Abstract
Argania spinosa is an evergreen tree endemic of southwestern Morocco. Many preparations have been used in traditional Moroccan medicine for centuries to treat several illnesses including diabetes. However, scientific evidence supporting these actions is lacking. Therefore, we prepared various extracts of the argan fruit, namely keel, cake and argan oil extracts, which we tested in the HTC hepatoma cell line for their potential to affect cellular insulin responses. Cell viability was measured by Trypan Blue exclusion and the response to insulin evaluated by the activation of the extracellular regulated kinase (ERK1/2), ERK kinase (MEK1/2) and protein kinase B (PKB/Akt) signaling components. None of the extracts demonstrated significant cytotoxic activity. Certain extracts demonstrated a bi-phasic effect on ERK1/2 activation; low doses of the extract slightly increased ERK1/2 activation in response to insulin, whereas higher doses completely abolished the response. In contrast, none of the extracts had any significant effect on MEK whereas only a cake saponin subfraction enhanced insulin-induced PKB/Akt activation. The specific action of argan oil extracts on ERK1/2 activation made us consider an anti-proliferative action. We have thus tested other transformed cell lines (HT-1080 and MSV-MDCK-INV cells) and found similar results. Inhibition of ERK1/2 activation was also associated with decreased DNA synthesis as evidenced by [3H]thymidine incorporation experiments. These results suggest that the products of Argania spinosa may provide a new therapeutic avenue against proliferative diseases.
Keywords: cancer, diabetes, MAPK, PKB, transformed cell lines
Introduction
The argan tree (Argania spinosa) is a thorny evergreen tree that plays an essential ecological and socio-economic role in southwestern parts of Morocco. A program aimed to increase the industrial value of Argania spinosa is currently carried out in Morocco (1,2). All the parts of the tree have value. Specially, the almond oil is largely used for cooking or in traditional medicine and represents the argan tree's most important product.
Various biological properties largely known by traditional medicine have received some scientific supports. It is notably the case for the traditional uses of almond oil to decrease blood cholesterol and hypertension (3–5), to treat skin diseases such as juvenile acne and chicken pox pustules (2), or for other beneficial effects of saponin derivatives, notably analgesic and anti-inflammatory (6), anti-microbial (2) and lipolytic properties (2).
Chemical analysis revealed that argan oil is particularly well balanced in terms of fatty acid composition (2,7). Argan oil is rich (80%) in unsaturated fatty acids, 43% of which are monounsaturated fatty acids (especially oleic acid). Essential fatty acids represent 36% (especially linoleic acid) (8). Studies with the unsaponifiable fraction of the oil highlighted antioxidant compounds present in relatively high concentrations (mainly tocopherols). Argan oil is also known to contain phenolic constituents like caffeic, vanillic and ferulic acids, as well as tyrosol, and sterols like schottenol, spinasterol and finally squalene (2,8,9).
Recently we became aware of claims for a potential anti-diabetic action of Argania spinosa almonds that has not been scientifically validated to date (Z. Charrouf, unpublished data). Diabetes has become a worldwide epidemic disease with over 170 million people suffering from it (10). The vast majority of cases are of the so-called Type 2 Diabetes Mellitus (T2DM). There exists a genetic predisposition to the disease that is precipitated by environmental factors such as a sedentary lifestyle and unhealthy eating habits, obesity being one of the most prominent risk factors. T2DM is characterized by an insensitivity of peripheral tissues to the action of insulin, termed insulin resistance.
Binding of insulin to its specific cell surface receptor induces receptor autophosphorylation that triggers the activation of two main intracellular signaling pathways: the phosphatidyl-inositol-3-kinase (PI3K) pathway involved in glucose transport and glycogen synthesis, and the extracellular regulated kinase (ERK) pathway involved in gene expression and cell proliferation mechanisms (11). Resistance to insulin can appear at various levels of these signaling pathways (11).
We therefore explored the claimed anti-diabetic potential of argan seeds by examining the effects of various extracts on an insulin-responsive cell line. More specifically we looked at the potential of our argan extracts to increase the in vitro PI3K and ERK responses to insulin in HTC cells, a cell line of hepatic origin. One of the saponin extracts had a stimulatory effect on the PI3K cascade, compatible with an anti-diabetic potential. However we observed an inhibitory action specifically on ERK for a majority of the extracts. This unexpected result prompted us to explore the anti-proliferative effect of pertinent argan seed extracts on transformed cells of human and canine origin.
Materials and Methods
Chemicals
Phospho-specific antibodies p44/42 MAPKThr202, PKB/AktSer473, MEK1/2Ser217 and corresponding non-phospho-specific antibodies corresponding kits were purchased from Cell Signaling Technology (Beverly, MA). Bovine pancreas insulin as well as other chemicals were obtained from Sigma-Aldrich Canada (Oakville, ON). Tritiated thymidine ([methyl-3H] TdR; 4.0 Ci/mmol) was procured from MP Biomedicals, LLC (Irvine, CA).
