Abstract
Wild-type Escherichia coli cells grow normally in 95% O2/5% CO2. In contrast, cells that cannot make polyamines because of mutations in the biosynthetic pathway are rapidly killed by incubation in 95% O2/5% CO2. Addition of polyamines prevents the toxic effect of oxygen, permitting cell survival and optimal growth. Oxygen toxicity can also be prevented if the growth medium contains an amino acid mixture or if the polyamine-deficient cells contain a manganese-superoxide dismutase (Mn-SOD) plasmid. Partial protection is afforded by the addition of 0.4 M sucrose or 0.4 M sorbitol to the growth medium. We also report that concentrations of H2O2 that are nontoxic to wild-type cells or to mutant cells pretreated with polyamines kill polyamine-deficient cells. These results show that polyamines are important in protecting cells from the toxic effects of oxygen.
It is well known from the classic studies of Fridovich and his group (1–5) and others (6–8) that Escherichia coli cells, grown in air, have mechanisms for the protection of the cells from the toxic effects of superoxide and of oxygen radicals. Of particular importance is the protective effect exerted by the superoxide dismutase that is present in these cells. It has also been observed that E. coli cells can grow at a normal rate in an atmosphere of increased oxygen, presumably by operation of these protective mechanisms (7).
Polyamines, the ubiquitous polycationic compounds, are associated with a variety of biological processes, such as nucleic acid biosynthesis, cell growth, and differentiation (9, 10). In this paper, we present data showing that another important factor in the protection of E. coli cells from the toxic effects of oxygen is the intracellular level of polyamines. We have found that polyamine-deficient cells are rapidly killed by incubation in oxygen, even though, as we previously reported, when incubated in air, these same cells are not killed and grow indefinitely (albeit at a somewhat reduced growth rate; ref. 11).
Materials and Methods
The strains used are listed in Table 1. All strains were maintained on LB plates. All incubations were at 37°C with vigorous shaking. The various mutants were grown on a purified polyamine-free Vogel–Bonner (VB) medium (Figs. 1–4; ref. 16) or M63 medium (Fig. 5; ref. 7) [supplemented with glucose and the auxotrophic requirements for EWH319 and HT306 (thiamin, pantothenate, proline, and threonine)] for at least 20–25 generations to deplete any polyamines remaining from the original LB medium. The cultures then were grown overnight in limiting glucose (0.025%) to an OD600 of 0.1–0.2. Additional glucose (0.4%) was then added, plus polyamines where indicated, and the cultures were incubated for another 2 h. The cultures were then diluted to an OD600 of 0.001 into the same medium with and without polyamines.† In some of the experiments an amino acid mixture (Fig. 3A), sucrose or sorbitol (Fig. 3B) was added at this point. For experiments presented in Fig. 5 (with pDT1.5, sodA plasmid), the cultures were grown in M63 medium containing 100 μg/ml ampicillin. The diluted cultures were then incubated in an atmosphere of air or 95% O2/5% CO2, as indicated. Periodically, aliquots were diluted into VB medium and spread on LB plates for viable cell counts.
Table 1.
Strain list and polyamine content
| Strain designation | References or sources | Mutations in polyamine biosynthetic pathway* | Amine content, μmol/g wet weight†
|
|
|---|---|---|---|---|
| Putrescine | Spermidine | |||
| 71-18 | 12 | None | 7 | 1.2 |
| EWH319 | 13 | Δ(speAspeB) ΔspeC Δ(speDspeE) | 0‡ | 0‡ |
| HT306 | 11 | Δ(speAspeB) ΔspeC Δ(speDspeE)cadA | 0‡ | 0‡ |
| EWH319/pDT1.5 | This study§ | Δ(speAspeB) ΔspeC Δ(speDspeE) | ¶ | ¶ |
| HT551 | 14 | Δ(speDspeE) | 12 | 0‡ |
speA, arginine decarboxylase; speB, agmatine ureohydrolase; speC, ornithine decarboxylase; speD, S-adenosylmethionine decarboxylase; speE, spermidine synthase (putrescine aminopropyltransferase).
