Abstract
Des2 (degenerative spermatocyte 2) is a bifunctional enzyme that produces phytoceramide and ceramide from dihydroceramide. The molecular mechanism involved in C-4-hydroxylation has not been studied in detail. In the present paper, we report that C-4-hydroxylation requires an electron-transfer system that includes cytochrome b5 and that the hydroxylase activity is reconstituted in an in vitro assay with purified recombinant Des2. FLAG-tagged mouse Des2 was expressed in insect Sf9 cells and was purified by solubilization with digitonin and anti-FLAG antibody affinity column chromatography. The activity of dihydroceramide:sphinganine C-4-hydroxylase was reconstituted with the purified FLAG–Des2, mb5 (the membrane form of cytochrome b5) and bovine erythrocyte membrane. The apparent Km and Vmax of Des2 for the substrate N-octanoylsphinganine were 35 μM and 40 nmol·h−1·mg of protein−1 respectively. The Km of the hydroxylase for mb5 was 0.8 μM. Interestingly, mb5 was not replaced with the soluble form of cytochrome b5, which lacks the C-terminal membrane-spanning domain. The erythrocyte membrane was separated into Triton X-100-soluble and -insoluble fractions, and the detergent-soluble fraction was replaced by the soluble or membrane form of b5R (NADH-cytochrome b5 reductase). The Triton-X-100-insoluble fraction contained trypsin-resistant factors. The Des2 protein is found in the endoplasmic reticulum and is assumed to have three membrane-spanning domains. The findings of the present study indicate that the hydroxylation requires complex formation between Des2 and mb5 via their membrane-spanning domains and electron transfer from NADH to the substrate via the reduction of mb5 by b5R.
Keywords: cytochrome b5, degenerative spermatocyte 2 (Des2), dihydroceramide:sphinganine C-4-hydroxylation, microvillous membrane, N-acyl-4-hydroxysphinganine, phytoceramide
Abbreviations: b5R, NADH-cytochrome b5 reductase; BCA, bicinchoninic acid; Des1, degenerative spermatocyte 1; des-1, dihydroceramide Δ4-desaturase; Des2, degenerative spermatocyte 2; ER, endoplasmic reticulum; HPTLC, high performance thin layer chromatography; mb5, membrane form of cytochrome b5; MOI, multiplicity of infection; N-octanoyl-C18-phytosphingosine, N-octanoyl-D-ribo-1,3,4-trihydroxy-2-amino-octadecane; sb5, soluble form of cytochrome b5
INTRODUCTION
Cell membranes are composed of phospholipids, cholesterol, sphingolipids, glycosphingolipids and proteins. Sphingolipids and glycosphingolipids are involved in signal transduction [1,2] and support membrane protein functions by providing the membrane microdomain environment [3–5]. The molecular mechanisms of how these membrane components support the various functions remain unclear. Previous studies have shown that the microvillous membranes of epithelial cells in the small intestine of mice are very rich in glycosphingolipids containing 4-hydroxylsphinganine and α-hydroxy fatty acid [6–8], and this characteristic is conserved in various mammals [9–13]. One function of the microvillous membrane is to absorb digested nutrients. Therefore the functions of various transporter proteins must be supported by microvillous membrane-specific lipid components. We aim to understand the biological functions of the hydroxy group at the sphinganine C-4 position of hydroxyceramide.
After the Drosophila melanogaster des-1 (dihydroceramide Δ4-desaturase) gene product [14,15] and its mouse homologue [degenerative spermatocyte 1 (Des1)] were identified as sphingolipid Δ4-desaturase, another mouse homologue, Des2 (degenerative spermatocyte 2), was demonstrated to have both sphingolipid C-4-hydroxylase and Δ4-desaturase activities, suggesting that Des2 is involved in sphingolipid C-4-hydroxylation [16]. We have reported previously that Des2 is responsible for the biosynthesis of glycosphingolipids containing 4-hydroxysphinganine in the small intestine of mice, on the basis of the following. First, homogenates from COS-7 cells transfected with mouse Des2 cDNA exhibited C-4-hydroxylase activity in an in vitro assay with N-octanoyl-sphinganine as the substrate. Secondly, the Des2 mRNA content was regulated in a tissue-specific manner and was high in the mouse small intestine. Thirdly, in situ hybridization detected Des2 mRNA in mouse crypt cells. Fourthly, immunohistochemistry using an anti-(Des2 peptide) antibody stained mouse crypt cells and the adjacent epithelial cells [17].
Glycolipids with 4-hydroxysphinganine are found only in the intestine, kidney and skin, with the highest content observed in the small intestine [6–13,18]. We measured dihydroceramide:sphinganine C-4-hydroxylase activity in the homogenates or microsomal fractions of mouse small intestine and kidney. We were able to detect the C-4-hydroxylase activity in the kidney but not in the small intestine owing to very high ceramidase activity. We chose to express Des2 as a recombinant protein and to reconstitute the C-4-hydroxylase activity in vitro using purified components. We have discussed previously that, as a preliminary result, the addition of cytochrome b5 to a C-4-hydroxylase assay mixture increased the hydroxylase activity, suggesting the involvement of the electron-transfer system that includes cytochrome b5 [17]. To determine how the structure of 4-hydroxysphinganine supports microvillous membrane functions, it would be useful to manipulate the 4-hydroxysphinganine content of glycosphingolipids in cultured cells or experimental animals. To do this, we studied the molecular mechanism of C-4-hydroxylation. In the present study, we reconstituted the C-4-hydroxylase activity in vitro using purified FLAG-tagged Des2, cytochrome b5 and bovine erythrocyte membrane. A novel interesting finding is that Des2 requires the mb5 (membrane form of cytochrome b5) for C-4-hydroxylation and that the sb5 (soluble form of cytochrome b5) cannot support electron transfer from NADH to Des2. In addition, we demonstrated that the erythrocyte membrane was the source of b5R (NADH-cytochrome b5 reductase) and contained trypsin-resistant factors that support the C-4-hydroxylation.
