Abstract
Channels of the C-type transient receptor potential (TRPC) are involved in agonist-stimulated and capacitative calcium entry. There are seven TRPCs, all of which have a Ca2+-dependent calmodulin (CaM)-binding domain in their C termini. We now tested binding of CaM to TRPC N termini and show that only that of TRPC2 binds CaM in a Ca2+-dependent manner. Four TRPC2 cDNAs have been reported: a (also clone 14), b (also clone 17), α, and β. Sequences responsible for CaM binding in TRPC2 a and b are absent from the α and β isoforms. The α and β cDNAs of TRPC2 were reported as alternative forms, when recloning of TRPC2 a and b proved impossible. Here we analyzed total RNA samples from brain and testis for presence of TRPC2 a and b and describe the splicing patterns responsible for their formation, as well as those leading to the α and β forms of TRPC2. We re-assert existence of RNA encoding the TRPC2 a and b, encoded in 21 exons with an initiator ATG in exon 2 for TRPC2a and in exon 4 for TRCP2b. The analysis of α and β TRPC2 cDNAs indicates that although the TRPC2β mRNA may exist, the TRPC2α cDNA is derived from an incompletely processed TRPC2a mRNA: It includes in its presumed 5′-untranslated sequence, 713 nt of TRPC2a cDNA fused to 291 nt of an incompletely excised intron. While encoding an active channel in the mouse, the human TRPC2 appears to be a pseudogene. We searched for the human gene in the data bank and located approximately one-half of it in a chromosomal region syntenic to that of the mouse, with similar intron–exon structure. We conclude that the human TRPC2 gene may never have been an active gene because of incomplete ancestral duplication or, if it was complete at one point, that it became inactive upon loss of chromosomal sequences.
Keywords: cation channel|store-operated channel|gene structure|splice variants|pseudogene
Channels of the C-type transient receptor potential (TRPC) are the founding subfamily of the superfamily of TRP channels. Other subfamilies include the TRPV and TRPM channels (1). TRPs encode cation channels with widely varying cation selectivity and widely differing mechanisms of activation (ref. 2 and http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2001/90/re1). Some of the TRPC channels, e.g., TRPC1 (3, 4), TRPC2 (5), and TRPC4 (6, 7), but not all, e.g., TRPC3 (8), are involved in mediating capacitative entry triggered by depletion of endogenous calcium stores without concomitant activation of a phospholipase C, be it through activation of a G protein or secondary to activation of a tyrosine kinase. Studies on the mechanism of TRPC activation are complex, not only because of the nature of the putative regulatory mechanism under investigation, but also because of existence of splice variants. Moreover, in some cases the reported splice variants (9) appear to be difficult to be verified by other laboratories, one of which reported instead the identification by molecular cloning of two novel alternate variants that appear to be selectively retained in the endoplasmic reticulum (10). In the present report, we concentrated on defining the existence and mode of origination of the clone 14 (1,172 aa) and clone 17 (1,072 aa) N-terminal variants of TRPC2, cloned by Vannier et al. (9) and the origins of the α and β TRPC2 transcripts cloned by Hofmann et al. (10). Furthermore, although previous studies on the binding of Ca2+/calmodulin (CaM) concentrated only on presence of such sites in the C termini, we now extended these studies to the N termini. TRPC2a and TRPC2b, but none of the other TRPCs, contain Ca2+-dependent CaM-binding sites at their N terminus. Thus, TRPC2a and TRPC2b join TRPC4α (11, 12) in having more than one CaM-binding site.
Materials and Methods
Protein–Protein Interaction Tests.
Standard procedures reported previously were used to construct plasmids encoding CaM and N termini as GST fusion proteins for expression in Escherichia coli or under the control of the T7 promoter for expression and labeling with [35S]Met and [35S]Cys in reticulocyte lysates by using Promega's coupled transcription and translation reagents (TNT) (11, 13, 14). Protein–protein interactions were then performed as described in these same reports.
RT-PCR.