The cells used for these assays were grown in polyamine-deficient VB medium to an OD600 of 0.4–0.5, collected by centrifugation, and extracted with 5% trichloroacetic acid. The supernatant was assayed for putrescine and spermidine by HPLC chromatography (14).
Not detected by HPLC analysis.
JI171, an E. coli strain containing pDT1.5 overexpressing E. coli Mn-SOD (sodA) (6), was kindly provided by J. Imlay (University of Illinois, Urbana). Plasmid DNA was isolated and used to transform EWH319 with standard procedures.
Not determined.
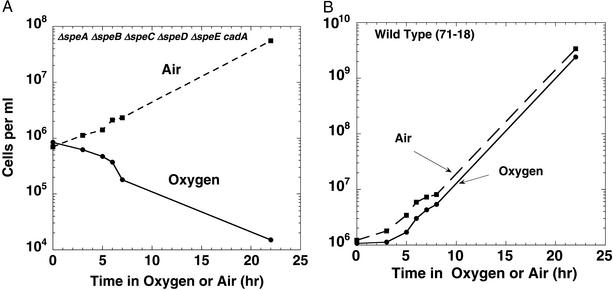
Figure 1.
(A) Oxygen toxicity and cell death of polyamine-deprived E. coli cells. E. coli HT306 was grown on polyamine-free VB medium for 20–25 generations and diluted to an OD600 of 0.001, as described in Materials and Methods. Replicate cultures were incubated in either air or 95% O2/5% CO2 and assayed periodically for viable cell counts. (B) Wild-type E. coli are resistant to oxygen-induced cell death. Wild-type E. coli (71–18) cells were grown in amine-free VB medium as described above, diluted to an OD600 of 0.001, and incubated in either air or 95% O2/5% CO2.
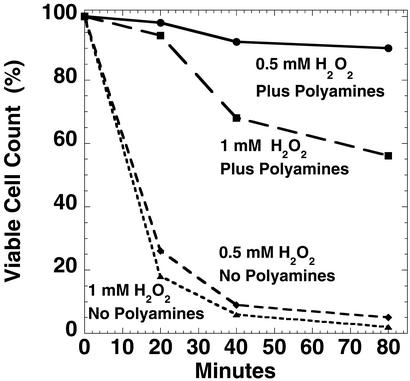
Figure 4.
Polyamine deficiency results in H2O2 hypersensitivity. HT306 cells were grown in amine-deficient VB medium to deplete cellular amines. The cultures were diluted 1:50 and divided into two parts. To one part of the culture, a mixture of 10−5 M of spermidine and 10−4 M of putrescine was added; to another part, no amines were added. The cultures were grown overnight to an OD600 of 0.15–0.20, the cells were washed twice and resuspended in K-PO4 buffer (pH 7), and different concentrations of hydrogen peroxide were added. Periodically, aliquots were taken for viable cell counts. The data are plotted as the percent survival after the H2O2 treatment; the initial cell count was 1.1 × 108 cells per ml.
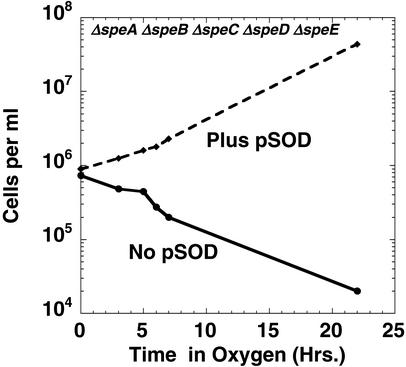
Figure 5.