EXPERIMENTAL
Materials
N-Octanoyl-D-erythro-[4,5-3H]C18-sphinganine was purchased from American Radiolabeled Chemicals; N-octanoyl-D-erythro-C18-sphingenine and N-octanoyl-D-erythro-C18-sphinganine were from BIOMOL Research Laboratories; N-octanoyl-C18-phytosphingosine (N-octanoyl-D-ribo-1,3,4-trihydroxy-2-amino-octa-decane) was from Avanti Polar Lipids. The protease-inhibitor cocktail tablets were from Roche, HPTLC (high performance thin layer chromatography) plates were from Merck; and the recombinant human His6-tagged mb5 was from PanVera, Invitrogen [19]. sb5 was purified from horse erythrocyte lysate using a method described previously [20]. The soluble form of b5R from human erythrocyte lysate and the membrane form of b5R from bovine erythrocyte membranes were purified using DEAE- and AMP-Sepharose column chromatography following methods described previously [21–23].
Construction and production of recombinant Des2
The full-length mouse Des2 cDNA, as described previously [17], was subcloned into the pFLAG-MAC vector (Sigma), adding a nucleotide sequence encoding the FLAG epitope (DYKDDDDDK) to the 5′-end of the Des2 cDNA. The region coding for FLAG–Des2 (∼1 kb) was subcloned into the pFastBac1 vector and the recombinant plasmid was used to transform DH10Bac™ competent Escherichia coli cells according to the manufacturer's protocol (Invitrogen). Recombinant Bacmid DNA was transfected into Sf9 cells. To amplify the viruses, the viruses harvested 72 h after transfection were used first to infect Sf9 insect cells (1×106 cells/ml) using an MOI (multiplicity of infection) of 1–10. The viral stocks were stored at 4 °C.
Cell culture, virus infection and preparation of cell homogenates
Sf9 cells were cultured in Sf900II serum-free medium with 10% (v/v) fetal bovine serum at 28 °C. The recombinant baculoviruses obtained from the first infection were subsequently used to infect Sf9 cells (1×106 cells/ml) at an MOI of 0.1–0.5. Cells derived from the second infection were harvested 48 h later. After washing with PBS, the cells were stored at −80 °C until use. The harvested cells (6×108 cells) were resuspended in 1 vol. (0.5 ml) of buffer A (0.1 M Tris/HCl, pH 7.5, 0.25 M sucrose, 1 mM EDTA, 5 mM dithiothreitol, 1 mM PMSF and the protease-inhibitor cocktail) and disrupted using a Physcotron Polytron-type microhomogenizer (NITI-ON) in an ice-bath with 30 s of homogenization and 30 s of rest five times. The cell homogenates were stored at −80 °C until use.
Enzymatic activity assay
The C-4-hydroxylase activity of Des2 was measured using the method described in [17] with slight modifications. Briefly, the reaction mixture was composed of 118 μM N-octanoyl-D-erythro-C18-sphinganine containing 1 μCi of N-octanoyl-D-erythro-[4,5-3H]C18-sphinganine, 0.3% octylglucoside, 50 mM Tris/HCl, pH 7.5, the indicated amount of cytochrome b5, and fractions containing FLAG–Des2 (cell homogenates, 600 g supernatant, detergent solubilized fractions and fractions collected before and after ultracentrifugation) or purified FLAG–Des2 in a total volume of 50 μl. The reactions were initiated by the addition of 100 mM NADH dissolved in 10 mM Tris/HCl, pH 7.5, and 1 mM EDTA at a final concentration of 5 mM, and were allowed to proceed for 2 h at 37 °C. Reactions were terminated by the addition of 150 μl of 50 mM Tris/HCl, pH 7.5, and 570 μl of chloroform/methanol (2:1, v/v). Lipids were recovered from the lower organic phase. The dried lipids collected at the bottoms of conical tubes were dissolved in 20 μl of chloroform/methanol (1:1, v/v). All the lipids were applied to borate-impregnated HPTLC plates using a microsyringe. Chloroform/methanol/water (60:20:2, by vol.) was used as the developing solvent. The lipids produced by the enzyme reaction were visualized and quantified by autoradiography with a Fuji Bas 2500 bio-imaging analyser. To identify spots by autoradiography, unlabelled N-octanoyl-D-erythro-C18-sphingenine, N-octanoyl-D-erythro-C18-sphinganine and N-octanoyl-C18-phytosphingosine were separated using HPTLC and detected by spraying the HPTLC plates with 3% (w/v) cupric acetate in an 8% (v/v) phosphoric acid solution and heating them to 180 °C for 10 min. The amount of reaction product was calculated as described previously [17].
Solubilization and purification of FLAG–Des2
The cell homogenates were centrifuged at 600 g for 10 min. The supernatant obtained was then solubilized with 1% (v/v) of each of the following detergents: digitonin, deoxycholate, octylglucoside or Triton X-100 at a protein concentration of 2 mg/ml for 1 h on ice and centrifuged at 50000 rev./min in a Beckman TLA110 rotor for 1 h at 4 °C. Samples (volume dependent on experiment) of the supernatants and pellets were analysed by Western blotting with the anti-FLAG M2 monoclonal antibody (Sigma). To purify FLAG–Des2, the supernatant obtained from 6×108 cells by centrifugation at 600 g for 10 min was solubilized with 1% digitonin for 1 h on ice and centrifuged at 50000 rev./min in a Beckman TLA110 rotor for 1 h at 4 °C. The resulting supernatant was adjusted to 0.2 M NaCl and 0.2% digitonin in buffer A, and applied to an anti-FLAG M2 antibody-conjugated agarose column (2 ml; Sigma) pre-equilibrated with buffer B (buffer A containing 0.2 M NaCl and 0.2% digitonin). The column was washed with 5 vol. (10 ml) each of buffers B, C (buffer A containing 0.2% digitonin) and D (buffer A containing 0.2% Triton X-100), and finally with 8 vol. of buffer C. The bound FLAG–Des2 was eluted with 5 vol. of buffer E (buffer C containing 100 μg/ml FLAG peptide). The fractions containing active enzyme were collected, concentrated using an Ultra-4 centrifugal filter (Millipore) and stored at −80 °C in a 1 M sucrose solution. The purified FLAG–Des2 was analysed by SDS/PAGE and Western blotting (see below). All procedures were performed at 4 °C unless stated otherwise. Protein concentrations were determined using the Bradford reagent or the BCA (bicinchoninic acid) method with BSA as the reference. The amount of purified FLAG–Des2 was estimated by densitometry of silver-stained SDS/PAGE gels with FLAG-tagged bacterial alkaline phosphatase (Sigma) as a reference.
