Abstract
Light-induced production of superoxide (O2•−) in spinach PSII (photosystem II) membrane particles was studied using EPR spin-trapping spectroscopy. The presence of exogenous PQs (plastoquinones) with a different side-chain length (PQ-n, n isoprenoid units in the side-chain) enhanced O2•− production in the following order: PQ-1>PQ-2≫PQ-9. In PSII membrane particles isolated from the tobacco cyt (cytochrome) b559 mutant which carries a single-point mutation in the β-subunit and also has a decreased amount of the α-subunit, the effect of PQ-1 was less than in the wild-type. The increase in LP (low-potential) cyt b559 content, induced by the incubation of spinach PSII membrane particles at low pH, resulted in a significant increase in O2•− formation in the presence of PQ-1, whereas it had little effect on O2•− production in the absence of PQ-1. The enhancement of O2•− formation induced by PQ-1 was not abolished by DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea]. Under anaerobic conditions, dark oxidation of LP cyt b559 increased, as pH was decreased. The presence of molecular oxygen significantly enhanced dark oxidation of LP cyt b559. Based on these findings it is suggested that short-chain PQs stimulate O2•− production via a mechanism that involves electron transfer from Pheo− (pheophytin) to LP cyt b559 and subsequent auto-oxidation of LP cyt b559.
Keywords: cytochrome b559 (cyt b559), electron paramagnetic resonance (EPR), plastoquinone (PQ), photosystem II (PSII), spin-trapping, superoxide radical
Abbreviations: chl, chlorophyll; cyt, cytochrome; DCMU, 3-(3,4-dichlorophenyl)-1,1-dimethylurea; desferal, deferoxamine mesylate; EMPO, 2-ethoxycarbonyl-2-methyl-3,4-dihydro-2H-pyrrole-1-oxide; HP, high-potential; LP, low-potential; P680, photosystem II electron donor formed by chl a molecules; pheo, pheophytin; PQ, plastoquinone; PQ-n, PQ with n isoprenoid units in the side-chain; PSII, photosystem II; QA, primary quinone electron acceptor in PSII; QB, secondary quinone electron acceptor in PSII
INTRODUCTION
PSII (photosystem II) is a pigment–protein complex embedded in the thylakoid membranes of higher plants, algae and cyanobacteria. It is involved in the conversion of light energy into chemical energy by the transfer of electrons from water to PQ (plastoquinone) [1,2]. Cyt b559 (cytochrome b559), which is an integral part of PSII, mediates cyclic electron flow around PSII [3,4]. An important feature of cyt b559 is its occurrence in different potential forms. Typically, these are a HP (high-potential) form of cyt b559 with a midpoint redox potential of 330–400 mV, and an LP (low-potential) form of cyt b559, the midpoint redox potential of which varies from 20 to 80 mV [5,6]. Under strong light illumination, LP cyt b559 was found to accept electrons from Pheo (pheophytin), protecting PSII against acceptor side photoinhibition [7]. Under conditions when electron donation from a water-oxidizing complex to P680 [PSII electron donor formed by chl (chlorophyll) a molecules] is inhibited, HP cyt b559 reduces the oxidizing species and thus protects PSII against donor side photoinhibition [8].
Among several prenyl-lipids found in thylakoid membranes, the long-chain PQs [PQ-9 (PQ-n, PQ with n isoprenoid units in the side-chain), PQ-B and PQ-C] only are believed to participate in photosynthetic electron transport [9,10]. Free PQs act as electron and proton carriers across the thylakoid membrane, whereas permanently (QA) and transiently (QB-binding site) bound PQs serve as primary and secondary quinone electron acceptors respectively. In our previous study, with synthetic short-chain PQs (PQ-1 and PQ-2), two specific binding sites in the vicinity of cyt b559 were proposed for natural long-chain PQs [11]. PQ bound to the first binding site was suggested to mediate reduction of LP cyt b559 via the semiquinone form, whereas the PQ molecule bound to the other binding site was proposed to oxidize LP cyt b559. Under aerobic conditions, molecular oxygen has also been proposed to serve as an electron acceptor from LP cyt b559 [12].
Although the main site of superoxide (O2•−) production in thylakoid membranes is PSI (photosystem I) [13,14], the light-induced production of O2•− in PSII membrane particles was also demonstrated by an assay involving cyt c reduction in the presence of xanthine/xanthine oxidase [15], and by EPR spin-trapping spectroscopy [16]. The primary electron acceptor (Pheo−) and the primary quinone electron acceptor (QA−) were proposed to reduce O2 [15–17].
In the present study, O2•− production was studied in PSII membrane particles using EPR spin-trapping spectroscopy in the presence of synthetic short-chain PQs that are not normally present in the thylakoid membrane. In agreement with our previous work [11], we show that short-chain PQs facilitate electron transfer from Pheo− to LP cyt b559. In the present study, direct evidence is given that synthetic short-chain PQs (PQ-1, PQ-2) enhance O2•− generation, supporting the suggestion that LP cyt b559 reduces molecular oxygen to form O2•−.
MATERIALS AND METHODS
Plant material
Spinach (Spinacia oleracea L.) plants were obtained from a local market. Tobacco (Nicotiana tabacum L. cv. Petit Havana) plants were grown in a greenhouse in soil during summer. The tobacco plant mutant grew considerably more slowly than the wild-type plant.
