Abstract
Forkhead proteins comprise a highly conserved family of transcription factors, named after the original forkhead gene in Drosophila. To date, over 100 forkhead genes have been identified in a large variety of species, all sharing the evolutionary conserved ‘forkhead’ DNA-binding domain, and the cloning and characterization of forkhead genes have continued in recent years. Forkhead transcription factors regulate the expression of countless genes downstream of important signalling pathways in most, if not all, tissues and cell types. Recent work has provided novel insights into the mechanisms that contribute to their functional diversity, including functional protein domains and interactions of forkheads with other transcription factors. Studies using loss- and gain-of-function models have elucidated the role of forkhead factors in developmental biology and cellular functions such as metabolism, cell division and cell survival. The importance of forkhead transcription factors is underlined by the developmental defects observed in mutant model organisms, and multiple human disorders and cancers which can be attributed to mutations within members of the forkhead gene family. This review provides a comprehensive overview of current knowledge on forkhead transcription factors, from structural organization and regulatory mechanisms to cellular and developmental functions in mice and humans. Finally, we will discuss how novel insights gained from involvement of ‘Foxes’ in the mechanisms underlying human pathology may create new opportunities for treatment strategies.
Keywords: cell cycle, development, forkhead, Fox, immunoregulation, transcription factor
Abbreviations: CBP, CREB (cAMP-response-element-binding protein)-binding protein; CCNB, cyclin B; CDK, cyclin-dependent kinase; CKI, CDK inhibitor; DYRK1A, dual-specificity tyrosine-phosphorylated and -regulated kinase 1A; ER, oestrogen receptor; FHA, forkhead-associated domain; FM, FoxH1 motif; Fox, Forkhead box; GADD45a, growth arrest and DNA-damage-inducible protein 45α; HDAC, histone deacetylase; IκB, inhibitory κB; IKKβ, IκB kinase β; MH domain, mothers against decapentaplegic homology domain; NF-κB, nuclear factor κB; NLS, nuclear localization signal; PKB, protein kinase B; Plk-1, Polo-like kinase 1; SCF, Skp2/cullin/F-box; SGK, serum- and glucocorticoid-induced protein kinase; Smad, similar to mothers against decapentaplegic; SID, Smad-interaction domain; SIM, Smad-interaction motif; TGFβ, transforming growth factor β
FORKHEAD TRANSCRIPTION FACTORS
Spatial and temporal gene expression patterns are tightly regulated by tissue-specific transcription factors that bind to regulatory DNA sequence elements located proximal or distal to the promoter region. Such transcription factors are often assigned to different families on the basis of the conservation of their DNA-binding domain. One such family comprises the forkhead family of transcription factors, named after the first gene identified containing this highly conserved DNA-binding domain in Drosophila [1–3]. Mutations in this gene cause homoeotic transformation of certain gut structures, resulting in replacement of both fore- and hind-gut by ectopic spike-formed head structures, hence the name forkhead [1]. Since the existence of a forkhead family was acknowledged, over 100 forkhead genes have been identified in species ranging from yeast to human, although not in plants. Interestingly, the number of forkhead genes in different species rises with increased anatomical complexity, from four known family members in Saccharomyces cerevisiae to over 35 in mouse and human. In 2000, a unified nomenclature was introduced which reflects the phylogenetic origin for all known chordate forkhead genes [4], and has replaced diverse names such as fkh (forkhead), FREAC (forkhead-related activator), HFH (hepatocyte nuclear factor 3/forkhead homologue) and FKHR (forkhead in rhabdomyosarcoma). In this nomenclature, each family member is designated, e.g. Foxd2, whereby Fox reflects the presence of the forkhead box, and the trailing letter refers to the subfamily, currently ranging from A to S, while the number distinguishes between subfamily members. Species differences are indicated by the use of uppercase letters for human (e.g. FOXD2), a capital F for mouse (e.g. Foxd2) and the first and subclass letters uppercase for all other chordate species (e.g. FoxD2). The classification into subfamilies is based on the structural similarities and conservation levels in the forkhead DNA-binding domain (Figure 1).
