Abstract
System A and N amino acid transporters are key effectors of movement of amino acids across the plasma membrane of mammalian cells. These Na+-dependent transporters of the SLC38 gene family are highly sensitive to changes in pH within the physiological range, with transport markedly depressed at pH 7.0. We have investigated the possible role of histidine residues in the transporter proteins in determining this pH-sensitivity. The histidine-modifying agent DEPC (diethyl pyrocarbonate) markedly reduces the pH-sensitivity of SNAT2 and SNAT5 transporters (representative isoforms of System A and N respectively, overexpressed in Xenopus oocytes) in a concentration-dependent manner but does not completely inactivate transport activity. These effects of DEPC were reversed by hydroxylamine and partially blocked in the presence of excess amino acid substrate. DEPC treatment also blocked a reduction in apparent affinity for Na+ (K0.5Na+) of the SNAT2 transporter at low external pH. Mutation of the highly conserved C-terminal histidine residue to alanine in either SNAT2 (H504A) or SNAT5 (H471A) produced a transport phenotype exhibiting reduced, DEPC-resistant pH-sensitivity with no change in K0.5Na+ at low external pH. We suggest that the pH-sensitivity of these structurally related transporters results at least partly from a common allosteric mechanism influencing Na+ binding, which involves an H+-modifier site associated with C-terminal histidine residues.
Keywords: allosteric regulation, amino acid transport, hydrogen ion, System A, System N
Abbreviations: DEPC, diethyl pyrocarbonate; GABA, γ-aminobutyric acid; MBM, modified Barth's medium; SNAT2, System A transporter 2; SNAT5, System N transporter 2; TMACl, tetramethylammonium chloride
INTRODUCTION
Systems A and N are major Na+-dependent transport systems for zwitterionic amino acids in mammalian cells. The two transporter activities are mediated by structurally similar carrier proteins of a transporter family (SLC38) related to the vesicular GABA (γ-aminobutyric acid) transporter VGAT (see [1] for a review). Three distinct isoforms of System A transporter have been cloned to date (SNAT1,2 and 4; previously also known as ATA1–3 and SAT1–3 respectively) [2–6], alongside two isoforms of System N (SNAT3,5; previously also known as SN1 and 2 respectively) [7–10]. System A transporters mediate the uptake of a wide range of neutral amino acids, coupled to the uptake of Na+ with a stoichiometry of 1:1. System A is the principal insulin-regulated amino acid transporter in mammalian cells and has a ubiquitous tissue distribution; SNAT2 appears to be the major regulated isoform in response to endocrine and nutrient stimuli [2,4,6]. The System N transporter is reported to have a narrower substrate specificity, with preference for transport of glutamine, histidine and asparagine [7–10]. The molecular mechanism of System N is distinct from that of System A, being coupled to counter-transport of H+ as well as co-transport of Na+ [7,11,12]. All isoforms of the System A and N transporters identified to date exhibit marked pH-sensitivity, with influx depressed as external pH is lowered within the physiological range (pH 7.0–7.8) (see [1] for a review). Such dependence on external pH may have important physiological implications for tissue amino acid and protein metabolism, as well as for regulation of glutamatergic and GABAergic neurotransmission (see [1,13] for reviews).
The mechanism by which external pH influences the activity of System N transporters has been interpreted as a direct effect of elevated external [H+] in reducing the overall driving force for amino acid substrate uptake [7,11]. However, this proposed mechanism does not explain the marked similarity between pH-sensitivity of System A (SNAT1,2,4) and System N (SNAT3,5) isoforms, which we believe is suggestive of a common mechanism not requiring H+ flux through the transport cycle. One possibility, suggested recently for System A transporters at least, is that H+ competes with Na+ for the cation-binding site without being transported [14]. An alternative or complementary possibility is that H+ exerts allosteric effects on transport activity [15], perhaps related to the functionality of histidine residues within the transporter structures [16]. The electrical charge on histidine residues is significantly altered within the physiological pH range and histidine residues are known to have key roles in the function of a number of membrane proteins involved in H+ sensing or H+ transport (e.g. [17–21]). We have therefore investigated the hypothesis that conserved histidine residues within the transporter protein structures are at least partly responsible for the pH-sensitivity of the SLC38 family transporters, using cloned rat SNAT2 (SLC38A2) and SNAT5 (SLC38A5) overexpressed in Xenopus oocytes as our representative experimental system.
EXPERIMENTAL
Materials
Female toads (Xenopus laevis) were obtained from the South African Xenopus facility (Noordhoek, South Africa). Collagenase A was purchased from Roche Diagnostics (Mannheim, Germany). The QuikChange site-directed mutagenesis kit was obtained from Stratagene (La Jolla, CA, U.S.A.). L-[3H]Serine and L-[3H]glutamine were obtained from NEN (PerkinElmer Life Sciences, Cambridge, U.K.). All other chemicals were obtained from Sigma (Poole, Dorset, U.K.).
Preparation of cRNA
Plasmid DNA was linearized with HincII (pTLN2-rat SNAT2) [6] or HindIII (pSPORT1-rat SNAT5) [10] and cRNA was then synthesized in vitro using the T7 or SP6 mMessage mMachine® kit (Ambion, Austin, TX, U.S.A.), as appropriate. The cRNA was purified by phenol/chloroform/3-methylbutan-1-ol extraction, followed by precipitation with sodium acetate and ethanol.
Site-directed mutagenesis
Mutagenesis was performed using the QuikChange site-directed mutagenesis kit. Conserved histidine codons (CAC or CAT) were changed to alanine codons (GCC or GCT) in SNAT2 and SNAT5. Mutagenic primers were designed, and the PCR-based mutagenesis was carried out following the kit protocol. All mutations were confirmed by DNA sequencing using DYEnamic ET terminator chemistry (Amersham Biosciences) on Applied Biosystems automated DNA sequencers (School of Life Sciences, University of Dundee).