Sample preparation
PSII membrane particles from spinach and tobacco were prepared by using Triton X-100 purification according to the method of Berthold et al. [18] with modifications as described in Ford and Evans [19]. PSII membrane particles were stored in resuspension medium containing 400 mM sucrose, 15 mM NaCl, 5 mM MgCl2 and 40 mM Mes (pH 6.5) at −80 °C. Mn-depleted PSII membranes were prepared by incubation of PSII membrane particles in 0.8 M Tris (pH 8.0) for 20 min [20]. After this treatment, PSII membrane particles were washed several times in resuspension buffer and stored at −80 °C. PQs were added to the samples before illumination in an ethanol solution (the final concentration of ethanol in the sample did not exceed 1%). Short-chain PQs (PQ-1 and PQ-2) were a gift from Professor H. Koike (Department of Life Sciences, Himeji Institute of Technology, Hyogo, Japan), and PQ-9 was obtained as described in Kruk [21].
Oxygen uptake
Oxygen uptake by PSII membrane particles (150 μg of chl·ml−1) was measured using a Clark-type electrode (Hansatech) under saturating white light in the presence of 400 mM sucrose, 15 mM NaCl, 5 mM MgCl2 and 40 mM Mes (pH 6.5). To eliminate O2 evolution, the measurements were performed in the absence of artificial electron acceptors.
Absorption spectroscopy
To monitor the redox state of cyt b559, absorption difference spectra were measured in the range 520–590 nm under continuous stirring using an SLM Aminco DW2000 spectrophotometer. The absorption changes at 559–570 nm were used to determine the level of individual cyt b559 forms. Absorption spectra and kinetic measurements were acquired in split-beam and dual-wavelength modes respectively. During kinetic measurements, photoreduction of cyt b559 was achieved using white light from a halogen lamp passed through a 630 nm cut-off filter. An interference filter [λmax=564 nm, Tmax=30%, FWHM (full width at half-maximum)=12 nm] was used to protect the photomultiplier from the scattered light. To stabilize the baseline and ensure O2 consumption by the oxygen trap, all samples were incubated for 10 min before measurement under continuous stirring.
EPR spin-trapping spectroscopy
The spin-trapping was accomplished by using EMPO, 2-ethoxy-carbonyl-2-methyl-3,4-dihydro-2H-pyrrole-1-oxide (Alexis Biochemicals, Lausen, Switzerland). PSII membrane particles (150 μg of chl·ml−1) were illuminated in a glass capillary tube (Blaubrand® intraMARK, Brand, Germany) in the presence of 25 mM EMPO, 100 μM desferal (deferoxamine mesylate), 40 mM Mes (pH 6.5) or acetate buffer (pH 4.5). Illumination was performed with continuous white light (900 μE·m−2·s−1) using a halogen lamp with a light guide (KL 1500 electronic, Schott, Germany), and spectra were recorded using an EPR spectrometer MiniScope MS200 (Magnettech GmbH, Germany). Signal intensity was evaluated as the relative height of the central doublet of the first derivative of the absorption spectrum. EPR conditions were as follows: microwave power, 10 mW; modulation amplitude, 1 G; modulation frequency, 100 kHz; sweep width, 100 G; scan rate, 1.62 G·s−1.
RESULTS
Production of O2•− in PSII membrane particles
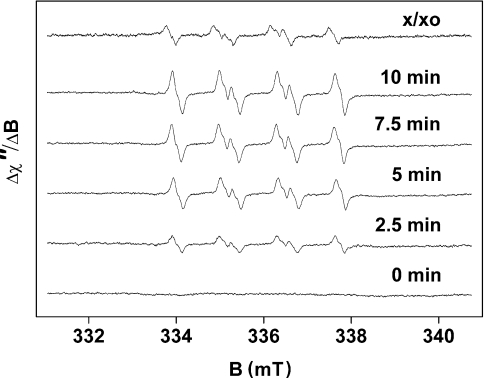
The light-induced formation of O2•− in PSII membrane particles was measured by EPR spin-trapping spectroscopy with an EMPO spin-trap compound. Due to high stability of the EMPO-OOH adduct [5-fold more stable than the O2•− adduct of the commonly used spin trap, DMPO (5,5-dimethylpyrroline N-oxide)], EMPO is suitable for O2•− detection on a time-scale of several minutes [22]. In the dark, addition of EMPO to PSII membrane particles did not induce an EPR signal, whereas illumination of the sample in the presence of EMPO led to the appearance of an EPR signal of the EMPO-OOH adduct (Figure 1). The model EPR spectrum of the EMPO-OOH adduct obtained using the xanthine/xanthine oxidase system is presented in Figure 1. The four-line spectra exhibit all the characteristics of EPR spectra of the EMPO-OOH adduct as published in the literature [22].
Figure 1. Light-induced EPR spectra of the EMPO-OOH adduct measured in spinach PSII membrane particles after illumination for the time indicated.
EPR spectra were obtained after illumination of PSII membrane particles with white light (900 μE·m−2·s−1) in the presence of 25 mM EMPO, 100 μM desferal, 150 μg of chl·ml−1 and 40 mM Mes (pH 6.5). The upmost trace labelled x/xo shows the EPR signal of the EMPO-OOH adduct generated by xanthine/xanthine oxidase system.
