Abstract
An increasing death rate due to cardiovascular disease in patients with rheumatoid arthritis (RA) has been reported. Keishibukuryogan (KBG) is a traditional Chinese/Japanese (Kampo) formula that has been administered to patients with blood stagnation, e.g. thrombotic disease and atherosclerosis. The objective of this study was to evaluate the efficacy of KBG on disease activity and endothelial dysfunction in RA patients. Sixteen RA patients were enrolled and administered KBG (12 g per day) for 12 weeks in addition to continuing other drugs. The disease activity of RA was assessed by modified disease activity scores for 28 joints (DAS28). Plasma levels of adhesion molecules, soluble E-selectin (sE-selectin), soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) were evaluated. C-reactive protein (CRP), inflammatory cytokines (IL-1β, IL-6 and TNF-α) and lipid peroxide (LPO) were also evaluated. Fourteen patients completed the study. The disease activity of RA, tender joint count, swollen joint count and DAS28 decreased significantly. Among adhesion molecules, only sVCAM-1 decreased significantly. LPO also decreased significantly, whereas CRP and inflammatory cytokines remained unchanged. These results suggest that KBG has insufficient anti-inflammatory or immunomodulating effect but does have a beneficial effect on articular symptoms and a protective effect against endothelial dysfunction in RA patients.
Keywords: antioxidant, lipid peroxide, vascular cell adhesion molecule-1
Introduction
Rheumatoid arthritis (RA) is a chronic articular inflammatory disease and leads to tenderness, swelling and destruction of systemic joints. Shortening of life span and increasing death rate due to cardiovascular and cerebrovascular diseases in RA patients have been reported (1,2). The high incidence of cardiovascular events in RA patients cannot be entirely explained by only traditional cardiac risk factors (3), and increased levels of adhesion molecules are associated with inflammatory activity in RA patients (4).
In the early phase of atherosclerosis, leucocytes migrate trans-endothelially into the vascular wall (5). This process is mediated by adhesion molecules, which are expressed on the vascular endothelium, and circulating leukocytes in response to several inflammatory stimuli (6,7). Selectins and their ligands are involved in the rolling and tethering of leukocytes on the vascular wall. Intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-1 (VCAM-1), as well as some integrins, induce firm adhesion of inflammatory cells to the vascular surface (8–10). In most of the cell adhesion molecules, soluble forms have been identified in the circulation. Several lines of evidence have supported a crucial role of cell adhesion molecules in the development of atherosclerotic plaque, and plasma levels of soluble E-selectin (sE-selectin), soluble ICAM (sICAM-1) and soluble VCAM-1 (sVCAM-1) have been postulated to be useful risk predictors of the progression of atherosclerosis and cardiovascular events (6,7).
Cell adhesion molecules also play an important role in the process of destruction of synovial tissue in RA patients. The activation, circulation and migration of mononuclear cells to inflammatory sites are regulated by cell adhesion molecules (11,12). In fact, the plasma levels of sE-selectin, sICAM-1 and sVCAM-1 reflect the activity of RA (13,14). Together with the fact of the increasing death rate due to cardiovascular diseases in RA patients, cell adhesion molecules might be associated not only with the pathological progression of synovial tissue but also with the atherosclerotic progression of vascular tissue in RA patients.
Currently, Western medicine is commonly used, but traditional Chinese/Japanese (Kampo) medicine also has a long history of contributing to medical care in Japan (15). In essence, Kampo formulas are prescribed according to the characteristic pathological concept of Kampo medicine (16). Keishibukuryogan (KBG) (Gui-Zhi-Fu-Ling-Wan in Chinese) is a Kampo formula composed of five kinds of medicinal plants, Cinnamomum cassia Blume (Cinnamomi cortex), Paeonia lactiflora Pallas (Paeoniae radix), Paeonia suffruticosa Andrews (Moutan cortex), Prunus persica Batsch (Persicae semen) and Poria cocos Wolf (Hoelen) (Table 1). In Japan, KBG has been administered to patients with blood stagnation, e.g. thrombotic disease and atherosclerosis. The contemporary practice of Kampo medicine is required to be evidence based (17). We have previously reported that KBG improves microcirculation in patients with multiple old lacunar infarction (18) and that it prevents the progression of atherosclerosis and preserves endothelium-dependent vasorelaxation in cholesterol-fed rabbit by its antioxidant activity (19,20). Therefore, it is expected that KBG might prevent the progression of vascular endothelial activation in RA patients. We conducted this clinical study for the purpose of evaluating the effects of KBG on endothelial dysfunction and disease activity in RA patients.