SDS/PAGE, silver staining and Western blotting
SDS/PAGE was performed using 15% polyacrylamide gels under reducing conditions. The gels were stained with 2D-Silver Stain II (Daiichi Pure Chemicals), and the proteins blotted on to PVDF membranes were probed first with anti-FLAG M2 monoclonal antibody and then with horseradish-peroxidase-conjugated goat anti-mouse antibody. The immunoreactive blots were detected with ECL® (enhanced chemiluminescence) reagents (Amersham Biosciences) using the chemiluminescence detector LAS 1000 plus (Fuji Photo Film).
Preparation of bovine erythrocyte membrane
Bovine erythrocytes were haemolysed by a hypotonic treatment and the erythrocyte membranes were washed by centrifugation at 18500 g for 30 min at 4 °C with 5 mM Tris/HCl, pH 7.6, 1 mM 2-mercaptoethanol and 1 mM EDTA, according to the method described in [20]. The membranes were stored at −80 °C until use. To assay the enzymatic activity, the membrane pellet was homogenized with 1/3 vol. of buffer A in a Teflon homogenizer.
Reconstitution of the enzymatic activity and kinetic analysis with purified FLAG–Des2
The reaction mixtures contained, as standard assay conditions, 0.22 μg of purified FLAG–Des2, 1.4 μM of mb5 and 85 μg of bovine erythrocyte membrane protein. Substrate, FLAG–Des2 and mb5 saturation isotherms were determined, and Km and Vmax values were calculated using Lineweaver–Burk plots.
Solubilization of bovine erythrocyte membrane with Triton X-100
The bovine erythrocyte membrane was resuspended in 1/4 vol. of 0.1 M Tris/HCl, pH 7.5, 0.25 M sucrose and 1 mM EDTA and solubilized by the addition of a 10% (v/v) Triton X-100 adjusted to a final concentration of 1% (v/v) for 1 h on ice. Following the incubation, the samples were centrifuged at 50000 rev./min in a Beckman TLA110 rotor for 1 h at 4 °C. The supernatant fraction was used as the Triton X-100-soluble erythrocyte membrane fraction. A Triton X-100-insoluble fraction was prepared by the centrifugation of discontinuous sucrose layers composed of the Triton X-100 solubilized solution and 0.4 and 2 M sucrose solutions at 23500 rev./min in a Coulter JS-24 rotor for 1 h at 4 °C. The Triton X-100-insoluble fraction was recovered on the 2.0 M sucrose layer. The Triton X-100-soluble and -insoluble fractions were treated with trypsin (0.5 μg of trypsin per 30 μg of protein) for 2 h at 37 °C. The digestion was stopped by the addition of AEBSF [4-(2-aminoethyl)benzenesulphonyl fluoride] at a final concentration of 100 μM.
Double immunofluorescence staining of Des2 and cytochrome b5
Mouse small intestine was fixed in 4% (w/v) paraformaldehyde and 0.1 M phosphate buffer, pH 7.4. The tissue samples were immersed in 30% (w/v) sucrose and 0.1 M phosphate buffer, pH 7.4, frozen in OCT (optimal cutting temperature) compound at −80 °C and sliced into 3 μm thick sections. The frozen sections were incubated with 10% (v/v) goat serum in PBS to prevent non-specific antibody adsorption and then rabbit anti-Des2 serum (diluted 1:100) [17] and chicken anti-(cytochrome b5) antibody (diluted 1:500), which was kindly given by Dr J. B. Schenkman (Department of Pharmacology, University of Connecticut, Farmington, CT, U.S.A.). Goat anti-rabbit IgG conjugated to Alexa Fluor® 488 (Molecular Probes) and goat anti-chicken IgG conjugated to Alexa Fluor® 546 (Molecular Probes) were used as the secondary antibodies. The sections were examined using a Zeiss LSM 510 laser confocal microscope.
RESULTS
Requirement for mb5 in the C-4-hydroxylation and solubilization of FLAG–Des2
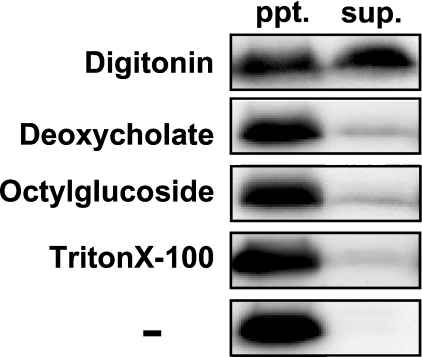
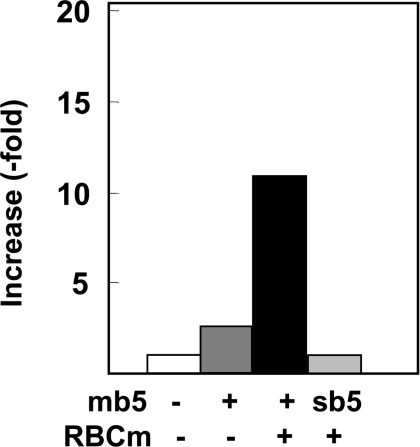
The addition of mb5 to an incubation mixture containing NADH and the homogenates of Sf9 cells transfected with FLAG–Des2 increased the hydroxylase activity in an mb5-dose-dependent manner up to 1.4 μM, whereas an inhibitory effect was observed at the highest concentration tested, 2.8 μM. By contrast, sb5 had no effect, suggesting that the hydrophobic domain of mb5 is required for the hydroxylation reaction, probably owing to the hydrophobic nature of the substrate. These results are not shown, but refer to the results obtained using the purified FLAG–Des2, as described below. To solubilize FLAG–Des2, the supernatants from lysed cells were treated with 1% digitonin, deoxycholate, octylglucoside or Triton X-100 and were then cleared by ultracentrifugation of the solubilized fractions. Western blotting of the cleared, solubilized supernatants with anti-FLAG antibody showed that digitonin solubilized FLAG–Des2 effectively (Figure 1). Since digitonin supported hydroxylase activity in an in vitro assay, we selected digitonin as the detergent for the purification of FLAG–Des2. The cleared digitonin-solubilized supernatant was used as an enzyme source and the effect of mb5 was also examined, which was the same compared with the cell homogenates. The involvement of mb5 suggested that b5R is also required for the hydroxylation. Therefore we tested this possibility using liver microsomes and bovine erythrocyte membrane as the source of the reductase. Mouse liver microsomes increased the hydroxylase activity (9-fold increase with 85 μg of microsomal protein) and bovine erythrocyte membrane was equally effective. Figure 2 shows the effect of bovine erythrocyte membrane on the hydroxylase activity in the presence of mb5. The addition of the membrane increased C-4-hydroxylase activity ∼12-fold. We used the bovine erythrocyte membrane instead of liver microsomes for further analysis because liver microsomes had a low level of C-4-hydroxylase activity and a high level of Des1, whereas the bovine erythrocyte membrane did not show any detectable C-4 hydroxylase or Des1 activities and contained much fewer proteins than the microsomes, which would be an advantage for purifying factors, if required. We used bovine erythrocyte membrane instead of microsomes in the C-4-hydroxylase assay in the process of FLAG–Des2 purification.
