Abstract
Royal jelly (RJ) has been used worldwide for many years as medical products, health foods and cosmetics. Since RJ contains testosterone and has steroid hormone-type activities, we hypothesized that it may have beneficial effects on osteoporosis. We used both an ovariectomized rat model and a tissue culture model. Rats were divided into eight groups as follows: sham-operated (Sham), ovariectomized (OVX), OVX given 0.5% (w/w) raw RJ, OVX given 2.0% (w/w) RJ, OVX given 0.5% (w/w) protease-treated RJ (pRJ), OVX given 2.0% (w/w) pRJ, OVX given 17β-estradiol and OVX given its vehicle, respectively. The Ovariectomy decreased tibial bone mineral density (BMD) by 24%. Administration of 17β-estradiol to OVX rats recovered the tibial BMD decrease by 100%. Administration of 2.0% (w/w) RJ and 0.5–2.0% (w/w) pRJ to OVX rats recovered it by 85% or more. These results indicate that both RJ and pRJ are almost as effective as 17β-estradiol in preventing the development of bone loss induced by ovariectomy in rats. In tissue culture models, both RJ and pRJ increased calcium contents in femoral-diaphyseal and femoral-metaphyseal tissue cultures obtained from normal male rats. However, in a mouse marrow culture model, they neither inhibited the parathyroid hormone (PTH)-induced calcium loss nor affected the formation of osteoclast-like cells induced by PTH in mouse marrow culture system. Therefore, our results suggest that both RJ and pRJ may prevent osteoporosis by enhancing intestinal calcium absorption, but not by directly antagonizing the action of PTH.
Keywords: bone mineral density, bone tissue culture, ovariectomized rats, post-menopausal osteoporosis model, protease-treated RJ, royal jelly
Introduction
With the growing aged population, we urgently need to develop new and effective treatments for chronic and protracted diseases, e.g. chronic pain, diabetes, arthritis, osteoporosis, cancer as well as cardiovascular and brain ailments. Recently, more and more unconventional therapies have been used for treating these diseases (1–3).
The peak bone mass of women is less than that of men because of the decrease in bone mass owing to menopause. Therefore, women are vulnerable to bone loss known as post-menopausal osteoporosis (4,5). The primary cause for this symptom is an estrogen deficiency. An ovariectomized rat model, which artificially produces estrogen deficiency, has been used for the study of post-menopausal osteoporosis. Both aged and mature rats have been employed as animal models for experimentally induced osteoporosis. The mature rat model has characteristics which are seen in early post-menopausal bone loss (6). Using this model, several compounds were screened and found to be effective for the therapeutic management of osteoporosis; namely, estrogen (5,6), bisphosphonate (6,7), calcitonin (6,8), calcium products (6,9) and anabolic steroids (10). Recently, efficacy of traditional Chinese (Kampo) medicines has been reevaluated by clinicians (11). Kampo medicines have less side effects, and therefore, seem to be more suitable for a long-term medication. We reported their inhibitory effects on both the progression of tibial bone loss and the alteration of periodontium induced by ovariectomy in rats (12–15).
Royal jelly (RJ) is the principal food for the honeybee queen. This is produced in the hypopharyngeal and mandibular glands of worker honeybees and has been demonstrated to possess several pharmacological activities, such as life-span-elongating (16), antifatigue (17), antiallergic (18), antitumor (19), antihypercholesterolemic (20), antihypertensive (21) and anti-inflammatory effects (22). Indeed, RJ has been used worldwide as commercial medical products, health foods and cosmetics. The chemical composition of RJ was reported to be proteins, sugars, lipids and vitamins (23). It contains many bioactive substances such as 10-hydroxy-2-decenoic acid (23), proteins mentioned above (17,18) and antibacterial protein (24). RJ contains testosterone and has activities similar to other steroid hormones (20,25,26). Therefore, RJ may be beneficial for osteoporosis. Although RJ has been traditionally known to improve post-menopausal symptoms, to our knowledge, no one has studied whether or not it prevents the decrease of bone mass, which is one of the deleterious symptoms for post-menopausal women.
Therefore, we undertook this study to evaluate the efficacy of RJ and protease-treated RJ (pRJ) in preventing osteoporosis using the mature rat model. We included pRJ because this is known to enhance intestinal absorption of calcium (27). In order to analyze the mechanism of action of these compounds, their in vitro effects on the bone formation were also investigated using a bone tissue culture model. We also examined whether these compounds could antagonize the osteoporotic actions of parathyroid hormone (PTH) in in vitro experiments.
Materials and Methods
In Vivo Experiments
RJ and Chemicals
Both dried powders of raw RJ and pRJ were supplied by Yamada Apiculture Center, Inc., (Okayama Prefecture, Japan). The constituents and contents of both RJ and pRJ used in this study are shown in Table 1. 17β-Estradiol was purchased from Sigma–Aldrich Co. (St Louis, MO, USA). All other reagents were purchased from ABIOZ Co. Ltd (Osaka, Japan).
Table 1.
