Abstract
The degree to which memory is enhanced by estrogen replacement in postmenopausal women may depend on environmental factors such as education. The present study utilized an animal model of environmental enrichment to determine whether environmental factors influence the mnemonic and neural response to estrogen. Female mice were raised in standard (SC) or enriched (EC) conditions from weaning until adulthood (7 months). All mice were ovariectomized at 10 weeks, and tested in object recognition and water-escape motivated radial arm maze (WRAM) tasks at 6 months. Each day at the completion of training, mice received injections of 0.1 mg/kg cyclodextrin-encapsulated 17-β-estradiol (E2), 0.2 mg/kg E2, or cyclodextrin vehicle (VEH). At the completion of behavioral testing, hippocampal levels of the presynaptic protein synaptophysin and of brain-derived neurotrophic factor (BDNF) were measured. Enrichment effects were evident in VEH-treated mice; relative to SC-VEH females, EC-VEH females committed fewer working memory errors in the WRAM and exhibited increased hippocampal synaptophysin levels. Estrogen effects depended on environmental conditions. E2 (0.2 mg/kg) improved object memory only in SC females. The same dose improved working memory in SC females, but somewhat impaired working memory in EC females. Furthermore, both doses reduced hippocampal synaptophysin levels in EC, but not SC, females. In contrast, E2 reduced hippocampal BDNF levels in SC, but not EC, females.
This study is the first to compare the effects of estrogen on memory and hippocampal function in enriched and non-enriched female mice. The results suggest that: (1) estrogen benefits object and working memory more in mice raised in non-enriched environments than in those raised in enriched environments, and (2) the changes induced by estrogen and/or enrichment may be associated with alterations in hippocampal synaptic plasticity.
Keywords: estradiol, environment, memory consolidation, hippocampus, synaptophysin, BDNF
Recent data indicate that conjugated equine estrogen in combination with medroxyprogesterone acetate provides no protection from mild cognitive impairment in postmenopausal women (Rapp et al., 2003; Shumaker et al., 2003). In contrast, estrogen alone can improve several types of memory (Duff and Hampson, 2000; Duka et al., 2000;Resnick et al., 1998; Verghese et al., 2000), although these benefits are also not universally reported (Carlson and Sherwin, 1998; Hogervorst et al., 2000). These inconsistencies may be attributed to differences in subject pools including the source of menopause (natural vs. surgical), duration of estrogen replacement prior to testing, and the delay between menopause onset and start of estrogen treatment. Furthermore, variables such as level of education, age, health, or socioeconomic status of the subjects, which may influence the extent to which cognitive improvements are observed, have not been systematically manipulated. Indeed, recent work in humans suggests that level of education can significantly influence the cognitive response to estrogen replacement (Matthews et al., 1999). Because the implications of this finding are difficult to address in humans, animal models may be useful for examining the interactions between estrogen and environmental factors such as cognitive enrichment.
Estrogen reportedly enhances memory in young and aging female rats (e.g. Frick et al., 2002; Gibbs, 2000;Luine and Rodriguez, 1994; Vaucher et al., 2002; although see Chesler and Juraska, 2000; Fugger et al., 1998;Holmes et al., 2002). However, because studies such as these administer estrogen prior to training, they cannot distinguish between the effects of estrogen on mnemonic processes versus the effects on other performance factors present during training. In contrast, post-training estrogen administration can pinpoint the effects on memory consolidation in the absence of confounding effects on non-mnemonic factors such as motivation, attention, and motor ability. Post-training systemic administration of estradiol immediately after training improves memory consolidation in young female rats tested in spatial water maze (Packard and Teather, 1997b) and non-spatial object recognition (Luine et al., 2003) tasks, as does post-training intrahippocampal estradiol administration in young female rats tested in the spatial water maze (Packard and Teather, 1997a). Because these memory improvements are not observed when estrogen is administered 2 h after training, the possibility that the mnemonic benefits of immediate post-training treatment are due to performance factors can be eliminated (Luine et al., 2003; Packard and Teather, 1997a,b).
One way in which estrogen may improve mnemonic function is by enhancing synaptic plasticity in the hippocampus, a brain region critical for learning and memory. For example, increases in synaptophysin, a presynaptic protein located in the membranes of neurotransmitter-containing vesicles (Calhoun et al., 1996; Schlaf et al., 1996) have been noted following estrogen treatment. Estradiol increases synaptophysin expression in cultured hippocampal neurons and elevates synaptophysin immunoreactivity in the cornu ammonis (CA) 1 region of the rat hippocampus (Brake et al., 2001; Murphy and Segal, 1996;Pozzo-Miller et al., 1999b). Furthermore, estrogen increases levels of synaptophysin in aged female mice, a finding which has been associated with enhanced hippocampal-dependent learning (Frick et al., 2002).
Estrogen also modulates hippocampal levels of the neurotrophin BDNF (brain-derived neurotrophic factor). BDNF has been shown to increase synaptic plasticity in numerous ways, including enhancing synaptogenesis (McAllister et al., 1999), promoting basal synaptic transmission in excitatory synapses of CA1 (Kang and Schuman, 1995; although see Gottschalk et al., 1998), and inducing LTP (see Lu and Chow, 1999; Thoenen, 1995 for reviews). Estrogen replacement in ovariectomized female rodents has been shown to increase, decrease, or not affect hippocampal BDNF mRNA expression (Berchtold et al., 2001; Cavus and Duman, 2003; Gibbs, 1999; Liu et al., 2001; Singh et al., 1995). Cavus and Duman (2003) have recently shown that the duration of estrogen depletion prior to replacement or of estrogen replacement following ovariectomy affect whether or not changes in BDNF expression are observed in vivo. These factors may contribute to current discrepancies in the literature. Nevertheless, in vitro preparations have proven useful in identifying a mechanism through which estrogen-induced changes in BDNF promote synaptic plasticity. Specifically, Murphy et al. (1998a,b) have shown in hippocampal cultures that estrogen-induced decreases in BDNF reduce the inhibition of hippocampal pyramidal neurons, which leads to increased hippocampal CA1 spine density. Studies in cycling rats further support this idea, as decreases in BDNF mRNA expression have been reported when estrogen levels and CA1 spine density peak (Cavus and Duman, 2003;Gibbs, 1998; Woolley et al., 1990; Woolley and McEwen, 1992). Taken together, enhanced hippocampal synaptic plasticity, as indicated by decreased BDNF or increased synaptophysin expression, may contribute to estrogen-induced mnemonic improvements.
Environmental enrichment, like estrogen, may also improve memory by altering synaptophysin and BDNF levels. Enrichment provides animals with a complex combination of social and cognitive stimulation through interactions with cage-mates and toys (Rosenzweig et al., 1978). Enrichment-induced improvements in both spatial and non-spatial (e.g. object recognition) memory tests (Daniel et al., 1999; Nilsson et al., 1999; Rampon et al., 2000; Teather et al., 2002; Williams et al., 2001) have been consistently noted throughout the lifespan, in young (Kempermann et al., 1997), middle-aged (Frick et al., 2003; Kempermann et al., 1998), and aged animals (Frick and Fernandez, 2003;Kempermann et al., 1998). Environmental enrichment has been shown to attenuate age-related decreases in hippocampal synaptophysin expression in rats (Saito et al., 1994). Furthermore, a recent study in aging female mice found that enrichment-induced increases in hippocampal synaptophysin levels were associated with enhanced spatial memory (Frick and Fernandez, 2003). Additionally, exposure to enrichment increases hippocampal BDNF protein in rats (Ickes et al., 2000). Interestingly, one aspect of environmental enrichment, voluntary exercise, has also consistently increased hippocampal BDNF protein and mRNA levels in rats (Berchtold et al., 2001; Neeper et al., 1996; van Praag et al., 2000; Widenfalk et al., 1999).