Isolation of oocytes and cRNA expression
Xenopus laevis oocytes were isolated by collagenase treatment [22]. Stage V–VI (prophase-arrested) oocytes were selected and maintained at 18 °C in MBM (modified Barth's medium) as described previously [22]. Oocytes were injected with 50 ng of cRNA into the cytoplasm using a positive displacement micro-injector (World Precision Instruments, Sarasota, FL, U.S.A.). Oocytes were incubated at 18 °C for 2–3 days to allow full expression of the cRNA before investigation of overexpressed transport activity.
Amino acid transport assays
The influx of radiolabelled amino acid tracer in Xenopus oocytes was measured as described previously [22] at 22 °C in Na+ transport buffer (100 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 3 mM Hepes, 3 mM Tris, 3 mM Mes; pH adjusted to 6.0–8.0 by altering the amounts of Hepes, Tris and Mes). NaCl was replaced by TMACl (tetramethylammonium chloride) in certain experiments. Oocytes were treated with hydroxylamine (50 mM) prepared in a modified Na+ transport buffer containing only 50 mM NaCl adjusted to pH 8. Individual measurements on eight to eleven oocytes were made for each uptake experiment. The vehicle control for the DEPC studies (1% ethanol) had no significant independent effect on transport activity with the protocols used.
Statistical analysis
Results are means±S.E.M. for n measurements or experiments. To test for statistical significance, the difference between mean values was assessed using Student's unpaired t test, with significance assigned at P<0.05. Where multiple comparisons were required ANOVA was performed and differences were determined using least significant difference. Significance was assigned at P<0.05.
RESULTS
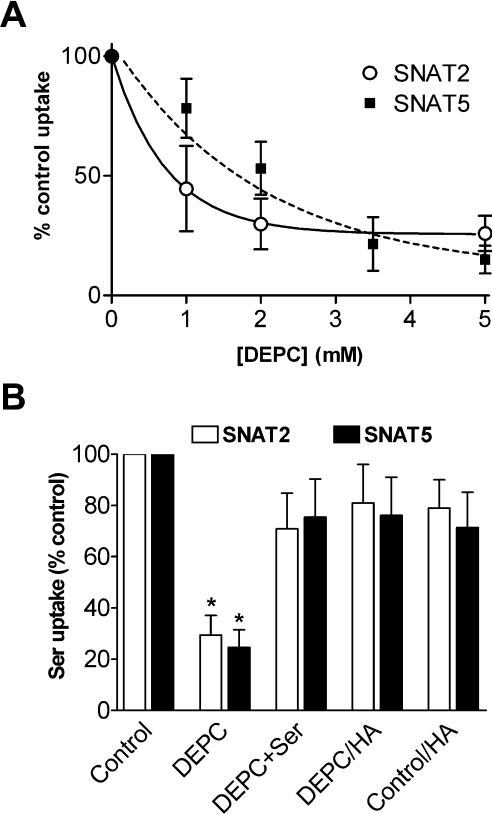
Serine was used to study transport activity in the present study as it is a preferred substrate for both SNAT2 and SNAT5 and has a similar apparent Km value for the two transporters (∼0.8 mM in both cases) [23]. Overall expression of functional transport activity (measured as 0.5 mM serine uptake at the optimum pH value of 8.0) in different oocyte batches ranged from 120 to 560 pmol·oocyte−1·(30 min)−1 for SNAT2 and 110 to 380 pmol·oocyte−1·(30 min)−1 for SNAT5. Histidine has an imidazole side chain with a pK value of 6.0, which enables ionization of histidine residues in proteins within the physiological pH range and allows them to form hydrogen bonds within proteins [20]. DEPC reacts with histidine residues to form N-carbethoxyhistidine residues and is used to investigate the functionality of histidine residues in peptide chains [24]. DEPC is considered to be specific for histidine within the pH range 5.5–7.5 [24] as used here. DEPC pre-treatment of SNAT2- and SNAT5-expressing oocytes substantially, but not completely, inactivates the mechanism(s) for uptake of amino acid substrates (Figure 1A). The K0.5 for DEPC-induced inactivation was approx. 0.5 mM for SNAT2 and 1.5 mM for SNAT5 with DEPC pre-incubation times of 10 min (Figure 1A); no further inhibition of transport was seen after longer pre-incubation periods of up to 30 min (results not shown). Hydroxylamine (50 mM for 60 min) was able to reverse the inactivating effect of DEPC (Figure 1B), as expected if DEPC was acting at a histidine residue(s) [25]. DEPC also reacts with protein tyrosine and cysteine residues under certain conditions, but we excluded the possibility that either type of residue was of significance here by showing (i) that pre-treatment of oocytes with either the tyrosine-modifying agent N-acetylimidazole (3 mM in MBM for 10 min) or the cysteine-specific reagent N-ethylmaleimide (2 mM in MBM for 15 min) had no significant independent effect on SNAT2 or SNAT5 transport activity and (ii) that the thiol-restoring agent DTT (dithiothreitol) (10 mM in MBM for 10 min) was unable to reverse the effects of DEPC (results not shown). Pre-incubation of oocytes with DTT alone had no significant effect on substrate influx (results not shown). Addition of the transporter substrate serine at 5 mM to the preincubation medium offered substantial protection from the inactivating effects of DEPC (see Figure 1B), averaging 73±14% retention of DEPC-inhibitable SNAT2 transport activity (n=3 oocyte preparations) and 65±11% retention of DEPC-inhibitable SNAT5 transport activity (n=6 oocyte preparations) under the experimental conditions described in the legend to Figure 1(B). In contrast, removal of the co-substrate Na+ (by replacement of NaCl with TMACl transport buffer under experimental conditions described for Figure 1B) gave no protection to the inactivating effects of DEPC on either SNAT2 or SNAT5 (results not shown).
Figure 1. Effects of pre-treatment with DEPC on serine influx in SNAT2- and SNAT5-expressing oocytes.