Constituents and contents in royal jelly and protease-treated royal jelly
| Constituents | Contents | Royal jelly | Protease-treated royal jelly |
|---|---|---|---|
| Water | % (w/w) | 1.5 | 4.0 |
| Proteins | % (w/w) | 40.4 | 37.7 |
| Carbohydrates | % (w/w) | 47.1 | 45.3 |
| Lipids | % (w/w) | 8.0 | 4.4 |
| Ashes | % (w/w) | 3.0 | 8.6 |
| Minerals | |||
| Sodium | mg per 100 g | 38.7 | 3000 |
| Phosphorus | mg per 100 g | 661 | 619 |
| Calcium | mg per 100 g | 18.7 | 30.5 |
| Magnesium | mg per 100 g | 91.7 | 83.9 |
| Zinc | mg per 100 g | 6.3 | 7.6 |
| Iron | mg per 100 g | 3.0 | 3.1 |
| Free amino acids | mg per 100 g | 1569 | 4780 |
Ovariectomy and Administration of RJ
Forty-eight female Sprague–Dawley rats, aged 9 weeks, were purchased from Seac Yoshitomi Ltd (Fukuoka Prefecture). Twenty-seven days later [90-days-old; a mature rat model (6) was used], six rats were given a sham operation (control rats; first group) and 42 rats were bilaterally ovariectomized under nembutal (pentobarbital sodium; 50 mg kg−1 body weight: Abbott Lab., IL, USA) anesthesia. They were also equally divided into the second to the eighth groups. The second group received orally MF powdered pellets (Oriental Yeast Co. Ltd, Tokyo, Japan), which are designed for feeding rodents. Their contents are 24% (w/w) proteins, 5.1% (w/w) lipids, 2.9% (w/w) fibers and 6.1% (w/w) ashes. The third and fourth groups orally received MF powdered pellets containing 0.5% (w/w) (0.5 g RJ was mixed with 100 g MF powdered pellets; RJ-1) and 2.0% (w/w) RJ (RJ-2). The fifth and sixth groups orally received MF powdered pellets containing 0.5% (w/w) (pRJ-1) and 2.0% (w/w) pRJ-2. Each ovariectomized rat in the seventh group was injected subcutaneously with 17β-estradiol [in 20% (v/v) polyethylene glycol 400] 5 days per week at a dose of 10 μg kg−1 body weight (12,14). Rats in the eighth group were injected with 20% (v/v) polyethylene glycol 400 (vehicle) in the same manner as the seventh group.
To develop bone loss in ovariectomized rats, all animals were maintained for 7 weeks under regulated 12 h/12 h light/dark illumination cycles at constant temperature (24 ± 0.5°C) and humidity (45–50%). Food and drinking water (tap water) were supplied ad libitum.
The time for measuring daily food consumption and body weight was the same during the entire period. After euthanizing the rat with cervical dislocation under chloroform anesthesia, the right leg was dissected from each animal. The uterus of each rat was dissected and weighed (12,14).
Measurement of Bone Mineral Density
After removing adherent soft tissues, longitudinal section of right tibiae was prepared by manual grinding with whetstones (#600). All longitudinal specimens were dehydrated by a stepwise application of 70 and 99.9% (v/v) ethanol solutions. These samples were used for both bone mineral density (BMD) measurements and scanning electron microscope (SEM) observations.
The BMD was quantitatively determined by the computed X-ray densitometry (CXD) method (Bonalyzer; Teijin Company, Tokyo, Japan) (12,14). The radiographs of the longitudinal sections of tibiae were taken along with an aluminum step-wedge using an X-ray apparatus (Model CSMW-2 Specific; Softex Co., Tokyo, Japan) set at 5 mA, 40 cm, 30 s, 50 V and 55 kVp. The densitometric pattern of the proximal tibia on an X-ray picture was read by a personal computer (PC-9801; NEC, Tokyo, Japan) using a software program for rat bone density (Version 2.10A-M; Teijin Company). ΣGS (mm Al) and ΣGS/D were used as the indices of bone mineral content (BMC) and BMD, respectively. Here, the value for ΣGS was obtained by integrating the pattern area, which was obtained opticodensitometrically and was converted into a number of steps in an aluminum step-wedge. D represents the bone width. The value for ΣGS/D was calculated for the areas that cover the epiphysis and a part of metaphysis in the proximal tibia. The determination of the area and the precision of the measurements were described by Hidaka et al. (12).
Electron Microscopic Observations
Longitudinal sections of tibiae were shadowed with carbon before examination in a SEM (JSM-6330F; Nihon Denshi, Tokyo, Japan) at 10 kV.
In Vitro Experiments
Chemicals
Dulbecco's modified Eagle's medium (DMEM) (high glucose, 4.5 g dl−1) and a penicillin–streptomycin solution (penicillin 5000 U mg−1; streptomycin 5000 μg ml−1) were purchased from Gibco Laboratories (Grand Island, NY, USA). PTH [synthetic human PTH (1–34)] was purchased from Sigma–Aldrich Co. All other reagents were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Male Rats or Mice for Bone Tissue Cultures
Male Wistar rats weighing 90–100 g (4 weeks old) or male mice (ddY strain; 6 weeks old) were obtained from Japan SLC (Hamamatsu, Japan). The animals were fed with a commercial laboratory chow containing 1.1% calcium and 1.1% phosphorus at a room temperature of 25°C, with free access to distilled water.
Bone Culture Experiments
Under ether anesthesia with cervical dislocation, the femurs of male rats were removed aseptically after bleeding and were then soaked in ice-cold 0.25 M sucrose solution. The soft tissue and marrow were cleaned-off from the femur, and the diaphysis and metaphysis (not containing epiphyseal tissue) were separated by a morphological tool. The femoral-diaphyseal and femoral-metaphyseal tissues were cut into small pieces (the size of about 2 × 2 mm) by a pair of scissors. Diaphyseal or metaphyseal fragments (pieces of 3 or 4) were cultured for 48 h in a 35 mm dish in 2.0 ml of medium consisting of Minimum essential medium (MEM) (high glucose, 4.5 g dl−1) supplemented with antibiotics (penicillin 100 units and streptomycin 100 μg ml−1) (28). In order to determine the effects of RJ and pRJ on bone calcium content, bone tissues were cultured in a medium containing either 10–100 μg ml−1 RJ or 50–100 μg ml−1 pRJ in the presence or absence of 10−7 M PTH. Both RJ and pRJ were soluble in the culture medium.