Argania spinosa Extracts
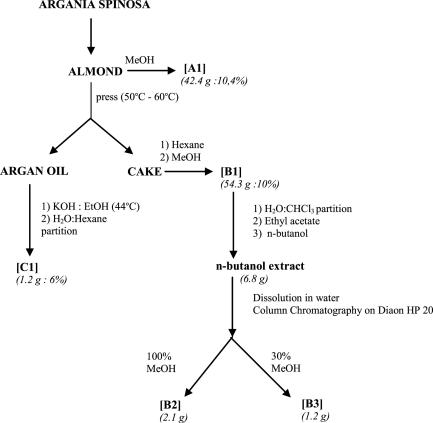
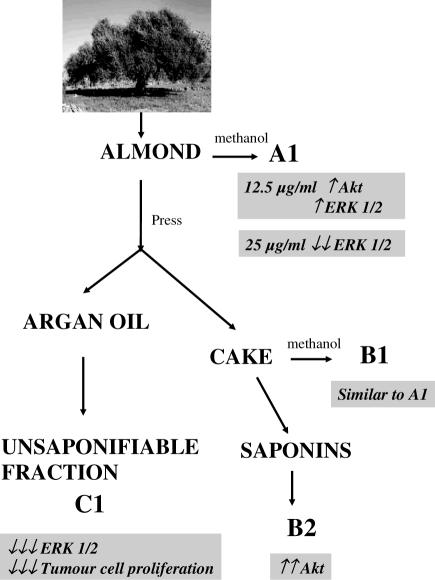
The Argania spinosa extracts were prepared at the Chemistry Laboratory of Plants Chemistry, Organic and Bioorganic Synthesis headed by Prof. Z. Charrouf. The argan fruit almonds, cake and oil were kindly provided by the Argan Oil Womens Cooperative (Targanine), Morocco, and prepared according to procedures previously described (8,12,13). Figure 1 summarizes the preparation of different extracts. All extraction procedures were carried out under normal lighting conditions. Almond extract A1 was obtained by macerating ground argan almonds (407 g) at room temperature in 80% methanol for 36 h with agitation. The solvent was then evaporated and the residue dried to obtain a fine whitish powder (42.4 g: 10.4% yield; Fig. 1). Almonds were also mechanically pressed at 50–60°C in order to separate argan oil and press cake. The press cake (543 g starting material) was first defatted with hexane and extracted with methanol at room temperature giving extract B1 (54.3 g: 10% yield). Additionally, two saponin fractions were obtained from the press cake following extraction with methanol as follows. The methanol extract B1 was partitioned between water and chloroform; the water soluble portion was successively extracted with ethyl acetate and n-butanol. The n-butanol extract (6.8 g) was dissolved in minimum water and chromatographed on diaon HP20 column (80 × 2 cm) sequentially with water, 30% MeOH and 100% MeOH. After evaporation of MeOH from the last fraction, the polar fraction of press cake saponins B2 (2.1 g) and a less polar fraction from the 30% methanol B3 extract (1.2 g) were obtained, the latter being collected after treating the water soluble portion with n-butanol. Finally, an unsaponifiable fraction C1 was obtained from argan oil. The oil (20 g starting material) was well mixed with 200ml of ethanol and potassium hydroxide (KOH2N/EtOH analysis) and heated (at ∼44°C) with backward flow for 1 h. Following addition of distilled water (200 ml) and cooling to room temperature, hexane was added (150 ml). This mixture was shaken vigorously for 1 h and the partitioned hexane layer was collected. The remaining aqueous phase was extracted twice more with hexane (150 ml). The hexane extracts were pooled, washed with water/ethanol (50 : 50), dehydrated over anhydrous Na2SO4 and filtered. The solvent was removed using a rotavapor. The residue (1.2 g) was heated at 103°C for 1 h. Cake saponin extracts B2 and B3 were dissolved in water. The three other extracts namely A1, B1 and C1 were dissolved in DMSO. The maximal DMSO content in culture medium was set at 0.1 % (v/v).
Figure 1.
Steps involved in the preparation of various argan almond extracts. Details are described in the Materials and Methods section. The different extracts are as follows: A1, methanolic almond extract; B1, methanolic press cake extract; B2, more polar saponin press cake fraction (extracted with 100% MeOH); B3, less polar saponin press cake fraction (extracted with 30% MeOH); and C1, unsaponifiable argan oil extract. All extraction procedures were carried out at room temperature, except where indicated.
Cell Culture
HTC cells express insulin receptors and signaling pathways similar to those found in primary rat hepatocytes (14,15). HTC cells were cultured in minimal essential medium (MEM) (Sigma, St Louis), supplemented with 5% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C under a humidified atmosphere of 5% CO2/95% air.
Other cells used were the HT-1080 (human fibrosarcoma) cell line, and the invasive variant of Moloney Sarcoma Virus transformed Madin-Darby Canine Kidney cells (MSV-MDCK-INV) (16) possessing motile and invasive properties. They were cultured in Dubelcco's minimal essential medium (DMEM) containing 10% FBS, 2 mM glutamine, 1% non-essential amino acids, 0.5% vitamins, 1% penicillin and streptomycin under 5% CO2 atmosphere at 37°C (16,17).
Cell Viability Studies
Confluent cells were incubated in MEM + 0.5% FBS with different doses of extracts. Following 6 or 21 h incubation periods, cells were rinsed with calcium-free PBS (137 mM NaCl, 2.68 mM KCl, 1.47 mM KH2PO4 and 8.1 mM Na2HPO4, pH 7.4) and loosened with trypsin (0.05% trypsin and 0.53 mM EDTA) for 5 min at 37°C. Cell viability was then determined by Trypan Blue exclusion (0.2% final concentration).
Treatments
Confluent HTC cells were rinsed and incubated in MEM containing 0.5% FBS and extracts at different doses (12.5, 25, 50 and 100 μg ml−1) for periods of 5 or 20 h. Additional 1 h incubation with fresh extracts was done in serum-free medium. This procedure ensured that intracellular signaling pathways activated by growth factors resumed baseline activity. Following treatments with extracts, insulin (0–100 nM final concentration) was added to the medium for 5 min at 37°C. The stimulation was stopped by quickly removing the medium and washing the cells twice with ice-cold phosphate-buffered saline (PBS). Cells were solubilized in lysis buffer [25 mM Tris–HCl, pH 7.4, 25 mM NaCl, 0.5 mM EDTA, 1% Triton X-100, 0.1% SDS, protease inhibitors (2 mM benzamide, 1 mM phenylmethanesulfonyl fluoride and 10 μg ml−1 aprotinine) and the phosphatase inhibitors (10 mM sodium fluoride, 1 mM sodium orthovanadate and 10 mM sodium pyrophosphate)]. Lysis was carried out for 5 min on ice followed by scraping. Lysates were recovered and insoluble material was removed by centrifugation (4500 g for 10 min) at 4°C. The supernatant was kept at −80 °C until analyzed.
HT-1080 and MSV-MDCK-INV cells were seeded at 1.80 × 105 cells per 60 mm plate and cultured as indicated for ∼24 h before use. About 18 h before the beginning of the incubation, at 60–70% confluence, the initial medium was changed for a DMEM medium containing 0.5% FBS. Cells were then incubated with serum-free medium containing Argania spinosa extracts (doses ranging from 0.79 to 12.5 μg ml−1 as indicated) for 6 h. Cells were washed three times with PBS/CM (PBS/0.1 mM CaCl2, 1 mM MgCl2), and lysed with 3× Laemmli sample buffer (15% β-mercaptoethanol, 7.5% SDS, 30% glycerol, 300 mM Tris–HCl pH 6.8 and bromophenol blue) heated at 95°C. The cell lysates were harvested and kept at −80°C pending immunoblot assays.