Total RNA from either mouse brain or mouse testis were isolated by using reagents and protocols of the RNeasy RNA isolation kit (Qiagen). Reverse transcripts were prepared by a two-step procedure. In the first step, 0.2 μg of total RNA was incubated with 50 ng of random hexamers in a final volume of 12 μl at 65°C for 5 min, followed by a chill on ice. For the second step, the product of the first step was incubated at 25°C for 10 min in a final volume of 20 μl by addition of 4 μl of 5× cDNA synthesis buffer (250 mM Tris-acetate, pH 8.4/375 mM K acetate/40 mM Mg acetate), 1 μl 100 mM DDT, 1 μl of RNaseOUT (40 units), 2 μl 10 mM dNTPs, 1 μl of RNase H− reverse transcriptase (ThermoScript, Invitrogen, 15 units), and 1 μl of diethyl pyrocarbonate-treated H20 followed by 50 min at 55°C, 5 min at 85°C, cooling to room temperature, and a final incubation with 1 μl of RNase H (2 units) at 37°C for 20 min. Reverse transcripts were amplified by PCR by adding 2 μl of the previous reaction products to 48 μl of PCR reaction mixture containing 5 μl of 10× PCR buffer (100 Tris⋅HCl, pH 8.3/500 mM KCl/0.01% gelatin), 1.5 μl of 50 mM MgCl2, 1 μl of 10 mM dNTPs, 1 μl of 10 μM sense and 10 μM antisense primers, 0.4 μl of Taq polymerase (5 units/μl), and diethyl pyrocarbonate-treated water. The cycling protocol used was 60 sec at 94°C, then 60 sec at 94°C, 60 sec at 52°C, and 120 sec at 72°C for 34 cycles, and finally 5 min at 72°C and a minimum of 10 min at 15°C. Reaction products were separated by standard agarose gel electrophoresis (15) and cloned into pCR-2.1 TOPO (Invitrogen) by using a protocol supplied by the vendor. The identity of the inserts was then confirmed by dideoxy sequencing (16).
PCR Primer Composition.
The TRPC2 primers used to test for presence of specific transcripts in total brain and testis RNA (vide infra) were designed with the help of primer3 software (www-genome.wi.mit.edu/cgi-bin/primer/primer3.cgi). The composition of PCR primers used was: primer A, 5′-TGGAGTTTCAGCTGGAGAGG; primer B, 5′-GCTAATGTCCCGCACTGACT; primer C, 5′-GTTCTCCGTGGCTGTTGTTT; primer D, 5′-ACGTCATCAGTCCTTTGGCCT; and primer E, 5′-GGTTCTCCGTGGCTGTTGTTT.
The wisconsin package (Version 10.3, Accelrys, San Diego) was used for standard nucleotide and amino acid sequence manipulations and analysis.
Results
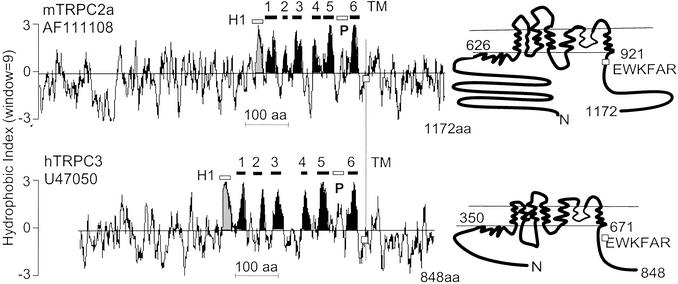
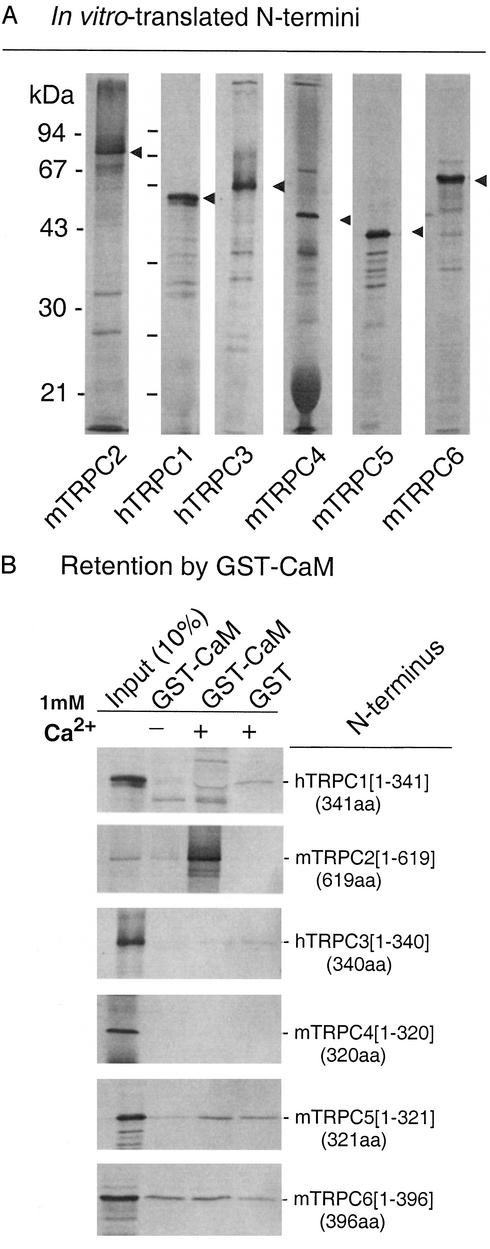