Figure 1. Solubilization of FLAG–Des2 from the 600 g supernatant of transfected Sf9 cells.
The fractions solubilized with various detergents at a final concentration of 1% (v/v) (see the Experimental section for details) were analysed by Western blotting with anti-FLAG antibody. The precipitates (ppt.) and supernatants (sup.) were separated by ultracentrifugation. − indicates no detergent used.
Figure 2. Effects of mb5 and bovine erythrocyte membrane on the C-4-hydroxylase activity in the fraction solubilized with 1% (v/v) digitonin.
The solubilized protein (25 μg) was incubated in the presence of 5.6 μM mb5 alone, or with 85 μg of bovine erythrocyte membrane (RBCm). The addition of 5.5 μM sb5 did not increase the activity. Results are the means for duplicate assays.
Purification of FLAG–Des2 expressed in Sf9 cells
The digitonin-solubilized fraction was applied to an anti-FLAG antibody-conjugated affinity column in buffer B (see the Experimental section for details). After the column was washed with buffer C, then with buffer D and then again with buffer C, the FLAG–Des2 was successfully eluted with FLAG peptide and 0.2% digitonin in buffer A (see Experimental section for buffer compositions). Table 1 summarizes the purification process; the final yield of enzyme activity of the purified FLAG–Des2 from the homogenates was 3%. Figure 3 shows the purified FLAG–Des2 detected by silver staining of an SDS/PAGE gel and by Western blotting with the anti-FLAG M2 monoclonal antibody. The observed molecular mass of the purified FLAG–Des2 was ∼35 kDa, which matches the estimated molecular mass of FLAG–Des2 of 38503 Da.
Table 1. The purification of FLAG–Des2.
The C-4 hydroxylase activity was measured in the presence of mb5 (5.7 μM) and bovine erythrocyte membrane (85 μg of protein) in a 50-μl incubation mixture. The proteins in the homogenates and ultracentrifugation-cleared supernatants were determined using Bradford reagent or the BCA method and the amount of purified Des2 was determined using densitometry, as described in the Experimental section, using FLAG-tagged bacterial alkaline phosphatase as a reference.
| Total protein (mg) | Total activity (pmol/h) | Specific activity (pmol·h−1·mg−1 of protein) | Yield (%) | |
|---|---|---|---|---|
| Homogenate | 30.6 | 22.57 | 0.737 | 100 |
| Cleared supernatant | 14.0 | 10.93 | 0.781 | 48.4 |
| Purified Des2 | 0.0064 | 0.72 | 113.57 | 3.2 |
Figure 3. SDS/PAGE and Western blotting of the purified FLAG–Des2.
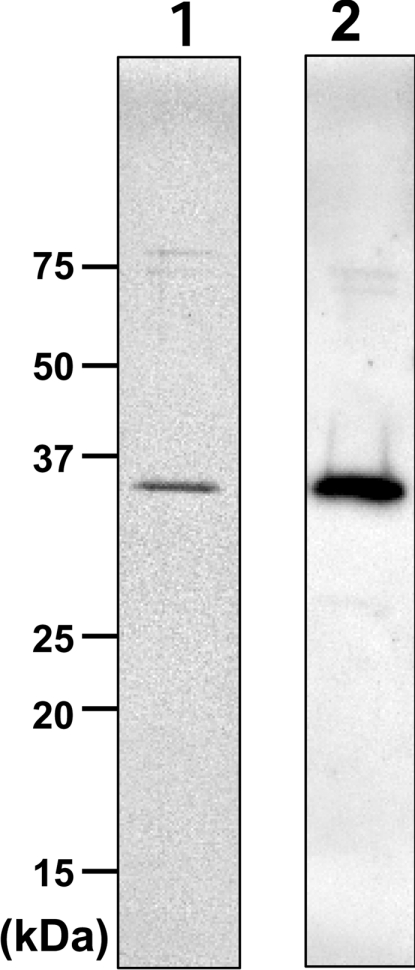
Lane 1, silver staining; lane 2, Western blotting with anti-FLAG antibody. Molecular masses are indicated in kDa.