Effect of PQs on O2•− production
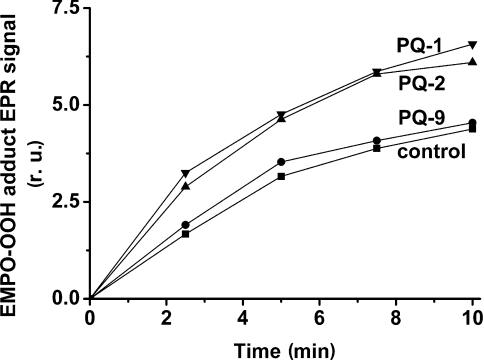
To test the involvement of PQs in O2•− production, time-dependence of EPR signal intensity of the EMPO-OOH adduct was measured in PSII membrane particles in the presence of exogenous PQs. As evident from Figure 2, the intensity of the EPR signal of the EMPO-OOH adduct was enhanced in the presence of PQs in the following order: PQ-1>PQ-2≫PQ-9. In the presence of PQ-9, production of O2•− increased only slightly, whereas short-chain PQs stimulated O2•− production by 53% after 10 min of illumination.
Figure 2. Time dependence of EPR signal intensity of the EMPO-OOH adduct measured in spinach PSII membrane particles in the presence of PQs with different side-chain length.
PSII membrane particles were illuminated with white light (900 μE·m−2·s−1) in the presence of 25 mM EMPO, 100 μM desferal, 150 μg of chl·ml−1 and 40 mM Mes (pH 6.5). PQs at 33 μM were added before illumination. r.u., relative units.
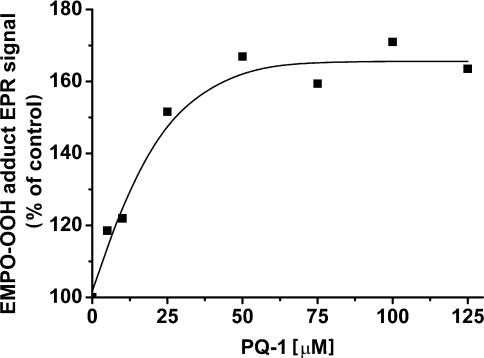
The effect of PQ-1 concentration on O2•− production is shown in Figure 3. The increase in PQ-1 concentration resulted in the enhancement of the EPR signal of the EMPO-OOH adduct. The production of O2•− was saturated at a PQ-1 concentration of approx. 30–40 μM, which corresponds to a prenyl-lipid/chl molar ratio of approx. 1:5.
Figure 3. Effect of various PQ-1 concentrations on EPR signal intensity of the EMPO-OOH adduct measured in spinach PSII membrane particles.
PSII membrane particles were illuminated with white light (900 μE·m−2·s−1) for 5 min in the presence of 25 mM EMPO, 100 μM desferal, 150 μg of chl·ml−1, 40 mM Mes (pH 6.5) and PQ-1 as indicated.
Effect of PQ-1 on O2•− production in the cyt b559 tobacco mutant
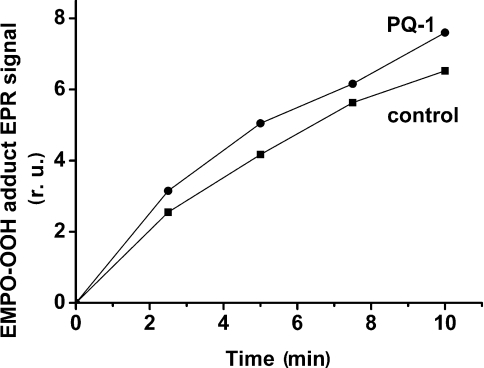
The tobacco mutant, in which phenylalanine at position 26 in the β-subunit was changed to serine [23], was used to study the involvement of cyt b559 in O2•− production in the presence of PQ-1. It has been shown that the tobacco mutant has a decreased amount of cyt b559 [24]. We have determined that in PSII membrane particles isolated from the tobacco mutant, the total amount of cyt b559 is one-seventh of that in the wild-type tobacco plant (the molar ratio of chl:cyt b559 is approx. 1850). In PSII membrane particles isolated from the tobacco mutant, PQ-1 caused only an approx. 15% increase in O2•− production after 10 min of illumination (Figure 4), whereas in PSII membrane particles isolated from wild-type tobacco, the effect of stimulation was similar to that in spinach PSII membrane particles (results not shown). However, the overall level of O2•− production in PSII membrane particles of the tobacco mutant was higher compared with wild-type tobacco and spinach PSII membranes. The increased leakage of electrons to O2 in PSII membrane particles in the mutant might be explained by altered PSII assembly due to changes in the hydrophobic stretch of transmembrane helix in the β-subunit of cyt b559.
Figure 4. Time dependence of EPR signal intensity of the EMPO-OOH adduct measured in tobacco mutant PSII membrane particles in the presence or absence of 33 μM PQ-1.
Experimental conditions were as described in Figure 2. r.u., relative units.