Table 1.
Herbal medicines composing KBG and their ratio
| Herbal medicine | Ratio (g) | ||
|---|---|---|---|
| Cinnamomi cortex | Cinnamomum cassia Blume | Gui-Zhi | 0.2 |
| Paeoniae radix | Paeonia lactiflora Pallas | Shao-Yao | 0.2 |
| Moutan cortex | Paeonia suffruticosa Andrews | Mu-Dan-Pi | 0.2 |
| Persicae semen | Prunus persica Batsch | Tao-Ren | 0.2 |
| Hoelen | Poria cocos Wolf | Fu-Ling | 0.2 |
These five herbal powders were mixed with boiled honey (1 g) and rolled up into balls (2 g each).
Methods
Patients
The patients enrolled in the study were diagnosed as suffering from RA according to the 1987 diagnostic criteria for RA of the American Rheumatism Association (21) and the revised criteria of the American College of Rheumatology (22). Diagnoses were made at the Department of Japanese Oriental Medicine, Toyama University Hospital, between September 2002 and January 2004. At this point, patients had been receiving constant treatment with non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), corticosteroid (prednisolone < 5mg per day) or other drugs for at least 3 months and had been in a stable condition in terms of the disease activity of RA. Smokers were excluded from entry. No patients had clinical or laboratory signs of liver or kidney disease, infection, diabetes mellitus, malignancy, previous stroke or myocardial infarction.
Drug
KBG consists of five dried herbal medicines: Cinnamomi cortex, Peoniae radix, Moutan cortex, Persicae semen and Hoelen. These herbal powders were mixed with boiled honey in the ratio shown in Table 1 and rolled up into balls (2 g each). All these herbal medicines and honey were purchased from Uchida Wakanyaku (Tokyo, Japan).
Study Design
The recruited RA patients were administered KBG (12 g per day) after meals three times a day for 12 weeks in addition to their prescribed drugs. During the study, other medications could not be newly introduced. Clinical and laboratory assessments were performed at week 0 and week 12. The study design was approved by the Human Subjects Committee, University of Toyama. All patients provided written informed consent in accordance with the ethical guidelines set forth in the 1975 Declaration of Helsinki.
Assessment of RA Activity
For the assessment of the disease activity of RA, disease activity scores for 28 joints (DAS28) (23) were evaluated by rheumatologists. In detail, tender joint count (0–28) (T28), swollen joint count (0–28) (S28), erythrocyte sedimentation rate (ESR) and the patients' global assessment of disease activity [general health (GH)] using a visual analog scale (VAS; 0–100 mm) were evaluated. Then, DAS28 was calculated using the following equation:
Clinical Laboratory Assessment
Blood was collected after overnight fasting, and separated plasma was frozen at −80°C immediately and stored until assay. As markers of endothelial dysfunction, levels of sE-selectin, sICAM-1 and sVCAM-1 were measured by enzyme-linked immunosorbent assay (ELISA) at a commercial laboratory (SRL, Tokyo, Japan). Inflammatory cytokines, interleukin-1β (IL-1β; R&D Systems Inc., Minneapolis, MN), interleukin-6 (IL-6; Endogen Inc., Woburn, MA) and tumor necrosis factor-α (TNF-α; Endogen Inc.) were measured by ELISA kit according to the manufacturer's instructions. As a marker of oxidative stress, lipid peroxide (LPO) was measured by lipid peroxidation assay kit (Determina LPO; Kyowa Medics, Tokyo, Japan). C-reactive protein (CRP), total cholesterol (T-chol), high-density lipoprotein cholesterol (HDL-chol), low-density lipoprotein cholesterol (LDL-chol) and triglyceride were measured using standard laboratory techniques in our hospital.
