factor FOXP3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 46.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 1994;299:1237–40. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 47.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 48.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–30. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 50.Belghith M, Bluestone JA, Barriot S, Megret J, Bach J, Chatenoud L. TGF-β-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–8. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 51.Nardelli DT, Nurchill MA, England DM, Torrealba J, Callister SM, Schell RF. Association of CD25+CD4+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged g-interferon-deficient mice treated with anti-interleukin-17 antibody. Clin Diag Lab Immunol. 2004;11:1075–84. doi: 10.1128/CDLI.11.6.1075-1084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang SH, Bretz JD, Phelps E, Mezosi E, Arscott PL, Utsugi S, et al. A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms nondestructive thyroiditis in experimental autoimmune thyroiditis. J Immunol. 2002;168:2470–4. doi: 10.4049/jimmunol.168.5.2470. [DOI] [PubMed] [Google Scholar]
- 53.Gangi E, Vasu C, Cheaten D, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in GM-CSF-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–13. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 54.Verginis P, Li HS, Carayannoitis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol. 2005;174:7433–9. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- 55.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 56.Mukherjee R, Chaturvedi P, Qinh Y, Singh B. CD4+CD25+ regulatory T cells generated in response to insulin B:9–23 peptide prevent adoptive transfer of diabetes by diabetogenic T cells. J Autoimmun. 2003;21:221–7. doi: 10.1016/s0896-8411(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 57.Szabo SK, Hammerberg C, Yoshida Y, Bata-Csorgo Z, Cooper KD. Identification and quantitation of interferon-gamma producing T cells in psoriatic lesions: localization to both CD4+ and CD8+ subsets. J Invest Dermatol. 1998;111:1072–8. doi: 10.1046/j.1523-1747.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 58.Cooper KD. Skin-infiltrating lymphocytes in normal and disordered skin: activation signals and functional roles in psoriasis and mycosis fungoides-type cutaneous T cell lymphoma. J Dermatol. 1992;19:731–7. doi: 10.1111/j.1346-8138.1992.tb03770.x. [DOI] [PubMed] [Google Scholar]
- 59.Vollmer S, Menssen A, Trommler P, Schendel D, Prinz JC. T lymphocytes derived from skin lesions of patients with psoriasis vulgaris express a novel cytokine pattern that is distinct from that of T helper type a and T helper type 2 cells. Eur J Immunol. 1994;24:2377–82. doi: 10.1002/eji.1830241018. [DOI] [PubMed] [Google Scholar]
- 60.Prens EP, van Joost T, Hegmans JP, Hooft-Benne K, Ysselmuiden OE, Benner R. Effects of cyclosporine on cytokines and cytokine receptors in psoriasis. J Am Acad Dermatol. 1995;33:947–53. doi: 10.1016/0190-9622(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 61.Bata-Csorgo Z, Cooper KD, Ting KM, Voorhees JJ, Hammerberg C. Fibronectin and a5 integrin regulate keratinocyte cell cycling: a mechanism for increased fibronectin potentiation of T cell lymphokine-driven keratinocyte hyperproliferation in psoriasis. J Clin Invest. 1998;101:1509–18. doi: 10.1172/JCI171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mrowietz U, Zhu K, Christophers E. Treatment of severe psoriasis with anti-CD25 monoclonal antibodies. Arch Dermatol. 2000;136:675–6. doi: 10.1001/archderm.136.5.675. [DOI] [PubMed] [Google Scholar]
- 63.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–87. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prinz JC, Gross B, Vollmer S, Trommler P, Strobel I, Meurer M, et al. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. Eur J Immunol. 1994;24:593–8. doi: 10.1002/eji.1830240315. [DOI] [PubMed] [Google Scholar]
- 65.Bata-Csorgo Z, Hammerberg C, Voorhees JJ, Cooper KD. Kinetics and regulation of human keratinocyte stem cell growth in short-term primary ex vivo culture: cooperative growth factors from psoriatic lesional T lymphocytes stimulate proliferation among psoriatic uninvolved, but not normal, stem keratinocytes. J Clin Invest. 1995;95:317–27. doi: 10.1172/JCI117659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ichiki Y, Bowlus CL, Shimoda S, Ishibashi H, Vierling JM, Gershwin ME. T cell immunity and graft-versus-host disease (GVHD) Autoimmun Rev. 2006;5:1–9. doi: 10.1016/j.autrev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Adeegbe D, Bayer AL, Levy RB, Malek TR. Cutting edge: allogeneic CD4+CD25+Foxp3+ T regulatory cells suppress autoimmunity while establishing transplantation tolerance. J Immunol. 2006;176:7149–53. doi: 10.4049/jimmunol.176.12.7149. [DOI] [PubMed] [Google Scholar]
- 68.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2001;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 69.Sakaguchi S. Naturally arising FOXP3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 70.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 1994;299:1237–40. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 71.Maizels RM. Infections and allergy—helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–61. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]