A sequence similarity analysis of mammalian TRPC channels yields a phylogenetic tree that groups these channels into four subgroups (9): TRPC1 and TRPC2 belong to subgroups I and II, TRPC3, TRPC6, and TRPC7 belong to subgroup III, and TRPC4 and TRPC5 belong to subgroup IV. Fig. 1 shows typical Kyte and Doolittle plots of TRPC2 and TRPC3, highlighting eight hydrophobic domains. Of these, the first was shown to be intracellular by glycosylation scanning mutagenesis and epitope insertion mapping (17). One of the remaining seven comprises a region that forms the selectivity pore of the channel. The remaining six, five prior and one after the pore region, form the transmembrane domain of these channels. Previous studies had shown that TRPC1–7 have a conserved Ca2+-dependent CaM-binding site in their C termini (11–13), and that TRPC4 also has three CaM-binding sites in its C terminus. Among these three CaM-binding sites, two are encoded in the facultative exon 12 (252 nt) of TRPC4α variant. Binding of CaM to the N termini of TRPCs on the other hand has not been systematically evaluated. Fig. 2 shows, that among TRPC1–6, TRPC2 is unique in that its N terminus strongly binds CaM and does so in a Ca2+-dependent manner. Subdividing the TRPC2 N terminus, showed that its CaM-binding activity is located in a region formed by amino acids 104–195, possibly just 109–161 (Fig. 3, TRPC2a numbering), a little more than 400 aa from the first hydrophobic region. We failed to find amino acid sequences similar to those comprising the TRPC2 N-terminal CaM-binding domain in the N (or C) termini of other TRPC channels, indicating that they are unique to TRPC2.
Figure 1.
Kyte and Doolittle plots and predicted transmembrane topology of TRPC2a and TRPC3. The figure highlights eight hydrophobic regions of which the regions 2–5 and 8 form the transmembrane segments. The seventh encodes the pore region and the first is wholly intracellular. □, Location of EWKFAR motif. The hydrophobic domain clusters extend from amino acids 626 to 921 in TRPC2a and from amino acids 350 to 671 in TRPC3.
Figure 2.
The N terminus of TRPC2 associates with CaM in a Ca2+-dependent manner. (A) N termini of TRPC1–6 were translated in vitro in a reticulocyte lysate in the presence of 35S-labeled methionine and cysteine. Aliquots were subjected to SDS/PAGE and the gels were autoradiographed. (B) Ca2+-dependent binding of the TRPC2 N terminus, but not that of other TRPCs, to CaM fused to GST. When present, Ca2+ was 1 mM.
Figure 3.
The CaM-binding activity of the TRPC2 N terminus resides in a fragment encoded in exon 5. (A) Diagram of exons encoding the N terminus of TRPC2. (B) Binding of CaM synthesized in a reticulocyte lysate in the presence of 35S-labeled cysteine and methionine to fragments of the N terminus of TRPC2. Bound CaM was separated from free by washing and SDS/PAGE. When present Ca2+ was 1 mM; 1 μg of fragment B fused to GST retained ≈5% of the [35S]CaM.