Cultures were maintained at 37°C in a water-saturated atmosphere containing 5% CO2 and 95% air. After culture, the diaphyseal or metaphyseal tissues were removed, washed with ice-cold 0.25 M sucrose solution and dried for 16 h at 110°C. The calcium content was determined by atomic absorption spectrophotometry (28,29). The concentration of calcium in the medium containing RJ or pRJ was in the range of 0.05–1.0 μg ml−1 of medium. The calcium content in the bone tissue was expressed as mg g of dry bone. All experimental data are mean ± SE for six animals.
Marrow Culture Experiments
Bone marrow cells isolated from male mice were used. Briefly, both bone ends of the femur were cut-off, and the marrow cavity was flushed with 1 ml of α-MEM. The marrow cells obtained from six animals were washed with α-MEM and cultured in the same medium containing 10% heat-inactivated fetal bovine serum at 1.0 × 107 cells per ml in 24-well plates (0.5 ml per well) in a water-saturated atmosphere containing 5% CO2 and 95% air at 37°C. The cells were cultured for 3 days, then 0.2 ml of the old medium was replaced with fresh medium and the cultures maintained for an additional 4 days (30,31). RJ or pRJ was added to the culture medium containing either the vehicle or PTH (10−7 M) (or the vehicle) at the beginning of the culture and at the time of medium change.
Enzyme Histochemistry
After being cultured for 7 days, cells which adhered to the 24-well plates were stained for tartrate-resistant acid phosphatase (TRACP), a marker enzyme of osteoclasts (32,33). Briefly, cells were washed with the Hank's balanced salt solution and fixed with 10% neutralized formalin-phosphate (pH 7.2) for 10 min. After the culture dishes were dried, TRACP staining was applied according to the method of Burstone (32). The fixed cells were incubated for 12 min at room temperature (25°C) in an acetate buffer (pH 5.0) containing 10 mM sodium tartrate and naphthol AS-MX phosphate (Sigma) as a substrate, and red violet LB salt (Sigma) as a stain for the reaction product. TRACP-positive multinucleated cells (MNCs) containing three or more nuclei were counted as osteoclast-like cells.
Ethics
The ovariectomy analyses were performed at the Fukuoka Dental College. The tissue culture experiments were carried out at the University of Shizuoka. The study was approved by the Institutional Use and Care of Animal Committee of the respective university. All procedures were in accordance with the Guidelines on Animal Experiments in either Fukuoka Dental College or University of Shizuoka of Japan, and performed following the Government Law Concerning the Protection and Control of Animals (No. 221) and Japanese Government Notification on Feeding and Safekeeping of Animals (No. 6).
Satistics
Data were obtained from three measurements and expressed as means ± standard deviations. Data of tissue cultures were expressed as means ± standard errors. Statistical comparisons were made by ANOVA with Fisher's Protected Least Significant Difference test using a statistical software program. The difference was considered significant when P < 0.05.
Results
Food Consumption and Body Weight
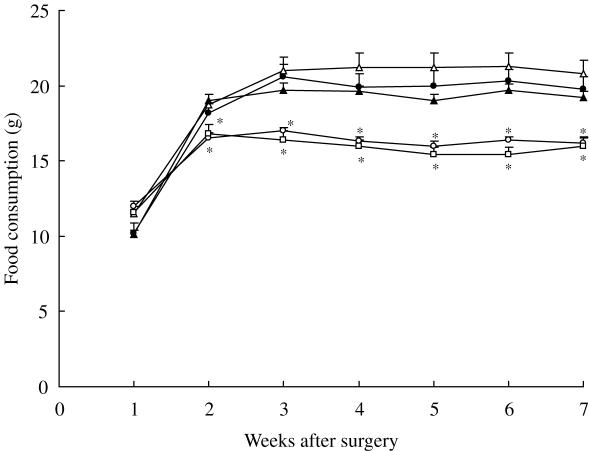
As shown in Fig. 1, the mean daily food consumption of the Sham, OVX + Est and OVX + pRJ-2 rats increased and reached a plateau at Week 2 after surgery, while that of food consumption of the remaining five groups increased until Week 3 after surgery and then leveled off (data of OVX + RJ-1,+ pRJ-1 and + Peg rats were not shown).
Figure 1.
Changes in the food consumption of rats. Rats were sham-operated (open circles), ovariectomized (OVX) (closed circles); ovariectomized and administered with 2.0% (w/w) RJ (open triangles), 2.0% (w/w) protease-treated RJ (closed triangles) and 17β-estradiol (open squares). Data of OVX administered 0.5% (w/w) RJ, 0.5% (w/w) protease-treated RJ and 20% (v/v) polyethylene glycol 400 were not shown. Each point represents the mean ± SD (n = 6). *P < 0.05, significant difference from the ovariectomized group at the respective time.
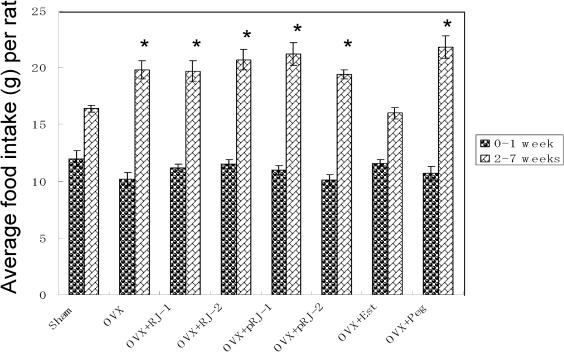
Since food consumption in Week 1 was particularly smaller than that in the following weeks, two values (Week 1 and Weeks 2–7) were examined separately. As shown in Fig. 2, the mean daily food consumption of the OVX rats in Week 1 was comparable with all other groups. However, that of OVX rats in Weeks 2–7 was higher than that of Sham rats by 21%.