The impact of environmental enrichment on the cognitive and neural response to estrogen is not well understood. The influence of environmental factors has been particularly difficult to assess in women, because most patients taking hormone replacement are highly educated (Keating et al., 1999) and are healthier (Matthews et al., 1996) than women who do not elect treatment. However, one recent study found that estrogen improved memory more effectively in women with less education than in those with more education (Matthews et al., 1999). Because no study to date has examined estrogen-enrichment interactions in animal models, it is unclear whether estrogen and enrichment affect cognitive and neural function in an additive or contradictory fashion. Thus, the present study was designed to determine whether environmental enrichment in mice from weaning through adulthood influences the mnemonic response to estrogen (measured in object recognition and radial arm maze tasks) and to identify whether changes in hippocampal plasticity (assessed by changes in synaptophysin and BDNF levels) contribute to this effect.
EXPERIMENTAL PROCEDURES
Subjects
Female (N=60) C57BL/6J mice were obtained at 3 weeks of age from Taconic (Germantown, NY). Upon arrival, all mice were handled for 5 days (5 min/day) in order to acclimate them to being picked up by the experimenter. Five mice/cage were housed in a room with a 12-h light/dark cycle (lights on at 07:00 h). All behavioral testing occurred during the light phase of the cycle. Food and water were available ad libitum for the duration of testing. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of Yale University. All efforts were made to minimize the number of animals used and their suffering.
Design
Mice were raised in standard (SC) or enriched (EC) conditions for the duration of the study (7 months). A water-soluble form of estrogen was administered each day following the completion of object recognition and water-escape motivated radial arm maze (WRAM) training. Because this form of estrogen is rapidly metabolized, it is only in the circulation during memory consolidation and not during retrieval 24 or 48 h later (Packard and Teather, 1997b). Post-training estrogen has previously been shown to facilitate spatial memory consolidation in the Morris water maze (Packard et al., 1996; Packard and Teather, 1997b) and non-spatial object recognition (Luine et al., 2003).
Rearing environment and enrichment
Upon arrival, mice were assigned to one of two rearing environments. Mice in the SC were group housed in their home cage but were not exposed to enriching stimuli at any time. Mice in the EC were group housed and exposed to both cognitive and physical stimulation in enrichment chambers (56.6×41.5×22 cm high) separate from the home cage through interactions with cage-mates, toys (e.g. Legos, PVC pipe fittings), and running wheels for 3 h/day. All EC mice from one home cage were placed together in an enrichment chamber. Each chamber always contained three or four different toys, a running wheel, food pellets, and a weigh boat filled with water. To maintain interest in the toys for the duration of the study, toys and wheels were changed daily. At the completion of the enrichment session, the mice were returned to their home cage. Daily enrichment began the day after arrival and continued for the duration of the study. A detailed description of the enrichment chambers and procedures is provided elsewhere (Frick and Fernandez, 2003).
Ovariectomy (OVX)
All mice underwent OVX between 9 and 11 weeks of age, as previously described (Frick and Berger-Sweeney, 2001), to ensure that the mice reached sexual maturity prior to removal of endogenous sources of estrogen. Furthermore, surgery at this time ensured that all mice would be without estrogen for several months prior to estrogen replacement. Mice were anesthetized with 2% isoflurane gas in 100% oxygen. Two incisions were made on the dorsal surface of the body, just above the upper left and right pelvic bones. The ovary, oviduct, and tip of the uterine horn were isolated, clamped off and removed. The rest of the uterine horn was returned to the abdominal cavity. Mice were housed singly for 1 week, during which time pediatric Tylenol (approximately 1 ml/100 ml of water) was added to each water bottle. During recovery, a small toy which changed daily was placed in the home cages of EC mice. After recovery, mice were re-housed with their original cage-mates in groups of five/cage. Daily enrichment sessions resumed for EC mice after re-housing.
Estrogen administration
SC and EC mice were injected intraperitoneally with either estrogen or vehicle. Injections were administered each day at the completion of training (see below). Cyclodextrin-encapsulated 17β-estradiol (E2; Sigma, St. Louis, MO, USA), in a dose of either 0.1 mg/kg or 0.2 mg/kg, was dissolved in physiological saline in a volume of 4 ml/kg. The cyclodextrin complex acts as a solubility-enhancing carrier for the estradiol and this type of estrogen is metabolized within 24 h (Pitha and Pitha, 1985;Taylor et al., 1989). Cyclodextrin-encapsulated estrogen has previously improved memory in a post-training Morris water maze paradigm (Packard and Teather, 1997b) when injected immediately, but not 2 h, after training. The vehicle (VEH), hydroxypropyl-β-cyclodextrin, was dissolved in an equal volume of saline and contained the same amount of cyclodextrin present in the 0.2 mg/kg dose of cyclodextrin-encapsulated E2. Because techniques for assaying serum estradiol levels in mice are currently unreliable, we could not accurately measure circulating levels in our mice. Nevertheless, it has previously been reported that 1 μg and 10 μg of β-estradiol produce levels in the mouse similar to those seen during estrus and proestrus, respectively (Akinci and Johnston, 1997). Based on their weights (ranging from 25 to 45 g), mice in the present study were injected with E2 in volumes ranging from 0.10 to 0.18 ml, corresponding to a concentration of E2 ranging from 2.5 μg (for the low dose) to 9 μg (for the high dose). Thus, the doses used in the present study were within the physiological range.
Object recognition
Object recognition testing began 12 weeks after surgery. The object recognition task was conducted in a wooden open field box (58×58×46 cm high) painted white and located in a quiet room under dim lighting. A video camera was mounted on the ceiling above the box and connected to a video recorder, monitor, and computer in an adjoining room. Throughout testing, the door to the testing room was closed and mice were observed on the monitor.
The experimental protocol was based on that reported previously (Clark et al., 2000; Ennaceur and Delacour, 1988) and was similar to a previously published description by our laboratory (Frick and Gresack, 2003). The task takes advantage of the natural affinity of mice for novelty. In brief, the task consisted of three phases, habituation, sample and choice, which were completed on separate test days. During habituation, each mouse was placed in the empty open field box and allowed to explore for 5 min. Locomotor activity was assessed during habituation by recording the number of crossings of a 5×3 grid laid over the field on the computer monitor. The next day, all mice completed the sample phase. Following a 1-min rehabituation to the box, two identical objects were placed in the northwest and northeast corners of the box (approximately 5 cm from the walls) and the mice explored the objects until they accumulated 30 s of exploration. Each mouse was then removed from the box, immediately injected with VEH, 0.1 mg/kg E2, or 0.2 mg/kg E2, and returned to its home cage. Mice were tested in the choice phase 48 h after injection. During this phase, one familiar object (identical to that which was used in the sample phase) and one novel object were placed in the same corners of the box occupied during the sample phase. The location of the novel object was counterbalanced across mice in each group. Mice remained in the box until they accumulated 30 s of object exploration. Objects used during this task differed significantly from objects used during enrichment in terms of shape, size, material (e.g. metal vs. plastic). Furthermore, unlike enrichment objects, those used during the recognition task could neither be stood upon, nor crawled through. During all phases of testing, the box was always cleaned with 70% ethanol in between each mouse. Time spent exploring each object was recorded during both the sample and choice phases using the video tracking system and a custom-written computer program. Object exploration was scored only when the mouse’s nose or front paws touched the object. In addition, the time needed to accumulate 30 s of exploration (i.e. elapsed time) during both the sample and choice phases was recorded.
WRAM
The protocol for this task was adapted from that described by Bimonte et al. (2000) and has been previously described by our laboratory (Gresack and Frick, 2003). Briefly, the center of the maze (diameter, 44 cm) was opaque plastic and the eight arms radiating from the center (38×12 cm) were constructed of clear Plexiglas. The maze was placed in a large pool of water (24 ± 2 °C) made opaque with white non-toxic tempera paint. Four of the eight arms in the WRAM contained hidden platforms submerged approximately 1 cm below the water surface at the end of the arms. The sequence of arms with platforms was randomized between mice but remained unchanged within a mouse for all sessions.