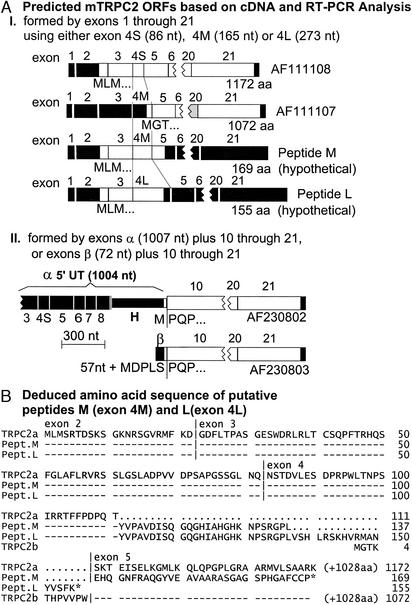
Four variants, apparently having their origin in alternative splicing of the primary transcripts have been reported for TRPC2. They have been named TRPC2a, TRPC2b (9), TRPC2α, and TRPC2β (10). The a and b forms correspond to the cDNAs originally reported as clones 14 and 17, respectively. They code for proteins of 1,172 and 1,072 aa, respectively, differing in their N termini. The shorter, TRPC2b (clone 17), differs from TRPC2a in its first 11 aa, with amino acids 12 –1,072 being identical to amino acids 112–1,172 of TRPC2a. Thus, both TRPC2a and TRPC2b have the N-terminal CaM-binding site.
There are examples in the literature of proteins with differing N termini, each regulated by a separate promoter, i.e., each exon 1 being preceded by an independent promoter. This allows for different tissue specific or developmentally conditioned expression of a single gene product. To determine whether this might be the case for the a and b forms of TRPC2, we mapped the respective mRNAs onto the genomic sequence to delineate their intron–exon organization. Genome analysis software accessible from the National Center for Biotechnology Information web site predicts not only the exons as they are defined by the cDNA sequence, but its model maker subroutine predicts one additional exon (complement to the region 19,937,673–19,937,815 of the supercontig NW_000328), where splice donor site is 2,570 nt 5′ to the exon encoding Met-1 of TRPC2a. We, thus tested for the presence of sequences in reverse transcripts of brain and testis total RNA that would start in either putative exon 1 or in exon 2 by PCR analysis where partnering reverse primer is designed to anchor in exon 5, the first exon common to TRPC2a and TRPC2b (Fig. 4A).
Figure 4.
RT-PCR of brain and testis RNA confirms existence of predicted exon 1 upstream of the exon harboring Met-1 of TRPC2 and reveals existence of three forms of exon 4. (A Upper) Scheme of TRPC2a mRNA with location of exon boundaries. Open boxes, exons; filled boxes, untranslated sequences. The numbers above the cDNA are exons as predicted by National Center for Biotechnology Information's map viewer software by using the complement to region 19,929,800–19,960,915 of supercontig NW_000328. Diagonally hatched areas denote transmembrane segments 1–6 and pore region (P). (Lower) Design of RT-PCR analysis for identification of putative exon 1 (pink box) and presumed composition of exon 4 variants between exons 3 and 5 (graded blue boxes) (B) Agarose gel electrophoresis of RT-PCR products. (Left) Products obtained with primer pairs AC and BC. (Right) Agarose gel electrophoresis of products obtained with primer pairs D and E. Note that instead of the two bands with exon 4 predicted by clones 17 and 14, there exist three distinct forms of exon 4: 4S, 4M, and 4L.
Sequencing of the A-C and B-C PCR products (Fig. 4B Left) revealed that putative exon 1 indeed existed (A-C products were extensions of B-C products) and, furthermore, that each set contained three distinct products that differ in the length of exon 4. Direct PCR amplification of exon 4 with primers D and E anchored in exons 3 and 5, respectively, yielded three distinct bands (Fig. 4B Right). These bands coincided with their length as predicted from A-C and B-C PCR products and defined exons 4S, 4M, and 4L, for short, medium, and long, all arising from the use of a common splice acceptor site at the beginning of exon 4, and either 2 different splice donor sites in exon 4, or, in the case of exon 4L, from a failure to excise the intron between exon 4 and 5. As shown in Fig. 5AI, the ORF initiated by the first Met of exon 2 (MLM motif) is preserved to give that of clone 14 in the transcripts with exon 4S, but terminate either 73 codons into exon 4L or 32 codons into exon 5 in transcripts made with exon 4M. Transcripts with exon 4S therefore encode TRPC2a. Exon 4M, on the other hand, has in its 3′ extension (with respect to 4S) an ORF with a Met-encoding ATG codon (MGT motif), which upon joining exon 5 uses as its extension the same ORF used by the MLM of exon 2 in transcripts made with exon 4M. Thus, the ORF initiated in 4M continues throughout exons 5–21 to encode TRPC2b. TRPC2a and TRPC2b are thus identical from exon 5 onwards (stop codon in exon 21). Transcripts made with exon 4M and 4L encode in addition two shortened forms of the TRPC2a, peptides M (mRNA with exon 4M) and L (mRNA with exon 4L; Fig. 5AI). Peptides M and L are predicted to have 169 and 155 aa, respectively (Fig. 5B).