Figure 2.
Mean daily food consumption in sham-operated and ovariectomized rats treated with various compounds for 7 weeks. Rats were divided into eight groups, sham-operated (Sham), ovariectomized (OVX), OVX given 0.5% (w/w) RJ (OVX + RJ-1), OVX given 2.0% (w/w) RJ (OVX + RJ-2), OVX given 0.5% (w/w) pRJ (OVX + pRJ-1), OVX given 2.0% (w/w) pRJ (OVX + pRJ-2), OVX given 17β-estradiol (OVX + Est) and OVX given polyethylene glycol 400 (OVX + Peg), each having six rats. *Significant difference (P < 0.05) when compared with the Sham rats.
The daily food consumption for OVX + RJ-2 rats in Weeks 2–7 was 20.7 g (Fig. 2). The food for this group contains 2.0 g RJ per 100 g [2.0% (w/w)]. Therefore, the daily consumption of RJ was 0.41 g. Since the body weight of a rat varied from 280 to 378 g (Figs 3 and 4A), the daily dose of RJ was between 1.08 and 1.46 g kg−1 (an average 1.27 g kg−1) body weight. The daily dose taken by RJ-1 rats was about one-fourth (0.32 g kg−1) of that in RJ-2 rats. Similarly, the daily food consumption for OVX + pRJ-2 rats in Weeks 2–7 was 19.4 g (Fig. 2). The food for this group contains 2.0 g pRJ per 100 g [2.0% (w/w)]. The daily consumption of pRJ was 0.39 g. Since the body weight of a rat varied from 280 to 370 g (Figs 3 and 4A), the daily dose of pRJ was between 1.05 and 1.39 g kg−1 (an average 1.22 g kg−1) body weight. The daily dose taken by pRJ-1 rats was about one-fourth (0.31 g kg−1) of that in pRJ-2.
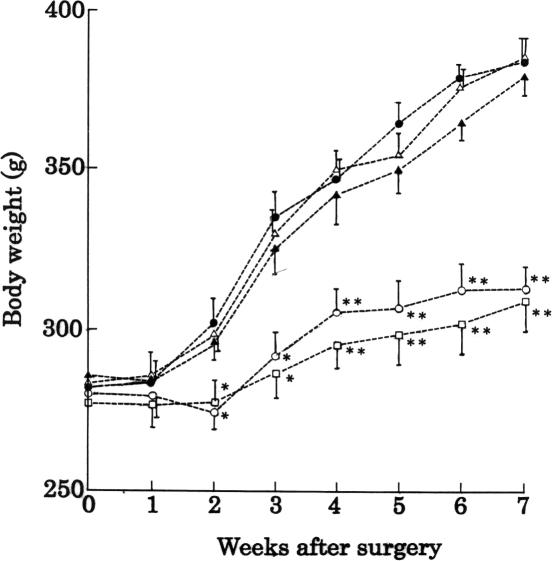
Figure 3.
Changes in the body weight of rats. Rats were sham-operated (open circles), ovariectomized (OVX) (closed circles); ovariectomized and administered with 2.0% (w/w) RJ (open triangles), 2.0% (w/w) pRJ (closed triangles) and 17β-estradiol (open squares). Data of OVX administered 0.5% (w/w) RJ, 0.5% (w/w) pRJ and 20% (v/v) polyethylene glycol 400 were not shown. Each point represents the mean ± SD (n = 6). *P < 0.05 and **P < 0.01, significant difference from the ovariectomized group at the respective time.
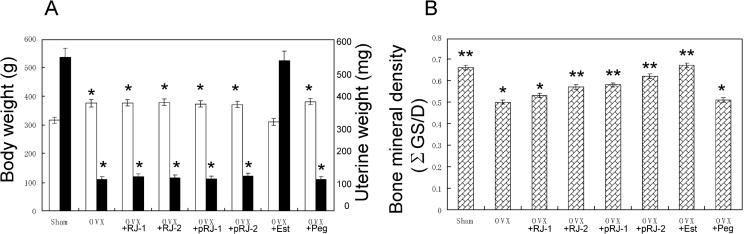
Figure 4.
(A) Either body weight (white bar) and uterine weight (black bar) or (B) tibial BMD of sham-operated rats and those of ovariectomized rats treated with various compounds for 7 weeks. Abbreviations were the same as those shown in Fig. 2. *Significant difference (P < 0.05) when compared with Sham rats. **Significant difference (P < 0.05) when compared with OVX rats.
The increase of body weight in ovariectomized (OVX) rats was significantly higher than that in sham control (Sham) rats (Fig. 3). After Week 7, the mean body weight ± SD in Sham and OVX rats were 315 ± 11 (n = 6) and 375 ± 13 (n = 6), respectively (Fig. 4A). The increases in the body weight of OVX + RJ-1, + RJ-2, + pRJ-1, + pRJ-2 and + Peg rats were similar to that in OVX rats (data of OVX + RJ-1, + pRJ-1 and + Peg were not shown). With the administration of the 17β-estradiol to OVX rats (OVX + Est), the increase was reduced to almost the same level as that for Sham rats (Figs 3 and 4A).
Uterine Weight
As shown on the right side of Fig. 4A, the uterine weight of OVX rats decreased significantly from that of the Shams (P < 0.01). The weight in the OVX + RJ-1, + RJ-2, + pRJ-1 or + pRJ-2 group was almost the same as that in the OVX group. The weight of the group injected with 17β-estradiol was similar to that of the Sham rats. The weight of the group injected with the vehicle was almost as high as that of the OVX rats.