Prior to the first day of testing, each mouse completed a four-trial shaping procedure as previously described (Gresack and Frick, 2003). During testing, all mice completed four trials/day for 15 days. At the start of trial 1, the mouse was released from a designated start arm and given 2 min to locate and climb onto a submerged platform. If the mouse did not find a platform within this time, it was gently guided to the nearest one and allowed to remain there for 15 s, after which time it was removed and placed in a holding cage for a 30 s inter-trial interval (ITI). During the ITI, the platform that had been found was removed from the maze, leaving three platforms in the pool. The mouse was then returned to the start arm for trial 2. This procedure was repeated until all four platforms were located (one platform/trial). At the end of the fourth trial, the mouse was removed from the maze, dried off, injected with VEH, 0.1 mg/kg E2, or 0.2 mg/kg E2, and returned to its home cage. The next test session began approximately 24 h following injection. Despite the fact that mice were injected with E2 every day for 15 consecutive days, the encapsulated estradiol used in the present study was metabolized within hours (Pitha and Pitha, 1985) and thus estrogen effects on this task may be due to modulation of memory consolidation rather than to effects on non-mnemonic performance factors.
Three types of errors were recorded during each trial of the 15 test sessions (Bimonte et al., 2000; Gresack and Frick, 2003). Entries into arms from which a platform had been removed during a daily session were considered working memory errors. First entries in a session into arms that never contained a platform were considered initial reference memory errors. Finally, repeated entries into arms that never contained a platform were considered repeated reference memory errors. In addition to determining the total number of working memory errors committed in each session, the number of working memory errors committed in trials 2-4 of each session was determined (it was not possible to make a working memory error in trial 1). This allowed working memory errors to be assessed as the trials progressed and as the amount of working memory information to be remembered (i.e. the working memory load) increased (Hyde et al., 1998).
Enzyme-linked immunosorbent assays (ELISA)
Immediately following the completion of Session 15 in the WRAM, mice were injected with VEH or estradiol. Approximately 24 h later, mice were sedated briefly with CO2 and decapitated. The hippocampus was dissected bilaterally on ice, weighed and stored at -70 °C until homogenization. The protein content of the samples was measured using a Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA) protein assay (Bradford, 1976; Frick and Berger-Sweeney, 2001).
Synaptophysin was measured using an ELISA adapted from Schlaf et al. (1996) for use in mice (Frick et al., 2003). Because purified synaptophysin was not available for use as a standard, synaptophysin levels in the samples were expressed as ‘equivalents’ relative to synaptophysin immunoreactivity from whole mouse brain homogenate [termed mouse brain standard (MBS)]. An antibody sandwich ELISA using two different anti-synaptophysin antibodies (monoclonal anti-synaptophysin Clone SY 38 and polyclonal rabbit anti-synaptophysin; DAKO Corporation, Carpinteria, CA, USA) was used to determine the relative amounts of synaptophysin in the samples. A Labsytems Multiskan Plus microplate reader set at a wavelength of 405 nm was used to measure optical density.
To calculate the relative amount of synaptophysin in the samples, the absorbance of each of four MBS concentrations was plotted versus the log of the total protein concentration. The equation of the straight line that resulted and the absorbance of each sample were used to determine the concentration of MBS which would have the absorbance exhibited by the sample. This apparent MBS concentration of the sample was divided by the total protein concentration of the sample to determine the relative amount of synaptophysin in the sample versus the amount of synaptophysin in the MBS homogenate (termed ‘MBS synaptophysin equivalent’). A detailed description of the procedure used to measure synaptophysin in mice has been reported previously (Frick and Fernandez, 2003; Frick et al., 2003).
BDNF levels were analyzed using a Promega Emax Immuno-Assay System ELISA kit (Promega, Madison, WI, USA). Prior to running the assay, samples were resuspended 1:10 w/v in Tris/Triton X-100, homogenized and centrifuged for 10 min at 10,000×g, diluted 1:20 w/v in a lysis buffer (0.137 mM NaCl, 20 mM Tris-HCl, 1% Tergitol NP-40, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 mM sodium meta-vanadate), and re-centrifuged at 10,000×g. On the day of assay, 96 well Nunc-Immuno Plates with Maxisorp surface were coated with a carbonate coating buffer containing an anti-BDNF monoclonal antibody and incubated overnight at 4 °C. The next day, the plates were washed with TBST wash buffer, blocked with block and sample buffer, and incubated at room temperature for 1 h. The samples were diluted 1:50 in DPBS (2.7 mM KCl, 0.137 mM NaCl, 1.47 nM KH2PO4, 8.1 mM Na2HPO4, 0.5 mM MgCl2, 0.9 mM CaCl2, pH 7.4), acid-treated and neutralized, and diluted 1:200 in block and sample buffer. Standards (0-500 pg/ml) and samples were run in triplicate using anti-human BDNF polyclonal antibody and anti-IgY HRP conjugate. The plates were then incubated with TMB One Solution, the reaction terminated with 1 N HCl, and optical density was measured at 450 nm on the microplate reader. The concentration of BDNF was divided by the total protein concentration of each sample to yield a value of ng BDNF/mg total protein.
Data analysis
A two (Environment)×three (Treatment) analysis of variance (ANOVA) was used to analyze grid crossings completed during the habituation phase of object recognition. A preference for the novel object during the choice phase was assessed using one-sample t-tests to determine whether time spent with the novel object differed significantly from the chance value of 15 s (Baker and Kim, 2002; Frick and Gresack, 2003). This type of t-test was used because time spent with the novel object is not independent from time spent with the familiar object, as the total time exploring must equal 30 s. For the same reason, one-sample t-tests were used to determine whether time spent with the northwest object during the sample phase differed significantly from 15 s.
Working memory errors, initial reference memory errors, and repeated reference memory errors committed in the WRAM were analyzed separately using repeated-measures ANOVA with two between-subject (Environment and Treatment) and one within-subject (Sessions) factors. Planned contrasts were performed to analyze treatment effects within an environmental condition. The effect of increasing working memory load on errors was first analyzed using repeated-measures ANOVA with two between-subject (Environment and Treatment) and one within-subject (Trials) factors. Next, working memory errors within each trial were analyzed using ANOVA with two between-subject (Environment and Treatment) factors. This was followed by planned contrasts to identify treatment effects within an environmental condition.
Data from session 1 of the WRAM were excluded from all analyses, as described previously, because performance in this session does not accurately measure memory. In addition, by conducting separate ANOVAs on the first (sessions 2-8) and second (sessions 9-15) halves of testing, previous studies have demonstrated that the greatest amount of learning in the WRAM task occurs during acquisition (i.e. sessions 2-8; Bimonte et al., 2000; Gresack and Frick, 2003; Hyde et al., 2000). In order to more closely examine effects in the present study during task acquisition, we also conducted separate ANOVAs on the first (sessions 2-8) and second (sessions 9-15) halves of testing.
Hippocampal synaptophysin and BNDF data were analyzed separately using ANOVA with two between-subject (Environment and Treatment) factors. Tukey-Kramer post hocs were performed to delineate between-group differences.
RESULTS
Subjects
In general, all mice remained in good health for the duration of the study. However, one non-EC mouse died within days of arrival of unknown causes. In addition, four mice (one EC and three non-EC) were killed prior to behavioral testing because of postoperative complications. One additional mouse (non-EC) was excluded from all data analyses because, in addition to weighing significantly less than her cage-mates, she exhibited clear signs of hyperactivity during several behavioral tasks. Thus, the final sample sizes for each group were: SC-VEH (n=8), SC-0.1 E2 (n=9), SC-0.2 E2 (n=8), EC-VEH (n=9), EC-0.1 E2 (n=10), EC-0.2 E2 (n=10).
Object recognition
Grid crossings. No effects of enrichment or estrogen treatment were noted on general locomotor activity measured during the habituation phase of the object recognition task (Table 1). Neither Environment (F(1,48)=0.83, P=0.35) nor Treatment (F(2,48)=0.93, P=0.40) significantly affected the number of grid crossings completed during the 5 min habituation period, nor did these factors interact (F(2,48)=1.85, P=0.15).
Table 1.