Figure 5.
(A) Diagrams of ORF in mature TRPC2 transcripts based on RT-PCR results of Fig. 4 and on α and β cDNAs reported by Hofmann et al. (10). (B) Alignment of deduced amino acid sequences of ORFs encoded in TRPC2 transcripts with exons 4S, 4M, and 4L. Top line of alignment is master sequence; -, amino acids identical to TRPC2a in TRPC2b, peptide L, and peptide M; ⋅, gap; *, stop; |, exon boundaries.
Amino acids that form the N-terminal CaM-binding domain of Fig. 3 are encoded in exon 5 and are thus present in TRPC2a and TRPC2b. Amino acids encoding the C-terminal CaM-binding site of TRPC2 are encoded in exon 20.
The α and β forms of TRPC2, reported by Hofmann et al. (10), toward the end of 2000, are identical to amino acids 287–1,172 of the TRPC2a reported by us at the beginning of 1999. Codon 283 codes for the first P of the PQP motif located at the 5′-end of exon 10. Thus, the α, β, and a forms share sequences encoded in exons 10–21, differing upstream of exon 10. Amino acids upstream of PQP in TRPC2α and β are Met- and Met-Asp-Pro-Leu-Ser (MDPLS), respectively. The TRPC2α cDNA was reported with 1,004 nt of 5′-untranslated sequence. Sequence alignment of the TRPC2a and TRPC2α cDNAs, revealed that the first 713 nt of the 5′-untranslated sequence of TRPC2α are identical to those sections of the TRPC2a cDNA that are derived from exons 3–8, the remaining 291 nt just before the initiator ATG of TRPC2α are identical to the 5′-end of the intron H between exons 8 and 9 (Fig. 5AII). We conclude that the TRPC2α cDNA, rather than representing an independent splice variant of the murine TRPC2 gene, resulted from the molecular cloning of a misprocessed TRPC2a mRNA, due to use of the ATGgt sequence in intron H as a splice donor site. An in-frame TAA stop codon in the intronic sequence at position −108 from this ATG gave the impression of having isolated a complete cDNA. In contrast to TRPC2α, there is no evidence in favor of or against TRPC2β being derived from a correctly spliced mRNA. The 5′-end of its ORF codes for MDPLS proceeded by 57 nt of presumptive 5′-untranslated sequence. This sequence is wholly represented within the intron between exons 9 and 10, the latter beginning with codons coding for the PQP motif that marks the beginning of TRPC2β's identity to the TRPC2a (or b) sequence. Moreover, the mouse TRPC2β is nearly identical to the rat TRPC2 cDNA, which was reported to be expressed in the vomeronasal auxiliary olfactory bulb (18). The ORF of the rat TRPC2β cDNA codes for a protein that is highly homologous to TRPC2a from amino acid 289–1,172, beginning with MDPLSP, where the P corresponds to the second P of the PQP motif of mouse exon 10. As is the case for mouse TRPC2β, the upstream nucleotides reported for rat TRPC2 are wholly represented within the genomic sequence upstream of exon 10, giving no further information as to whether the cDNA is derived from a bonafide mRNA or not. Further tests will be required to settle this issue. It is noteworthy to comment here that, neither the murine TRPC2α nor the murine TRPC2β cDNAs encodes a protein capable of forming active channels. Instead, when observed by confocal microscopy, TRPC2α and TRPC2β failed to reach the plasma membrane and to form active influx channels (10). Finally, analysis of the same batch of reverse transcripts that allowed for PCR amplification of the fragments shown in Fig. 4, failed to yield amplified fragments with primers based on the nucleotide sequence preceding that encoded in exon 10 (data not shown). Further studies are therefore required to determine the molecular origin of TRPC2β. Particularly, it will be of interest to determine whether, as suggested by the data of Liman et al. (18), this form is expressed exclusively in the auxiliary olfactory bulb where loss of the channel it encodes causes loss of sexual discrimination and promotes aggressive behavior in male mice (19).