(A) Oocytes were pre-incubated for 10 min in MBM (pH 7.5) containing the indicated concentrations of DEPC or vehicle control, then rinsed before 0.5 mM L-[3H]serine influx was measured over 30 min in transport buffer (pH 8.0). Influx of serine in uninjected control oocytes has been subtracted from the values for SNAT2/5-expressing oocytes and results are percentages of the control value (±S.E.M. for nine to twelve oocytes) for amino acid influx. (B) Oocytes were pre-incubated for 10 min in NaCl transport buffer (pH 7.5) containing 2 mM DEPC (SNAT2), 3.5 mM DEPC (SNAT5) or vehicle control with or without 5 mM serine (Ser), followed where indicated by treatment with 50 mM hydroxylamine (HA) for 60 min. Oocytes were then rinsed in NaCl transport buffer and 0.5 mM L-[3H]serine influx was measured over 30 min. Pre-incubation of oocytes with serine alone had no significant independent effect on substrate influx. Each point represents the mean±S.E.M. for eight to eleven oocytes from one batch. Influx of serine in appropriately treated uninjected oocytes was subtracted from the values for SNAT2/5-expressing oocytes. *P<0.05 compared with the respective control value.
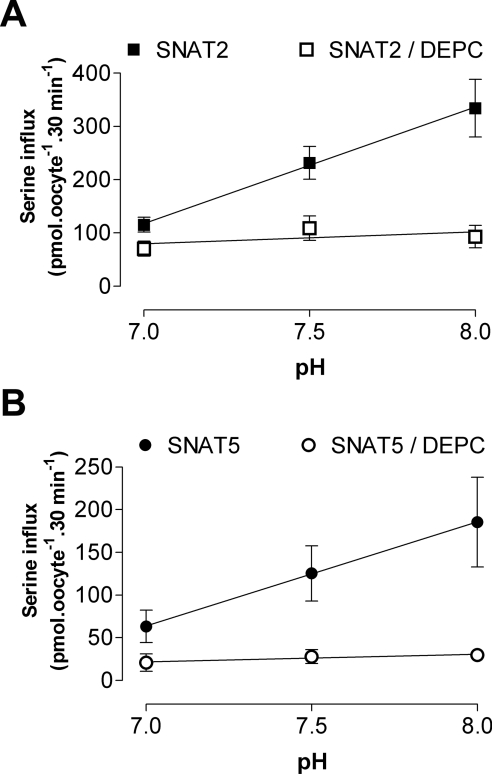
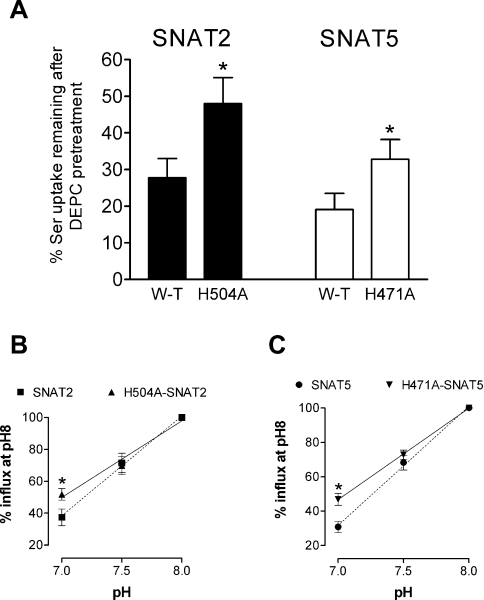
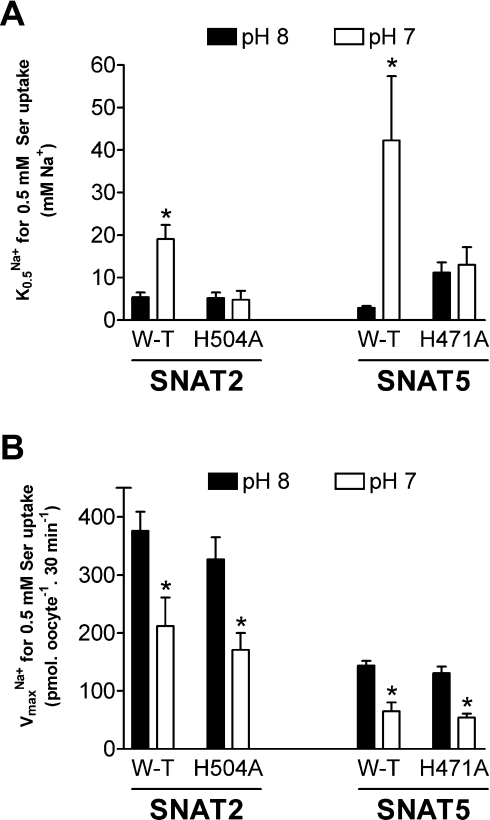
Both SNAT2 and SNAT5 exhibit markedly pH-sensitive transport activity, with influx of amino acid substrate increasing with increased external pH over a physiologically relevant pH range (for example, see Figure 2). DEPC pre-treatment markedly reduced this pH-sensitivity of substrate influx through both transporters (Figure 2), consistent with our suggestion that the pH-dependence of SLC38 transporters of both System A-type (such as SNAT2) and System N-type (such as SNAT5) share a common mechanism involving histidine residues. We therefore progressed to examine how specific conserved histidine residues in the polypeptide chains of System A and N transporters might contribute to their shared sensitivity to external pH and DEPC. There are three histidine residues conserved throughout the five functionally characterized members of the SLC38 transporter family, corresponding to His304/His411/His504 of rat SNAT2 and His271/His375/His471 of rat SNAT5 respectively. Conserved histidine residues of SNAT2 and SNAT5 were mutated individually to alanine and functional properties of the mutant transporters (identified here in the form H504A-SNAT2, for example, representing His504→Ala mutation of SNAT2) were investigated. As an internal control, we also studied histidine to alanine mutations of a specific residue common to rat SNAT2 (His120) and rat SNAT5 (His99) but not conserved throughout the SLC38 family. The majority of mutant transporters (seven out of eight) were functionally competent, but of these, only H504A-SNAT2 and the corresponding H471A-SNAT5 mutant presented a transport phenotype with significantly reduced sensitivity to inactivation by DEPC pre-treatment (Figure 3A; results not shown for other mutant transporters) and were therefore chosen to study in greater detail. Both H504A-SNAT2 and H471A-SNAT5 were found to have a reduced pH-sensitivity compared with their respective wild-types within the physiological range (from pH 8 to 7, with a progressive increase in effect towards pH 7; Figures 3B and 3C, and Table 1) and unlike the wild-types, their degree of pH-sensitivity was not modified by DEPC pre-treatment (Table 1). These observations revealed that the conserved C-terminal histidine residue equivalent to His504 of rat SNAT2 and His471 of rat SNAT5 contributed significantly to DEPC-dependent pH-sensitivity of System A and N transport function.