Effect of pH on O2•− production
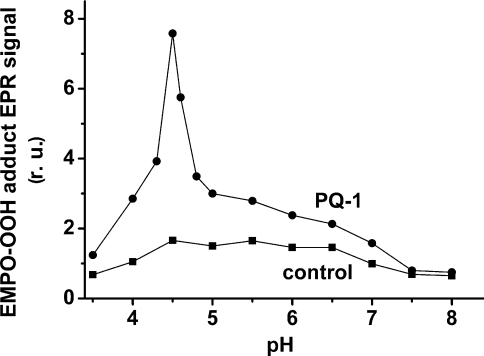
In order to identify the actual electron donor to O2, the effect of pH on EPR signal intensity of the EMPO-OOH adduct was studied (Figure 5). As demonstrated in Table 1, control spinach PSII membrane particles at pH 6.5 contain 28% LP-form cyt b559, which is in agreement with the literature [12,25]. When the pH was lowered to 4.5, the amount of LP cyt b559 increased to approximately half of the total content of cyt b559 (Table 1). In the presence of PQ-1, the fall in pH to values close to 4.5 caused the enhancement of O2•− formation, whereas in the absence of exogenous PQs, O2•− production was unaffected within a pH range from 4.5 to 6.5 (Figure 5). The decrease in EPR signal intensity of the EMPO-OOH adduct observed below pH 4.5 and above pH 6.5 was caused by a decrease in PSII electron transport activity (results not shown).
Figure 5. Effect of pH on EPR signal intensity of the EMPO-OOH adduct measured in spinach PSII membrane particles in the presence or absence of 33 μM PQ-1.
PSII membrane particles were illuminated with white light (900 μE·m−2·s−1) for 2.5 min in the presence of 25 mM EMPO, 100 μM desferal, 150 μg of chl·ml−1, 40 mM acetate buffer (pH 3.5–5.0), or 40 mM Mes (pH 5.5–6.5) or 40 mM Hepes (pH 7.0–8.0).
Table 1. Effect of various PQs on oxygen uptake measured in control, low-pH-incubated and Tris-treated spinach PSII membrane particles.
Oxygen uptake was measured under saturating white light in the presence of either 400 mM sucrose, 15 mM NaCl, 5 mM MgCl2 and 40 mM Mes (pH 6.5) (control and Tris-treated PSII membrane particles), or 400 mM sucrose, 15 mM NaCl, 5 mM MgCl2 and 40 mM acetate buffer (pH 4.5) (low-pH incubation). In Tris-treated PSII membranes, electron transport was supported by the addition of 1 mM DPC (diphenylcarbazide). The values in parentheses are the percentage of O2 uptake measured in the absence of PQs. The amount of HP cyt b559 and LP cyt b559 was determined from difference absorption spectra at 559–570 nm. The amount of HP cyt b559 was calculated from the absorbance change after the addition of potassium ferricyanide (control-FeCy), whereas the oxidized HP and LP cyt b559 content was estimated from the absorbance change after sequential reduction by hydroquinone (HQ-control) and sodium ascorbate (Asc-HQ) respectively. No oxidized HP cyt b559 was found.
| O2 uptake [μmol of O2·(mg of chl)−1·h−1] | ||||
|---|---|---|---|---|
| LP cyt b559 | PSII… (%)… | Control 28 | Low-pH-incubated 49 | Tris-treated 75 |
| −PQ | 2.18 (100%) | 5.69 (100%) | 6.01 (100%) | |
| +33 μM PQ-9 | 2.45 (112%) | 6.86 (121%) | 7.99 (133%) | |
| +33 μM PQ-2 | 2.66 (122%) | 8.56 (150%) | 16.43 (274%) | |
| +33 μM PQ-1 | 2.76 (126%) | 18.48 (324%) | 20.00 (333%) | |
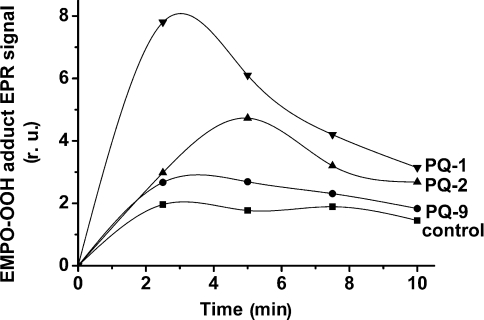
The effect of various PQs on the time-dependence of the EPR signal intensity of the EMPO-OOH adduct was measured at pH 4.5 (Figure 6). During 2–3 min of illumination, a greater than 3-fold increase in O2•− production was observed in the presence of PQ-1, whereas in the presence of PQ-9, the EPR signal of the EMPO-OOH adduct was increased by approx. 50%. The decrease in EPR signal intensity of the EMPO-OOH adduct that was observed for a longer period of illumination is probably caused by reduction of the adduct by an unspecified reductant generated in the sample during illumination.
Figure 6. Time dependence of EPR signal intensity of the EMPO-OOH adduct measured at pH 4.5 in spinach PSII membrane particles in the presence of PQs with different side-chain length.
PSII membrane particles were illuminated with white light (900 μE·m−2·s−1) in the presence of 25 mM EMPO, 100 μM desferal, 150 μg of chl·ml−1 and 40 mM acetate buffer (pH 4.5). PQs at 33 μM were added before illumination. r.u., relative units.