Bone Mineral Density
As shown in Fig. 4B, the tibial BMD of OVX rats (0.50 ± 0.01) was 24% lower than that of Sham (0.66 ± 0.01). The administration of the 2.0% (w/w) RJ and 0.5–2.0% (w/w) pRJ to the rats significantly recovered the BMD. However, the administration of 0.5% (w/w) RJ to OVX rats did not recover the BMD. The recoveries with 2.0% (w/w) RJ, 0.5% (w/w) pRJ and 2.0% (w/w) pRJ were 85, 87 and 93% of that with 17β-estradiol. The injection of 17β-estradiol to OVX rats restored BMD almost completely to the level of the Sham rats. The vehicle had no effect.
Scanning Electron Microscopic Analyses
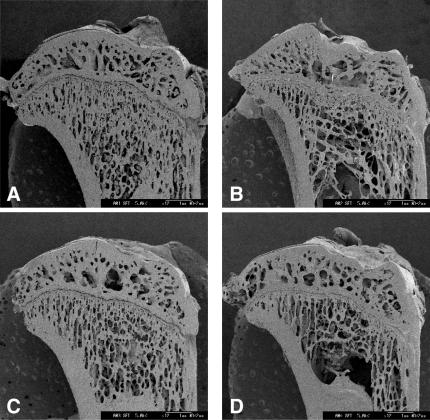
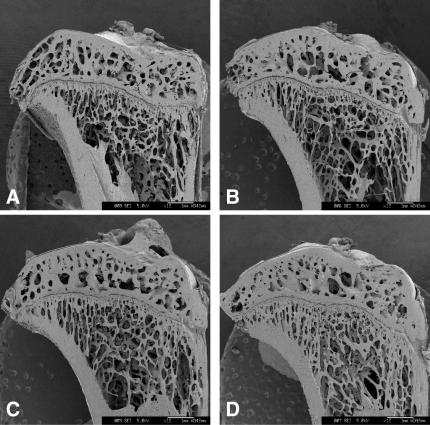
The representative scanning electron micrograph of the proximal tibiae taken at 7 weeks after the ovariectomy is shown in Fig. 5B. Compared to that of Sham rats (Fig. 5A), the connectivity of cancellous bone in the epiphysis and that of trabecular bone in the metaphysis exhibited greater loss. The administration of 17β-estradiol restored the connectivity in the metaphysis to those of the Sham rats, whereas the vehicle had no effect (Fig. 5C and D). The administration of 2.0% (w/w) RJ and 0.5% (w/w) pRJ slightly restored the connectivity (Fig. 6B and C), but 0.5% (w/w) RJ did not (Fig. 6A). The administration of 2.0% (w/w) pRJ (Fig. 6D) restored the connectivity in the metaphysis to the level of those of 17β-estradiol rats.
Figure 5.
Representative scanning electron micrographs of the longitudinal sections of the proximal tibia sampled at Week 7 after ovariectomy from (A) sham-operated, (B) ovariectomized (OVX), (C) OVX given 17β-estradiol and (D) OVX given the vehicle.
Figure 6.
Representative scanning electron micrographs of the longitudinal sections of the proximal tibia sampled at Week 7 after ovariectomy of rats. (A) Ovariectomized (OVX) given 0.5% (w/w) RJ, (B) OVX given 2.0% (w/w) RJ, (C) OVX given 0.5% (w/w) pRJ and (D) OVX given 2.0% (w/w) pRJ.
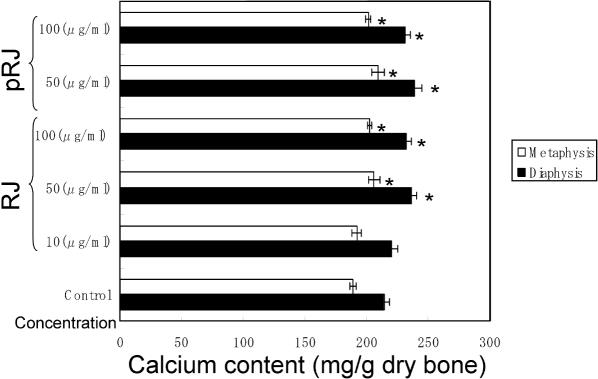
Calcium Contents in Bone Tissues
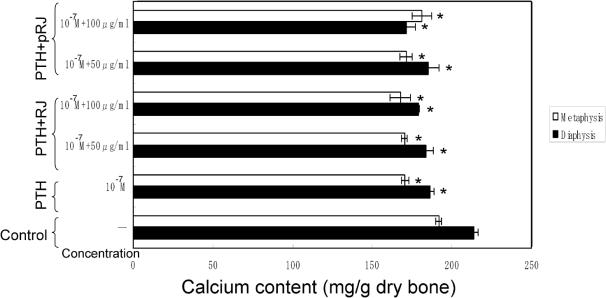
As shown in Fig. 7, the calcium content in the diaphyseal or metaphyseal tissues significantly increased when the bone tissues were cultured in the presence of 50–100 μg ml−1 RJ or 50–100 μg ml−1 pRJ. However, as shown in Fig. 8, the presence of PTH (10−7 M) induced a significant decrease in calcium content in the diaphyseal or metaphyseal tissues. This decrease was not significantly prevented in the presence of RJ (50–100 μg ml−1) or pRJ (50–100 μg ml−1).
Figure 7.
RJ and pRJ on the calcium contents in the femoral-diaphyseal and femoral-metaphyseal tissues obtained from normal rats in vitro. Bone tissues were cultured for 48 h in a medium containing vehicle, RJ or pRJ. Each value is the mean ± SE for six rats. RJ, royal jelly; pRJ, protease-treated royal jelly. *Significant difference (P < 0.05) when compared with the control.
Figure 8.