Figure 2. Effects of DEPC pre-treatment on the pH-sensitivity of serine influx through (A) SNAT2 and (B) SNAT5 overexpressed in Xenopus oocytes.
Oocytes were subjected to a 10 min pre-incubation period in MBM (pH 7.5) containing 2 mM DEPC (SNAT2), 5 mM DEPC (SNAT5) or vehicle control. Oocytes were washed and influx of 0.5 mM L-[3H]serine was measured over 30 min at the pH values indicated. Values for uninjected oocytes have been subtracted from the values for SNAT2/5-expressing oocytes. Each point represents the mean±S.E.M. for five batches of oocytes. Regression lines fitted using the method of least-squares to experimental data are shown: regression-line slopes are significantly different from zero (P<0.05) for untreated SNAT2/5-expressing oocytes, but are not significantly different from zero after DEPC treatment.
Figure 3. Serine influx through wild-type and mutant SNAT2 and SNAT5 transporters overexpressed in oocytes.
(A) Reduced effect of DEPC pre-treatment on serine influx through H504A-SNAT2 and H471A-SNAT5 compared to wild-type transporters. Oocytes were subjected to a 10 min pre-incubation period in MBM (pH 7.5) containing 2 mM DEPC (SNAT2), 5 mM DEPC (SNAT5) or vehicle control. Oocytes were then washed and influx of 0.5 mM L-[3H]serine was measured over 30 min at pH 8. Values for uninjected oocytes have been subtracted from the values for SNAT2/5-expressing oocytes. Mean values±S.E.M. for five (SNAT2) or seven (SNAT5) batches of oocytes are shown. *P<0.05 compared with respective value for wild-type (W-T) transporter. (B, C) The pH relations of serine influx through wild-type and mutant SNAT2 and SNAT5 transporters overexpressed in oocytes: (B) SNAT2 relative to H504A-SNAT2, (C) SNAT5 relative to H471A-SNAT5. Influx of 0.5 mM L-[3H]serine was measured over 30 min at the pH values indicated for oocytes overexpressing transporters of interest. Values are presented as a percentage (mean±S.E.M) of flux measured at pH 8, after subtraction of respective values measured for control (uninjected) oocytes. In (B), pH 8 fluxes were 375±49 and 378±65 pmol of serine·oocyte−1·(30 min)−1 for SNAT2 and H504A-SNAT2 respectively (n=5 oocyte batches). In (C), pH 8 fluxes were 225±40 and 265±36 pmol of serine·oocyte−1·(30 min)−1 for SNAT5 and H471A-SNAT5 respectively (n=9 oocyte batches). *P<0.05 compared with value for wild-type transporter at same pH.
Table 1. pH-sensitivity of wild-type and mutant SNAT2 and SNAT5 expressed in Xenopus oocytes: effects of DEPC pre-treatment, expressed as a ratio of pH 7/pH 8 uptakes.
DEPC pre-treatment was for 10 min at 2 or 5 mM DEPC (SNAT2 and SNAT5 respectively) before measurement of 0.5 mM [3H]serine uptake as described in the text. Values shown are means±S.E.M. (number of oocyte batches).
| Transporter under study | pH 7/pH 8 uptake; no pre-treatment | pH 7/pH 8 uptake; DEPC pre-treatment |
|---|---|---|
| SNAT2 | 0.378±0.051 (9) | 0.811±0.132 (6)* |
| H504A-SNAT2 | 0.525±0.041 (8)# | 0.513±0.033 (4) |
| SNAT5 | 0.315±0.032 (10) | 0.715±0.16 (7)* |
| H471A-SNAT5 | 0.469±0.035 (9)# | 0.51±0.093 (6) |
#Significantly different from respective wild-type value (no pre-treatment column only; P<0.05; Student's t test).
*Significantly different from respective value with no pre-treatment (P<0.05; Student's t test).
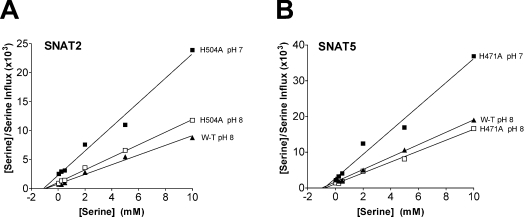
We subsequently undertook a more detailed comparison of the kinetic characteristics of mutant compared with wild-type transporters. The Km values for serine transport by H504A-SNAT2 and H471A-SNAT5 were not significantly different from that of wild-type and were also unaffected by a change in external pH from 8 to 7 (Figure 4; [12,14] have previously described the lack of effect of pH on Km for amino acids in wild-type SLC38 transporters). Both mutant transporters showed a substantial reduction in Vmax for serine transport going from pH 8 to 7 (Figure 4), consistent both with the reductions in 0.5 mM serine transport described above and with previous reports for wild-type transporters [12,14,15]. Albers et al. [15] and Chaudhry et al. [14] have shown, for SNAT1 and SNAT2 respectively, that decreasing external pH reduces the strength of interaction between Na+ and the transporter as judged both by a progressive increase in the external Na+ concentration required to produce half-maximal activation of transport activity (K0.5Na+) and a progressive decrease in the rate of amino acid transport supported at saturating external Na+ concentrations (VmaxNa+). We confirmed these observations for SNAT2 (see Figures 5 and 6, and Table 2) and extended them to include SNAT5 (Figure 6), noting similar proportional changes in VmaxNa+ when lowering external pH from 8 to 7 (see legend to Figure 6). In contrast, H504A-SNAT2 and H471A-SNAT5 did not display any significant increase in K0.5Na+ on reduction of external pH from 8 to 7 (Figure 6A), although pH 7/pH 8 ratios of VmaxNa+ remained similar to wild-type (Figure 6B, Table 2). DEPC pre-treatment also prevented the increase in K0.5Na+ of serine transport by SNAT2 after switching from pH 8 to 7 (Figure 5 and Table 2), while substantially reducing the maximal rate to which saturating [Na+] activated 0.5 mM serine transport (VmaxNa+) by SNAT2 at pH 8 as well as the proportional reduction in this value when switching from pH 8 to 7 (Figure 5 and Table 2). A minor residual DEPC-resistant component to the pH-sensitivity of SNAT2 transport activity was confirmed by these experiments (note a small but significant difference between VmaxNa+ values shown at the bottom of Table 2).