Immunoblot Analysis
Cell lysates were subjected to electrophoresis on 10% SDS-polyacrylamide gels, transferred to nitrocellulose membranes and blotted with phospho-specific p42/44 MAPKThr202 (1 : 2000), PKB/AktSer473 (1/1000) or MEK1/2Ser217 (1 : 2000) antibody. Equal protein loading was routinely ascertained following transfer to nitrocellulose membranes by Ponceau Red staining. Moreover non-phosphorylated forms of the proteins were detected by following antibodies stripping and incubation of membranes with pan-specific antibodies, namely anti-Akt(1 : 1000), anti-ERK1/2 (1 : 1000) or anti-MEK1/2 (1 : 1000). The antibody-antigen complexes were detected with anti-mouse or anti-rabbit antibodies coupled with horseradish peroxidase and detected using an enhanced chemiluminescence kit (Perkin Elmer, Boston, MA).
Anti-proliferative Activity
HT-1080 and MSV-MDCK-INV cells were seeded into 24-well plates at a density of 1 × 105 cells per well and cultured 24 h in 10% FCS supplemented medium. They were starved in serum-free medium for another 24 h and then incubated for 16 h with fresh serum-free medium containing different concentrations (0.195, 0.39, 0.78, 1.56, 3.12, 6.25 and 12.5 μg ml−1) of C1. Medium was then removed and replaced with serum-free medium supplemented with fresh extract and [3H]thymidine (1 μCi ml−1) for 4 h at 37°C. Proteins were precipitated with cold 5% trichloroacetic acid (TCA), washed with water and the cells lysed with NaOH 0.1 N. The radioactivity incorporated was counted in a liquid-scintillation beta counter. The results were expressed as a function of maximal incorporation, and each value was normalized for respective protein content.
Results
Argania spinosa Extracts are not Cytotoxic
We first examined the effects of the different argan seed extracts on HTC viability. As shown in Table 1 the extracts do not exhibit any cytotoxic activity toward HTC cells. This was true for concentrations as high as 50 μg ml−1 and for incubation periods of 6 or 21 h.
Table 1.
In vitro cytotoxicity of argan extracts on HTC cells
| Treatment | % Viability | |||
|---|---|---|---|---|
| 6 h | 21 h | |||
| DMSO | 98.6 | ±0.10 | 99.0 | ±0.16 |
| H2O | 98.7 | ±0.15 | 99.6 | ±0.21 |
| A1 | 97.5 | ±0.55 | 97.0 | ±0.52 |
| B1 | 98.7 | ±0.46 | 97.4 | ±0.47 |
| B2 | 97.3 | ±0.98 | 98.5 | ±1.47 |
| B3 | 97.8 | ±0.12 | 97.5 | ±0.10 |
| C1 | 98.8 | ±0.48 | 95.3 | ±0.44 |
The cells were exposed to 50 μg ml−1 of each extract for 6 or 21 h and viability was then determined by Trypan Blue (0.2%) exclusion. The suitable controls (DMSO or H2O) were carried out as indicated in the Materials and Methods section.
Methanol Extracts of A. spinosa Almonds and Cake Exhibit Bi-phasic Modulation of Insulin Signaling in HTC Cells
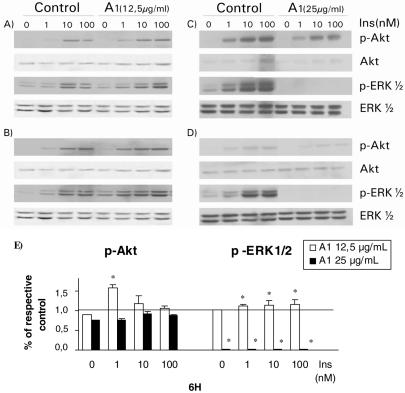
Next, HTC cells were treated with various argan seed extracts for 6 or 21 h and their action on the cells' responsiveness to insulin was assessed by measuring phosphorylation of ERK1/2 and Akt/PKB. Treatment of HTC cells with the methanolic extract of argan almond (A1) or cake (B1) induced a bi-phasic effect on the response of ERK1/2 and, to lesser extent of Akt, to insulin stimulation. Indeed, short- or long-term exposure of cells to 12.5 μg ml−1 of A1 (Fig. 2A and B, respectively) and B1 (Fig. 3A and B) enhanced slightly the activation of ERK1/2. However increasing A1 concentration to 25 μg ml−1 completely abolished ERK1/2 response and diminished that of Akt after either 6 or 21 h incubation (Fig. 2C and D). This bi-phasic behavior is highlighted in Fig. 2E, where Akt and ERK1/2 activation is presented in relation to respective DMSO controls for both concentrations of A1 extract used over a 6 h period. Similar results were observed with B1 at 25 μg ml−1 (results not illustrated).
Figure 2.
Concentration-dependent effects of methanolic almond extract on major insulin-signaling pathways. HTC cells cultured for 6 h (A and C) and 21 h (B and D) in the presence of 12.5 μg ml−1 (A and B) or 25 μg ml−1 (C and D) of A1 extract. Treated or control cells were then stimulated with indicated concentrations of insulin (Ins) after treatment by A1 extract. The cells were lysed and 20 μg of SDS-lysis protein extract were used for western blot analysis as described in the Materials and Methods section. Proteins immunoreactive to antibodies raised against the phosphorylated forms of ERK1/2 (p-ERK1/2) and PKB/Akt (p-Akt) were revealed by enhanced chemiluminescence as described in the Materials and Methods section. The non-phosphorylated proteins were revealed on stripped membrane as loading controls. Results are representative of three separate determinations. Densitometric analysis of results with 6 h treatment with both concentrations of A1 extract is presented in panel E. Values obtained with A1 are presented relative to their respective DMSO controls at each insulin concentration. Statistically different from respective DMSO control, *P < 0.05.
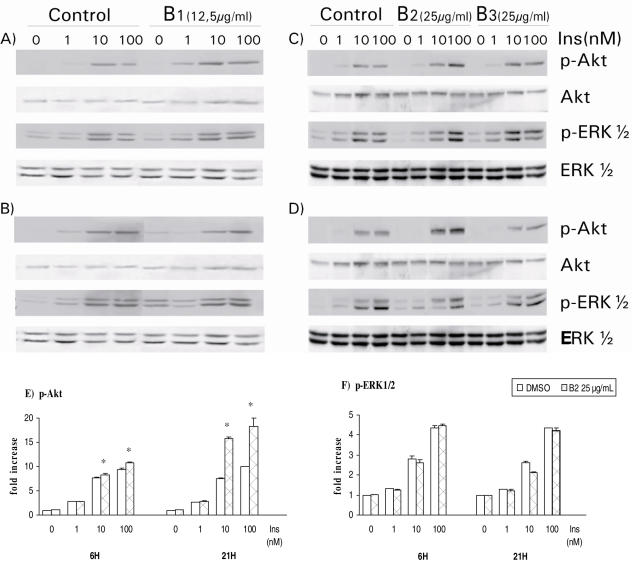
Figure 3.