Statistical Analysis
Statistical analysis was performed with Stat View J-4.5 (Abacus Concept, Berkley, CA). Data were expressed as mean ± SE. The difference between week 0 and week 12 was analyzed using the Wilcoxon signed-rank test. A value of P < 0.05 was considered statistically significant.
Results
Patient Characteristics
Sixteen patients were initially enrolled in this study. Two patients dropped out because of acute bronchitis and bad compliance with treatment. Finally, 14 patients completed the study with no adverse events. The characteristics of the patients analyzed are shown in Table 2.
Table 2.
Patient characteristics
| Age (years) | 59.1 ± 2.7 |
| Duration of RA (years) | 12.1 ± 1.7 |
| Sex (male/female) | 0/14 |
| BMI (kg m−2) | 21.8 ± 0.7 |
| Anatomical stage of RA | |
| Stage I | 0 |
| Stage II | 3 |
| Stage III | 2 |
| Stage IV | 9 |
| Functional class of RA | |
| Class I | 5 |
| Class II | 6 |
| Class III | 3 |
| Class IV | 0 |
| Complication | |
| Hypertension | 6 |
| Hyperlipidemia | 2 |
| Concomitant drugs | |
| NSAIDs | 9 |
| Corticosteroid | 3 |
| DMARDs | |
| Bucillamine | 5 |
| Salazosulfapyridine | 4 |
| Methotrexate | 3 |
| Antihypertensive | 2 |
| HMG-CoA reductase inhibitor | 1 |
| Kampo medicines except KGB | 13 |
Data represent mean ± SE (n = 14) or number of cases. RA, rheumatoid arthritis; BMI, body mass index; NSAIDs, non-steroidal anti-inflammatory drugs; DMARDs, disease-modifying anti-rheumatic drugs; HMG-CoA, 2-hydroxyl-3-methylglutaryl coenzyme A; KBG, keishibukuryogan.
Activity of RA
Disease activity of RA is shown in Table 3. T28 (P < 0.05), S28 (P < 0.05) and DAS28 (P < 0.05) were significantly decreased at week 12 by administration of KBG compared with baseline, but GH and ESR were not changed.
Table 3.
Disease activity of RA evaluated by disease activity score for 28 joints at baseline and after 12 weeks of KBG treatment
| Week 0 | Week 12 | P-value | |
|---|---|---|---|
| T28 (0–28) | 3.6 ± 1.7 | 0.9 ± 0.3 | <0.05 |
| S28 (0–28) | 2.5 ± 0.8 | 1.2 ± 0.4 | <0.05 |
| GH (VAS; 0–100 mm) | 46.0 ± 5.6 | 41.3 ± 5.5 | NS |
| ESR (mm h−1) | 67.9 ± 9.9 | 71.1 ± 8.1 | NS |
| DAS28 | 4.7 ± 0.3 | 4.1 ± 0.3 | <0.05 |
Data represent mean ± SE (n = 14). T28, tender joint count (0–28); S28, swollen joint count (0–28); GH, patient's global assessment of disease activity (general health); VAS, visual analog scale; ESR, erythrocyte sedimentation rate; DAS28, disease activity score for 28 joints; NS, not significant.