Kinetics of the hydroxylase activity reconstituted with purified FLAG–Des2, mb5 and bovine erythrocyte membrane
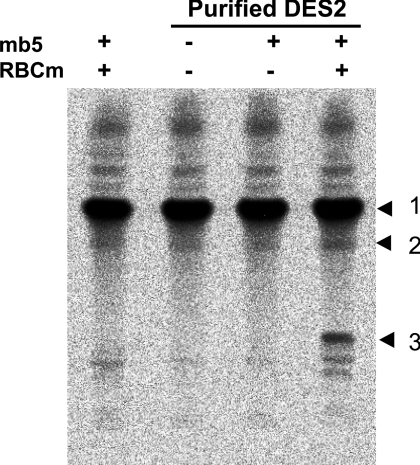
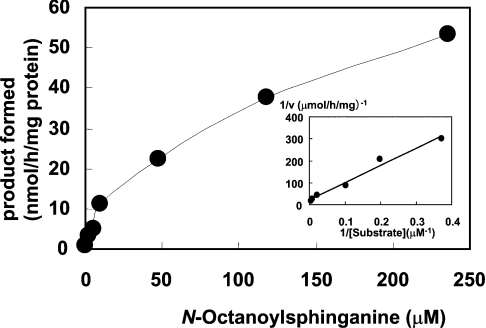
The purified FLAG–Des2, mb5 and bovine erythrocyte membrane were able to reconstitute the C-4-hydroxylase activity, as shown in Figure 4. The purified FLAG–Des2 alone, the combination of the purified FLAG–Des2 and mb5, or the combination of mb5 and the bovine erythrocyte membrane had no detectable hydroxylase activity. Almost no Δ4-desaturase activity was seen with the complete set of FLAG–Des2, mb5 and the bovine erythrocyte membrane. The apparent Km and Vmax of Des2 for N-octanoylsphinganine substrate calculated from a Lineweaver–Burk plot were 35 μM and 40 nmol·h−1·mg of protein−1 respectively, as shown in Figure 5. When 118 μM substrate and 0.22 μg FLAG–Des2 protein were used, the addition of mb5 up to 1.4 μM stimulated the hydroxylase activity linearly, whereas concentrations higher than 1.4 μM had an inhibitory effect (Figure 6). The apparent Km of the hydroxylase for mb5 calculated from a Lineweaver–Burk plot was 0.8 μM. The soluble form of b5 did not have any effects.
Figure 4. Requirement of the bovine erythrocyte membrane for the C-4 hydroxylase activity.
TLC autoradiography indicates that the C-4-hydroxylase activity requires Des2 (82 ng of protein), mb5 (5.7 μM) and the bovine erythrocyte membrane (85 μg of protein; RBCm). TLC was developed with chloroform/methanol/water (60:20:2, by vol.) and the radioactivity was visualized using a bio-imaging analyser. The numbers to the right of the Figure indicate the positions of the substrate and reaction products: 1, substrate N-octanoyldihydroceramide; 2, N-octanoylceramide, the product of δ4-desaturase; 3, N-octanoylphytoceramide, the product of C-4-hydroxylase.
Figure 5. Substrate isotherm and Lineweaver–Burk plot.
The reaction mixture contained purified FLAG–Des2 (0.22 μg of protein), mb5 (1.4 μM) and bovine erythrocyte membrane (85 μg of protein). Other conditions were as described in the Experimental section. Km and Vmax of the hydroxylase for N-octanoylsphinganine were 34.8 μM and 40 nmol·h−1·mg of Des2 protein−1 respectively. Points are means for duplicate assays.
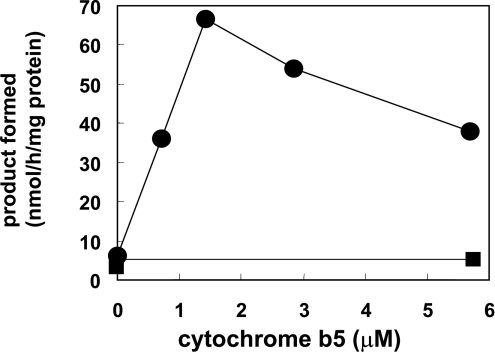
Figure 6. Effects of mb5 and sb5 on the C-4-hydroxylase activity.
The reaction mixture contained purified FLAG–Des2 (0.3 μg of protein), bovine erythrocyte membrane (85 μg protein) and various amounts of mb5 or sb5. Results are means for duplicate assays for mb5 (●) and sb5 (■). The same effects of cytochrome b5 were observed when the digitonin-solubilized fraction or the Sf9 homogenates were used in place of the purified FLAG–Des2.
Other cofactors required for hydroxylation
Another cofactor required for the hydroxylation reaction is b5R, which was recovered in the 1% (v/v) Triton X-100-soluble fraction of the bovine erythrocyte membrane. Therefore we tested reconstitution conditions that included 1% (v/v) Triton X-100-soluble and -insoluble fractions of bovine erythrocyte membrane. The Triton X-100-soluble fraction was unable to reconstitute the hydroxylase activity in the presence of the 1% (v/v) Triton X-100-insoluble fraction, which was prepared as a pellet after ultracentrifugation for 1 h (see the Experimental section), but was able to reconstitute the activity when the insoluble fraction was recovered on a 2 M sucrose cushion after ultracentrifugation for 1 h (see the Experimental section and Figure 7). Neither the Triton X-100-soluble nor the -insoluble fraction alone could reconstitute the hydroxylase activity; both fractions were required. The soluble fraction was trypsin-sensitive and the insoluble fraction was trypsin-resistant. As the Triton X-100-soluble fraction contains the membrane form of b5R, we purified it using DEAE–Sepharose and AMP–Sepharose affinity column chromatography. The purified membrane form of b5R was able to reconstitute the hydroxylase activity, indicating that the membrane form of b5R is an essential component for hydroxylation. The purified soluble form of b5R was tested to determine whether it could replace the membrane form, and indeed the soluble form supported hydroxylation, as shown in Figure 7. The results show clearly that C-4-hydroxylation requires Des2, mb5, the membrane or soluble form of b5R and the Triton X-100-insoluble fraction.
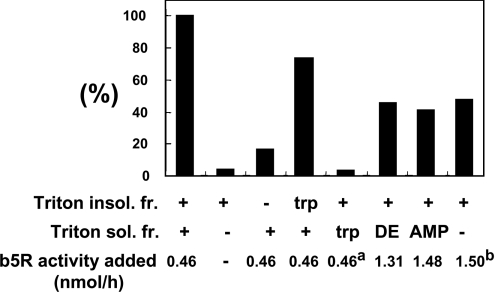
Figure 7. Restoration of C-4-hydroxylase activity with purified FLAG–Des2, mb5, b5R and the Triton X-100-insoluble fraction of the bovine erythrocyte membrane.