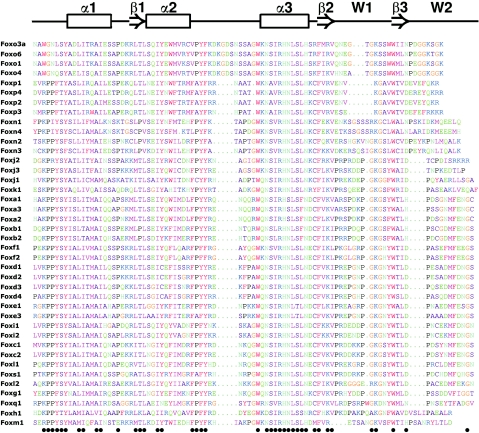
Figure 1. Alignment of murine forkhead domains showing the high degree of amino acid conservation.
Above the alignment, the approximate position of the structural features are depicted, taken from the human FOXK2 factor [9]. Colour coding refers to the relative similarity between amino acid residues based on their physicochemical properties (e.g. the aromatic residues phenylalanine, tyrosine and tryptophan are depicted in red). Below the alignment, dots indicate residues that are highly conserved (similar in >75% of cases). Note the high degree of conservation in the third α-helix (α3), also referred to as the recognition helix. The short insertion in the Foxo subclass between helices 2 and 3 has little effect on domain structure [12]. α1–α3, α-helices 1–3; β1–β3, β-sheets 1–3; W1–W2, wings 1 and 2. Dots in the alignment represent gaps.
The importance of forkhead transcription factors in embryonic development was quickly recognized (reviewed in [5,6]), and our knowledge of their function in developmental biology has expanded since then through the increased availability of mouse mutants. However, a wealth of data have accumulated in recent years that demonstrate an important role for forkhead proteins in the regulation of cellular processes such as metabolism and cell-cycle regulation. Also, the signalling pathways that govern forkhead function are starting to be elucidated, and provide insights into the mechanisms that contribute to their functional diversity. In this review, we provide an overview of current knowledge about forkhead transcription factors and their roles in the biology of mice and humans.
FORKHEAD ARCHITECTURE AND ORGANIZATION
Forkhead domain
All forkhead transcription factors share a highly conserved DNA-binding domain, named after the Drosophila forkhead gene. The three-dimensional structure of the forkhead domain has been characterized by both X-ray crystallography and NMR spectroscopy. Its apparent resemblance to the shape of butterflies, in addition to the comparable composition with the helix–turn–helix motif, resulted in the label ‘winged-helix domain’ [7]. Not all winged-helix proteins are forkhead transcription factors, however, and other evolutionarily unrelated winged-helix proteins have been identified [8]. The canonical forkhead domain consists of three α-helices, three β-sheets and two loops or wings, typically arranged in an α1-β1-α2-α3-β2-W1-β3-W2 order (Figure 1). Variations on this order include additional α-helices [9,10] and missing β-sheets or wings [11,12], and, although DNA binding generally does not cause major structural changes, an additional helix is formed in Foxd3 upon DNA binding [13]. Conservation of the functional structure of the forkhead domain is mirrored at the amino acid level over approx. 100 residues (Figure 1). High sequence similarity exists in the α-helices and β-sheets, particularly in the third helix, but lower identity exists in the wings and in between the second and third helices, which are notably the locations of most structural variations.
Information on how binding specificity is conferred comes from work on the three-dimensional structure of forkhead domains bound to matching DNA sequences. Although multiple contacts with the sugar–phosphate backbone occur, the primary binding interaction is through the third α-helix, dubbed the recognition helix, which binds target DNA directly in the major groove [7,14]. Interactions with the minor groove through binding of the second wing can also influence binding stability and specificity. The first wing, although contacting DNA, does not contribute significantly to the protein–DNA interactions [8,13]. Considering the high degree of sequence and structural conservation, especially in the recognition helix (Figure 1), all forkhead transcription factors are thought to bind in a similar fashion. Since most residues that are in contact with the target DNA are highly conserved, additional features must exist that govern sequence specificity, and variations in amino acid sequence N-terminal to the recognition helix and in the second wing are thought to be involved [9,15,16]. Differences in electrostatic distribution are also thought to contribute [12], but the mechanisms of DNA-binding specificity clearly require additional research. Unlike certain other transcription factor families, forkhead transcription factors usually bind target DNA sequences as monomers. The FoxP subfamily is an exception, however, as recent findings suggest that Foxp1, Foxp2 and Foxp4 require dimerization for DNA binding [17]. This dimerization, mediated by a leucine zipper domain, leads to swapping of part of the forkhead domain, including the recognition helix [10]. Interestingly, the three-dimensional structure of FOXP2 bound to DNA implies that FoxP dimers cannot bind adjacent DNA elements, suggesting that FoxP factors might require or regulate more intricate protein–DNA complexes and chromatin structure.