Mean ± S.E.M. activity levels during object recognition testing
| Group | Grid crossings |
Elapsed time(seconds) |
|
|---|---|---|---|
| Habituation | Sample | Choice | |
| SC-VEH | 131.0±11.7 | 184.8±35.8 | 205.0±38.3 |
| SC-0.1 E2 | 128.4±12.8 | 193.9±33.1 | 192.5±24.1 |
| SC-0.2 E2 | 105.4±7.4 | 203.4±40.6 | 174.8±21.8 |
| EC-VEH | 98.4 ±11.8 | 228.5±36.6 | 281.9±50.9 |
| EC-0.1 E2 | 123.5±14.6 | 280.3±37.7 | 234.0±20.6 |
| EC-0.2 E2 | 117.2±7.3 | 192.8±24.1 | 186.8±20.9 |
Sample phase. Only mice raised in SC and treated with the high dose of estrogen showed a preference for one of the identical objects during the sample phase (t(7)=2.67, P<0.05), although this preference was very modest (17.8 ± 2.9 s for the northwest object relative to chance). No other group showed a preference for either object (time with northwest object for each group: 13.2±1.6-16.7±1.3 s). Time to accumulate 30 s during the sample phase was unaffected by Environment (F(1,48)=1.96, P=0.16) and Treatment (F(2,48)=0.72, P=0.49), and these factors did not interact (F(2,48)=1.00, P=0.37), indicating that neither enrichment nor estrogen affected activity level in the sample phase (Table 1).
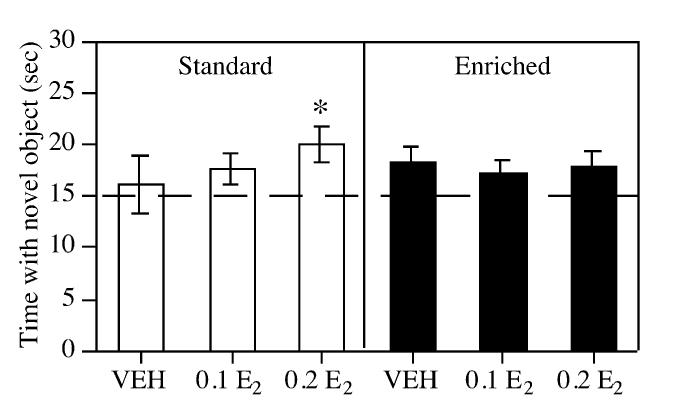
Choice phase. Only mice raised in SC and treated with the high dose of estrogen showed a significant preference for the novel object during the choice phase (Fig. 1). Specifically, neither the SC-VEH (t(7)=0.40, P=0.70) nor the SC-0.1 E2 group (t(8)=1.71, P=0.12) spent significantly more time exploring the novel object relative to chance. In contrast, the SC-0.2 E2 group showed a significant preference for the novel object (t(7)=2.95, P<0.03). Among EC mice, the EC-VEH group tended to prefer the novel object (t(8)=2.16, P=0.06). Treatment with either dose of estrogen did not, however, increase the magnitude of this preference (ts(9)=1.74-2.02, Ps>0.05). Neither enrichment nor estrogen affected activity level during the choice phase as indicated by the nonsignificant main effects of Environment (F(1,48)=2.93, P=0.09) and Treatment (F(2,48)=1.98, P=0.14) and the nonsignificant Environment×Treatment interaction (F(2,48)=0.53, P= 0.59) for time to accumulate 30 s (Table 1).
Fig. 1.
Time spent with the novel object during the choice phase (48 h following estrogen injection). Each bar represents the mean (±S.E.M.) of each group. The dashed line represents chance performance (15 s). SC-VEH females did not show a preference for the novel object. Among the SC group, estrogen dose-dependently improved performance, such that SC-0.2 E2 females spent significantly more time with the novel object relative to chance (* P<0.03). Among the EC females, there was a trend for a preference for the novel object relative to chance in the VEH group. However, estrogen did not increase this preference.
WRAM
Working memory errors. Overall, mice raised in EC conditions committed fewer errors than mice raised in SC. The number of working memory errors made by all groups significantly decreased across sessions 2-15 (F(13,624)=4.36, P<0.0001), as well as in the first (sessions 2-8) and second (sessions 9-15) halves of testing considered separately (Fs(6,288)>2.34, Ps<0.04). There was a significant main effect of Environment (F(1,48)=6.37, P<0.02) across sessions 2-15, with EC mice committing fewer working memory errors than standard mice. This effect was driven mainly by the non-estrogen treated groups, as the EC-VEH group made significantly fewer working memory errors than the SC-VEH group across all sessions (F(1,15)=5.31, P<0.04; Fig. 2A). Interestingly, this effect was most robust during the first, but not the second, half of testing (sessions 2-8: F(1,15)=16.85, P<0.001; sessions 9-15: F(1,15)=0.07, P=0.79; Fig. 2B, C).
Fig. 2.
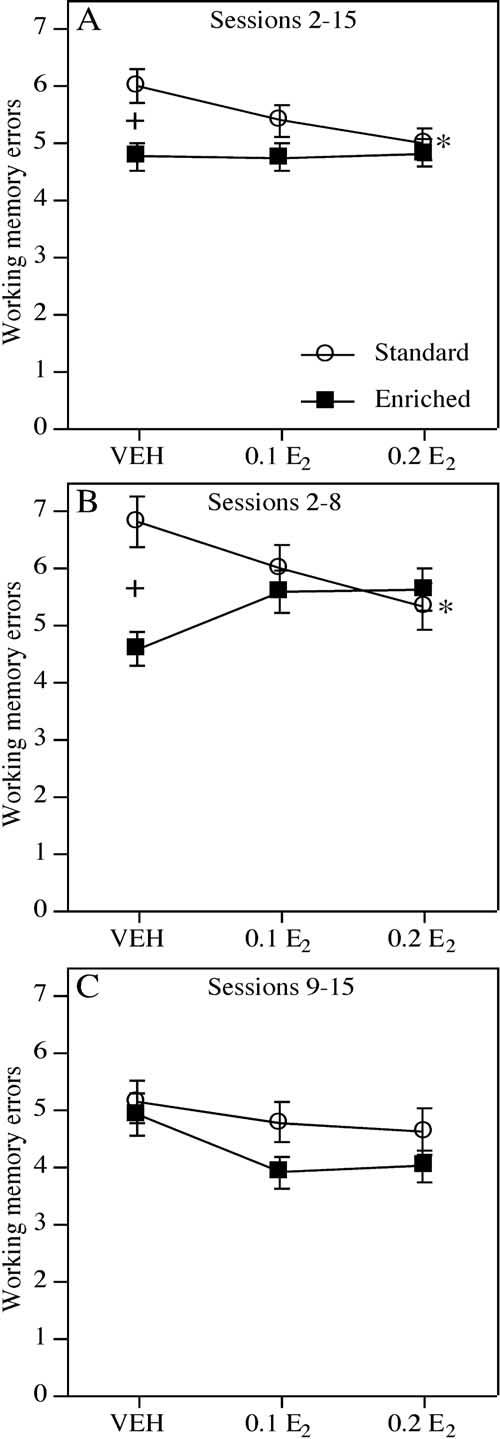
The mean (±S.E.M.) number of working memory errors committed by SC and EC mice during all sessions. (A) During sessions 2-15, EC-VEH females made significantly fewer errors than SC-VEH females (+ P<0.04). Among SC females, those treated with 0.2 E2, but not 0.1 E2, committed fewer errors relative to VEH (* P<0.05). Among EC females, neither the 0.1 E2 nor the 0.2 E2 group differed from VEH. (B) During sessions 2-8, enrichment reduced working memory errors in VEH-treated females (+ P<0.001, EC-VEH relative to SC-VEH). In addition, 0.2 E2 significantly reduced working memory errors in SC mice (* P<0.03, SC-0.2 E2 relative to SC-VEH), but tended to increase the number of errors in EC mice. (C) No environment or treatment effects were observed during sessions 9-15.