Each reaction mixture contained purified FLAG–DES2 (0.37 μg), mb5 (1.4 μM), the Triton X-100-soluble fraction (14 μg; Triton sol. fr.) and the Triton X-100-insoluble fraction (12.8 μg; Triton insol. fr.). The amounts of these two fractions added to the assay contained proteins equivalent to the amount obtained from the same amount of bovine erythrocyte membrane (32 μg of protein). b5R activity added refers to the amount of b5R activity in the assay mixtures. The reductase activity was measured by monitoring the absorbance at 424 nm owing to the reduction of mb5 with NADH, and calculated using a molar absorption coefficient of 120 mM−1·cm−1 for the difference between the reduced and oxidized forms of mb5. trp, trypsin treatment; DE, DEAE column-bound fraction containing the membrane form of b5R; AMP, the membrane form of b5R purified using DEAE- and AMP–Sepharose column chromatography. a indicates the activity of the membrane form of b5R in the Triton X-100-soluble fraction before the trypsin treatment; b indicates the activity of the purified soluble form of b5R. Results are presented as means for duplicate assays. Note that the Triton X-100-soluble fraction contains trypsin-sensitive factors that can be replaced with the purified membrane or soluble forms of b5R and the Triton X-100-insoluble fraction contains a trypsin-resistant factor or factors. The activity reconstituted with all the purified proteins and the Triton X-100-insoluble fraction of the bovine erythrocyte membrane was still half of that obtained with the purified FLAG–DES2, mb5 and crude Triton X-100-soluble and -insoluble fractions, suggesting that the Triton X-100-soluble fraction contains trypsin-sensitive activation factors in addition to b5R.
The results in Figure 7 indicate that the level of hydroxylase activity using purified Des2, mb5, b5R and the Triton X-100-insoluble fraction did not reach the level of that with purified Des2, mb5 and the Triton X-100-soluble and -insoluble fractions, although a high level of b5R was used in the assay and that the Triton X-100-soluble fraction was trypsin-sensitive. These results suggest that the Triton X-100-soluble fraction contains a trypsin-sensitive activator protein(s), in addition to b5R.
Co-localization of Des2 and cytochrome b5
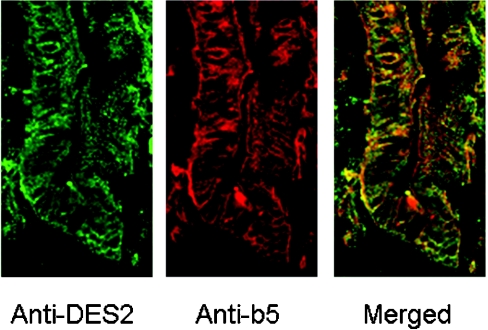
The kinetic results suggest that mb5 is required for the hydroxylase activity. Therefore we examined the co-localization of Des2 and cytochrome b5 in epithelial cells of the mouse small intestine. As shown in Figure 8, double immunofluorescence staining shows Des2 and cytochrome b5 co-localized in epithelial cells near crypts in the mouse small intestine, indicating that the epithelial cells expressing Des2 and cytochrome b5 maintain the ability to biosynthesize hydroxyceramide.
Figure 8. Immunohistochemistry of the mouse small intestine using anti-(cytochrome b5) and anti-Des2 antibodies.
Mouse small intestine was fixed and sectioned to thicknesses of 3 mm, as described in the Experimental section, and the frozen sections were stained with anti-(cytochrome b5) and anti-Des2 antibodies. Cytochrome b5 is evenly distributed in the epithelial cells and Des2 is localized in the epithelial cells near the crypt, indicating that the epithelial cells containing Des2 and cytochrome b5 possess the ability to biosynthesize phytoceramide (N-acyl-4-hydroxysphinganine).
DISCUSSION
Ternes et al. [16] identified Des2 as a bifunctional enzyme with dihydroceramide or sphinganine Δ4-desaturase and C-4-hydroxylase activities. They found that the transformation of Sur2-defective yeast, which produces dihydroceramide and sphinganine, but not 4-hydroxylceramide, ceramide or 4-hydroxysphinganine, with Des2 cDNA produced sphingenine and 4-hydroxysphinganine, indicating that Des2 is involved in the Δ4-desaturation and C-4-hydroxylation of dihydroceramide or sphinganine. Understanding the molecular mechanisms of both reactions is of interest, and a prerequisite for this understanding is biochemical analyses to determine substrate and factor requirements. Previously, we reported that mouse Des2 hydroxylated dihydroceramide, but not sphinganine, in an in vitro assay and suggested that cytochrome b5 supported the hydroxylation [17].
In the present study, we report that C-4-hydroxylation of dihydroceramide is catalysed by Des2 with NADH, mb5, b5R and the Triton X-100-insoluble fraction of the bovine erythrocyte membrane as essential components. Des2 is considered to be a tri-spanning ER (endoplasmic reticulum) protein; interestingly, the membrane-binding domain of cytochrome b5 is required to support the Des2 hydroxylase activity. These results suggest that the tertiary structure of the complex formed by Des2, mb5 and the substrate via the membrane-spanning domains, and the hydrophobic structure of the substrate, are essential for hydroxylation. We did not study the conditions required for the desaturase activity of Des2 in detail, but it is clear that the factors supporting the desaturase activity differ from those required for the hydroxylase activity, because the Δ4-desaturase activity of the purified FLAG-tagged Des2 under the optimal conditions for C-4-hydroxylase activity was negligible. Therefore factors supporting the two reactions are quite different. Michel et al. [24] reported that preincubation of rat liver microsomes with anti-(cytochrome b5) antibodies inhibited Δ4-desaturase activity by up to 82%, indicating that cytochrome b5 is involved in δ4-desaturation by Des1. These results suggest that the affinities of Des1 and Des2 to cytochrome b5 or cytochrome b5-related kinetic parameters differ markedly. Therefore further studies of the optimal conditions for desaturation by Des2 are required and comparison with the optimal conditions for Δ4-desaturation and C-4-hydroxylation by Des2 will provide us with invaluable information concerning reaction molecular mechanisms. Interestingly, the recently cloned α-hydroxylase of free-fatty-acid [25] or the acyl-chain of ceramide [26] each contain the cytochrome b5 domain within the molecules themselves. This type of hydroxylase is a primitive form taken from an evolutionary viewpoint.