Clinical Laboratory Parameters
For the plasma levels of adhesion molecules, markers of endothelial activation, sE-selectin (77.8 ± 9.2 versus 74.1 ± 9.6 ng ml−1) and sICAM-1 (291.7 ± 32.0 versus 295.0 ± 31.5 ng ml−1) were unchanged, but sVCAM-1 was decreased significantly by 12 week KBG administration (857.1 ± 51.5 versus 744.6 ± 47.3 ng ml−1; P < 0.01) (Fig. 1). The plasma level of LPO, a marker of oxidative stress, also significantly decreased at week 12 compared with baseline (P < 0.05), whereas the values of the inflammatory marker CRP and the inflammatory cytokines IL-1β, IL-6 and TNF-α were unaltered (Table 4). T-chol, HDL-chol, LDL-chol and triglyceride also remained unchanged (data not shown).
Figure 1.
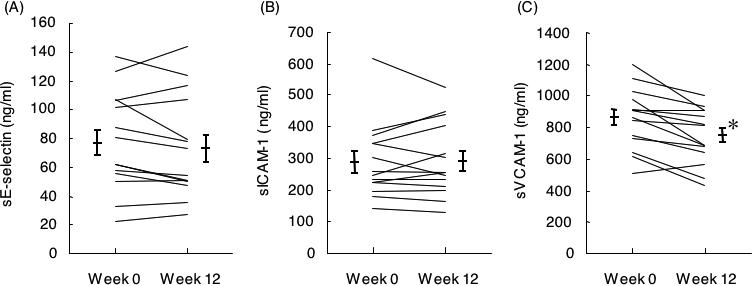
Plasma levels of sE-selectin (A), sICAM-1 (B), and sVCAM-1 (C) at baseline and 12 weeks after administration of KBG. Data represent mean ± SE (n = 14), *P < 0.01 compared with baseline.
Table 4.
Plasma levels of LPO, CRP and inflammatory cytokines at baseline and after 12 weeks of KBG treatment
| Week 0 | Week 12 | P-value | |
|---|---|---|---|
| LPO (nmol ml−1) | 2.95 ± 0.67 | 1.46 ± 0.25 | <0.05 |
| CRP (mg dl−1) | 1.82 ± 0.51 | 2.14 ± 0.58 | NS |
| IL-1β (pg ml−1) | 0.59 ± 0.09 | 0.54 ± 0.06 | NS |
| IL-6 (pg ml−1) | 3.40 ± 0.95 | 2.37 ± 0.58 | NS |
| TNF-α (pg ml−1) | 1.07 ± 0.48 | 1.10 ± 0.71 | NS |
Data represent mean ± SE (n = 14). LPO, lipid peroxide; CRP, C-reactive protein; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; NS, not significant.
Discussion
In the present study, the administration of KBG for 12 weeks did not reduce inflammatory markers or inflammatory cytokines in RA patients. From this, it is suggested that KBG does not possess sufficient anti-inflammatory or immunomodulating effect. In essence, we think that Kampo medicines should be used for RA patients in combination with common Western medicines, particularly when the RA disease activity is high. In this study, constant treatment regimens using NSAIDs, DMARDs, corticosteroids and so on were followed for at least 3 months before the KBG administration, and only patients who had been in a stable condition in terms of disease activity of RA during this period were enrolled. In fact, the ESR and CRP levels of our patients at 3 months prior to, and right before, KBG administration were not significantly different (ESR, 63.8 ± 9.5 versus 67.9 ± 9.9 mm h−1; CRP, 1.90 ± 0.51 versus 1.82 ± 0.51 mg dl−1). Common Western medicines might already have modulated the plasma levels of inflammatory cytokines prior to KBG administration. Indeed, the plasma inflammatory cytokine levels in our patients were relatively low compared with those of RA patients in another report (24). We cannot rule out the possibility that KBG, when used alone in RA patients, will reduce inflammatory cytokine levels.
In our study, KBG decreased tender joint count, swollen joint count and disease activity of RA as evaluated by DAS28, and also a marker of oxidative stress, the plasma level of LPO. RA patients are exposed to oxidative stress (25,26), and it was reported that antioxidants decrease pain parameters, improve RA activity and decrease oxidative stress markers (27,28). KGB consists of the phenol-containing herbal medicines Cinnamomi cortex, Paeoniae radix and Moutan cortex, and it has antioxidant activity (19,20). We speculate that the antioxidant effect of KBG might have contributed to the reduction in articular symptoms of the RA patients observed in this study.