Discussion
In this study, we have concentrated on the intron–exon organization of murine TRPC2 and the available evidence for the expression of splice variants as deduced from RT-PCR analysis of mRNA extracted from two tissues, brain and testis. At the amino acid level, a comparison of TRPC2 to the other six members of the TRPC subfamily of TRP channels shows TRPC2 to be unique in that it has an extension, N-terminal to the location of the three ankyrin repeats found in all TRPCs. This extension shows no evolutionary relation to sequences elsewhere in the other TRPCs. Sequence similarities that define TRPCs as such, stretch from the beginning of the ankryin repeats through the region of the six transmembrane segments and an immediately following group of six amino acids, EWKFAR. This group of amino acids is referred to as the TRP motif, and is present not only in the C-type but, in a slightly degenerated form, also in the V- and M-type TRPs. Although being clear structural landmarks, neither the role of the ankyrin repeats nor that of the TRP motif have been elucidated. Ankyrin repeats are presumed to play a role in protein–protein interactions, the TRP motif may play no practical role. The highest sequence similarity among the TRP-C, -V, and -M subfamilies lie within their transmembrane regions, especially sequences associated with S3–S6 regions. These, more than the TRP motif, contribute to the structural definition of TRP channels. Indeed, the TRPP subfamily, that includes polycystic kidney disease 2, lacks the TRP motif, but shows sequence similarity at the level of the transmembrane segments that classifies it and its congeners as TRP channels. C-terminal to the TRP motif, TRPC channels share very limited sequence identity, except for the presence of a CaM and inositol trisphosphate receptor-binding domain, that has been implied in both feedback inhibition of channel activity by calcium, and a mechanisms for activation associated with store depletion. A comparison of the intron–exon organization of the seven TRPC channels and relative location of exons coding for ankyrin repeats, the TRP motif and the various CaM-binding domains is shown in Fig. 6. They are aligned along the EWKFAR TRP motif found after transmembrane segment 6. Exons encoding the common C-terminal CaM-binding domains are in cyan, and exons containing the three ankyrin motifs are in green. A careful inspection of the boundaries (not shown) reveals no overriding conservation of boundaries associated with certain transmembrane segments, the pore, or the EWKFAR motif. For example although the end of the S6 segment and the EWKFAR are in the same exon in TRPC1, the TRP motif is found in a dedicated exon in all other TRPCs; although the three ankyrin repeats are in a single exon in TRPC2–7, in TRPC1 they are in three exons, and one of the TRPC1 splice variants, TRPC1β, lacks the middle of these exons. The transmembrane segments on the other hand are encoded in four exons in all TRPCs except in TRPC2 and TRPC1, in which they are encoded in five and six exons, respectively. The most intriguing C-terminal splice variants is that of TRPC4. The α form results from failure to excise the intron between exons 11 and 12 so that it's last exon spans from the beginning of exon 11 to the end of the transcript. The β form is made up of 12 exons as shown in Fig. 6. The spliced out segment codes for 84 aa with two CaM-binding sites. RT-PCR analysis showed that both splice forms are coexpressed; the full-length α form is inactive and plays a dominant negative role (11, 12, 19).
Figure 6.
Comparison of exon boundary locations in TRPC cDNAs highlighting in addition transmembrane and pore regions, the TRP motifs and exons coding for ankyrin repeats and CaM-binding domains. All cDNAs are human except TRPC2, which is murine. The spliced (β) form of TRPC1 differs from the unspliced (α) form in that it lacks exon 3 (turquoise) and its attendant middle ankyrin repeat. The β form of TRPC4, able to form active channels, is shown; the α form, which does not form active channels, incorporates the 252-nt exon 12 (21). This exon harbors two additional CaM-binding domains (11, 12).