The stereochemistry of the enzymatic reactions of a bifunctional dihydroceramide desaturase/hydroxylase of Candida albicans has been reported [27] and this serves as a model of mammalian DES2. That report stated, “since the hydroxylation, like the desaturation process, removes the same C(4)-HR [the H of the (R) configuration at the C4 position] from the chiral precursor, both processes apparently generate the same reactive intermediate, namely a C(4)-centred radical or radicaloid that is channelled predominantly (approx. 93%) into desaturation or recombines with an active-site bound oxygen atom to give an alcohol (approx. 7% formal insertion of oxygen into the C(4)-HR bond)” [27]. On the basis of this discussion and the results of the present study, we can draw a scheme in which the C(4)-centred radical or radicaloid recombines with active-site bound oxygen with the aid of mb5 and the complex formed receives electrons from NADH via the action of b5R. Recently, we identified an amino-acid sequence (XAFGY) of mouse Des2, which is essential for expressing C-4-hydroxylase activity, by demonstrating the loss of hydroxylase activity when the Des2 sequence was replaced with the corresponding sequence of Des1 (63% sequence identity with Des2), which exhibits only Δ4-desaturase activity [28]. This led us to hypothesize that the sequence XAFGY, which is located on the C-terminal side of the first His-box of Des2, plays a critical role in the process of complex formation that is essential for hydroxylation.
Since Des2 is an ER protein, the FLAG–Des2 purification required special attention. The solubilization with digitonin and antibody-mediated affinity chromatography with a series of buffers containing different detergents were effective at removing other membrane proteins. The purified FLAG–Des2 stored in 1 M sucrose at −80 °C was stable for at least 2 months. This fundamental knowledge of Des2 increases the feasibility of studying the protein using a structural biology approach; although the required detergent, digitonin, would need to be replaced by other detergents for crystallization.
In the present study, we first suggest that hydroxylation by Des2 requires trypsin-resistant factors in the Triton X-100-insoluble fraction. Considering the hydrophobic nature of the substrate and the requirement for mb5, we speculated that the component is a membrane lipid. However, the lipids that we tested did not replace the Triton X-100-insoluble fraction. The finding that there is at least one activator in the Triton X-100-soluble fraction is also new. Further studies are required to identify the factors in the Triton X-100-soluble and -insoluble fractions.
Previously, we reported that NADH and NADPH support hydroxylation by Des2 equally, and do not have a synergistic effect [17]. We examined whether NADPH–cytochrome P450 reductase supports hydroxylation by Des2 and mb5 in the presence of NADPH. A commercially available recombinant human NADPH–cytochrome P450 reductase with a membrane-spanning domain did not support the hydroxylation, suggesting that the assay conditions are not optimal or a new type of NADPH–cytochrome b5 reductase supports the hydroxylation; this therefore requires further investigation.
Histological findings confirmed that cytochrome b5 is co-localized with Des2 in epithelial cells near the crypt of the small intestine in mice. The tissue-specific localization of Des2 has been shown previously [17]. The tissue-specific regulation of the level of C-4-hydroxylated sphinganine containing glycolipids depends on the expression of Des2. However, transfection experiments using mammalian cells indicated that the in vitro hydroxylase activity requires co-transfection of b5R cDNA, suggesting that the level of b5R also regulates the hydroxylase activity.
The results described in the present paper were obtained using in vitro reconstitution experiments and one can argue that there is a difference between in vitro and in vivo conditions. One critical experiment would be to determine whether sphingolipids or glycosphingolipids containing 4-hydroxysphinganine are produced on transfecting the cells with Des2, mb5 and b5R cDNAs.
Further studies are required to understand the molecular mechanism of dihydroceramide:sphinganine C-4-hydroxylase and Δ4-desaturase, and the physiological functions of the products, sphingolipids containing 4-hydroxysphinganine.
Acknowledgments
This study was supported by funds for the Frontier Research System, RIKEN, Wako 351-0198, Japan and a RR (Research Revolutions) grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Smith W. L., Merrill A. H., Jr Sphingolipid metabolism and signaling minireview series. J. Biol. Chem. 2002;277:25841–25842. doi: 10.1074/jbc.R200011200. [DOI] [PubMed] [Google Scholar]
- 2.Merrill A. H., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S. I. The glycosynapse. Proc. Natl. Acad. Sci. U.S.A. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simons K., Vaz W. L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 5.Holthuis J. C., Pomorski T., Raggers R. J., Sprong H., Van Meer G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 2001;81:1689–1723. doi: 10.1152/physrev.2001.81.4.1689. [DOI] [PubMed] [Google Scholar]
- 6.Umesaki Y., Suzuki A., Kasama T., Tohyama K., Mutai M., Yamakawa T. Presence of asialo GM1 and glucosylceramide in the intestinal mucosa of mice and induction of fucosyl asialo GM1 by conventionalization of germ-free mice. J. Biochem. (Tokyo) 1981;90:1731–1738. doi: 10.1093/oxfordjournals.jbchem.a133650. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A., Yamakawa T. The different distribution of asialo GM1 and Forssman antigen in the small intestine of mouse demonstrated by immunofluorescence staining. J. Biochem. (Tokyo) 1981;90:1541–1544. doi: 10.1093/oxfordjournals.jbchem.a133622. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A., Umesaki Y., Yamakawa T. Localization of asialo GM1 and Forssman antigen in the small intestine of mouse. Adv. Exp. Med. Biol. 1982;152:415–424. [PubMed] [Google Scholar]