Traditionally, KBG has been used for improvement of not only blood stagnation but also dysmenorrhea. For this reason, KBG is more likely to be administered to females than males. Further, the morbidity rate of RA in females is higher than in males. By coincidence, and the reason that smokers were excluded from entry, all of the patients in this study were females. Thus, we cannot rule out the possibility that male RA patients might exhibit a different response to KBG than female RA patients.
In this study, KBG did not decrease the plasma levels of sE-selectin and sICAM-1, but it reduced sVCAM-1, one of the markers of vascular endothelial activation. The plasma level of sVCAM-1 is higher in RA patients than in osteoarthritis patients (13) and decreases by a reduction in disease activity (13,14). VCAM-1 is expressed on impaired endothelium and is regulated by various factors such as inflammatory cytokines (29). In this study, KBG did not change the plasma cytokine levels in RA patients, suggesting that KBG downregulates sVCAM-1 through a certain pathway not involving cytokines. It is reported that vitamin E, an antioxidant, downregulates sVCAM-1 in patients with hyperlipidemia (30). Therefore, we speculate that the antioxidant effect of KBG might contribute to the downregulation of VCAM-1.
It was reported that among adhesion molecules, VCAM-1 plays a major role in the progression of atherosclerosis (31), can be a useful marker reflecting vascular endothelial activation (24,30,32,33) and is associated with future cardiovascular events (34). We reported that KBG inhibits the progression of atherosclerosis in cholesterol-fed rabbit (19) and improves endothelial function in cholesterol-fed rabbit and spontaneously hypertensive and diabetic rat (20,35,36), and that Cinnamomi cortex and Paeoniae radix, medicinal herbs used in KBG, have a vascular endothelial relaxation effect through nitric oxide production (37,38). These effects of KBG also suggest the benefit of this drug for vascular endothelial function. There is another aspect concerning VCAM-1, as it plays an important role as angiogenic mediator in the pathogenesis of RA by recruiting leukocytes to synovial tissue, leading to proliferative synovitis (39,40). We speculate that the reduction of sVCAM-1 might be one of the reasons for the improving arthritic scores in this study.
The results obtained in the present study suggest that KBG does not possess particular anti-inflammatory or immunomodulating effects but that it has a beneficial effect on articular symptoms and a protective effect against endothelial activation through the reduction of VCAM-1, and it may thereby contribute to the prevention of the progression of cardiovascular disorders in RA patients. Our hypothetical representation of the effects of KBG on RA is summarized in Fig. 2. This was a pilot study, and only patients with stable RA activity achieved by common Western medicines were enrolled, so the number of patients was limited. Further studies based on randomized controlled trials or comparative studies will be needed to validate the benefit of KBG in RA patients.
Figure 2.
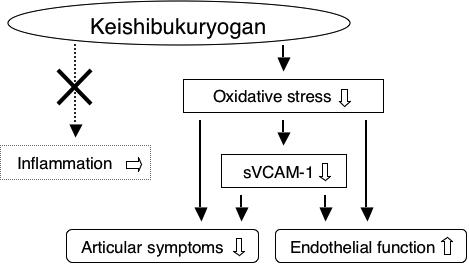
Hypothetical representation of the effects of KBG on RA.