Effect of methanolic press cake extract B1 and press cake saponins B2 and B3 on insulin response in HTC cells. HTC cells were treated for 6 h (A and C) and 21 h (B and D) with B1 (A and B), B2 and B3 (C and D) extracts followed by stimulation with indicated concentrations of insulin. The cells were lysed and 20 μg of SDS-lysis protein extract were used for western blot analysis as described in the Materials and Methods section. Proteins immunoreactive to antibodies raised against the phosphorylated forms of ERK1/2 (p-ERK1/2) and PKB/Akt (p-Akt) were revealed by enhanced chemiluminescence as described in the Materials and Methods section. The non-phosphorylated proteins were revealed on stripped membrane as loading controls. Results are representative of three separate determinations. Densitometric analysis of results for p-Akt and p-ERK1/2 in response to B2 treatment for 6 and 21 h is presented in E and F, respectively. Statistically different from respective DMSO control, *P < 0.05.
A. spinosa Cake Polar Saponins Selectively Enhance Insulin-dependent Akt Activation in HTC Cells
In contrast, B2 saponin extract increased Akt phosphorylation without affecting that of ERK1/2. As illustrated in Fig. 3E, the positive effect of B2 extract on Akt phosphorylation was more pronounced after a 21 h incubation period (immunoblot in Fig. 3D) as compared with 6 h (immunoblot in Fig. 3C, see also Fig. 3E). On the other hand, B3 extract did not have any significant effect on either ERK1/2 or Akt/PKB activation by insulin, be it after 6 or 21 h incubations (Fig. 3C and D, respectively).
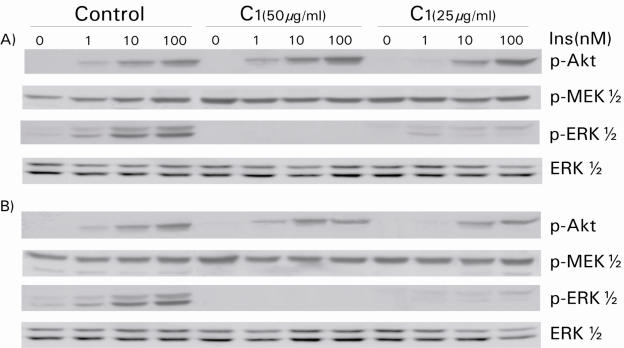
The Unsaponifiable Fraction of A. spinosa Oil Extract Selectively and Potently Inhibits Insulin-dependent and Insulin-independent ERK1/2 Activation in HTC Cells
The unsaponifiable extract of argan oil, C1, had a different pattern of action on HTC liver cells. At a concentration of 25 μg ml−1, C1 treatment reduced the ability of ERK1/2 to respond to increasing doses of insulin, whereas the Akt response remained undisturbed. This effect became more prominent at a concentration of 50 μg ml−1. The ERK1/2 response was abolished completely when C1 incubation time was increased from 6 to 21 h (Fig. 4). Cell lysates were then probed for phosphorylated MEK1/2, the kinases lying immediately upstream of ERK1/2 in this insulin-signaling cascade. As shown in Fig. 4, insulin-induced activation of MEK1/2 was not decreased by C1 extract irrespective of the dose or incubation period used. If anything, treatment with C1 extract appeared to slightly increase basal MEK1/2 phosphorylation.
Figure 4.
Unsaponifiable fraction C1 exerts a different action on MEK1/2 and ERK1/2 activity in response to insulin in HTC cells. HTC cells were treated with 25 and 50 μg ml−1 of C1 extract for 6 h (A) and 21 h (B) then the stimulated with insulin for 5 min before lysates were prepared. The cells were lysed and 20 μg of SDS-lysis protein extract were used for western blot analysis as described in the Materials and Methods section. Proteins immunoreactive to antibodies raised against the phosphorylated forms of ERK1/2 (p-ERK1/2) and PKB/Akt (p-Akt) were revealed by enhanced chemiluminescence as described in the Materials and Methods section. The non-phosphorylated proteins were revealed on stripped membrane as loading controls. Results are representative of three separate determinations.
The Unsaponifiable Fraction of A. spinosa Oil Extract Inhibits Proliferation of Several Transformed Cell Lines in a Dose-dependent Manner through ERK1/2 Inactivation
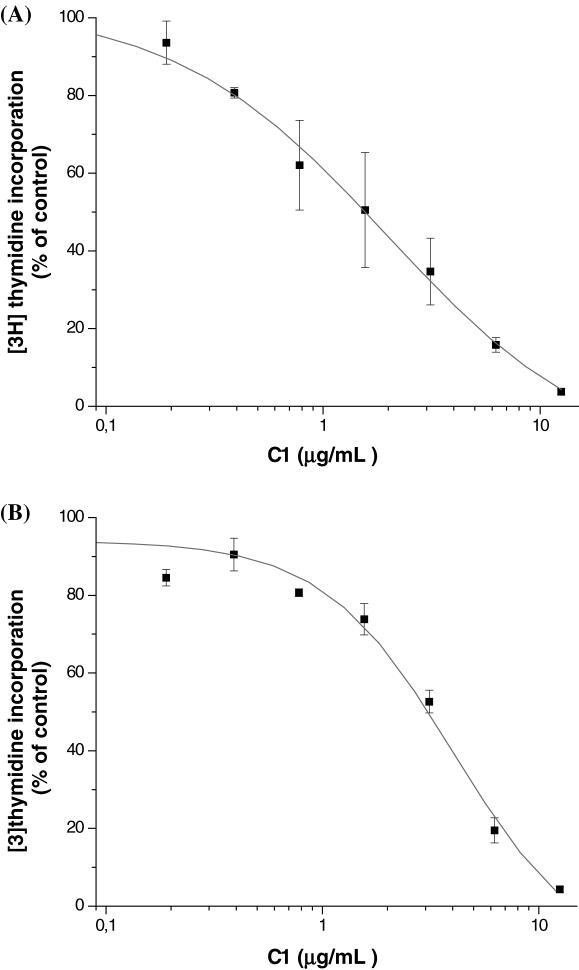
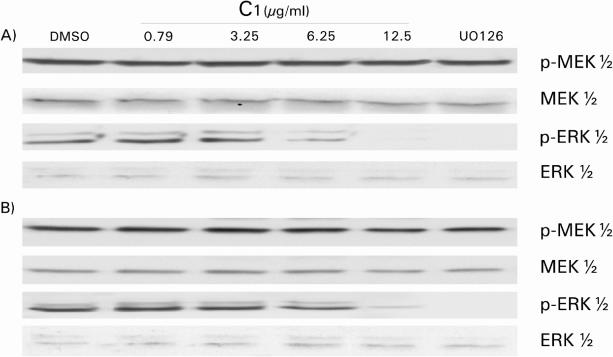
These intriguing results indicated that C1 extract could specifically interrupt the insulin-signaling cascade between MEK1/2 and ERK1/2. In view of the fact that this signaling cascade is involved in the proliferative action of insulin (also shared by growth factors), it seemed appropriate to examine the anti-proliferative effect of C1. For this purpose, we selected two cell lines of tumorigenic origin, namely the human HT-1080 fibrosarcoma cell line and the transformed and invasive canine MSV-MDCK-INV cells. As shown in Fig. 5, exposure to increasing concentrations of C1 extract inhibited DNA synthesis in both cell types in a dose-dependent manner. At a dose of 12.5 μg ml−1, C1 completely abolished [3H]thymidine incorporation. When the activation states of MEK1/2 and ERK1/2 were examined, a dose of 12.5 μg ml−1 of C1 strongly inhibited ERK1/2 without affecting MEK1/2 (Fig. 6). This behavior is similar to that seen previously in HTC cells (Fig. 4). Furthermore, the effect of C1 extract was comparable to that of U0126 a well-known inhibitor of MEK1/2 activity. Hence, C1 possesses strong anti-proliferative activity that appears to be mediated by the interruption of signaling cascades at the MEK1/2–ERK1/2 interface.