- 9.Smith E. L., McKibbin J. M., Karlsson K. A., Pascher I., Samuelsson B. E. Characterization by mass spectrometry of blood group A active glycolipids from human and dog small intestine. Biochemistry. 1975;10:2120–2124. doi: 10.1021/bi00681a012. [DOI] [PubMed] [Google Scholar]
- 10.Bouhours J. F., Glickman R. M. Rat intestinal glycolipids. III. Fatty acids and long chain bases of glycolipids from villus and crypt cells. Biochim. Biophys. Acta. 1977;487:51–60. doi: 10.1016/0005-2760(77)90043-1. [DOI] [PubMed] [Google Scholar]
- 11.Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H. Studies on differentiating epithelial cells of rat small intestine: alterations in the lipophilic part of glycosphingolipids during cell migration from crypt to villus tip. Biochim. Biophys. Acta. 1982;710:415–427. doi: 10.1016/0005-2760(82)90125-4. [DOI] [PubMed] [Google Scholar]
- 12.Breimer M. E., Falk K. E., Hansson G. C., Karlsson K. A. Structural identification of two ten-sugar branched chain glycosphingolipids of blood group H type present in epithelial cells of rat small intestine. J. Biol. Chem. 1982;257:50–59. [PubMed] [Google Scholar]
- 13.McKibbin J. M., Spencer W. A., Smith E. L., Mansson J. E., Karlsson K. A., Samuelsson B. E., Li Y. T., Li S. C. Lewis blood group fucolipids and their isomers from human and canine intestine. J. Biol. Chem. 1982;257:755–760. [PubMed] [Google Scholar]
- 14.Endo K., Akiyama T., Kobayashi S., Okada M. Degenerative spermatocyte, a novel gene encoding a transmembrane protein required for the initiation of meiosis in Drosophila spermatogenesis. Mol. Gen. Genet. 1996;253:157–165. doi: 10.1007/s004380050308. [DOI] [PubMed] [Google Scholar]
- 15.Endo K., Matsuda Y., Kobayashi S. Mdes, a mouse homolog of the Drosophila degenerative spermatocyte gene is expressed during mouse spermatogenesis. Dev. Growth Differ. 1997;39:399–403. doi: 10.1046/j.1440-169x.1997.00015.x. [DOI] [PubMed] [Google Scholar]
- 16.Ternes P., Franke S., Zahringer U., Sperling P., Heinz E. Identification and characterization of a sphingolipid Δ4-desaturase family. J. Biol. Chem. 2002;277:25512–25518. doi: 10.1074/jbc.M202947200. [DOI] [PubMed] [Google Scholar]
- 17.Omae F., Miyazaki M., Enomoto A., Suzuki M., Suzuki Y., Suzuki A. DES2 protein is responsible for phytoceramide biosynthesis in the mouse small intestine. Biochem. J. 2004;379:687–695. doi: 10.1042/BJ20031425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekine M., Suzuki M., Inagaki F., Suzuki A., Yamakawa T. A new extended globoglycolipid carrying the stage specific embryonic antigen-1 (SSEA-1) determinant in mouse kidney. J. Biochem. (Tokyo) 1987;101:553–562. doi: 10.1093/jb/101.3.553. [DOI] [PubMed] [Google Scholar]
- 19.Holmans P. L., Shet M. S., Martin-Wixtrom C. A., Fisher C. W., Estabrook R. W. The high-level expression in Escherichia coli of the membrane-bound form of human and rat cytochrome b5 and studies on their mechanism of function. Arch. Biochem. Biophys. 1994;312:554–565. doi: 10.1006/abbi.1994.1345. [DOI] [PubMed] [Google Scholar]
- 20.Kozutsumi Y., Kawano T., Kawasaki H., Suzuki K., Yamakawa T., Suzuki A. Reconstitution of CMP-N-acetylneuraminic acid hydroxylation activity using a mouse liver cytosol fraction and soluble cytochrome b5 purified from horse erythrocytes. J. Biochem. (Tokyo) 1991;110:429–435. doi: 10.1093/oxfordjournals.jbchem.a123598. [DOI] [PubMed] [Google Scholar]
- 21.Yubisui T., Takeshita M. Purification and properties of soluble NADH-cytochrome b5 reductase of rabbit erythrocytes. J. Biochem. (Tokyo) 1982;91:1467–1477. doi: 10.1093/oxfordjournals.jbchem.a133838. [DOI] [PubMed] [Google Scholar]
- 22.Kitajima S., Yasukochi Y., Minakami S. Purification and properties of human erythrocyte membrane NADH-cytochrome b5 reductase. Arch. Biochem. Biophys. 1981;210:330–339. doi: 10.1016/0003-9861(81)90196-x. [DOI] [PubMed] [Google Scholar]
- 23.Tamura M., Yubisui T., Takeshita M. Microsomal NADH-cytochrome b5 reductase of bovine brain: purification and properties. J. Biochem. (Tokyo) 1983;94:1547–1555. [PubMed] [Google Scholar]
- 24.Michel C., van Echten-Deckert G., Rother J., Sandhoff K., Wang E., Merrill A. H., Jr Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 1997;272:22432–22437. doi: 10.1074/jbc.272.36.22432. [DOI] [PubMed] [Google Scholar]
- 25.Alderson N. L., Rembiesa B. M., Walla M. D., Bielawska A., Bielawski J., Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 2004;279:48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- 26.Eckhardt M., Yaghootfam A., Fewou S. N., Zoller I., Gieselmann V. A mammalian fatty acid hydroxylase responsible for the formation of α-hydroxylated galactosylceramide in myelin. Biochem. J. 2005;388:245–254. doi: 10.1042/BJ20041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckmann C., Rattke J., Sperling P., Heinz E., Boland W. Stereochemistry of a bifunctional dihydroceramide Δ4-desaturase/hydroxylase from Candida albicans; a key enzyme of sphingolipid metabolism. Org. Biomol. Chem. 2003;1:2448–2454. doi: 10.1039/b303939k. [DOI] [PubMed] [Google Scholar]
- 28.Omae F., Miyazaki M., Enomoto A., Suzuki A. Identification of an essential sequence for dihydroceramide C-4 hydroxylase activity of mouse DES2. FEBS Lett. 2004;567:63–67. doi: 10.1016/j.febslet.2004.08.060. [DOI] [PubMed] [Google Scholar]