The highest dose of estrogen improved memory in mice raised in SC, but impaired working memory in mice raised in EC conditions, particularly during the first half of testing. During sessions 2-15, the main effect of Treatment (F(2,48)=1.02, P=0.36) and Environment×Treatment interaction (F(2,48)=1.31, P=0.28) were not significant. However, contrasts revealed that the SC-0.2 E2, but not the SC-0.1 E2 group, committed significantly fewer errors across all sessions than SC-VEH females (SC-0.2 E2: t(14)=2.17, P<0.05; SC-0.1 E2: t(15)=1.05, P=0.31). Estrogen-treated EC groups did not significantly differ from EC-VEH (ts(17)=0.03-0.15, P>0.05;Fig. 2A). The environment-dependent effect of estrogen was particularly evident in the first half of testing as indicated by the significant Environment×Treatment interaction in sessions 2-8 (F(2,48)=4.82, P<0.02; Fig. 2B), but not in sessions 9-15 (F(2,48)=0.27, P=0.76;Fig. 2C). Contrasts for sessions 2-8 indicated that 0.2 mg/kg E2 significantly decreased working memory errors among SC females (SC-VEH vs. SC-0.2 E2: t(14)=2.58, P<0.03), whereas this dose tended to increase working memory errors among EC females (EC-VEH vs. EC-0.2 E2: t(17)=2.01, P=0.06). Mice receiving 0.1 mg/kg E2 did not differ significantly from their respective VEH groups (SC: t(15)=1.39, P=0.18; EC: t(17)=1.65, P=0.11). Contrasts for sessions 9-15 indicated that no dose of E2 decreased working memory errors among SC females (SC-VEH vs. SC-0.1 E2: t(15)=0.45, P=0.66; SC-VEH vs. SC-0.2 E2: t(14)=0.72, P=0.48) or EC females (ts(17)=1.47-1.74, P>0.10).
Working memory load. Mice raised in EC conditions made fewer errors than mice raised in SC at medium and high working memory loads. As working memory load increased during trials 2-4, the number of working memory errors increased in all phases of testing (Fs(2,96)=190.26-382.65, Ps<0.0001 for sessions 2-15, 2-8, and 9-15). Enrichment significantly reduced errors made throughout testing (sessions 2-15: F(1,48)=6.33, P<0.02), but this effect was particularly evident during the first half of testing (sessions 2-8: F(1,48)=5.40, P<0.03; sessions 9-15: F(1,48)=2.37, P=0.13). Specifically, EC-VEH females made fewer errors than SC-VEH females in trial 3 of sessions 2-15 (F(1,15)=11.33, P<0.004; Fig. 3B), whereas EC-VEH made fewer errors than SC-VEH females in both trial 3 (F(1,15)=14.77, P<0.002) and trial 4 of sessions 2-8 (F(1,15)=8.67, P<0.01). No effect of enrichment was observed in VEH-treated females in any trial during sessions 9-15 (F(1,15)=0.072, P=0.79).
Fig. 3.
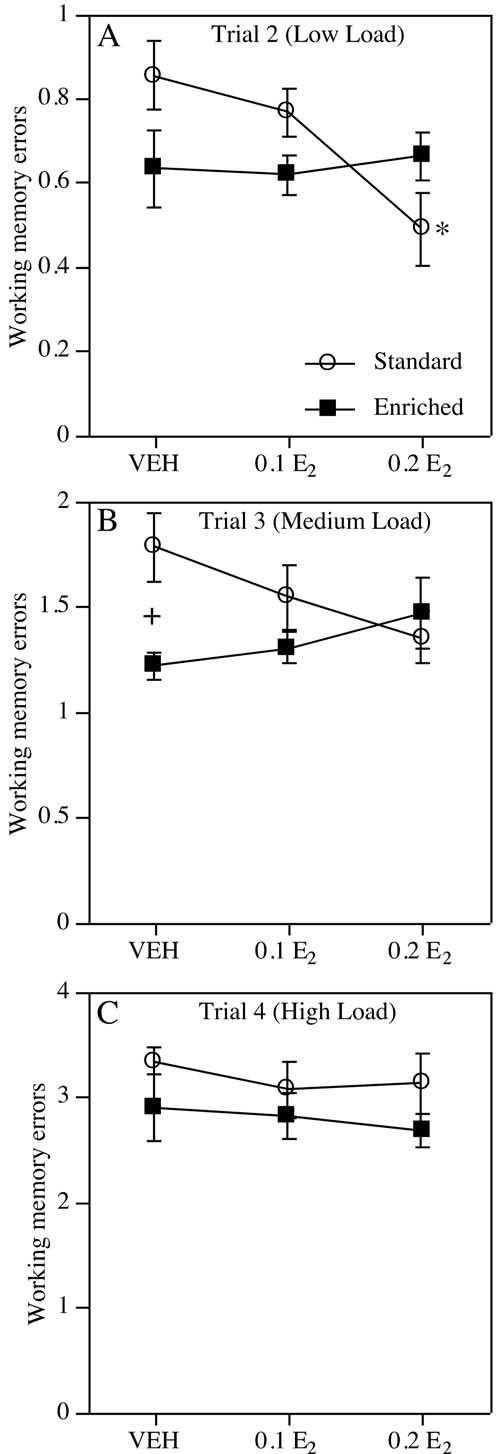
Working memory errors (mean ± S.E.M.) committed by all groups within each trial across all sessions. Note that the number of working memory errors increased across trials 2-4 with increased working memory load. (A) During trial 2, SC, but not EC, mice treated with 0.2 E2 committed significantly fewer working memory errors than those treated with VEH or 0.1 E2 (* P<0.02, SC-0.2 E2 relative to SC-VEH and SC-0.1 E2). (B) The Environment×Treatment interaction remained significant during trial 3. EC-VEH mice made fewer errors relative to SC-VEH mice (+ P<0.004). (C) During trial 4, estrogen did not improve performance in either SC or EC mice.
In general, estrogen dose-dependently reduced errors at low loads in SC, but not EC, females. When all trials were analyzed together, neither the Treatment effect (F(2,48)=1.02, P=0.36) nor the Environment×Treatment interaction was significant (F(2,48)=1.31, P=0.28). Although the main effect of Treatment was not significant for any specific trial in any phase of testing (Fs(2,48)=0.31-1.60, Ps>0.05), treatment effects were nearly significant for trial 2 in sessions 2-15 (F(2,48)=2.92, P=0.06) and sessions 2-8 (F(2,48)=2.97, P=0.06). Furthermore, the Environment×Treatment interaction was significant in trials 2 and 3 across all sessions (Fs(2,48)=3.25-4.37, Ps<0.02, Fig. 3A, B) and in the first half analyzed separately (F(2,48)=3.68-4.55, P<0.04). This interaction was not significant for trial 4 of any phase of testing (Fs(2,48)=0.11-1.60, Ps>0.05; Fig. 3C) or for any trial in sessions 9-15 (F(2,48)=0.27, P=0.77). Contrasts were performed to further decompose the significant Environment×Treatment interactions in trials 2 and 3 across all sessions. In trial 2, SC-0.2 E2 females made significantly fewer errors than both SC-VEH (t(14)=-3.06, P<0.01) and SC-0.1 E2 (t(15)=2.77, P<0.02) females (Fig. 3A). In contrast, neither EC-0.1 E2 nor EC-0.2 E2 females differed significantly from EC-VEH females in trial 2 (ts(17)=-0.15-0.28, P>0.05). In trial 3, SC-0.2 E2 females (t(14)=-2.09, P=0.06), but not SC-0.1 E2 females (t(15)=-1.06, P=0.31), tended to commit fewer errors than SC-VEH females. No effect of estrogen was observed among EC females (ts(17)=0.83-1.34, Ps>0.05).
Initial reference memory errors. No effects of enrichment or estrogen were noted for initial reference memory errors. Initial reference memory errors decreased significantly during sessions 2-15 (F(13,624)=7.11, P<0.0001), indicating improved memory for arms not containing platforms. The main effect of Environment approached significance (F(1,48)=3.66, P=0.06); however, neither the main effect of Treatment (F(2,48)=1.40, P=0.25), nor the Environment×Treatment interaction (F(2,48)=0.18, P=0.83) was significant (Table 2). These results did not change when data were analyzed separately for sessions 2-8 and 9-15, except that the decrease in number of initial reference memory errors across sessions 2-8 was no longer significant (F(6, 288)=2.05, P=0.06).