Figure 5.
[3H]thymidine incorporation by HT-1080 and MSV-MDCK-INV cells following treatment with C1 extract. HT-1080 (A) and MSV-MDCK-INV (B) cells were seeded in 24-well plates and serum removed after 24 h. Cells were then incubated in serum-free medium in the presence of the extract for 6 h then in the presence of extract and 1 μCi ml−1 [3H]thymidine 1 μCi ml−1)for another 4 h. Radioactivity was counted on NaOH extracts. The results are expressed according to maximal incorporation in the presence of DMSO; each value having been corrected for the quantity of proteins in each well. Measurements were done in triplicate and are the mean of two separate experiments.
Figure 6.
ERK1/2 and MEK1/2 phosphorylation status in HT-1080 and MSV-MDCK-INV cells after C1 treatment. Lysates obtained from HT-1080 (A) and MSV-MDCK-INV (B) cells after exposition to unsaponifiable fraction C1 at the indicated concentrations or to 10 μM UO126 were blotted against the phosphorylated or non-phosphorylated forms of MEK1/2 and ERK1/2, respectively.
Discussion
It recently came to our attention through Moroccan folk medicine that argan almond may be benefical for the treatment of diabetes (Z. Charrouf, unpublished data). This study was thus carried out to examine the potential anti-diabetic action of various fractions of the seed of the argan tree, Argania spinosa, and to begin exploring their mechanism of action. Methanolic extracts of the almonds (A1), and cake (B1), were prepared as well as two fractions of cake saponins (B2 and B3) and an unsaponifiable fraction of argan oil (C1). None of the fractions exhibited any cytotoxic activity toward a model liver cell line, HTC cells.
Being an insulin-responsive cell type, HTC cells were used to test the hypothesis that some of the Argania spinosa seeds extract may exert some insulin-sensitizing action. We measured two key enzymes involved in mediating the physiological responses to insulin in target cells. Akt/PKB lies downstream of phosphatidyl-inositol-3-Kinase (PI3K) and mediates the effect of insulin to enhance glucose transport in muscle and adipose tissues (11). The enzyme ERK1/2, on the other hand, is implicated in the action of insulin on gene transcription and cell division (11). In the liver, both enzymes participate in insulin-stimulated glycogen synthesis (18). Figure 7 summarizes the results obtained in the present studies.
Figure 7.
Summary of the biological effects of Argania spinosa extracts.
Firstly, total methanolic extracts of the almond (A1) and press cake (B1) had a bi-phasic (concentration-dependent) effect on ERK1/2 and Akt activation by insulin (Fig. 7). A concentration of 12.5 μg ml−1 of A1 had a small stimulating action on both enzymes, whereas 25 μg ml−1 strongly inhibited ERK 1/2. When a saponin-rich fraction of the pressed cake was extracted with 100% methanol (B2 extract), a more potent stimulatory effect on insulin-induced Akt activation was uncovered and modulation of ERK1/2 was lost. In contrast, isolating the unsaponifiable fraction from argan oil C1 extract yielded the loss of enhanced Akt activation but was characterized with a powerful inhibitory action at the level of ERK1/2. This result along with the bi-phasic nature of the effects of cruder almond and pressed cake extracts argues for the presence of at least two active components in seeds of Argania spinosa.
A first component appears to be present in the B2 saponin fraction of the pressed cake and could underlie an insulin-sensitizing action that offers a possible mechanism for the alleged anti-diabetic potential of Argania spinosa almonds. Indeed, previous work by Alaoui et al. (19) demonstrated a hypoglycaemic effect in rats treated with 100 mg kg−1 per day of an oral mixture of press cake saponins that occurred within one month. The hypoglycaemic effect could be related to enhanced transport of glucose into adipose or skeletal muscle tissues, which are known to be activated through the PI3K pathway. Few studies describe hypoglycaemic properties of the triterpenic type saponins. Yoshikawa et al. (20) have found that the structure requirements for hypoglycaemic activity were the olean-12-ene 3, 28-acylated bidesmoside. These saponins inhibited the increase of serum glucose levels in glucose-loaded rats; the transfer of glucose from stomach to small intestine (21,22); and glucose transport at the small intestinal brush border (21,23). Charrouf et al. (13) have elucidated the structure of saponins present in the pressed cake of Argania spinosa which are present in the extracts tested herein. Using high performance liquid chromatography (HPLC) we have found seven saponins, five of which are novel molecules. All are similar to saponins used by Yoshikawa and Matsuda (20). The results of the present study suggest that such saponins enhance insulin sensitivity in an insulin-responsive cell line of hepatic origin. Taken together, this evidence supports the use of Argania spinosa almonds for the prevention or treatment of diabetes, albeit clinical confirmation is still required.