RJ and pRJ on the PTH-induced decrease in calcium content in the femoral-diaphyseal or femoral-metaphyseal tissues of the rat obtained from normal rats in vitro. Bone tissues were cultured for 48 h in a medium containing vehicle, RJ or pRJ in the presence or absence of PTH (10−7 M). Each value is the mean ± SE for six rats. PTH, parathyroid hormone; RJ, royal jelly; pRJ, protease-treated royal jelly. *Significant difference (P < 0.05) when compared with the control.
Osteoclast-like Cell Formation
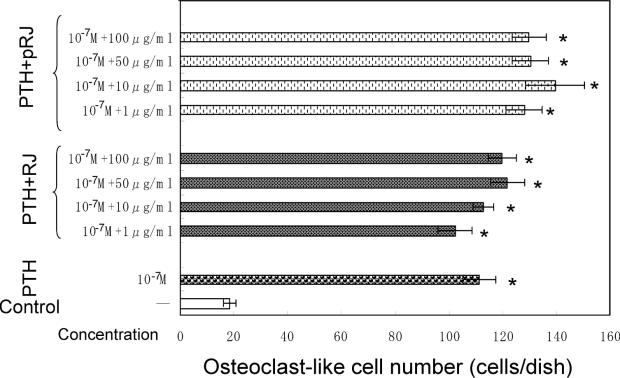
As shown in Fig. 9, the presence of PTH caused a significant increase in osteoclast-like cell formation in mouse marrow culture system in vitro. This increase was not significantly suppressed by either RJ (1–100 μg ml−1) or pRJ (1–100 μg ml−1).
Figure 9.
RJ and pRJ on the PTH-induced osteoclast-like cell formation in mouse marrow cultures in vitro. Mouse marrow cells were cultured for 7 days in a medium containing vehicle, RJ or pRJ in the presence or absence of PTH (10−7 M). Each value is the mean ± SE for four wells. PTH, parathyroid hormone; RJ, royal jelly; pRJ, protease-treated royal jelly. *Significant difference (P < 0.01) when compared with the control.
Discussion
It has been reported that ovariectomy (OVX) increased food intake and body weight and these changes could be inhibited or reversed by treatment with estradiol (12,14,34). In fact, the mean daily food consumption of OVX rats in 2–7 weeks was higher than that of Sham rats by 21% (Fig. 2). The administration of 17β-estradiol preserved the level of normal food consumption (Figs 1 and 2). Since neither RJ nor pRJ caused a decrease in food consumption in ovariectomized rats (during Weeks 2–7), their effect seems to be different from that of estrogen. However, it is noticed that 2% (w/w) pRJ seems to have a greater effect than its lower concentration [0.5% (w/w)] or both RJ's concentrations. Both values of the food intake and the weight gain of OVX + pRJ-2 rats were below those for OVX rats (Figs 1 and 3). Their food consumption reached a plateau at 2 weeks similar to that of the Sham rats.
OVX also caused an increase in body weight (Figs 2 and 4A), which had been postulated not only to be associated with regulatory effect of estrogen on the adipose tissue (35) but also to provide a partial protection against the development of osteoporosis (36). The increase in food consumption in ovariectomized rats (21% increase as described above) may in part contribute to the increase in body weight (12,14). However, OVX rats gained more weight than Sham control rats in the pair-fed condition (37,38). Therefore, we conclude that OVX rats seem to make more efficient use of their food (39).
A marked atrophy of the uterus has been used as evidence of the success of ovariectomy, because estrogen directly influences uterine weight (12). Indeed, ovariectomy also resulted in a dramatic decrease in uterine weight (Fig. 4B). Since both RJ and pRJ did not increase uterine weight, it is suggested that these agents do not influence the actions of estrogen or its receptor (40).
The primary proof for an effective antiosteoporotic agent is believed to be a prominent inhibitory effect against bone loss. The administration of 2.0% (w/w) RJ, 0.5% (w/w) pRJ and 2.0% (w/w) pRJ to the OVX rats recovered BMD by 14, 16 and 24%, after 7 weeks, while that of 17β-estradiol recovered BMD by 34%. The efficacy of the inhibition of bone loss by 2.0% (w/w) RJ, 0.5% (w/w) pRJ and 2.0% (w/w) pRJ was 41, 47 and 71% of that of 17β-estradiol. The efficacy of pRJ on the tibial BMD was greater than that of RJ.
Ovariectomy resulted in a decrease in the connectivity of cancellous bones in the epiphysis and that of the trabecular bones in the metaphysis (Fig. 5A and B). Our data show that pRJ was more effective than RJ in inhibiting the elution of bone calcium, because the connectivity in OVX rats was restored by the administration of 2.0% (w/w) pRJ. The appearance was comparable with that of the Sham rats (Figs 5A and 6D).
After the protease treatment of RJ, the contents of sodium, calcium and free amino acids increased by 7.8, 1.6 and 3.0 times (Table 1). Their increased content of sodium was derived from the addition of NaOH, which was used to adjust pH during the protease treatment. The reason for the increase of calcium content is not known. However, the effects of these increases may be small because food itself contains large amounts of sodium and calcium, and because the percentages of RJ and pRJ in the food are very small.
Nakasa et al. (27) reported that pRJ significantly promoted calcium absorption in the intestine as compared to that of raw RJ. Therefore, it is conceivable that the greater effect of pRJ may be caused by the enhanced digestibility (or utility) and by its promotion of calcium absorption in the intestine. The protease treatment of RJ eliminates the protein fractions above 10 000 molecular weight from RJ, leaving the peptides of 500–3000 molecular weight (MW) (27). Phosphoserine or acidic amino acids contained in peptides in pRJ could selectively bind calcium and facilitate calcium transport across the intestinal membranes.