Table 2.
Mean ± S.E.M. initial reference memory (IRM) and repeated reference memory (RRM) errors in sessions 2-15
| Group | IRM error | RRM errora |
|---|---|---|
| SC-VEH | 4.0±0.3 | 1.1±0.2 |
| SC-0.1 E2 | 4.5±0.2 | 1.3±0.2 |
| SC-0.2 E2 | 3.8±0.3 | 1.2±0.2 |
| EC-VEH | 3.6±0.2 | 0.7±0.1 |
| EC-0.1 E2 | 3.7±0.2 | 0.8±0.1 |
| EC-0.2 E2 | 2.8±0.2 | 0.5±0.1 |
Main effect of Environment.
Repeated reference memory errors. Overall, mice raised in EC conditions made fewer repeated reference memory errors than mice raised in SC. No effect of estrogen was noted for this type of error. Repeated reference memory errors significantly decreased during sessions 2-15 (F(13,624)=3.16, P<0.0001), suggesting that mice learned not to re-enter arms that did not contain a platform. In addition, the main effect of Environment was significant (F(1,48)=15.12, P<0.0004), with the EC group committing fewer repeated reference memory errors than the SC group (Table 2). Neither the main effect of Treatment (F(2,48)=0.61, P=0.54) nor the Environment×Treatment interaction (F(2,48)=0.17, P=0.84) was significant across all sessions. These effects did not change when sessions 2-8 and 9-15 were analyzed separately, although the decline in repeated reference memory errors across each block of sessions individually did not reach significance (Fs(6,288)=1.00-1.08, Ps>0.05).
Neurochemical assays
Environmental enrichment increased, and estrogen decreased, synaptophysin levels in the hippocampus, as indicated by significant main effects of Environment (F(1,47)=86.91, P<0.0001) and Treatment (F(2,47)= 6.57, P<0.005). Although the Environment×Treatment interaction was not significant (F(1,47)=2.14, P=0.12), post hoc analyses suggested that estrogen-mediated decreases in hippocampal synaptophysin levels were limited to EC mice. Among EC females, both estrogen-treated groups exhibited lower synaptophysin levels relative to VEH (Ps<0.05), but among SC females, neither E2 group exhibited altered synaptophysin levels relative to their VEH-treated counterparts (Ps>0.05; Fig. 4A).
Fig. 4.
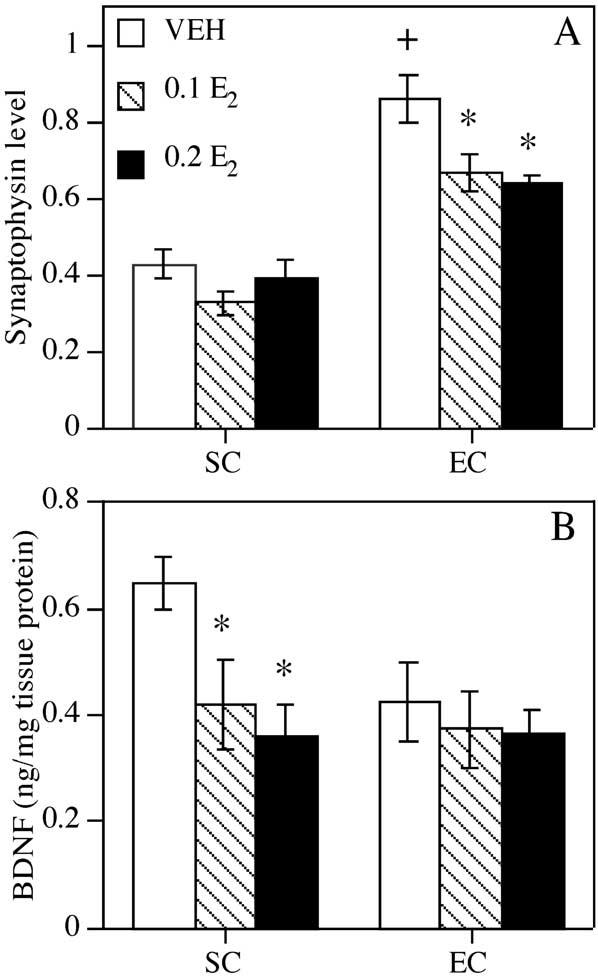
Synaptophysin and BDNF levels in the hippocampus. (A) Synaptophysin values represent mean (±S.E.M.) ‘MBS synaptophysin equivalents’ expressed as sample immunoreactivity relative to that of an equal amount of MBS. Hippocampal synaptophysin levels were increased in EC-VEH mice relative to SC-VEH mice (+ P<0.0001). Among SC females, estrogen did not alter hippocampal synaptophysin levels. However, both doses of estrogen, reduced synaptophysin immunoreactivity in EC females (* Ps<0.05, relative to EC-VEH). (B) BDNF levels in the hippocampus, expressed in ng BDNF/mg tissue protein. Both doses of estrogen reduced hippocampal BDNF levels in SC, but not EC, mice (* Ps<0.05, relative to SC-VEH).
Although environmental enrichment did not alter hippocampal BDNF levels (F(1,41)=2.24, P=0.14), EC-VEH mice tended to have lower BDNF levels than SC-VEH mice (F(1,12)=4.21, P= 0.06). The nearly significant main effect of Treatment (F(2,41)=3.06, P=0.06) suggest a possible effect of estrogen. Although the Environment×Treatment interaction was not significant (F(2,41)=1.27, P=0.29), post hoc analyses indicate that estrogen decreased hippocampal BDNF in SC, but not EC, mice. Both doses of estrogen significantly reduced BDNF levels relative to VEH among SC (Ps<0.05), but not EC (Ps>0.05), females (Fig. 4B).
DISCUSSION
The results of the present study demonstrate that environmental factors present from early development significantly influence the mnemonic and neural response to estrogen. Environmental enrichment alone resulted in superior spatial working memory and increased levels of hippocampal synaptophysin. Estrogen treatment alone dose-dependently improved object memory and spatial working memory, and reduced hippocampal BDNF levels. However, when combined with enrichment, estrogen did not improve object recognition, impaired working memory acquisition, and reduced hippocampal synaptophysin immunoreactivity (Table 3).
Table 3.
Summary of estrogen effects on behavioral and neurochemical variablesa
| Behavioral or neurochemical variable | Environment |
|
|---|---|---|
| Standard | Enriched | |
| Object memory consolidation | — | |
| Working memory consolidation | ||
| Synaptophysin | — | |
| BDNF | — | |
Filled arrows indicate a significant increase or decrease by estrogen relative to same environment vehicle group. Open arrows indicate a moderate decrease by estrogen relative to vehicle. Horizontal lines represent no difference relative to vehicle.
Effects of enrichment alone
The finding that enrichment improved WRAM performance in non-estrogen-treated females is consistent with other reports of enhanced spatial memory following enrichment (Daniel et al., 1999; Kempermann et al., 1997; Nilsson et al., 1999; Williams et al., 2001). Enrichment also moderately improved object recognition, which is consistent with previous findings in adult mice of an improvement following a 24-h retention interval (Rampon et al., 2000). Our use of a 48-h retention interval may explain why the novelty preference exhibited by EC mice was not as robust as previously reported (Rampon et al., 2000). Nonetheless, the present findings suggest that enrichment can improve both spatial working and non-spatial object memory in adult female mice.
The aforementioned improvements in behavior may result from enrichment-mediated increases in hippocampal plasticity. Synaptophysin is a constituent of neurotransmitter-containing presynaptic vesicle membranes (Calhoun et al., 1996; Jahn et al., 1985; Schlaf et al., 1996; Wiedenmann and Franke, 1985), and alterations as assessed by ELISA could reflect numerous changes at the synapse (Frick and Fernandez, 2003) including increased neurotransmitter release (Ehrhart Bornstein et al., 1991). Our finding that enrichment increases hippocampal synaptophysin levels is consistent with our recent report that enrichment in aged female mice increases hippocampal synaptophysin levels (Frick and Fernandez, 2003), and suggests that enrichment can also increase hippocampal synaptic plasticity in younger females.