Effect of oxygen on dark oxidation of LP cyt b559
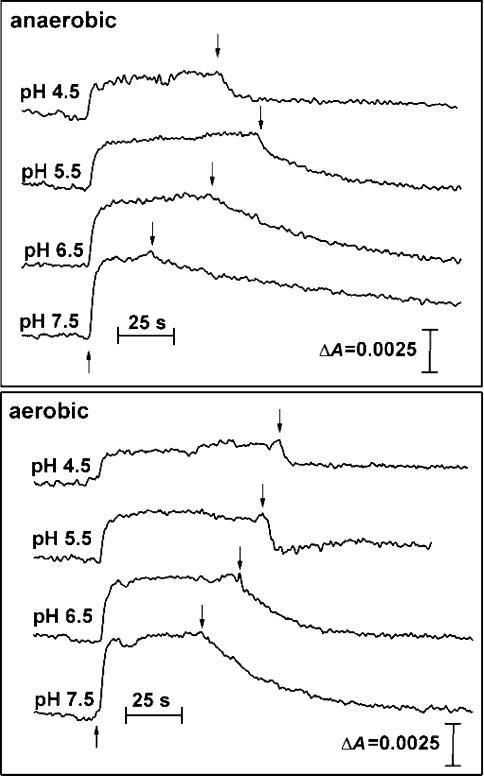
To test the effect of low pH on the oxidation of cyt b559, time-dependent absorption changes at 559–570 nm were measured in the presence of PQ-1 (Figure 7). Owing to the fact that photoreduction of LP cyt b559 was not detectable under aerobic conditions [11], it was measured in the presence of a glucose/glucose oxidase/catalase system, which is known to remove O2. When the pH was decreased, cyt b559 oxidation was enhanced (Table 2). The enhancement of cyt b559 oxidation at low pH is probably caused by an increase in the amount of LP cyt b559, as well as by the upshift of the redox potential of the PQ/PQH2 couple, which is the electron acceptor from LP cyt b559 under anaerobic conditions. It has been shown that, in contrast with HP cyt b559, the LP cyt b559 can be oxidized directly by molecular oxygen and PQs [12].
Figure 7. Effect of molecular oxygen on dark oxidation of cyt b559 measured in spinach PSII membrane particles in the presence of PQ-1 at different pHs.
The kinetics of reduction and oxidation of cyt b559 were measured by absorption changes at 559–570 nm after onset (↑) and offset (↓) of the illumination respectively. The measurements were performed using the oxygen trap (1 mM glucose, 50 units·ml−1 glucose oxidase, 500 units·ml−1 catalase) in the presence of 33 μM PQ-1, 1 mM MnCl2, 0.2% Triton, 40 mM acetate buffer (pH 4.5), or 40 mM Mes (pHs 5.5 and 6.5) or 40 mM Hepes (pH 7.5). In order to achieve aerobic conditions, H2O2 was injected into the measurement assay at the end of the illumination period to give a final concentration of 0.8 mM H2O2. Other experimental conditions were the same as under anaerobic conditions.
Table 2. Effect of oxygen on dark oxidation of LP cyt b559.
The rate of dark oxidation of LP cyt b559 expressed as half-time of absorption changes at 559–570 nm after offset of illumination.
| τ1/2 (s) | ||
|---|---|---|
| pH | Anaerobic | Aerobic |
| 7.5 | 107.5 | 21.7 |
| 6.5 | 36.1 | 11.4 |
| 5.5 | 12.6 | 3.5 |
| 4.5 | 6.7 | 1.8 |
In order to study the oxidation of LP cyt b559 under aerobic conditions, H2O2 was injected into the sample at the offset of illumination (Figure 7). Due to the presence of catalase in the sample, molecular oxygen was produced immediately after H2O2 injection. The amount of H2O2 injected was adjusted to give 250 μM oxygen in the sample, as checked by using a Clark-type electrode (results not shown). In the presence of O2, cyt b559 oxidation evidently increased when compared with anaerobic conditions (Table 2).
Effect of PQs on O2 uptake
To quantify the effect of PQs on O2•− production, O2 uptake was measured in PSII membrane particles. In the absence of PQs, the rate of oxygen consumption in control PSII membrane particles was approx. 2 μmol of O2·(mg of chl)−1·h−1 (Table 1). Low-pH incubation resulted in the enhancement of O2 uptake. In accordance with EPR spin-trapping data, a more-than-3-fold increase in the rate of oxygen consumption was observed in the presence of PQ-1 at pH 4.5. When PQ-2 was added, O2 consumption was enhanced by 50%, whereas PQ-9 caused only a 21% enhancement of O2 consumption at low pH. In further experiments, O2 uptake was measured in Tris-washed PSII membrane particles that contain a higher amount of LP cyt b559 as compared with control PSII membrane particles (Table 1). Similar to the low-pH incubation, PQ-1 caused a more than 3-fold increase in the rate of O2 consumption. An almost 3-fold increase in O2 uptake was also observed for PQ-2, whereas PQ-9 stimulated O2 uptake by 33% (Table 1).