Overexpression of Mn-SOD prevents oxygen toxicity of polyamine-deprived cells. EWH319 and EWH319/pDT1-5 were grown in M63 medium to deplete intracellular amines, as described in the previous figure legends. The plasmid-containing cells were grown in 100 μg/ml ampicillin. The amine-depleted cultures were diluted to an OD600 of 0.001 and incubated in an atmosphere of 95% O2/5% CO2; periodically, aliquots were taken for viable cell counts.
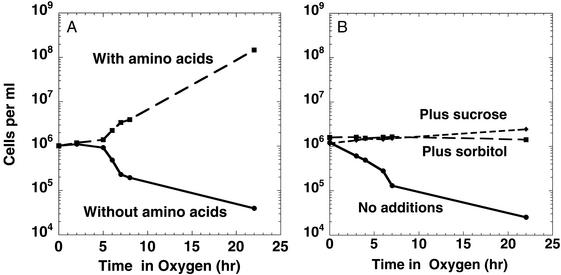
Figure 3.
Addition of amino acids (A) or of sucrose or sorbitol (B) prevents oxygen-induced cell death of polyamine-deficient cells. In each experiment, HT306 was grown in polyamine-free VB medium and diluted to an OD600 = 0.001, as described in the legend to Fig. 1A. (A) A portion of the culture contained a 12-amino acid mixture, as described in Materials and Methods. (B) Either sorbitol or sucrose (final concentration = 0.4 M) was added to portions of the culture. The cultures were incubated in a 95% O2/5% CO2 atmosphere; periodically, aliquots were taken for viable cell counts.
For the experiments studying the toxicity of hydrogen peroxide, the deprived cells were incubated overnight in limiting glucose with and without the addition of putrescine (10−4 M) and spermidine (10−5 M) to an OD600 of 0.15–0.2. Additional glucose (0.4%) was then added to each culture, and after a further incubation for 2 h, the cells were harvested by centrifugation and washed twice with 100 mM potassium phosphate (pH 7) to remove extracellular amines that might react directly with the hydrogen peroxide. The cells were resuspended in the same phosphate buffer. Different concentrations of hydrogen peroxide were added, and the mixtures were incubated in air with shaking at 37°C. At 20-min intervals, aliquots were diluted into 50 mM phosphate buffer (pH 7) and plated on LB plates for determination of survival rates. Results are presented as percent cell survival based on the cell count at zero time.
The amino acid mix used in Fig. 3A contained the 12 amino acids at the concentrations present in the standard MEM amino acid mix (17). The amino acid mix as well as the sorbitol and sucrose solutions used in Fig. 3B contained no measurable polyamine contamination, as measured by HPLC chromatography and by bioassays with polyamine-requiring mutants of Saccharomyces cerevisiae (18).
Results
Polyamine Deficiency Results in Oxygen Toxicity.
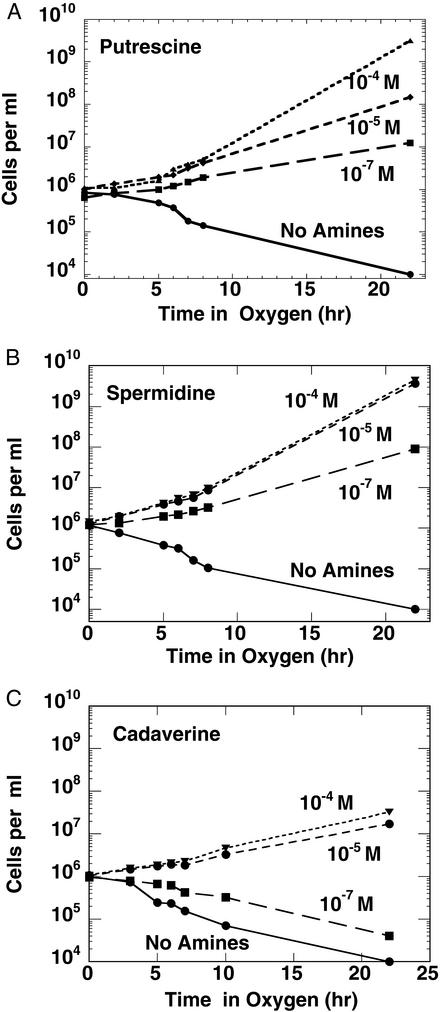
E. coli strain HT306 contains deletion mutations in the genes required for polyamine biosynthesis [Δ (speAspeB) Δ speC Δ (speDspeE) cadA] and, therefore, lacks putrescine and spermidine when grown in purified medium (11). As previously described, despite this lack of intracellular polyamines, cultures of this organism grow indefinitely in air (Fig. 1A), although the growth rate is 30–50% of the rate found in amine-supplemented media. In contrast, when these cultures were incubated in 95% O2/5% CO2, marked inhibition of growth and cell death occurred (Fig. 1A). Viable cell counts showed 40–50% cell death within 7–8 h; after 22 h of incubation under oxygen, only 0.1–0.5% cells survived. Comparable results