The conserved recognition helix binds to different DNA sequences, all complying with the core consensus sequence A/CA-A-C/T-A, which is required but not sufficient for binding [12,15,16]. Additional flanking sequences are essential for ensuring highly specific binding, complicating formulation of longer consensus sequences useful for, e.g. in silico genomic screening. Members of the same forkhead subfamily, however, have the ability to bind to highly similar DNA sequences, e.g. FoxO factors, which can all recognize and bind to the consensus sequence T/C-G/A-A-A-A-C-A-A [18].
In contrast with the highly conserved forkhead domain, few similarities exist between regions outside the DNA-binding domain of different forkhead family members. Still, several conserved domains and motifs have been identified in multiple forkhead factors, usually within specific subclasses. Often, common features have first been identified in one forkhead subfamily, usually the extensively studied FoxO subclass, after which similar properties have been progressively uncovered in other forkheads.
Transactivation domains
Forkhead factors differ in their transcriptional regulatory properties in that they can act as either activators or repressors of target gene expression. In the last few years, data have accumulated suggesting that several forkhead factors may direct gene expression either way, depending on recruitment and interaction with cofactors or co-repressors. Table 1 lists current knowledge on transactivational properties of most forkhead proteins. In general, members of the same subfamily share similar activating or repressing functions, although there are several clear exceptions to this rule. The inconsistencies in transactivational properties between forkhead factors are reflected by the lack of conserved conventional activation or repression domains, such as basic or acidic stretches or regions enriched for specific amino acid residues. Between subfamily members, however, non-canonical sequence similarities have been observed in the case of the FoxA and FoxF families [19–21]. In Foxd2 and Foxd3, an acidic patch has been identified N-terminal to the forkhead domain. Such acidic domains, however, have been identified as regions required for transactivation, whereas Foxd2 and Foxd3 generally function as repressors of gene expression. Other repressors, particularly Foxp1 and Foxp2, share a similar repressor domain N-terminal relative to the forkhead domain, which includes a zinc-finger motif [22]. They also contain a typical polyglutamine stretch that influences repressor activity and is lacking in a particular splice variant [23].
Table 1. Transcription regulatory properties of forkhead transcription factors.
Listed are forkhead factors for which evidence for transcriptional activation (+) or repression (−) is available (third column). Note that for some family members, both activation and repression have been reported (+/−). FoxH1 reportedly lacks any transcriptional activation properties and depends on interactions with Smad factors for target gene expression [24].
| Forkhead | Activation (+) or repression (−) | Reference(s) |
|---|---|---|
| FoxA | + | [22,200,201] |
| FoxC1 | + | [28] |
| FoxC2 | +/− | [202,203] |
| FoxD1 | + | [204] |
| FoxD2 | +/− | [137,202] |
| FoxD3 | − | [205] |
| FoxE1 | − | [206] |
| FoxF1–FoxF2 | + | [19,20] |
| FoxG1 | − | [207] |
| FoxH1 | + | [208] |
| FoxI1 | + | [209] |
| FoxJ1–FoxJ2 | + | [29,210] |
| FoxK1 | − | [211] |
| FoxK2 | + | [212] |
| FoxL2 | − | [213] |
| FoxM1 | + | [85] |
| FoxN1 | + | [214] |
| FoxN3 | − | [84] |
| FoxO1–FoxO6 | +/− | [30,215,216] |
| FoxP1–FoxP4 | − | [17,22,217] |
| FoxQ1 | − | [218] |
NLSs (nuclear localization signals)
For transcription factors to exert their gene-regulatory function, they must be located in the nucleus. NLSs have been identified in most gene family members, all confined to regions of the forkhead domain. A well-characterized bipartite NLS is shared by all members of the FoxO subfamily, located in the C-terminal end of the forkhead domain [25,26], whereas an additional NLS is present in FOXO1 [27]. Interestingly, a PKB (protein kinase B or Akt) phosphorylation motif located within the conserved NLS has a major impact on its functional abilities, acting in concert with other post-translational modifications to drive FoxO factors out of the nucleus (see below). In the case of FoxA2, FOXC1, FOXF2 and FOXJ2, nuclear localization is dependent on two amino acid stretches at both N-terminal (α1) and C-terminal (W2) ends of the forkhead domain ([19,21,28,29], and see Figure 2). Considering the high degree of sequence similarity within the forkhead domain, it is tempting to speculate that NLSs may be similarly arranged in other forkhead family members.