Effect of DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea] on O2•− production
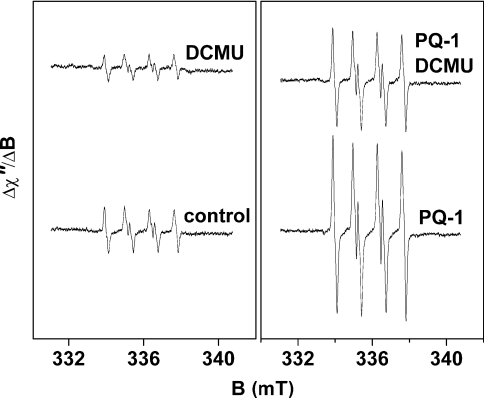
EPR spectra of the EMPO-OOH adduct were also measured in the presence of DCMU (Figure 8). In the absence of PQ-1, the addition of DCMU lowered EPR signal intensity of the EMPO-OOH adduct by 50% (Figure 8). Similarly, when PQ-1 was added, DCMU lowered the EPR signal intensity by 50% (Figure 8). It has been suggested that the decrease in O2•− production in the presence of DCMU is caused by the suppression of charge separation due to the electrostatic effect of negatively charged QA− (P. Pospíšil and A. W. Rutherford, unpublished work). These results suggest that the PQ-1-induced enhancement of O2•− formation is not connected with the QB binding site.
Figure 8. Effect of DCMU on EPR spectra of the EMPO-OOH adduct in spinach PSII membrane particles measured at pH 4.5 in the presence or absence of PQ-1.
PSII membrane particles were illuminated with white light (900 μE·m−2·s−1) for 2.5 min in the presence of 25 mM EMPO, 100 μM desferal, 150 μg of chl·ml−1 and 40 mM acetate buffer (pH 4.5). Prior to illumination, 33 μM PQ-1 and 10 μM DCMU were added to the reaction mixture.
DISCUSSION
Production of O2•− by PSII
In the present study, production of O2•− in PSII membrane particles was studied using EPR spin-trapping spectroscopy. As demonstrated in Figure 1, illumination of PSII membrane particles resulted in the generation of O2•−. It has previously been suggested that Pheo− and QA− may serve as electron donors to O2 [15–17]. Pheo− has the highest reducing power (Em=−610 mV, pH 7); however, the reduction of O2 by Pheo− is limited due to fast electron transfer from Pheo− to QA− (300–500 ps) [2]. On the other hand, reduction of O2 by QA− is less favourable from a thermodynamic point of view [the mid-point redox potential of the QA−/QA redox couple (Em)=−80 mV, pH 7]; however, it better fits the kinetic criteria (the time constant of electron transfer from QA− to QB is approx. 200–400 μs) [26]. Recently, we have proposed that the dominant reductant of O2 changes over the time course of photoinhibition [16]. It has been suggested that QA− serves as a reductant to O2 in the early phase, whereas later it is Pheo− that donates electrons to O2.
Role of PQs in O2•− production
The main objective of this study was to investigate the effect of PQs on O2•− production in PSII membrane particles. In our previous work, we have demonstrated that in the presence of shortchain PQs, LP cyt b559 is reduced upon illumination and reoxidized to its original level in the dark [11]. Based on these results, it has been suggested that short-chain PQs bound in the vicinity of cyt b559 facilitate electron flow from Pheo− to LP cyt b559. The recently determined crystal structure of PSII [27,28] reveals that the edge-to-edge distance between Pheo− and LP cyt b559 is too far to maintain direct electron transport. The structural data support our suggestion that PQs might sufficiently fill this gap and serve as electron carriers between Pheo− and LP cyt b559.
The finding that short-chain PQs are considerably more effective in the stimulation of O2•− production than long-chain PQ-9 (Figure 6) is in agreement with our previous observation that the rate of LP cyt b559 photoreduction increases with decreasing side-chain length of PQs [11].
Role of LP cyt b559 in O2•− production
It has been shown that site directed mutagenesis of cyt b559, which affects a highly hydrophobic stretch in the transmembrane helix of the β-subunit, results in a decrease of the cyt b559 content [23,24]. The observation that in PSII membrane particles isolated from tobacco mutant the stimulatory effect of PQ-1 on O2•− production was suppressed indicates that cyt b559 is involved in O2•− formation in the presence of PQ-1.
It is well known that in native PSII, cyt b559 is present mainly in the reduced HP-form [3]. Based on difference absorption spectra from cyt b559 in the α-band region, we have determined that our PSII membrane particles contain approx. 28% of cyt b559 in the LP form (Table 1) that is oxidizable by O2. In order to increase the level of LP cyt b559, PSII membrane particles were incubated at low pH. It has been demonstrated that the HP form of cyt b559 is unstable at low pH and is partially converted into the LP form [29]. After low-pH incubation of PSII membrane particles, the amount of LP cyt b559 increased to approximately half of the total content of cyt b559 (Table 1). The observation that, at a pH close to 4.5, production of O2•− was significantly enhanced in the presence of short-chain PQs, suggests the participation of LP cyt b559 in this stimulatory effect (Figures 5 and 6). In accordance with this finding, it has been demonstrated that in the absence of O2, the autoxidation of LP cyt b559 was increased at low pH (Figure 7). It is suggested that under anaerobic conditions it is PQ that oxidizes LP cyt b559. When O2 is present, the rate of LP cyt b559 autoxidation is enhanced even more due to electron transfer to O2 (Figure 7).
In Tris-treated PSII membrane particles, the effect of PQ-1 and PQ-2 on O2•− production was greatly increased compared with untreated PSII. In Tris-treated PSII, almost all of cyt b559 is in the LP form [30,31]. Our observation that the increase in the level of LP cyt b559 resulted in the enhancement of O2•− production supports the idea that LP cyt b559 is involved in O2•− formation.