were obtained with EWH319, which is similar to HT306, except for a normal cadA gene. Wild-type cells showed very little, if any, oxygen toxicity and grew at a nearly normal growth rate under similar conditions (Fig. 1B). Addition of putrescine or spermidine to the culture medium was completely effective in preventing the toxic effects of oxygen (Fig. 2 A and B). Considerable protection (but not complete) was obtained by the addition of higher (10−4 to 10−5 M) concentrations of cadaverine (Fig. 2C).
Figure 2.
Addition of exogenous putrescine (A), spermidine (B), or cadaverine (C) protects cells from oxygen toxicity. HT306 E. coli cells were grown in VB medium to deplete intracellular polyamines. The cultures were then incubated for 2 h with different concentrations of putrescine (A), spermidine (B), or cadaverine (C). These were then diluted to OD600 = 0.001 and incubated with or without the indicated concentrations of putrescine, spermidine, or cadaverine in 95% O2/5% CO2 and periodically assayed for viable cells.
Because these data showed that either putrescine or spermidine would protect the cells from oxygen, we next tested whether incubation in oxygen was toxic to HT551, a strain that can synthesize putrescine but lacked spermidine. This strain did not show any oxygen toxicity (data not shown).
Oxygen Toxicity to Polyamine-Deficient E. coli Cells Is Partially Prevented by Added Amino Acids or Sucrose.
Fridovich and others (7, 19–22) have shown that superoxide toxicity in E. coli results in a number of phenotypes, including amino acid auxotrophy and damage to the cell membrane. Their experiments were carried out in air with E. coli cells that had normal polyamine levels but that lacked superoxide dismutase. In the following experiments, we tested whether a similar phenotype occurs in the polyamine-deficient cells that are incubated in oxygen.
In the experiment presented in Fig. 3A, we showed that, if the medium contained a mixture of 12 amino acids (arginine, cystine, histidine, isoleucine, leucine, lysine, methionine, threonine, valine, phenylalanine, tyrosine, and tryptophane), essentially no toxicity of oxygen was observed. Thus, such cultures showed no cell death and grew at essentially the same rate in oxygen as in air. If the three aromatic amino acids were not included, the protective effect was less; the growth rate was only 40% of that seen with the 12-amino acid mix (data not shown). Combinations of leucine and valine, or leucine and isoleucine, or phenylalanine, tyrosine and tryptophane, or cystine and methionine did not protect the polyamine-deficient E. coli cells from oxygen toxicity (data not shown). Note that the addition of amino acids to the polyamine-deficient culture did not result in as high of a growth rate as was found when polyamines were added to the medium (Fig. 3A compared with Fig. 2 A and B); i.e., the addition of amino acids did not completely correct the need for polyamines for optimal growth. Similarly, in other experiments (data not shown), we have found that the addition of this amino acid mix to polyamine-deficient cells incubated in air did not result in any increase in growth rate.