Acknowledgments
This work was supported by a grant-in-aid for the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Myllykangas-Luosujärvi R, Aho K, Kautiainen H, Isomäki H. Shortening of life span and causes of excess mortality in a population-based series of subjects with rheumatoid arthritis. Clin Exp Rheumatol. 1995;13:149–53. [PubMed] [Google Scholar]
- 2.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 3.del Rincón I, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 4.Wållberg-Jonsson S, Cvetkovic JT, Sundqvist KG, Lefvert AK, Rantapää-Dahlqvist S. Activation of the immune system and inflammatory activity in relation to markers of atherothrombotic disease and atherosclerosis in rheumatoid arthritis. J Rheumatol. 2002;29:875–82. [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 7.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 8.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VACM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223–9. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerosis plaques: implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–82. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 11.Mojcik CF, Shevach EM. Adhesion molecules: a rheumatologic perspective. Arthritis Rheum. 1997;40:991–1004. doi: 10.1002/art.1780400602. [DOI] [PubMed] [Google Scholar]
- 12.Tokuhira M, Hosaka S, Volin MV, Hainess GK, Katschke KJ, Kim S, et al. Soluble vascular cell adhesion molecule 1 mediation of monocyte chemotaxis in rheumatoid arthritis. Arthritis Rheum. 2000;43:1122–33. doi: 10.1002/1529-0131(200005)43:5<1122::AID-ANR23>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik JP, Cylwik B, Skowronski J. Soluble adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and vascular endothelial growth factor (VEGF) in patients with distinct variants of rheumatoid synovitis. Ann Rheum Dis. 2002;61:804–9. doi: 10.1136/ard.61.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klimiuk PA, Sierakowski S, Domystawska I, Fiedorczyk M, Chwiecko J. Reduction of soluble adhesion molecules (sICAM-1, sVCAM-1, and sE-selectin) and vascular endothelial growth factor levels in serum of rheumatoid arthritis patients following multiple intravenous infusions of infliximab. Arch Immunol Ther Exp. 2004;52:36–42. [PubMed] [Google Scholar]
- 15.Terasawa K. Evidence-based reconstruction of Kampo medicine: part I—is Kampo CAM? Evid Based Complement Alternat Med. 2004;1:11–6. doi: 10.1093/ecam/neh003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terasawa K. Evidence-based reconstruction of Kampo medicine: part II—the concept of Sho. Evid Based Complement Alternat Med. 2004;1:119–23. doi: 10.1093/ecam/neh022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terasawa K. Evidence-based reconstruction of Kampo medicine: part III—how should Kampo be evaluated? Evid Based Complement Alternat Med. 2004;1:219–22. doi: 10.1093/ecam/neh046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hikiami H, Goto H, Sekiya N, Hattori N, Sakakibara I, Shimada Y, et al. Comparative efficacy of Keishi-bukuryo-gan and pentoxifylline on RBC deformability in patients with “oketsu” syndrome. Phytomedicine. 2003;10:459–66. doi: 10.1078/094471103322331395. [DOI] [PubMed] [Google Scholar]
- 19.Sekiya N, Tanaka N, Itoh T, Shimada Y, Goto H, Terasawa K. Keishi-bukuryo-gan prevents the progression of atherosclerosis in cholesterol-fed rabbit. Phytother Res. 1999;13:192–6. doi: 10.1002/(SICI)1099-1573(199905)13:3<192::AID-PTR412>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Sekiya N, Goto H, Tazawa K, Oida S, Shimada Y, Terasawa K. Keishi-bukuryo-gan preserves the endothelium dependent relaxation of thoracic aorta in cholesterol-fed rabbit by limiting superoxide generation. Phytother Res. 2002;16:524–8. doi: 10.1002/ptr.945. [DOI] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35:498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 23.Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther. 2005;7:634–43. doi: 10.1186/ar1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Öztürk HS, Çimen MY, Çimen ÖB, Kaçmaz M, Durak Î. Oxidant/antioxidant status of plasma samples from patients with rheumatoid arthritis. Rheumatol Int. 1999;19:35–7. doi: 10.1007/s002960050097. [DOI] [PubMed] [Google Scholar]