As opposed to the in vivo models, both RJ and pRJ were similarly effective in increasing the calcium contents in the rat femoral-diaphyseal and femoral-metaphyseal tissue culture models (Fig. 7). However, they did not inhibit the PTH-induced calcium loss in the femoral tissues and PTH-induced formation of osteoclast-like cells in mouse marrow culture system in vitro (Figs 8 and 9). These observations demonstrated that both RJ and pRJ have a stimulatory effect on bone formation, but that they do not have an inhibitory effect on bone resorption.
Combining these in vivo and in vitro results, we speculate that the mechanism of action of RJ or pRJ is to stimulate calcium absorption of bone. The greater efficacy of pRJ in the animal model may be derived from the enhancement of intestinal calcium absorption by pRJ. However, we cannot exclude the possibility that other factors, such as calcium-regulatory hormones or cytokines, are also involved.
Recently, estrogen replacement therapy (ERT) has raised serious concerns about its safety because of tumor-inducing side effects (41). Our initial study shows that the RJ products may be used as alternative agents for the treatment of osteoporosis without such side effects, may be different from that for Kampo medicines or isoflavones (12,14). Since RJ contains a male hormone testosterone (25), this agent may also be effective in men's osteoporosis that could be induced by a decrease of androgen (42).
In summary, both RJ and pRJ inhibited the progression of bone loss induced by ovariectomy in rats to a similar degree to that of 17β-estradiol. The results of in vitro tissue culture suggested that both nutrients increased calcium content of the femoral tissues. Our data indicated that pRJ seems to be more efficacious. We believe that pRJ may be useful in humans for the prevention of not only post-menopausal osteoporosis but also osteoporosis in general.
Acknowledgments
This work was supported by a research grant from Yamada Apiculture Center, Inc., Okayama, Japan.
References
- 1.Fugh-Berman A. Alternative Medicine: What Works. USA: Lippincott & Williams & Wilkinson Inc.; 1997. [Google Scholar]
- 2.Costa Rosa LFBP. Exercise as a time-conditioning effector in chronic disease: a complementary treatment strategy. Evid Based Complement Alternat Med. 2004;1:63–70. doi: 10.1093/ecam/neh018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao JCI, Zeltzer LK. Complementary and alternative medicine approaches for pediatric pain: a review of the state-of-the-science. Evid Based Complement Alternat Med. 2005;2:149–59. doi: 10.1093/ecam/neh092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riggs BL, Melton LJ., III . Osteoporosis: Etiology, Diagnosis, and Management. Philadelphia, USA: Lipincott-Raven; 1995. [Google Scholar]
- 5.Schmidt IU, Walkley GK, Turner RT. Effects of estrogen and progesterone on tibia histomorphometry in growing rats. Calcif Tissue Int. 2000;67:47–52. doi: 10.1007/s00223001096. [DOI] [PubMed] [Google Scholar]
- 6.Kalu DN. The ovariectomized rat model for postmenopausal bone loss. Bone Miner. 1991;15:175–92. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 7.Nishida A, Ito M, Uetani M, Nakayama T, Tanaka T. Effect of etidronate on three-dimensional trabecular structure in ovariectomized or sciatic neuretomized rats. J Bone Miner Metab. 2004;22:335–40. doi: 10.1007/s00774-003-0491-x. [DOI] [PubMed] [Google Scholar]
- 8.Mochizuki K, Inoue T. Effect of salmon calcitonin on experimental osteoporosis induced by ovariectomy and low-calcium diet in the rat. J Bone Miner Metab. 2000;18:194–207. doi: 10.1007/s007740070020. [DOI] [PubMed] [Google Scholar]
- 9.Morris HA, Need AG, Horowitz M, O'Leughlin PD, Nordin BEC. Calcium adsorption in normal and osteoporotic postmenopausal women. Calcif Tissue Int. 1991;48:240–43. doi: 10.1007/BF02556211. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama Y, Higuchi Y, Takeda S, Masaki T, Shira-Ishi A, Sato K, et al. ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone. 2002;30:582–8. doi: 10.1016/s8756-3282(02)00682-8. [DOI] [PubMed] [Google Scholar]
- 11.Terasawa K. In: Kampo: Japansese-Oritental Medicine—Insights from Clinical Cases. Bacowsky H, Gerz A, translators. Tokyo, Japan: Standard McIntyre K.K.; 1993. [Google Scholar]
- 12.Hidaka S, Okamoto Y, Nakajima K, Suekawa M, Liu SY. Preventive effects of traditional Chinese (Kampo) medicines on experimental osteoporosis induced by ovariectomy in rats. Calcif Tissue Int. 1997;61:239–46. doi: 10.1007/s002239900329. [DOI] [PubMed] [Google Scholar]
- 13.Hidaka S, Okamoto Y, Yamada Y, Kon Y, Kimura T. A Japanese herbal medicine, Chujo-to has a beneficial effect on the osteoporosis in rats. Phytother Res. 1999;13:14–9. doi: 10.1002/(SICI)1099-1573(199902)13:1<14::AID-PTR375>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 14.Hidaka S, Okamoto Y, Miyazaki K, Uesugi T. Evaluation of a soybean product Fujiflavone P40 as an antiosteoporotic agent in rats. Phytother Res. 2003;17:112–9. doi: 10.1002/ptr.1047. [DOI] [PubMed] [Google Scholar]