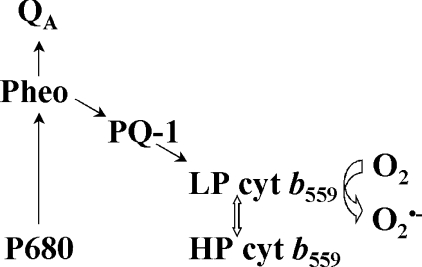
Our previous finding that under aerobic conditions no photoreduction of LP cyt b559 was detected indicates that LP cyt b559 is rapidly re-oxidized by O2 [12].The results presented in this study show that exogenously added short-chain PQs significantly enhance O2•− production by PSII. This finding indicates that one-electron reduced PQs are able to reduce LP cyt b559, which in turn undergoes spontaneous autoxidation resulting in O2•− formation (Scheme 1).
Scheme 1. The involvement of PQ-1 in O2•− production in PSII.
Physiological relevance
In the present study, the production of O2•− by LP cyt b559 is stimulated by synthetic short-chain PQs. Owing to their increased polarity and smaller molecular mass, they are able to bind in the vicinity of the haem group of LP cyt b559 at the relatively polar membrane region [32,33]. Even though short-chain PQs are not natural components of thylakoid membranes, the involvement of native long-chain PQs in O2•− production may have relevance under physiological conditions.
Acknowledgments
This work was supported by grant numbers 1K04103 and MSM 6198959215 from the Ministry of Education, Youth and Sports in the Czech Republic. This work was also financed (for the years 2005–2008) by the Polish Ministry of Science under project number 2 P04A 063 28. We thank to Dr H. Koike from Himeji Institute of Technology, Hyogo, Japan, for providing us with short-chain PQs. We are grateful to Dr Kvetoslava Burda (Institute of Physics, Jagiellonian University, Kraków, Poland) for providing us with tobacco wild-type and mutant seeds. We thank Dr Jan Hrbáč and Professor Jan Lasovský for stimulating discussion and support with respect to the EPR measurements.
References
- 1.Britt R. D. Oxygen Evolution. In: Ort D., Yocum C., editors. Oxygenic Photosynthesis: the Light Reactions. Dordrecht: Kluwer Academic Publishers; 1996. pp. 137–164. [Google Scholar]
- 2.Diner B. A., Rappaport F. Structure, dynamics, and energetics of the primary photochemistry of photosystem II of oxygenic photosynthesis. Annu. Rev. Plant Biol. 2002;53:551–580. doi: 10.1146/annurev.arplant.53.100301.135238. [DOI] [PubMed] [Google Scholar]
- 3.Whitmarsh J., Pakrasi H. B. Form and Function of Cytochrome b559. In: Ort D., Yocum C., editors. Oxygenic Photosynthesis: the Light Reactions. Dordrecht: Kluwer Academic Publishers; 1996. pp. 249–264. [Google Scholar]
- 4.Stewart D. H., Brudvig G. W. Cytochrome b559 of photosystem II. Biochim. Biophys. Acta. 1998;1367:63–87. doi: 10.1016/s0005-2728(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 5.Kaminskaya O., Kurreck J., Irrgang K. D., Renger G., Shuvalov V. A. Redox and spectral properties of cytochrome b559 in different preparations of photosystem II. Biochemistry. 1999;38:16223–16235. doi: 10.1021/bi991257g. [DOI] [PubMed] [Google Scholar]
- 6.Roncel M., Ortega J. M., Losada M. Redox and spectral properties of cytochrome b559 in different preparations of Photosystem II. Eur. J. Biochem. 2001;268:4961–4968. doi: 10.1046/j.0014-2956.2001.02427.x. [DOI] [PubMed] [Google Scholar]
- 7.Barber J., De Las Rivas S. A functional model for the role of cytochrome b559 in the protection against donor and acceptor side photoinhibition. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10942–10946. doi: 10.1073/pnas.90.23.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulson M., Samson G., Whitmarsh J. Evidence that cytochrome b559 protects photosystem II against photoinhibition. Biochemistry. 1995;34:10932–10938. doi: 10.1021/bi00034a027. [DOI] [PubMed] [Google Scholar]
- 9.Kruk J., Strzałka K. Identification of plastoquinone-C in spinach and maple leaves by reverse-phase high-performance liquid chromatography. Phytochemistry. 1998;49:2267–2271. [Google Scholar]
- 10.Kruk J., Burda K., Schmid G. H., Radunz A., Strzałka K. Function of plastoquinones B and C as electron acceptors in photosystem II. Fatty acids analysis of plastoquinone B. Photosynth. Res. 1998;58:203–209. [Google Scholar]
- 11.Kruk J., Strzałka K. Redox changes of cytochrome b559 in the presence of plastoquinones. J. Biol. Chem. 2001;276:86–91. doi: 10.1074/jbc.M003602200. [DOI] [PubMed] [Google Scholar]
- 12.Kruk J., Strzałka K. Dark reoxidation of the plastoquinone-pool is mediated by the low-potential form of cytochrome b-559 in spinach thylakoids. Photosynth. Res. 1999;62:273–279. [Google Scholar]