Figure 4. Effects of altered external pH on the apparent Km and Vmax of serine influx through H504A-SNAT2 and H471A-SNAT5 transporters overexpressed in oocytes.
Influx of L-[3H]serine at the indicated concentrations was measured over 30 min at external pH 7 or 8, alongside equivalent measurements for wild-type (W-T) transporters at pH 8 only (for comparative purposes). Results are Hanes plots for which each point is the mean value for eight to eleven oocytes (after subtraction of values measured in uninjected control oocytes) and lines were fitted using the method of least-squares: from each line Km was estimated as −(x-axis intercept) and Vmax as 1/line slope. (A) H504A-SNAT2. At pH 8: Km, 0.98 mM serine, Vmax, 920 pmol of serine·oocyte−1·(30 min)−1; at pH 7: Km, 1.05 mM serine, Vmax, 480 pmol of serine·oocyte−1·(30 min)−1 [for W-T SNAT2: Km, 0.94 mM serine, Vmax, 1200 pmol of serine·oocyte−1·(30 min)−1 at pH 8]. All results are from a single batch of oocytes. (B) H471A-SNAT5. At pH 8: Km, 0.74 mM serine, Vmax, 650 pmol of serine·oocyte−1·(30 min)−1; at pH 7: Km, 0.83 mM serine, Vmax, 300 pmol of serine·oocyte−1·(30 min)−1 [for W-T SNAT5: Km, 0.92 mM serine, Vmax, 580 pmol serine·oocyte−1·(30 min)−1 at pH 8]. All results are from a single batch of oocytes.
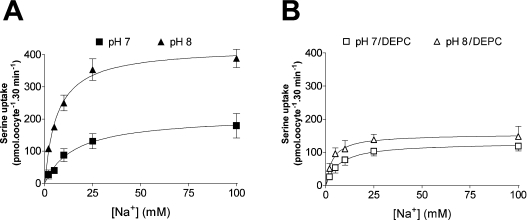
Figure 5. Effects of altering external pH on the Na+-dependence of serine influx through SNAT2 overexpressed in Xenopus oocytes.
(A) Oocytes without DEPC pre-treatment. Influx of 0.5 mM L-[3H]serine was measured over 30 min at the pH values indicated. Values for uninjected oocytes have been subtracted from the values for SNAT2-expressing oocytes. Each point represents the mean±S.E.M. for five batches of oocytes. (B) DEPC-pre-treated oocytes. Oocytes were subjected to a 10 min pre-incubation period in MBM (pH 7.5) containing 2 mM DEPC or vehicle control, washed and serine influx was measured as described above. Each point represents the mean±S.E.M. for three batches of oocytes. For (A) and (B), lines represent best-fit hyperbola to experimental data obtained by iterative curve-fitting (using GraphPad Prism software): kinetic characteristics describing all lines (apparent VmaxNa+; K0.5Na+) are presented in Table 2.
Figure 6. Effects of pH on the K0.5Na+ and VmaxNa+ of wild-type and mutant SNAT2 and SNAT5 transporters overexpressed in oocytes.
[3H]Serine uptake (0.5 mM) was performed with NaCl concentrations of 0–100 mM at the indicated pH (as illustrated in Figure 5 for SNAT2). Values shown are means±S.E.M calculated from iterative curve-fitting (using GraphPad Prism software) to experimental data of the type shown in Figure 5. (A) K0.5Na+ values refer to the [Na+] required for half-maximal activation of 0.5 mM serine transport and are shown as the means±S.E.M. from experiments with three (SNAT2) or four (SNAT5) batches of oocytes. *P<0.05 (Student's unpaired t test) compared with the value at pH 8. (B) VmaxNa+ values refer to the maximum rate of 0.5 mM serine transport supported at saturating external Na+ concentrations and are shown as the means±S.E.M. for nine to twelve oocytes from a representative experiment using a single batch of oocytes. *P<0.05 (Student's unpaired t-test) compared to value at pH 8. Similar proportional differences in VmaxNa+ measured at the two pHs were seen in experiments with other oocyte batches, giving mean pH 7/pH 8 ratios for this value of 0.49 for SNAT2, 0.51 for H504A-SNAT2 (n=3), 0.46 for SNAT5 and 0.43 for H471A-SNAT5 (n=4).
Table 2. Effects of pH and DEPC on the kinetics of the Na+-dependence of the SNAT2 transporter overexpressed in Xenopus oocytes.
[3H]Serine (0.5 mM) uptake was performed with NaCl concentrations of 0–100 mM at the indicated pH as illustrated in Figure 5. DEPC pre-treatment was for 10 min at 2 mM DEPC as described in the text. Values shown are means±S.E.M calculated from iterative curve-fitting (using GraphPad Prism software) to experimental data of the type shown in Figure 5, using data from four oocyte batches. K0.5Na+ values refer to the [Na+] required for half-maximal activation of 0.5 mM serine transport. VmaxNa+ values refer to the maximum rate of 0.5 mM serine transport supported at saturating external Na+ concentrations.
| Conditions | K0.5Na+±S.E.M. (mM Na+) | VmaxNa+±S.E.M. [pmol of serine·oocyte−1·(30 min)−1] |
|---|---|---|
| pH 8 | 5.5±0.9 | 422±16.9 |
| pH 7 | 19.8±2.9* | 209±11.8* |
| pH 6 | 68.4±26.5* | 123±38.2* |
| pH 8/DEPC | 3.7±1.1 | 164±9.5 |
| pH 7/DEPC | 6.1±1.8 | 132±10.2* |
*P<0.05 (Student's unpaired t test) compared with respective control (pH 8 or pH 8/DEPC).