Although the fact that enrichment tended to decrease BDNF levels may seem surprising, it should be noted that studies reporting increased BDNF expression following environmental enrichment or exercise utilized male or intact female rats (Berchtold et al., 2001; Ickes et al., 2000; Neeper et al., 1996). Importantly, however, estrogen deprivation resulting from OVX reduces hippocampal BDNF mRNA and protein relative to baseline levels (Berchtold et al., 2001; Singh et al., 1995). Furthermore, prolonged estrogen deprivation attenuates environment-mediated increases in BDNF (Berchtold et al., 2001). Thus, the 3-4- month estrogen deprivation in our VEH-treated mice may have diminished the response of BDNF to environmental stimuli. Interestingly, BDNF has previously been shown to promote presynaptic vesicle docking and increase the amount of synaptophysin per neuron (Lu and Chow, 1999; Pozzo-Miller et al., 1999a; Takei et al., 1997). Therefore, it is surprising that our enrichment-induced increases in synaptophysin did not positively correlate with changes in BDNF levels. It is important to note, however, that the synaptophysin ELISA does not allow us to differentiate whether increases in synaptophysin levels were in fact due to increases in number of synaptophysin vesicles per neuron or rather increases in the total number of presynaptic terminals. If the latter, then it is unclear whether the relationship between increased BDNF and increased synaptophysin would still be expected. Finally, it is possible that other neurotrophins implicated in synaptic plasticity, such as neurotophin-3 (Lu and Chow, 1999; McAllister et al., 1999), are responsible for enrichment-mediated increases in hippocampal synaptophysin observed in the present study.
In addition to enrichment, other types of environmental stimulation during development, such as neonatal handling, improve memory function in various behavioral tasks (Fernandez-Teruel et al., 2002). These improvements have been linked to a more adaptive hypothalamic-pituitary-adrenal axis response to stress and a faster return of corticosterone to basal levels following a stressful experience (Fernandez-Teruel et al., 2002; Meaney et al., 1988). Perhaps our EC mice performed better than the SC mice because they, like neonatally handled mice, exhibited an adaptive glucocorticoid response to the stress of behavioral testing. Because corticosterone levels were not measured, this possibility cannot be excluded. Alternatively, if handling in adulthood reduces anxiety, then the enrichment-induced improvements may have resulted from daily transfers from the home cage to enrichment chamber. However, studies in rats have shown that handled and non-handled control rats are similarly impaired relative to EC rats (Greenough et al., 1972). Furthermore, we have recently noted that although handled (transferred daily to and from home cage) and non-handled (never removed from home cage) C57BL/6 male mice perform similarly on object recognition and water maze tasks, both groups perform significantly worse than EC mice (K. M. Frick, unpublished observations). Therefore, we find the explanation that enrichment-induced improvements in the present study were due to increased handling unlikely. Finally, our grid crossing and elapsed time data from object recognition suggest that all mice were similarly aroused during testing.
Effects of estrogen in SC-housed mice
In the present study, estrogen alone significantly improved the performance of non-EC mice in both the object recognition and WRAM tasks. This finding is similar to previous estrogen-induced improvements in these tasks (Bimonte and Denenberg, 1999; Vaucher et al., 2002). However, these studies differ in that the present study administered estrogen post-training rather than prior to training. Administering a rapidly metabolized form of estrogen immediately after training enabled us to examine potential effects of estrogen on memory consolidation, particularly during the object recognition task. Previous post-training studies in rats tested in Morris water maze (Packard and Teather, 1997b) and object recognition (Luine et al., 2003) tasks have shown that when estrogen is injected 2 h post-training, the mnemonic improvements normally seen following immediate post-training injection disappear. Thus, improvements using an immediate post-training injection paradigm cannot be attributed to non-mnemonic (e.g. attentional, motoric) effects of estrogen that may persist even after metabolism. Whereas the effect of estrogen on object memory consolidation is clear, the 15-session nature of the WRAM task makes it difficult to definitively determine if estrogen improved spatial working memory consolidation, per se. Although we think it likely that estrogen improved memory for which arms contained platforms, estrogen could also have improved acquisition of the rules of the task. Nevertheless, the present report is the first to demonstrate that post-training estrogen enhances object memory consolidation and spatial working memory in SC-housed female mice.
Estrogen may have improved performance in SC mice by down-regulating BDNF. This study is the first to report estrogen-induced BDNF reductions in young mice. In young cycling rats, BDNF mRNA is significantly decreased in CA1, CA3, and dentate gyrus when estradiol levels peak (Cavus and Duman, 2003; Gibbs, 1998). BDNF expression in cultured hippocampal neurons is also decreased within 24 h after exposure to estradiol (Murphy et al., 1998b). In the hippocampus, BDNF is thought to promote the function of the GABAergic inter-neurons that inhibit pyramidal dendritic spine growth. In hippocampal cultures, estrogen-induced reduction of GABAergic function leads to increased dendritic spine density (Murphy et al., 1998a). Therefore, estrogen-induced reductions in BDNF, as seen in the present study, may reduce GABAergic neurotransmission and thereby promote spine growth and improve memory. This intriguing possibility will require further study. It should also be noted that although estrogen-induced increases in BDNF expression have been reported, estrogen replacement in these studies typically began shortly (3 weeks or less) after estrogen deprivation (OVX; Berchtold et al., 2001; Gibbs, 1999; Liu et al., 2001; Singh et al., 1995). The only study to begin estrogen replacement after extended deprivation (3 months) reported no change in BDNF expression following replacement (Cavus and Duman, 2003). In the present study, 3 1/2 months of estrogen deprivation prior to the first estrogen injection resulted in significant decreases in BDNF protein. Therefore, the way estrogen modulates BDNF may vary as a function of duration of estrogen deprivation. Systematically determining how estrogen-induced decreases and/or increases in BDNF may improve memory is an important question to be examined in future studies.
Finally, in SC mice, estrogen did not influence synaptophysin levels, suggesting that increases in presynaptic terminals or vesicles may not contribute to estrogen-induced memory enhancements. However, it is noteworthy that studies reporting estrogen-induced increases in synaptophysin immunoreactivity have used either aging mice, who exhibit substantial mnemonic and neural dysfunction (Frick et al., 2003), or have measured synaptophysin in discrete hippocampal subregions (Brake et al., 2001; Kadish and van Groen, 2002). Thus, the lack of an effect of estrogen on synaptophysin in this study may stem from already high levels of synaptophysin in the young adult brain or from changes in specific hippocampal subregions that were obscured by our analysis of the entire hippocampus.
Estrogen-enrichment interactions
Among EC females, estrogen had no effect on object recognition and impaired working memory during WRAM task acquisition. These findings may have been due to a ceiling effect; that is, there may have been little room for further improvement because EC females performed considerably better than SC females. Although this is possible, we do not think this is likely, as other C57BL/6 female mice in our laboratory have exhibited better performance in the object recognition (Frick and Gresack, 2003) and WRAM tasks (Gresack and Frick, 2003) than was observed in the EC-VEH mice. Furthermore, to minimize the likelihood of a ceiling effect in the object recognition task, a long delay (48 h) was used between the sample and choice phases. Thus, an estrogen-induced improvement in EC females should have been possible. In addition, it should be mentioned that whereas the beneficial effects of estrogen replacement among SC mice were observed in both the object recognition and WRAM tasks, the estrogen effects among EC mice were generally task-dependent. (That is, no effect was noted in object recognition whereas an impairing effect was observed in WRAM.) In light of these findings, it could be argued that the extent to which the beneficial effects of estrogen were minimized by enrichment depended on the task and subsequently the type of memory (e.g. non-spatial vs. spatial) being assessed. The combination of enrichment and estrogen appeared most detrimental to spatial working memory assessed during WRAM. Nevertheless, regardless of task, environmental enrichment consistently reduced the mnemonic benefits of estrogen previously noted in SC mice.