The TRPC2 channel is not only important in male sexual and social behavior (20, 21), but also in the process of fertilization, in which it is an intermediary step of the signaling pathway triggered by the oocyte's zona pellucida to elicit the sperm's acrosome reaction (4). Despite these central roles in the mouse, the sole human cDNA resembling TRPC2 (GenBank accession no. X89067) only codes for a protein that begins in the presumptive third transmembrane segment, lacks the fifth and half of the pore region, resuming thereafter to end with a C terminus highly similar to that of the mouse TRPC2. In addition, the ORF of this TRPC2-like sequence is interrupted by three stop codons. Thus, the human TRPC2-like cDNA appears to be derived from a pseudogene. We asked whether the TRPC2-like gene was unique or whether there might exist also a bonafide TRPC2 gene that had so far eluded detection through the molecular cDNA cloning approach. If there were such a true orthologous human TRPC2 gene, one would expect to find it in a chromosomal location that is syntenic to that in which the mouse gene is located. The mouse TRPC2 gene is on chromosome 7 in a region that is syntenic to human chromosome 11p15.3–15.4. Rather than finding a full TRPC2 gene in this location of the human chromosome 11, we found the genomic region encoding the human TRPC2-like transcript. Mapping the human cDNA to the human chromosome 11 sequence revealed an intron–exon structure similar to that of the mouse gene spanning from exons 14–21, with the exception that the sequence corresponding to exon 16, coding for the fifth transmembrane segment and half of the pore region, is absent (Fig. 7). A search for TRPC2 sequences elsewhere in the human genome was negative, except for locating sequences similar to that of corresponding to a fusion of mouse exons 2 and 3 ≈70 Mbp upstream of the sequences coding for the TRPC2-like transcript (Fig. 7). This reduces the number of functional human TRPC genes to 6. Further studies should clarify the involvement of TRPC2 channel(s) in mouse.
Figure 7.
Comparison of mouse chromosome 7 region, harboring the TRPC2 gene, to the syntenic region of human chromosome 11, harboring the TRPC2 pseudogene. (A) Intron–exon distribution along the chromosome is shown above the diagram of the cDNA with its exon boundaries. (B) Intron–exon distribution along the human chromosome is shown above the diagram of the human cDNA. Note that we leave as undecided (?) the existence of an additional exon with a separate promoter located 5′ to exon β (between exons 9 and 10). The human TRPC2 sequences can be found in the complements to regions 63,880–74,537 of genomic contig NT_035090 and 2,272,990–2,336,104 of contig NT_033927.
Abbreviations
- CaM
calmodulin
- TRP
transient receptor potential
- S
short
- M
medium
- L
long
- TRPC
C-type TRP
References
- 1.Montell C, Birnbaumer L, Flockerzi V, Bindels R J, Caterina M J, Clapham D E, Heller S, Julius D, Scharenberg A M, Schultz G, et al. Mol Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- 2.Montell C, Birnbaumer L, Fockerzi V. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Wang W, Singh B B, Lockwich T, Jadlowiec J, O'Connel B, Wellner R, Zhu M X, Ambudkar I S. J Biol Chem. 2000;275:9890–9891. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 4.Jungnickel M K, Marrero H, Birnbaumer L, Lémos J R, Florman H M. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- 5.Zitt C, Zobel A, Obukhov A G, Harteneck C, Kalkbrenner F, Lückhoff A, Schultz G. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- 6.Philipp S, Cavalie A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. EMBO J. 1996;15:6166–6171. [PMC free article] [PubMed] [Google Scholar]
- 7.Freichel M, Suh S H, Pfeifer A, Schweig U, Trost C P, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, et al. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 8.Zitt C, Obukhov A G, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann T, Schaefer M, Schultz G, Gudermann T. Biochem J. 2000;351:115–122. doi: 10.1042/0264-6021:3510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu M X. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trost C, Bergs C, Himmerkus N, Flockerzi V. Biochem J. 2001;355:663–670. doi: 10.1042/bj3550663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulay G, Brown D M, Qin N, Jiang M, Dietrich A, Zhu M X, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin N, Olcese R, Bransby M, Lin T, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:2435–2438. doi: 10.1073/pnas.96.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Sanger F, Nicklen S, Coulson A B. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vannier B, Zhu X, Brown D, Birnbaumer L. J Biol Chem. 1998;273:8675–8679. doi: 10.1074/jbc.273.15.8675. [DOI] [PubMed] [Google Scholar]
- 18.Liman E R, Corey D P, Dulac C. Proc Natl Acad Sci USA. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer M, Plant T D, Stresow N, Albrecht N, Schultz G. J Biol Chem. 2002;277:3752–3759. doi: 10.1074/jbc.M109850200. [DOI] [PubMed] [Google Scholar]
- 20.Stowers L, Holy T E, Meister M, Dulac C, Koentges G. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 21.Leypold B G, Yu C R, Leinders-Zufall T, Kim M M, Zufall F, Axel R. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]