To test whether the death of the polyamine-deficient cells in oxygen was caused by membrane damage and cell lysis, we incubated the polyamine-deficient cells in oxygen in the presence of either 0.4 M sucrose or 0.4 M sorbitol. As shown in Fig. 3B, under these conditions, the cells were not killed. However, even though the cells were not killed, the cultures did not show any growth, indicating that damage to the cell membrane was not the only deleterious effect of oxygen to polyamine-deficient cells. In other experiments (data not shown), we found that the addition of sorbitol or sucrose did not affect the growth rate of polyamine-deficient cells grown in air; i.e., these cultures still grew at 30–50% of the growth rate obtained when polyamines were added to the growth medium, indicating that cell lysis did not account for the decreased growth rate found in air with polyamine-deficient cells.
Overexpression of sodA Protects Cells from Oxygen Toxicity During Amine Deficiency.
To test whether superoxide was involved in the toxicity, we studied whether overexpression of a sod-containing plasmid (Mn-SOD, pDT1.5) would protect the polyamine-deficient cells from the toxic effects of oxygen. As shown in Fig. 5, overexpression of the sodA gene in polyamine-deficient cells prevented the toxic effects of oxygen; the polyamine-deficient cells containing the sod plasmid grew as well in oxygen as in air. Note that both the plasmid-containing cells and the control cells had intact sod genes in their chromosomes; i.e., the normal amount of superoxide dismutase was not sufficient to protect the polyamine-deficient cells from oxygen toxicity.
It is noteworthy that the presence of the sodA-containing plasmid did not affect the growth rate of the polyamine-deficient cells in air; i.e., the growth rate was still 30–50% of that seen with polyamine supplementation (data not shown), indicating that superoxide is not responsible for the decreased growth rate that we had observed in polyamine-deficient cells grown in air.
Al-Maghrebi et al. (23) have shown that the addition of Mn++ diminished the oxygen-dependent phenotypic deficits exhibited by sodA sodB E. coli; i.e., Mn++ mimics SOD activity. In our experimental condition, we found that the addition of 50 μM Mn++ reduced the lethal effect of oxygen, but did not permit any growth (data not shown). However, these experiments are hard to interpret because we have recently found that the addition of Mn++ (even at 25 μM) is particularly inhibitory to the growth of polyamine-deficient culture in air (data not shown).
Polyamine-Deficient Cells Are Hypersensitive to H2O2.
As shown in Fig. 4, polyamine-deficient cells were much more sensitive to H2O2 than polyamine-supplemented cells. Thus, a challenge with 0.5 or 1 mM of H2O2 resulted in the death of >96% of the amine-deficient E. coli cells within 40 min.
Discussion
The experiments reported in this paper clearly indicate that polyamines are important in protecting E. coli cells from the toxic effects of oxygen, superoxide, and hydrogen peroxide. These results are consistent with our earlier work (24) showing that paraquat, a known source of superoxide (1), is more toxic to polyamine-deficient cells than to cells containing polyamines. The present results are more definitive, however, because our results with paraquat might have been complicated by differences in the intracellular distribution of the paraquat in normal vs. polyamine-deficient cells or in the uptake of paraquat. In addition, some details of the protective action of polyamines against oxygen toxicity reported in this paper are different from those found in the paraquat experiments (24). Thus, in the current work, either putrescine, spermidine, or cadaverine protected the cells from oxygen toxicity, whereas in our experiments with paraquat, spermidine was much more protective than putrescine. In the current experiments, HT551, which was only defective in the biosynthesis of spermidine, showed no increase in oxygen toxicity (data not shown), whereas cultures of this strain did show increased toxicity of paraquat in our previous study (24).