DISCUSSION
Alteration of extracellular pH had similar effects on serine influx through the SNAT2 and SNAT5 transporters overexpressed in Xenopus oocytes; both were highly sensitive to pH changes, with maximum influx observed at pH 8. The present study has revealed two major components to the effect of pH on transport function: (i) progressive reduction in apparent affinity for Na+ binding (K0.5Na+) with decrease in extracellular pH, and (ii) progressive lowering of the Vmax for amino acid transport over the same pH range (pH 6–8; see Table 2, and also [14,15]). On switching from pH 8 to pH 7, these factors together translate into a marked reduction of transport activity to approx. 0.38 or 0.32 at pH 8 for SNAT2 or SNAT5 respectively (Table 1). Pre-treatment with the histidine-specific reagent DEPC greatly reduces the pH-sensitivity of substrate influx for both SNAT2 and SNAT5 whilst retaining a residual basal transport activity (Figure 2), consistent with our hypothesis that external H+ affects transport activity at least partly by interacting with conserved histidine residue(s) in the transporter protein structures. The inability of DEPC to completely block transport indicates that the histidine residues modified by DEPC are not located at any essential substrate-binding site(s). Nevertheless the protective effect of excess amino acid substrate on DEPC actions indicates that key target histidine residue(s) may lie in close proximity to the amino acid-binding site, or alternatively that their external accessibility is influenced at a distance by substrate binding.
The H504A-SNAT2 and H471A-SNAT5 mutations produced similar transport phenotypes characterized by (i) reduced extent of inactivation by DEPC, (ii) abolition of DEPC effects on the pH-sensitivity of amino acid transport, and (iii) no effect on serine transport Km. More specifically, the transporter mutants both show a loss of pH-sensitivity of K0.5Na+ between pH 7 and 8 (similar to that observed with DEPC treatment for SNAT2; see Figure 6A and Table 2), whilst retaining the 50–55% reduction in VmaxNa+ for serine transport observed with wild-type transporters. This translates to a pH 7/pH 8 ratio for serine uptake of approx. 0.5 for both H504A-SNAT2 and H471A-SNAT5 which is maintained after DEPC treatment (Table 1). The essential functionality of the H504A-SNAT2 and H471A-SNAT5 mutants precludes a direct role for the mutated histidine residues in binding or translocation of substrates (including H+ in the case of SNAT5). The loss of pH-sensitivity of Na+ binding (K0.5Na+) seen for SNAT2 both after DEPC treatment and following the His504 to alanine mutation appears most likely to result from the alleviation of an inhibitory allosteric effect of H+ on Na+ binding rather than the removal of competition between Na+ and H+ for binding to the same or overlapping sites on the transporter, because neither of the experimental manoeuvres appears to affect Na+–SNAT2 interactions significantly (note that K0.5Na+ at pH 8 is similar for SNAT2±DEPC and H504A-SNAT2). Mutation of His471 to alanine does affect K0.5Na+ for SNAT5 to a small extent, although again pH-sensitivity is lost. We therefore suggest that His504 of SNAT2 and His471 of SNAT5, while clearly not essential residues for basic functioning of the transport cycle, are important for determining pH-sensitivity either by direct involvement in the binding or ‘sensing’ of H+ ions at an allosteric site (of the type already postulated to exist on SNAT1 [15]) or in transmitting the effects of such H+ binding to the Na+-binding site. Protonation of the conserved His295 of excitatory amino acid carrier 1/excitatory amino acid transporter 3 has similarly been proposed to indirectly modulate substrate binding [26].
Despite their key role in modulation of K0.5Na+, His504 of SNAT2 and His471 of SNAT5 do not appear to be involved in the mechanism producing pH-dependent changes in VmaxNa+ (Figure 6B) or the overall Vmax for serine transport (Figure 4). Nevertheless, binding of DEPC to His504 of SNAT2 (rather than a specific property of the target His residue) appears to blunt the effect of external pH on VmaxNa+ (see Table 2), thus contributing to the appearance of a pH 7/pH 8 ratio for serine uptake of 0.81 (or similarly 0.71 for SNAT5) after DEPC pre-treatment (see Table 1). These observations may be explained on the basis that bound DEPC produces steric hindrance to a conformational change associated with regulation of Vmax for substrate transport by wild-type transporters, which is not reproduced with H504A-SNAT2 and H471A-SNAT5 owing to lack of DEPC binding at the mutated locus. This possibility is supported by the demonstration that transport activity of the prokaryotic Na+/H+ antiporter NhaA is regulated by a conformational change, altering the accessibility of substrate binding sites, induced by a change in local pH detected by an intramolecular H+-sensor [27]. Binding of DEPC to other accessible histidine residues of SNAT2 and SNAT5 also appears to hinder substrate translocation because neither H504A-SNAT2 nor H471A-SNAT5 are entirely resistant to inactivation by DEPC: rat SNAT2, for example, contains a total of 11 histidine residues to which DEPC may bind and thus impede transport activity.