Higher concentrations of the methanolic extracts (A1) of almond, of the pressed cake (B1) and of the unsaponifiable fraction (C1) slightly decreased the degree of phosphorylation of Akt in HTC cells, but induced a complete inhibition of ERK1/2 phosphorylation. The C1 extract specifically reduced the activation of ERK1/2 without affecting the degree of phosphorylation of MEK1/2 on two other transformed cell lines HT-1080 and MSV-MDCK-INV cells. At high concentration, its effect is comparable to that of U0126. This MEK inhibitor does not affect the phosphorylation status of MEK1/2 but inhibits its catalytic activity by binding to activated MEK in a non-competitive manner. This prevents the enzyme from phosphorylating and activating downstream ERK1/2. It is conceivable that C1 may inhibit MEK activity in a similar manner. Alternatively, C1 could inhibit the phosphorylation of ERK1/2 by another mechanism, such as activation of a specific phosphatase. Further studies will be necessary to address this point. Nevertheless, these results definitively point toward an anti-proliferative activity of A1 and C1 extracts rather than an anti-diabetic one. This was confirmed for C1 by a reduction of [3H]thymidine incorporation.
Argania spinosa almonds and press cake have not yet been subjected to detailed phytochemical analysis. In contrast, some phenolic compounds have been identified in argan oil including ferrulic acid, vanillic acid, tyrosol and syringic acid, carotenoids (8), cafeic acid and oleuropein (24). The unsaponifiable fraction of argan oil is rich in lipid-soluble vitamins, phytosterols, triterpenes, carotenoids and squalene components believed to confer cancer chemopreventive potential (8,25,26).
Phenolic or flavonoid compounds had long been recognized as potent antioxidants (27,28). Antioxidants are believed to play a role in cancer prevention (29,30), although the exact mechanisms have not been elucidated. In relation to the present studies, it must be noted that some flavonoids have shown an ability to inhibit the MEK/ERK cascade in rat hepatoma 5L cells (31). More specifically, the flavonoid naringenin impaired the PI3K and MAPK pathways; it inhibits the phosphorylation of ERK1/2 without affecting the upstream kinase, MEK (27). This is similar to the effect of A1, B1 (not shown) and C1 extract observed herein. Moreover the antitumor effect of another flavonoid, nobiletin, on human fibrosarcoma HT-1080 cells (28) was associated with the inhibition of MEK1/2 activity without influencing the activity of PI3K or the phosphorylation of Akt. Finally, ferulic acid exhibits a potent anti-proliferative effect through inhibition of ERK1/2 pathway (32). It is thus possible that flavonoids contained in the almond of Argania spinosa could participate in the inhibition of ERK1/2 pathway and be involved in the observed anti-proliferative activity. We do not expect the C1 fraction to contain flavonoids but it is possible that other components of the unsaponifiable fraction may have the same mechanism of action.
Components such as lipid-soluble vitamins, specifically vitamin E, may be involved in the action of C1 extract. Indeed, argan oil is rich in tocopherols (700 mg Kg−1) (8); principally γ-tocopherol (75%) and α-tocopherol (7%). γ-tocopherol is known for its cancero-preventive properties (33–36). α-tocopherol is also known to inhibit proliferation of cancer cells (37) and to interfere with signal transduction pathways such as PKC and ERK (38).
In addition, the unsaponifiable fraction of argan oil shows a particular wealth of phytosterols, principally in the form of schottenol (48%) and spinasterol (40%), with minor amounts of δ 8-22-stigmastadiene-3β-ol. These sterols are very rare in vegetable oils. Schottenol has been reported to exhibit anticarcinogenic, cytotoxic properties (39) and strong antitumor promoter activity (40). The anti-proliferative and anti-tumorigenic potential of spinasterol has also been demonstrated (41–43). Finally, some triterpenes, such as lupeol contained in argan oil unsaponifiable fraction, can target multiple signaling pathways leading to induction of apoptosis and inhibition of cell growth (44,45). Even if comparable to that of several natural compounds, such as certain flavonoids, the effect of these unsaponifiable components on signaling pathway enzymes is as yet unclear. Nonetheless, phytosterols, tocopherols and triterpenes in C1 extract could all contribute to its anti-proliferative activity.
Finally, squalene is another important component of argan oil (320 mg Kg−1) known as an excellent anti-hypercholesterolemic agent through suppression of HMG-CoA reductase activity, shown to intervene in the release of oncogenic activity (46,47). Squalene has also been reported to exert anti-tumorigenic (48) or anticarcinogenic effects (47) as well as glutathione-like cytoprotective activity (49). Finally, the anti-proliferative activity of squalene appears to involve inhibition of the Ras/Raf/MEK/ERK pathway (50).
Conclusion
While many single components of Argania spinosa seeds could be responsible for the effects observed in this study, synergy or antagonism between multiple components is also likely. However, using a fractionation approach can help unravel such complexities. Indeed, we prepared several extracts from argan seeds, its oil and remaining press cake. This allowed us to demontrate an insulin-sentitizing activity present in saponin-rich presscake fractions that lends support to the traditional use of argan almonds against diabetes. However, a bi-phasic modulation of ERK1/2 component of the insulin response by treatment with argan seed extract could be explained by anti-proliferative components concentrated in the unsaponifiable fraction of argan oil. Hence, our studies confirm in part the anti-diabetic and cancer chemopreventive potential of Argania spinosa seeds. They further suggest that active principles for each action may reside in different fractions and this may help their identification in future studies. Moreover, natural health products of vegetable origin were clearly identified as a promising avenue for the prevention of chronic diseases (51), as raised notably by a panel of experts that convened in Montreal in 2004 (52). Our study supports the concept that A. spinosa almonds, oil, and extracts thereof are part of such natural health products that can be useful in the prevention of serious chronic illnesses like cancer and diabetes.
Acknowledgments
HTC cells were kindly provided by Dr J. Gregory Fitz from the Colorado Health Science Center (Denver, CO). HT-1080 and MSV-MDCK-INV cell lines were both generously provided by Dr I. Robert Nabi from the University of British Columbia (Vancouver, BC). These studies were supported by grants from the Canadian Diabetes Association (P.S.H.) and the Canadian Institutes of Health Research (J.N.). S.S. received a partial graduate studentship from the Groupe d'étude des protéines membranaires. P.S.H. is a National Research Scientist and J.N. is a Senior Research Scholar of the Fonds de la Recherche en Santé du Québec. We thank Dr Louis Martineau for thoughtful discussion and help with immunoblot and statistical analyses. Ms Elisabeth Peres is gratefully acknowledged for her precious help in graphic support.