There have been many studies on the effect of oxygen stress in a variety of species and on the formation of O2−, H2O2, OH−, and singlet oxygen (1O2) in organisms growing in air (4, 7, 25, 26). These active species of oxygen can react with many cellular components, including DNA, RNA, proteins, and lipids, and can result in mutagenesis, decreased growth rates, and even cell death. Therefore, it is relevant to note that the protective effect of polyamines against oxygen toxicity is not restricted to polyamine-deficient E. coli. In earlier work, we reported that polyamine-deficient S. cerevisiae cells showed increased oxygen toxicity (27), but these results were somewhat different from the present results with E. coli, because in S. cerevisiae, only spermidine had a protective effect, and no protection was afforded by putrescine. E. coli is more convenient for these studies because cells completely deprived of polyamines can still grow and, thus, one can study the effect of oxygen in a steady-state situation, whereas with S. cerevisiae, the cells stop growing when completely polyamine-deficient. The results with S. cerevisiae were further complicated by the fact that S. cerevisiae lose mitochondria during amine deprivation (27).
The current experiments were designed to test the protective effects of polyamines in vivo. There have been several in vitro studies showing that polyamines can protect certain cell components such as DNA from the toxic effects of hydrogen peroxide or of singlet oxygen (28–31), or that the polyamines can directly react with oxygen radicals as scavengers in vitro (28, 32). However, it is somewhat difficult to evaluate whether these studies are applicable in vivo, especially because some of the studies used rather high concentrations of polyamines that might have reacted directly with the nascent oxygen species that was added or might have complexed with the copper used to generate the oxygen species (28, 29). A possible effect of polyamines in vivo was reported by Tkachenko et al. (33), who found that a large amount of polyamines added to wild-type E. coli cells up-regulated expression levels of oxyR/katG genes and increased cell survival after exposure to hydrogen peroxide. One might also speculate that putrescine might stimulate the activity of SOD. However, we have found that there was no difference in the SOD content of polyamine-deficient and wild-type cells. We also have added putrescine to the standard in vitro assay (34) for SOD (E. coli, Mn-SOD, Sigma) and have found that the addition of putrescine did not stimulate the activity of the enzyme (data not shown).
It is attractive to speculate that the polyamines protect the cells from the lethal effects of oxygen by protecting various cell moieties from being damaged by the reactive oxygen species. Such protection would be consistent with the various in vitro studies mentioned above, and would be comparable to previous reports from this and other laboratories on the protective effects of polyamines against DNA shearing or heat denaturation, ribosome dissociation, protoplast lysis, etc. (9, 10). The experiments showing the protective effect of amino acids or of sorbitol/sucrose (Fig. 3) are consistent with the findings of the Fridovich group (19, 20) on the protective effect of these agents in sodA sodB mutants, and indicate that, in the absence of polyamines, incubation in oxygen causes damage to some enzymes involved in amino acid biosynthesis, as well as damage to cell membranes (20). It is also possible, however, that the polyamines might react directly with the reactive oxygen species in vivo. However, our experiments do not permit us to distinguish between these alternatives.
Although the experiments in this paper with oxygen and with hydrogen peroxide clearly show that polyamines are important for protecting cells from oxidative stress, it should be emphasized that polyamines do have other functions, because the presence of SOD-containing plasmid or additions of amino acids or of sorbitol/sucrose did not affect the growth rate of polyamine-deficient cells in air.
Abbreviations
- SOD
superoxide dismutase
- VB
Vogel–Bonner
Footnotes
These experiments were carried out at a low optical density (initial OD600 = 0.001) to permit better oxygenation of the culture. In other experiments (data not shown), we used cultures that were more dense (initial OD600 = 0.03), but we found that these cultures required intensive aeration with a sparger to obtain comparable results.