- 26.Kamanli A, Naziroglu M, Aydilek N, Hacievliyagil C. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct. 2004;22:53–7. doi: 10.1002/cbf.1055. [DOI] [PubMed] [Google Scholar]
- 27.Edmonds SE, Winyard PG, Guo R, Kidd B, Merry P, Langrish-Smith A, et al. Putative analgesic activity of repeated oral doses of vitamin E in the treatment of rheumatoid arthritis: results of a prospective placebo controlled double blind trial. Ann Rheum Dis. 1997;56:649–55. doi: 10.1136/ard.56.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helmy M, Shohayeb M, Helmy MH, el-Bassiouni EA. Antioxidants as adjuvant therapy in rheumatoid disease: a preliminary study. Arzneim Forsch Drug Res. 2001;51:293–8. doi: 10.1055/s-0031-1300040. [DOI] [PubMed] [Google Scholar]
- 29.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–11. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 30.Desideri G, Marinucci MC, Tomassoni G, Masci PG, Santucci A, Ferri C. Vitamin E supplementation reduces plasma vascular cell adhesion molecule-1 and von Willebrand factor levels and increases nitric oxide concentrations in hypercholesterolemic patients. J Clin Endocrinol Metab. 2002;87:2940–5. doi: 10.1210/jcem.87.6.8545. [DOI] [PubMed] [Google Scholar]
- 31.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–62. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Caterina R, Basta G, Lazzerini G, Dell'Omo G, Petrucci R, Morale M, et al. Soluble vascular cell adhesion molecule-1 as a biohumoral correlate of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2646–54. doi: 10.1161/01.atv.17.11.2646. [DOI] [PubMed] [Google Scholar]
- 33.Desideri G, Croce G, Tucci M, Passacquale G, Broccoletti S, Valeri L, et al. Effects of bezafibrate and simvastatin on endothelial activation and lipid peroxidation in hypercholesterolemia: evidence of different vascular protection by different lipid-lowering treatments. J Clin Endocrinol Metab. 2003;88:5341–7. doi: 10.1210/jc.2003-030724. [DOI] [PubMed] [Google Scholar]
- 34.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–42. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 35.Kasahara Y, Goto H, Shimada Y, Sekiya N, Yang Q, Terasawa K. Effects of Keishi-bukuryo-gan (Gui-Zhi-Fu-Ling-Wan) on endothelial function in spontaneously hypertensive rats. J Trad Med. 2001;18:113–8. [Google Scholar]
- 36.Goto H, Shimada Y, Sekiya N, Yang Q, Kogure T, Mantani N, et al. Effects of Keishi-bukuryo-gan on vascular function and hemorheological factors in spontaneously diabetic (WBN/kob) rats. Phytomedicine. 2004;11:188–95. doi: 10.1078/0944-7113-00336. [DOI] [PubMed] [Google Scholar]
- 37.Goto H, Shimada Y, Akechi Y, Kohta K, Hattori M, Terasawa K. Endothelium-dependent vasodilator effect of extract prepared from the roots of Paeonia lactiflora on isolated rat aorta. Planta Med. 1996;62:436–9. doi: 10.1055/s-2006-957934. [DOI] [PubMed] [Google Scholar]
- 38.Tanikawa K, Goto H, Nakamura N, Tanaka N, Hattori M, Itoh T, et al. Endothelium-dependent vasodilator effect of tannin extract from Cinnamonomi Cortex on isolated rat aorta. J Trad Med. 1999;16:45–50. [Google Scholar]
- 39.van Dinther-Janssen AC, Horst E, Koopman G, Newmann W, Scheper RJ, Meijer CJ, et al. The VLA-4/VCAM-1 pathway is involved in lymphocyte adhesion to endothelium in rheumatoid synovium. J Immunol. 1991;147:4207–10. [PubMed] [Google Scholar]
- 40.Kitani A, Nakashima N, Izumihara T, Inagaki M, Baoui X, Yu S, et al. Soluble VCAM-1 induces chemotaxis of jurkat and synovial fluid T cells bearing high affinity very late antigen-4. J Immunol. 1998;161:4931–8. [PubMed] [Google Scholar]