References
- 1.Charrouf Z, Guillaume D. Chemistry of the secondary metabolites of Argania spinosa (L.) skeels. Curr Topics Phytochem. 2002a;5:99–102. [Google Scholar]
- 2.Charrouf Z, Guillaume D. Ethnoeconomical, ethnomedical, and phytochemical study of Argania spinosa (L.) Skeels. J Ethnopharmacol. 1999;67:7–14. doi: 10.1016/s0378-8741(98)00228-1. [DOI] [PubMed] [Google Scholar]
- 3.Berrada Y, Settaf A, Baddouri K, Cherrah A, Hassar M. Experimental evidence of an antihypertensive and hypocholesterolemic effect of oil of argan, Argania sideroxylon. Therapie. 2000;55:375–8. [PubMed] [Google Scholar]
- 4.Berrougui H, Ettaib A, Herrera Gonzalez MD, Alvarez de Sotomayor M, Benna-Kabchi N, et al. Hypolipidemic and hypocholesterolemic effect of argan oil (Argania spinosa L.) in Meriones shawi rats. J Ethnopharmacol. 2003;89:15–18. doi: 10.1016/s0378-8741(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 5.Drissi A, Girona J, Cherki M, Godas G, Derouiche A, El Messal M, et al. Evidence of hypolipemiant and antioxidant properties of argan oil derived from the argan tree (Argania spinosa) Clin Nutr. 2004;23:1159–66. doi: 10.1016/j.clnu.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Alaoui K, Lagorce JF, Cherrah Y, Hassar M, Amarouch H, Roquebert J. Analgesic and anti-inflammatory activity of saponins of Argania spinoza. Ann Pharm Fr. 1998b;56:220–8. [PubMed] [Google Scholar]
- 7.Chimi H, Cillard J, Cillard P. Autoxydation dec l'huile d'argan (Argania spinosa) sapotacée du Maroc. Sciences des Aliments. 1994;14:117–24. [Google Scholar]
- 8.Khallouki F, Younos C, Soulimani R, Oster T, Charrouf Z, Spiegelhalder B, et al. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur J Cancer Prev. 2003;12:67–75. doi: 10.1097/00008469-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Farines M, Charrouf M, Soulier J, Cave A. Etude de l'huile des graines d'Argania spinosa (L.) Sapotacea. II- Stérols, alcools triterpéniques et méthystérols de l'huile d'argan. Rev Franç Corps Gras. 1984;31:443–8. [Google Scholar]
- 10.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 11.Capeau J. Insulin signaling: mechanisms altered in insulin resistance. Med Sci (Paris) 2003;19:834–9. doi: 10.1051/medsci/20031989834. [DOI] [PubMed] [Google Scholar]
- 12.Alaoui A, Charrouf Z, Soufiaoui M, Carbone V, Malorni A, Pizza C, et al. Triterpenoid saponins from the shells of Argania spinosa seeds. J Agric Food Chem. 2002;50:4600–3. doi: 10.1021/jf0200117. [DOI] [PubMed] [Google Scholar]
- 13.Charrouf Z, Wieruszeski JM, Fkih-Tetouani S, Leroy Y, Charrouf M, Fournet B. Triterpenoid saponins from Argania spinosa. Phytochemistry. 1992;29:2629–35. doi: 10.1016/0031-9422(92)80367-n. [DOI] [PubMed] [Google Scholar]
- 14.Haddad PS, Vallerand D, Mathe L, Benzeroual K, Van de Werve G. Synergistic activation of mitogen-activated protein kinase by insulin and adenosine triphosphate in liver cells: permissive role of Ca2+ Metabolism. 2003;52:590–8. doi: 10.1053/meta.2003.50094. [DOI] [PubMed] [Google Scholar]
- 15.Lidofsky SD, Xie MH, Sostman A, Scharschmidt BF, Fitz JG. Vasopressin increases cytosolic sodium concentration in hepatocytes and activates calcium influx through cation-selective channels. J Biol Chem. 1993;268:14632–6. [PubMed] [Google Scholar]
- 16.Le PU, Nguyen TN, Drolet-Savoie P, Leclerc N, Nabi IR. Increased beta-actin expression in an invasive moloney sarcoma virus-transformed MDCK cell variant concentrates to the tips of multiple pseudopodia. Cancer Res. 1998;58:1631–5. [PubMed] [Google Scholar]
- 17.Vadnais J, Nault G, Daher Z, Amraei M, Dodier Y, Nabi IR, et al. Autocrine activation of the hepatocyte growth factor receptor/met tyrosine kinase induces tumor cell motility by regulating pseudopodial protrusion. J Biol Chem. 2002;277:48342–50. doi: 10.1074/jbc.M209481200. [DOI] [PubMed] [Google Scholar]
- 18.Carlsen J, Christiansen K, Vinten J. Insulin stimulated Glycogen synthesis in isolated rat hepatocytes: Effet of protein kinase inhibitors. Cell Signal. 1997;9:447–50. doi: 10.1016/s0898-6568(97)00035-1. [DOI] [PubMed] [Google Scholar]
- 19.Alaoui K, Belabbes M, Cherrah Y, Hassar M, Charrouf Z, Amarouch H, et al. Acute and chronic toxicity of saponins from Argania spinosa. Ann Pharm Fr. 1998a;56:213–9. [PubMed] [Google Scholar]
- 20.Yoshikawa M, Matsuda H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. Biofactors. 2000;13:231–7. doi: 10.1002/biof.5520130136. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda H, Li Y, Murakami T, Matsumura N, Yamahara J, Yoshikawa M. Antidiabetic principles of natural medicines. III. Structure-related inhibitory activity and action mode of oleanolic acid glycosides on hypoglycemic activity. Chem Pharm Bull (Tokyo) 1998;46:1399–403. doi: 10.1248/cpb.46.1399. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda H, Li Y, Murakami T, Yamahara J, Yoshikawa M. Structure-related inhibitory activity of oleanolic acid glycosides on gastric emptying in mice. Bioorg Med Chem. 1999;7:323–7. doi: 10.1016/s0968-0896(98)00207-7. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda H, Murakami T, Shimada H, Matsumura N, Yoshikawa M, Yamahara J. Inhibitory mechanisms of oleanolic acid 3-O-monodesmosides on glucose absorption in rats. Biol Pharm Bull. 1997;20:717–9. doi: 10.1248/bpb.20.717. [DOI] [PubMed] [Google Scholar]
- 24.Chimi H, Rahmani M, Cillard P. Etude de la fraction phenolique des huiles d'olives vierges et d'argan du Maroc. Actes de l'institut agronomique et vétérinaire. 1988;8:17–22. [Google Scholar]
- 25.Charrouf Z, Guillaume D. Secondary metabolites from Argania spinosa (L.) Skeels. Phytochem Rev. 2002b;1:345–54. [Google Scholar]