The involvement of histidine residues in the pH-sensitivity of the System A carrier in native liver cell membranes has been suggested previously on the basis of DEPC-induced inactivation [16]. SNAT4 has now been identified as the major System A isoform present in the liver [3], lending further support to the idea that a common mechanism underlies the shared pH-sensitivity of the SLC38 transporters. Histidine residues are known to be involved in binding and translocation of H+ ions by other transport proteins (e.g. His57 and His87 in PepT1 and PepT2 respectively) [17,21] and may also act as H+-sensing residues [18,19] or as part of a larger intramolecular ‘H+-sensor’ [28]. In this context, His504 of SNAT2 and His471 of SNAT5 are clearly important determinants of transporter sensitivity both to external pH and DEPC. The putative extracellular localization of these residues at the extreme C-terminus of the transport protein (according to the consensus 11-transmembrane domain model for these carriers [1]) renders them likely to be strongly influenced by changes in external pH and, assuming the C-terminus folds back within the core protein, they may conceivably form regulatory interactions with other intramolecular domains. The observation that mutation of His504 of SNAT2 or His471 of SNAT5 only partly reduces pH-sensitivity argues against the presence of a single H+-sensing histidine residue in SLC38 transporters of the type described for several other proteins (e.g. glycine transporter GLYT1b [18], P2X4 purinoceptor [29], HCN2 cation channel [19]) and it is more likely that a range of different residues contribute to at least two functionally distinct pH-sensors (e.g. lysine and threonine residues contribute to pH sensing in the renal outer medullary K+ channel 1 cation channel [30]).
At normal extracellular pH values, the SLC38/SNAT transporters are operating in the midrange of their capacity (measured maximally at pH 8.0) and thus respond to both acidic and alkaline shifts in extracellular pH. Substrate influx through SNAT1 [15] as well as the System A transporters present in skeletal muscle [31] (now known to be predominantly SNAT2 [32]) are also reduced by intracellular acidification: a trans-effect of this type is consistent with the idea that H+ may inhibit influx through allosteric influences on the transporter and raises the possibility that either distinct pH-sensors are present both intra- and extra-cellularly or that a single sensor is accessible from both sides of the plasma membrane. Several SNAT isoforms are reported to be involved in glutamine–glutamate cycling between different cell types within tissues such as brain and liver [13,23], where their pH-sensitivity may be a key modulator of flux through these important intercellular metabolic cycles, particularly during acidosis associated with pathophysiological [33] or neurological [34] conditions. Furthermore, inactivation of System A (SNAT2) may initiate the catabolic response of skeletal muscle cells to extracellular acidosis [35].
We conclude that the C-terminal histidine residue of SNAT2, SNAT5 and other SLC38 transporter isoforms is a pH-sensing residue which regulates substrate (Na+ plus amino acid) transport activity at least partly by allosteric effects on Na+ binding and which may therefore be important for physiological functioning of the transporters.
Acknowledgments
We are very grateful to Dr J. D. Erickson (Louisiana State University, New Orleans, LA, U.S.A.) for providing the pTLN2-rat SNAT2 DNA construct. We also thank Kevin Bett and Sophia Pardakis for technical assistance. This work was supported by the U.K. Medical Research Council, U.K. Biotechnology and Biological Sciences Research Council, Tenovus Tayside and University of Dundee.
References
- 1.Mackenzie B., Erickson J. D. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 2.Reimer R. J., Chaudhry F. A., Gray A. T., Edwards R. H. Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7715–7720. doi: 10.1073/pnas.140152797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugawara M., Nakanishi T., Fei Y., Martindale R. G., Ganapathy M. E., Leibach F. H., Ganapathy V. Structure and function of ATA3, a new subtype of amino acid transport system A, primarily expressed in the liver and skeletal muscle. Biochim. Biophys. Acta. 2000;1509:7–13. doi: 10.1016/s0005-2736(00)00349-7. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara M., Nakanishi T., Fei Y. J., Huang W., Ganapathy M. E., Leibach F. H., Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 5.Varoqui H., Zhu H., Yao D., Ming H., Erickson J. D. Cloning and functional identification of a neuronal glutamine transporter. J. Biol. Chem. 2000;275:4049–4054. doi: 10.1074/jbc.275.6.4049. [DOI] [PubMed] [Google Scholar]
- 6.Yao D., Mackenzie B., Ming H., Varoqui H., Zhu H., Hediger M. A., Erickson J. D. A novel system A isoform mediating Na+/neutral amino acid cotransport. J. Biol. Chem. 2000;275:22790–22797. doi: 10.1074/jbc.M002965200. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry F. A., Reimer R. J., Krizaj D., Barber D., Storm-Mathisen J., Copenhagen D. R., Edwards R. H. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell. 1999;99:769–780. doi: 10.1016/s0092-8674(00)81674-8. [DOI] [PubMed] [Google Scholar]
- 8.Fei Y. J., Sugawara M., Nakanishi T., Huang W., Wang H., Prasad P. D., Leibach F. H., Ganapathy V. Primary structure, genomic organization, and functional and electrogenic characteristics of human System N (SN1), a Na+- and H+-coupled glutamine transporter. J. Biol. Chem. 2000;275:23707–23717. doi: 10.1074/jbc.M002282200. [DOI] [PubMed] [Google Scholar]
- 9.Gu S., Roderick H. L., Camacho P., Jiang J. X. Identification and characterization of an amino acid transporter expressed differentially in liver. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3230–3235. doi: 10.1073/pnas.050318197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi T., Kekuda R., Fei Y. J., Hatanaka T., Sugawara M., Martindale R. G., Leibach F. H., Prasad P. D., Ganapathy V. Cloning and functional characterization of a new subtype of the amino acid transport system N. Am. J. Physiol. Cell. Physiol. 2001;281:C1757–C1768. [Google Scholar]