- 13.Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Mol. Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 14.Kruk J., Jemioła-Rzemińska M., Burda K., Schmid G. H., Strzałka K. Scavenging of superoxide generated in photosystem I by plastoquinol and other prenyllipids in thylakoid membranes. Biochemistry. 2003;42:8501–8505. doi: 10.1021/bi034036q. [DOI] [PubMed] [Google Scholar]
- 15.Ananyev G. M., Renger G., Wacker U., Klimov V. V. Photoreduction of superoxide radicals and the superoxide dismutase activity of Photosystem II. The possible involvement of cytochrome b559. Photosynth. Res. 1994;41:327–338. doi: 10.1007/BF00019410. [DOI] [PubMed] [Google Scholar]
- 16.Pospíšil P., Arató A., Krieger-Liszkay A., Rutherford A. W. Hydroxyl radical generation by photosystem II. Biochemistry. 2004;43:6783–6792. doi: 10.1021/bi036219i. [DOI] [PubMed] [Google Scholar]
- 17.Cleland R. E., Grace S. C. Voltammetric detection of superoxide production by photosystem II. FEBS Lett. 1999;457:348–352. doi: 10.1016/s0014-5793(99)01067-4. [DOI] [PubMed] [Google Scholar]
- 18.Berthold D. A., Babcock G. T., Yocum C. F. A highly resolved, oxygen evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett. 1981;134:231–234. [Google Scholar]
- 19.Ford R. C., Evans M. C. W. Isolation of a photosystem 2 preparation from higher plants with highly enriched oxygen evolution activity. FEBS Lett. 1983;160:159–164. [Google Scholar]
- 20.Ono T., Inoue Y. Mn-preserving extraction of 33-, 24- and 16-kDa proteins from O2-evolving PS II particles by divalent salt-washing. FEBS Lett. 1983;164:255–259. [Google Scholar]
- 21.Kruk J. Charge-transfer complexes of plastoquinone and α-tocopherol quinone in vitro. Biophys. Chem. 1988;30:143–149. doi: 10.1016/0301-4622(88)85011-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H., Joseph J., Vasquez-Vivar J., Karoui H., Nsanzumuhire C., Martásek P., Tordo P., Kalyanaraman B. Detection of superoxide anion using an isotopically labeled nitrone spin trap: potential biological applications. FEBS Lett. 2000;473:58–62. doi: 10.1016/s0014-5793(00)01498-8. [DOI] [PubMed] [Google Scholar]
- 23.Bock R., Kössel H., Maliga P. Introduction of a heterologous editing site into the tobacco plastid genome: The lack of RNA editing leads to a mutant phenotype. EMBO J. 1994;13:4623–4628. doi: 10.1002/j.1460-2075.1994.tb06784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bondarava N., De Pascalis L., Al-Babili S., Goussias C., Golecki J. R., Beyer P., Bock R., Krieger-Liszkay A. Evidence that cytochrome b559 mediates the oxidation of reduced plastoquinone in the dark. J. Biol. Chem. 2003;278:13554–13560. doi: 10.1074/jbc.M212842200. [DOI] [PubMed] [Google Scholar]
- 25.Ortega J. M., Hervás M., De la Rosa M., Losada M. pH-dependent photoreduction of the high- and low-potential forms of cytochrome b559 in spinach PSII-enriched membranes. Photosynth. Res. 1995;46:185–191. doi: 10.1007/BF00020429. [DOI] [PubMed] [Google Scholar]
- 26.Crofts A. R., Wraight C. A. The electrochemical domain of photosynthesis. Biochim. Biophys. Acta. 1983;726:149–185. [Google Scholar]
- 27.Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 28.Loll B., Kern J., Saenger W., Zouni A., Biesiadka J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature (London) 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 29.Crofts J., Horton P. Dissipation of excitation energy by Photosystem II particles at low pH. Biochim. Biophys. Acta. 1991;1058:187–193. [Google Scholar]
- 30.Gadjieva R., Mamedov F., Renger G., Styring S. Interconversion of low- and high-potential forms of cytochrome b559 in Tris-washed photosystem II membranes under aerobic and anaerobic conditions. Biochemistry. 1999;38:10578–584. doi: 10.1021/bi9904656. [DOI] [PubMed] [Google Scholar]
- 31.Roncel M., Boussac A., Zurita J. L., Bottin H., Sugiura M., Kirilovsky D., Ortega J. M. Factors determining the special redox properties of photosynthetic cytochrome b559. J. Biol. Inorg. Chem. 2003;8:206–216. doi: 10.1007/s00775-002-0406-7. [DOI] [PubMed] [Google Scholar]
- 32.Skowronek M., Jemioła-Rzemińska M., Kruk J., Strzałka K. Influence of the redox state of ubiquinones and plastoquinones on the order of lipid bilayers studied by fluorescence anisotropy of diphenyl-hexatriene and trimethylammonium-diphenyl-hexatriene. Biochim. Biophys. Acta. 1996;1280:115–119. doi: 10.1016/0005-2736(95)00264-2. [DOI] [PubMed] [Google Scholar]
- 33.Jemioła-Rzemińska M., Myśliwa-Kurdziel B., Strzałka K. Comparative studies on the effect of side chain length and the redox state of quinones on their interaction with phospholipid bilayers. Chem. Phys. Lipids. 2002;114:169–180. doi: 10.1016/s0009-3084(01)00207-9. [DOI] [PubMed] [Google Scholar]