One reason why the mnemonic and neural benefits of estrogen and enrichment disappeared when these two factors were combined may be tied to changes in circulating levels of the stress hormone, corticosterone. It is well established that optimal levels of corticosterone can enhance cognitive performance and that deviations above or below this level result in suboptimal cognitive performance (e.g. Larsson et al., 2002; Lupien and McEwen, 1997). Environmental enrichment and estrogen have independently been shown to increase corticosterone levels (Carey et al., 1995; Haemisch et al., 1994; Kempermann et al., 2002), and this may explain why these two factors individually enhanced memory in the present study. However, the combination of estrogen and enrichment may have elevated corticosterone levels beyond optimal levels, impairing memory as a result. Alternatively, estrogen-induced increases in the synthesis of corticosterone binding globulin may have blunted enrichment-mediated increases in basal free corticosterone, resulting in suboptimal elevations in this glucocorticoid and corresponding mnemonic deficits (Kempermann et al., 2002; McCormick et al., 2002). Because serum samples were not collected, corticosterone levels were not measured in the present study and thus the hypothesized effects of combined estrogen and enrichment on corticosterone-mediated changes in memory are speculative.
As indicated above, previous studies have shown that estrogen alone reduces hippocampal BDNF levels (Murphy et al., 1998b), whereas enrichment alone increases BDNF levels (Berchtold et al., 2001; Ickes et al., 2000). These competing effects may explain why no overall change in BDNF levels was observed in the estrogen-treated EC group. Furthermore, in the present study, estrogen had opposite effects on hippocampal plasticity depending on the environment in which mice were raised. In non-EC mice, estrogen reduced BDNF levels (beneficial effect). In EC mice, estrogen reduced synaptophysin levels (adverse effect). These findings illustrate the importance of environmental factors in evaluating the neurological basis of estrogen-induced mnemonic alterations.
CONCLUSIONS
The current study demonstrates for the first time that environmental factors significantly modulate the effects of estrogen on the brain and behavior. Although estrogen treatment improved memory consolidation in non-EC females, it was detrimental to memory and hippocampal plasticity in EC females. If similar relationships exist in older females, then this interaction may have implications for hormone therapy in menopausal women.
Acknowledgments
Acknowledgments—We would like to thank Heather Higgins and Lynne Achenbach for assistance with behavioral testing, Stephanie Fernandez for consulting about the neurochemical assays, and Drs. Mark Baxter, Marcia Johnson and Jeansok Kim for their helpful comments on versions of this manuscript. Funding was provided by an American Federation for Aging Research/Pfizer grant in Hormones and Aging and National Institutes of Health Grant MH065460.
Footnotes
- ANOVA
- analysis of variance
- BDNF
- brain-derived neurotrophic factor
- CA
- cornu ammonis
- E2
- 17-β-estradiol
- EC
- enriched condition
- ELISA
- enzyme-linked immunosorbent assay
- ITI
- inter-trial interval
- MBS
- mouse brain standard
- OVX
- ovariectomy
- SC
- standard condition
- VEH
- vehicle
- WRAM
- water-escape motivated radial arm maze.
REFERENCES
- Akinci M, Johnston G. Sex differences in the effects of gonadectomy and acute swim stress on GABAA receptor binding in mouse forebrain membranes. Neurochem Int. 1997;1:1–10. doi: 10.1016/s0197-0186(96)00143-x. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonists on recognition memory in rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;25:821–828. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB. Steroid hormones, memory and mood in a healthy elderly population. Psychoneuroendocrinology. 1998;23:583–603. doi: 10.1016/s0306-4530(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Cavus I, Duman RS. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm Behav. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Treiman M, Hansen GH, Schousboe A, Thorn NA, Frandsen A. Parallel expression of synaptophysin and evoked neurotransmitter release during development of cultured neurons. Int J Dev Neurosci. 1991;9:463–471. doi: 10.1016/0736-5748(91)90032-h. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats: 1. Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Gimenez-Llort L, Escorihuela RM, Gil L, Aguilar R, Steimer T, Tobena A. Early-life handling stimulation and environmental enrichment: are some of their effects mediated by similar neural mechanisms. Pharmacol Biochem Behav. 2002;73:233–245. doi: 10.1016/s0091-3057(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and synaptic plasticity in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan J-Y, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ER alpha on spatial learning. Horm Behav. 1998;34:163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Madden TC, Fleischmann TB. Effects of isolation, daily handling, and enriched rearing on maze learning. Psychon Sci. 1972;27:279–280. [Google Scholar]
- Gresack JE, Frick KM. Male mice exhibit better spatial working and reference memory than females in a water-escape radial arm maze task. Brain Res. 2003;982:98–107. doi: 10.1016/s0006-8993(03)03000-2. [DOI] [PubMed] [Google Scholar]
- Haemisch A, Voss T, Gartner K. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol Behav. 1994;56:1041–1048. doi: 10.1016/0031-9384(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Sherman GF, Hoplight BJ, Denenberg VH. Working memory deficits in BXSB mice with neocortical ectopias. Physiol Behav. 2000;70:1–5. doi: 10.1016/s0031-9384(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm A-C. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-Dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci USA. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish I, van Groen T. Low levels of estrogen significantly diminish axonal sprouting after entorhinal cortex lesions in the mouse. J Neurosci. 2002;22:4095–4102. doi: 10.1523/JNEUROSCI.22-10-04095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Keating NL, Cleary PD, Rossi AS, Zaslavsky AM, Ayanian JZ. Use of hormone replacement therapy by postmenopausal women in the United States. Ann Intern Med. 1999;130:545–553. doi: 10.7326/0003-4819-130-7-199904060-00002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol Biochem Behav. 2002;73:193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene protein in the forebrain of female prairie voles. J Comp Neurol. 2001;433:499–515. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58:76–87. [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers. Am J Epidemiol. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo D. Neurotrophins and synaptic plasticity. Ann Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–247. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effects of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998a;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci USA. 1998b;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997a;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997b;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Pitha J, Pitha J. Amorphous water-soluble derivatives of cyclodextrins: nontoxic dissolution enhancing excipients. J Pharmacol Sci. 1985;74:987–990. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng Z, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999a;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J Neurophysiol. 1999b;81:1404–1411. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang Y, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effects of estrogen plus progestin on global cognitive function in postmenopausal women: the women’s health initiative memory study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Herbert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Saito S, Kobayashi S, Ohashi Y, Igarashi M, Komiya Y, Ando S. Decreased synaptic density in aged brains and its prevention by rearing under enriched environment as revealed by synaptophysin contents. J Neurosci Res. 1994;39:57–62. doi: 10.1002/jnr.490390108. [DOI] [PubMed] [Google Scholar]
- Schlaf G, Salje C, Poethke R, Felgenhauer K, Mader M. A novel enzyme-linked immunosorbent assay for determination of synaptophysin as compared with other quantification procedures. J Neuroimmunol. 1996;67:59–65. doi: 10.1016/0165-5728(96)00049-5. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the women’s health initiative memory study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Takei N, Sasaoka K, Inoue K, Takahashi M, Endo Y, Hatanaka H. Brain-derived neurotrophic factor increases the stimulation-evoked release of glutamate and the levels of exocytosis-associated proteins in cultured cortical neurons from embryonic rats. J Neurochem. 1997;68:370–375. doi: 10.1046/j.1471-4159.1997.68010370.x. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: behavioral and physiological effects of episode-like pulses in rats. Pharm Res. 1989;6:641–646. doi: 10.1023/a:1015922019038. [DOI] [PubMed] [Google Scholar]
- Teather LA, Magnusson JE, Chow CM, Wurtman RJ. Environmental conditions influence hippocampus-dependent behaviours and brain levels of amyloid precursor protein in rats. Eur J Neurosci. 2002;16:2405–2415. doi: 10.1046/j.1460-9568.2002.02416.x. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–596. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KBJ. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Katz MJ, Sliwinski M, Crystal HA, Buschke H, Lipton RB. Cognitive performance in surgically menopausal women on estrogen. Neurology. 2000;55:872–874. doi: 10.1212/wnl.55.6.872. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats downregulate BDNF and trkB messages in the brain. Neurosci Res. 1999;34:125–132. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, McCoy JG. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol Behav. 2001;73:649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]