- 26.Khallouki F, Spiegelhalder B, Bartsch H, Owen RW. Secondary metabolites ofargan tree (Morocco) may have disease prevention properties. Afr. J. Boitechnol. 2005;4:381–88. [Google Scholar]
- 27.Harmon AW, Patel YM. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: a mechanism for impaired cellular proliferation. Breast Cancer Res Treat. 2004;85:103–10. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- 28.Miyata Y, Sato T, Yano M, Ito A. Activation of protein kinase C betaII/epsilon-c-Jun NH2-terminal kinase pathway and inhibition of mitogen-activated protein/extracellular signal-regulated kinase 1/2 phosphorylation in antitumor invasive activity induced by the polymethoxy flavonoid, nobiletin. Mol Cancer Ther. 2004;3:839–47. [PubMed] [Google Scholar]
- 29.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–18. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 30.Bartsch H, Nair J, Owen RW. Exocyclic DNA adducts as oxidative stress markers in colon carcinogenesis: potential role of lipid peroxidation, dietary fat and antioxidants. Biol Chem. 2002;383:915–21. doi: 10.1515/BC.2002.098. [DOI] [PubMed] [Google Scholar]
- 31.Reiners JJ, Jr, Lee JY, Clift RE, Dudley DT, Myrand SP. PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol Pharmacol. 1998;53:438–45. doi: 10.1124/mol.53.3.438. [DOI] [PubMed] [Google Scholar]
- 32.Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, Hatzoglou A, et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res. 2004;6:R63–R74. doi: 10.1186/bcr752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–22. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. Faseb J. 2002;16:1952–4. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–9. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shklar G, Oh SK. Experimental basis for cancer prevention by vitamin E. Cancer Invest. 2000;18:214–22. doi: 10.3109/07357900009031826. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Zu K, Ni J, Yeh S, Kasi D, James NS, et al. Cellular and molecular effects of alpha-tocopheryloxybutyrate: lessons for the design of vitamin E analog for cancer prevention. Anticancer Res. 2004;24:3795–802. [PubMed] [Google Scholar]
- 38.Clement SA, Tan CC, Guo J, Kitta K, Suzuki YJ. Roles of protein kinase C and alpha-tocopherol in regulation of signal transduction for GATA-4 phosphorylation in HL-1 cardiac muscle cells. Free Radic Biol Med. 2002;32:341–9. doi: 10.1016/s0891-5849(01)00802-4. [DOI] [PubMed] [Google Scholar]
- 39.Arizawa M, Kinghom A, Cordell G, Phebec C, Fansworth N. Plant anticancer agents: schottenol glucosides from Bacharis cordifolia and Ipnosis aggregatta. Planta medica. 1985;6:544–5. [PubMed] [Google Scholar]
- 40.Iwatsuki K, Akihisa T, Tokuda H, Ukiya M, Higashihara H, Mukainaka T, et al. Sterol ferulates, sterols, and 5-alk(en)ylresorcinols from wheat, rye, and corn bran oils and their inhibitory effects on Epstein-Barr virus activation. J Agric Food Chem. 2003;51:6683–8. doi: 10.1021/jf030371+. [DOI] [PubMed] [Google Scholar]
- 41.Jeon GC, Park MS, Yoon DY, Shin CH, Sin HS, Um SJ. Antitumor activity of spinasterol isolated from Pueraria roots. Exp Mol Med. 2005;37:111–20. doi: 10.1038/emm.2005.15. [DOI] [PubMed] [Google Scholar]
- 42.Villasenor IM, Domingo AP. Anticarcinogenicity potential of spinasterol isolated from squash flowers. Teratog Carcinog Mutagen. 2000;20:99–105. doi: 10.1002/(sici)1520-6866(2000)20:3<99::aid-tcm1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Jeong SI, Kim KJ, Choi MK, Keum KS, Lee S, Ahn SH, et al. alpha-Spinasterol isolated from the root of Phytolacca americana and its pharmacological property on diabetic nephropathy. Planta Med. 2004;70:736–9. doi: 10.1055/s-2004-827204. [DOI] [PubMed] [Google Scholar]
- 44.Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–14. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- 45.Saleem M, Kaur S, Kweon MH, Adhami VM, Afaq F, Mukhtar H. Lupeol, a fruit and vegetable based triterpene, induces apoptotic death of human pancreatic adenocarcinoma cells via inhibition of Ras signaling pathway. Carcinogenesis. 2006 doi: 10.1093/carcin/bgi157. in press. [DOI] [PubMed] [Google Scholar]
- 46.Chan P, Tomlinson B, Lee CB, Lee YS. Effectiveness and safety of low-dose pravastatin and squalene, alone and in combination, in elderly patients with hypercholesterolemia. J Clin Pharmacol. 1996;36:422–7. doi: 10.1002/j.1552-4604.1996.tb05029.x. [DOI] [PubMed] [Google Scholar]
- 47.Rao CV, Newmark HL, Reddy BS. Chemopreventive effect of squalene on colon cancer. Carcinogenesis. 1998;19:287–90. doi: 10.1093/carcin/19.2.287. [DOI] [PubMed] [Google Scholar]
- 48.Newmark HL. Squalene, olive oil, and cancer risk: a review and hypothesis. Cancer Epidemiol Biomarkers Prev. 1997;6:1101–3. [PubMed] [Google Scholar]
- 49.Das B, Yeger H, Baruchel H, Freedman MH, Koren G, Baruchel S. In vitro cytoprotective activity of squalene on a bone marrow versus neuroblastoma model of cisplatin-induced toxicity. implications in cancer chemotherapy. Eur J Cancer. 2003;39:2556–65. doi: 10.1016/j.ejca.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Kohl NE, Conner MW, Gibbs JB, Graham SL, Hartman GD, Oliff A. Development of inhibitors of protein farnesylation as potential chemotherapeutic agents. J Cell Biochem Suppl. 1995;22:145–50. doi: 10.1002/jcb.240590819. [DOI] [PubMed] [Google Scholar]
- 51.Haddad PS, Azar GA, Groom S, Boivin M. Natural Health Products, Modulation of immune function and preventaion of chronic diseases. Evidence-based Complem. Altern. Med. 2005;2:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haddad PS, Beauchamp G, Côté G, Boivin M. Maintenance of an efficient and equilibrated immune system through the novel use of natural health products: synopsis of a symphosium. Evidence-based Complem. Altern. Med. 2005;2:237–8. [Google Scholar]