- 15.Hidaka S, Okamoto Y, Yamada Y, Miyazaki K, Kimura T. Alterations in the periodontium after ovariectomy in rats: the effects of a Japanese herbal medicine, Chujo-to. Phytother Res. 2000;14:527–33. doi: 10.1002/1099-1573(200011)14:7<527::aid-ptr662>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Inoue S, Koya-Miyata S, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Royal jelly prolongs the life span of C3H/HeJ mice: correlation with reduced DNA damage. Exp Gerontol. 2003;38:965–9. doi: 10.1016/s0531-5565(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 17.Kamakura M, Mitani N, Fukuda T, Fukushima M. Antifatigue effect of fresh royal jelly in mice. J Nutr Sci Vitaminol. 2001;47:394–401. doi: 10.3177/jnsv.47.394. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto I, Taniguchi Y, Kunikita T, Kohno K, Iwaki K, Ikeda M, et al. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003;1:2029–45. doi: 10.1016/s0024-3205(03)00562-9. [DOI] [PubMed] [Google Scholar]
- 19.Bincoletto C, Eberlin S, Figueiredo CA, Luengo MB, Queiroz ML. Effects produced by royal jelly on haematopoiesis: relation with host resistance against Ehrlich ascites tumour challenge. Int Immunopharmacol. 2005;5:679–88. doi: 10.1016/j.intimp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Vittek J. Effect of royal jelly on serum lipids in experimental animals and humans with atherosclerosis. Experientia. 1995;51:927–35. doi: 10.1007/BF01921742. [DOI] [PubMed] [Google Scholar]
- 21.Matsui T, Yukiyoshi A, Doi S, Sugimoto H, Yamada H, Matsumoto K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J Nutr Biochem. 2002;13:80–6. doi: 10.1016/s0955-2863(01)00198-x. [DOI] [PubMed] [Google Scholar]
- 22.Fujii A, Kobayashi S, Kuboyama N, Furukawa Y, Kaneko Y, Ishihama S, et al. Augmentation of wound healing by royal jelly (RJ) in streptozotocin-diabetic rats. Jpn J Pharmacol. 1990;53:331–7. doi: 10.1254/jjp.53.331. [DOI] [PubMed] [Google Scholar]
- 23.Takenaka T. Chemical composition of royal jelly. Honeybee Sci. 1982;3:69–74. [Google Scholar]
- 24.Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K. A potent antibacterial protein in royal jelly. J Biol Chem. 1990;265:11333–7. [PubMed] [Google Scholar]
- 25.Vittek J, Slomiany BL. Testosterone in royal jelly. Experientia. 1984;40:104–6. [Google Scholar]
- 26.Kuratsu N, Isohama Y, Yamashita Y, Takei H, Tokuomi K, Inoue M, et al. A study on estrogen-like activity of royal jelly. Proc Med Pharm Soci WAKAN-YAKU, 2003;20(Suppl):161. (in Japanese) [Google Scholar]
- 27.Nakasa T, Okinaka O, Nakatsuka M. Promotion effect of calcium absorption by protease-treated royal jelly. Food Dev. 1999;34:42–4. (in Japanese) [Google Scholar]
- 28.Yamaguchi M, Oishi H, Suketa Y. Stimulatory effect of zinc on bone formation in tissue culture. Biochem Pharmacol. 1987;36:4007–12. doi: 10.1016/0006-2952(87)90471-0. [DOI] [PubMed] [Google Scholar]
- 29.Willis JB. Determination of calcium in blood serum by atomic absorption spectroscopy. Nature (London) 1960;186:249–50. doi: 10.1038/186249a0. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi N, Yamana H, Yoshiki S, Roodman GD, Mundy GR, Jones SJ, et al. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988;122:1373–82. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- 31.Mundy GR, Roodman GD. Osteoclast ontogeny and function. In: Peck WA, editor. Bone and Mineral Research. Amsterdam: Elsevier Science; 1987. pp. 209–79. [Google Scholar]
- 32.Burstone MS. Histochemical demonstration of acid phosphatases with naphthol AS-phosphates. J Natl Cancer Inst. 1958;21:523–39. [PubMed] [Google Scholar]
- 33.Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982;34:285–90. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- 34.Tarttelin MF, Gorski RA. The effect of ovarian steroids on food and water intake and body weight in the female rats. Acta Endocrinol. 1978;72:551–68. doi: 10.1530/acta.0.0720551. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa Y, Ikegami H, Sakata M, Mizutani T, Morishige K, Kurachi H, et al. Ovariectomy increases the level of estrogen receptor mRNA and estrogen receptor binding sites in female rat adipose tissue. J Endorinol Invest. 1993;16:579–83. doi: 10.1007/BF03347674. [DOI] [PubMed] [Google Scholar]
- 36.Roudebush RE, Magee DE, Benslay DN, Bendele AM, Bryant HU. Effect of weight manipulation on bone loss due to ovariectomy and the protective effects of estrogen in the rat. Calcif Tissue Int. 1993;53:61–4. doi: 10.1007/BF01352016. [DOI] [PubMed] [Google Scholar]
- 37.Morin LP, Fleming AS. Variation of food intake and body weight with estrous cycle, ovariectomy, and estradiol benzoate treatment in hamsters. J Comp Physiol Psychol. 1978;92:1–6. doi: 10.1037/h0077435. [DOI] [PubMed] [Google Scholar]
- 38.Mueller K, Hsiao S. Estrus- and ovariectomy-induced body weight changes: evidence for two estrogenic mechanisms. J Comp Physiol Psychol. 1980;94:1126–36. doi: 10.1037/h0077746. [DOI] [PubMed] [Google Scholar]
- 39.Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT. Energy balance in ovariectomized rats with and without estrogen replacement. Am J Physiol. 1980;238:R400–5. doi: 10.1152/ajpregu.1980.238.5.R400. [DOI] [PubMed] [Google Scholar]
- 40.Clark RC, Tarttelin MF. Some effects of ovariectomy and estrogen replacement on body composition in the rat. Physiol Behav. 1982;28:963–9. doi: 10.1016/0031-9384(82)90161-5. [DOI] [PubMed] [Google Scholar]
- 41.Writing group for the Women's Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 42.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]