References
- 1.Hassan H M, Fridovich I. J Biol Chem. 1978;253:8143–8148. [PubMed] [Google Scholar]
- 2.Fridovich I. Photochem Photobiol. 1978;28:733–741. doi: 10.1111/j.1751-1097.1978.tb07009.x. [DOI] [PubMed] [Google Scholar]
- 3.Fridovich I. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 4.Fridovich I. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 5.Fridovich I. J Exp Biol. 1998;201:1203–1209. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 6.Touati D. J Bacteriol. 1983;155:1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlioz A, Touati D. EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farr S B, D'Ari R, Touati D. Proc Natl Acad Sci USA. 1986;83:8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabor C W, Tabor H. Microbiol Rev. 1985;49:81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen S S. A Guide to the Polyamines. New York: Oxford Univ. Press; 1998. pp. 1–595. [Google Scholar]
- 11.Tabor H, Hafner E W, Tabor C W. J Bacteriol. 1980;144:952–956. doi: 10.1128/jb.144.3.952-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messing J, Gronenborn B, Muller-Hill B, Hans Hopschneider P. Proc Natl Acad Sci USA. 1977;74:3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafner E W, Tabor C W, Tabor H. J Biol Chem. 1979;254:12419–12426. [PubMed] [Google Scholar]
- 14.Tabor C W, Tabor H, Xie Q W. Proc Natl Acad Sci USA. 1986;83:6040–6044. doi: 10.1073/pnas.83.16.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamasaki-Katagiri N, Tabor C W, Tabor H. Gene. 1997;187:35–43. doi: 10.1016/s0378-1119(96)00660-9. [DOI] [PubMed] [Google Scholar]
- 16.Vogel H J, Bonner D M. J Biol Chem. 1956;219:97–106. [PubMed] [Google Scholar]
- 17.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1999. [Google Scholar]
- 18.Balasundaram D, Tabor C W, Tabor H. Proc Natl Acad Sci USA. 1991;88:5872–5876. doi: 10.1073/pnas.88.13.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benov L, Fridovich I. J Biol Chem. 1999;274:4202–4206. doi: 10.1074/jbc.274.7.4202. [DOI] [PubMed] [Google Scholar]
- 20.Benov L, Kredich N M, Fridovich I. J Biol Chem. 1996;271:21037–21040. doi: 10.1074/jbc.271.35.21037. [DOI] [PubMed] [Google Scholar]
- 21.Kuo C F, Mashino T, Fridovich I. J Biol Chem. 1987;262:4724–4727. [PubMed] [Google Scholar]
- 22.Liochev S I, Fridovich I. Free Radical Biol Med. 1994;16:29–33. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 23.Al-Maghrebi M, Fridovich I, Benov L. Arch Biochem Biophys. 2002;402:104–109. doi: 10.1016/S0003-9861(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 24.Minton K W, Tabor H, Tabor C W. Proc Natl Acad Sci USA. 1990;87:2851–2855. doi: 10.1073/pnas.87.7.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imlay J A, Chin S M, Linn S. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 26.Imlay J A, Linn S. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 27.Balasundaram D, Tabor C W, Tabor H. Proc Natl Acad Sci USA. 1993;90:4693–4697. doi: 10.1073/pnas.90.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha H C, Sirisoma N S, Kuppusamy P, Zweier J L, Woster P M, Casero R A J. Proc Natl Acad Sci USA. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha H C, Yager J D, Woster P A, Casero R A J. Biochem Biophys Res Commun. 1998;244:298–303. doi: 10.1006/bbrc.1998.8258. [DOI] [PubMed] [Google Scholar]
- 30.Khan A U, Di M P, Medeiros M H, Wilson T. Proc Natl Acad Sci USA. 1992;89:11428–11430. doi: 10.1073/pnas.89.23.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan A U, Mei Y H, Wilson T. Proc Natl Acad Sci USA. 1992;89:11426–11427. doi: 10.1073/pnas.89.23.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorlet G, Dumbroff E B, Legge R L, Thompson J E. Phytochemistry. 1986;25:367–371. [Google Scholar]
- 33.Tkachenko A, Nesterova L, Pshenichnov M. Arch Microbiol. 2001;176:155–157. doi: 10.1007/s002030100301. [DOI] [PubMed] [Google Scholar]
- 34.Crapo J D, McCord J M, Fridovich I. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]