- 11.Chaudhry F. A., Krizaj D., Larsson P., Reimer R. J., Wreden C., Storm-Mathisen J., Copenhagen D., Kavanaugh M., Edwards R. H. Coupled and uncoupled proton movement by amino acid transport system N. EMBO J. 2001;20:7041–7051. doi: 10.1093/emboj/20.24.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broer A., Albers A., Setiawan I., Edwards R. H., Chaudhry F. A., Lang F., Wagner C. A., Broer S. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J. Physiol. 2002;539:3–14. doi: 10.1113/jphysiol.2001.013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bode B. P. Recent molecular advances in mammalian glutamine transport. J. Nutr. 2001;131:2475S–2485S. doi: 10.1093/jn/131.9.2475S. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry F. A., Schmitz D., Reimer R. J., Larsson P., Gray A. T., Nicoll R., Kavanaugh M., Edwards R. H. Glutamine uptake by neurons: interaction of protons with system A transporters. J. Neurosci. 2002;22:62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albers A., Broer A., Wagner C. A., Setiawan I., Lang P. A., Kranz E. U., Lang F., Broer S. Na+ transport by the neural glutamine transporter ATA1. Pflugers Arch. 2001;443:92–101. doi: 10.1007/s004240100663. [DOI] [PubMed] [Google Scholar]
- 16.Bertran J., Roca A., Pola E., Testar X., Zorzano A., Palacin M. Modification of system A amino acid carrier by diethyl pyrocarbonate. J. Biol. Chem. 1991;266:798–802. [PubMed] [Google Scholar]
- 17.Fei Y. J., Liu W., Prasad P. D., Kekuda R., Oblak T. G., Ganapathy V., Leibach F. H. Identification of the histidyl residue obligatory for the catalytic activity of the human H+/peptide cotransporters PEPT1 and PEPT2. Biochemistry. 1997;36:452–460. doi: 10.1021/bi962058p. [DOI] [PubMed] [Google Scholar]
- 18.Aubrey K. R., Mitrovic A. D., Vandenberg R. J. Molecular basis for proton regulation of glycine transport by glycine transporter subtype 1b. Mol. Pharmacol. 2000;58:129–135. doi: 10.1124/mol.58.1.129. [DOI] [PubMed] [Google Scholar]
- 19.Zong X., Stieber J., Ludwig A., Hofmann F., Biel M. A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. J. Biol. Chem. 2001;276:6313–6319. doi: 10.1074/jbc.M010326200. [DOI] [PubMed] [Google Scholar]
- 20.Wiebe C. A., Dibattista E. R., Fliegel L. Functional role of polar amino acid residues in Na+/H+ exchangers. Biochem. J. 2001;357:1–10. doi: 10.1042/0264-6021:3570001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchiyama T., Kulkarni A. A., Davies D. L., Lee V. H. Biophysical evidence for His57 as a proton-binding site in the mammalian intestinal transporter hPepT1. Pharm. Res. 2003;20:1911–1916. doi: 10.1023/b:pham.0000008036.05892.e9. [DOI] [PubMed] [Google Scholar]
- 22.Peter G. J., Davidson I. G., Ahmed A., McIlroy L., Forrester A. R., Taylor P. M. Multiple components of arginine and phenylalanine transport induced in neutral and basic amino acid transporter-cRNA-injected Xenopus oocytes. Biochem. J. 1996;318:915–922. doi: 10.1042/bj3180915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baird F. E., Beattie K. J., Hyde A. R., Ganapathy V., Rennie M. J., Taylor P. M. Bidirectional substrate fluxes through the System N (SNAT5) glutamine transporter may determine net glutamine flux in rat liver. J. Physiol. 2004;559:367–381. doi: 10.1113/jphysiol.2003.060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- 25.Dzhandzhugazyan K. N., Plesner L. Diethyl pyrocarbonate inactivates CD39/ecto-ATPDase by modifying His-59. Biochim. Biophys. Acta. 2000;1466:267–277. doi: 10.1016/s0005-2736(00)00169-3. [DOI] [PubMed] [Google Scholar]
- 26.Tao Z., Grewer C. The conserved histidine 295 does not contribute to proton cotransport by the glutamate transporter EAAC1. Biochemistry. 2005;44:3466–3476. doi: 10.1021/bi047812i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunte C., Screpanti E., Venturi M., Rimon A., Padan E., Michel H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature (London) 2005;435:1197–1202. doi: 10.1038/nature03692. [DOI] [PubMed] [Google Scholar]
- 28.Cha B., Oh S., Shanmugaratnam J., Donowitz M., Yun C. C. Two histidine residues in the juxta-membrane cytoplasmic domain of Na+/H+ exchanger isoform 3 (NHE3) determine the set point. J. Membr. Biol. 2003;191:49–58. doi: 10.1007/s00232-002-1044-2. [DOI] [PubMed] [Google Scholar]
- 29.Clarke C. E., Benham C. D., Bridges A., George A. R., Meadows H. J. Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J. Physiol. 2000;523:697–703. doi: 10.1111/j.1469-7793.2000.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chanchevalap S., Yang Z., Cui N., Qu Z., Zhu G., Liu C., Giwa L. R., Abdulkadir L., Jiang C. Involvement of histidine residues in proton sensing of ROMK1 channel. J. Biol. Chem. 2000;275:7811–7817. doi: 10.1074/jbc.275.11.7811. [DOI] [PubMed] [Google Scholar]
- 31.Munoz P., Guma A., Camps M., Furriols M., Testar X., Palacin M., Zorzano A. Vanadate stimulates system A amino acid transport activity in skeletal muscle: evidence for the involvement of intracellular pH as a mediator of vanadate action. J. Biol. Chem. 1992;267:10381–10388. [PubMed] [Google Scholar]
- 32.Hyde R., Christie G. R., Litherland G. J., Hajduch E., Taylor P. M., Hundal H. S. Subcellular localization and adaptive up-regulation of the System A (SAT2) amino acid transporter in skeletal-muscle cells and adipocytes. Biochem. J. 2001;355:563–568. doi: 10.1042/bj3550563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haussinger D. Hepatic glutamine transport and metabolism. Adv. Enzymol. Relat. Areas Mol. Biol. 1998;72:43–86. doi: 10.1002/9780470123188.ch3. [DOI] [PubMed] [Google Scholar]
- 34.Chesler M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 35.Bevington A., Brown J., Butler H., Govindji S., M-Khalid K., Sheridan K., Walls J. Impaired system A amino acid transport mimics the catabolic effects of acid in L6 cells. Eur. J. Clin. Invest. 2002;32:590–602. doi: 10.1046/j.1365-2362.2002.01038.x. [DOI] [PubMed] [Google Scholar]