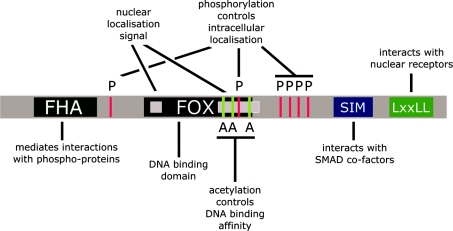
Figure 2. Domains and motifs in forkhead transcription factors.
A graphical display of the relative positions and functions of known regulatory elements in the primary protein structure of forkhead factors. FHA recognizes and binds phosphorylated threonine residues on partner proteins. FOX binds target DNA sequences in regulatory regions of target genes. In addition, it is involved in interactions with homeodomain proteins, although the subdomains responsible are as yet unknown. SIM binds Smad transcription factors at target promoters. LXXLL, lysine-rich amino acid motif (where X denotes any residue), implicated in the interaction with nuclear receptor proteins. White boxes within the forkhead domain reflect the presence of a bipartite NLS within the forkhead domain. P above the red lines refers to residues that can be phosphorylated by PKB, IKKβ, SGK, CK1 or DYRK1A, resulting in nuclear export of the protein. A below the green lines indicates the positions of lysine residues that can be acetylated, thereby attenuating DNA-binding affinity. Note that this is a schematic overview, and that these domains and motifs are not all present in any one particular forkhead protein. See text for details and references.
Phosphorylation motifs
Forkhead activity can be controlled by post-translational modifications in addition to gene expression levels. The most extensively studied modification is phosphorylation of FoxO proteins by PKB upon stimulation with insulin-like growth factors (reviewed in [26]). Three conserved PKB-recognition sites exist in FoxO subfamily members (Figure 2), one of which is lacking in FoxO6 [30]. A single PKB site is present in the N-terminus of the forkhead domain, which upon phosphorylation affects DNA binding and transactivational potential [31]. Phosphorylation of all three PKB motifs, in combination with SGK (serum- and glucocorticoid-induced protein kinase), CK1 and DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A) phosphorylation of serine residues adjacent to the C-terminal PKB site [32–34], results in nuclear exclusion and sequestration in the cytosol, thereby terminating FoxO functional activity [35–37]. Additionally, a C-terminally located serine residue in FOXO3a can be phosphorylated by IKKβ [IκB (inhibitory κB) kinase β] [38], a factor known to activate NF-κB (nuclear factor κB) signalling through inhibition of IκB. Subsequently, phosphorylated FoxO factors can be ubiquitinylated by Skp2 [39], a subunit of the SCF (Skp2/cullin/F-box) E3 ubiquitin ligase complex, targeting them for proteasomal degradation [40,41]. FoxA2 is the only forkhead factor not in the FoxO subfamily that is also regulated by such insulin-responsive, PKB-mediated translocation control [42], albeit by phosphorylation of a non-canonical PKB motif. Still, the consensus RXRXX{S/T} motif that is recognized by PKB [43] can also be found in other members, raising the possibility of PKB control of other forkhead factors.
Another potentially interesting feature consists of post-translational modifications under the control of the Ras–Ral pathway. Ral-dependent phosphorylation of C-terminal threonine residues by JNK (c-Jun N-terminal kinase) has been reported for FOXO4, which affects transcriptional competence independently of nuclear shuttling [44,45]. Whether such a regulatory pathway applies to all FoxO and other family members remains to be determined, especially since these phosphorylation sites do not appear to be highly conserved.
Acetylation motifs
In addition to regulation by PKB phosphorylation, a second pathway has been identified that regulates FoxO transcriptional activity. Direct acetylation of FoxO factors by CBP [CREB (cAMP-response-element-binding protein)-binding protein]/p300 or deacetylation by Sir2 can influence transcriptional regulation of downstream target genes [46–49]. The mechanisms whereby CBP/p300 and Sir2 bind and modify FoxO factors and how this influences FoxO functioning are still not entirely clear [50]. It has been proposed, however, that acetylation of positively charged lysine residues in the forkhead domain (Figure 2) attenuates the ability of FoxO proteins to bind target DNA, thereby reducing their transcriptional activity [51]. The same authors have also shown that the initial acetylation sensitizes FoxO factors to phosphorylation by PKB [51]. The widespread occurrence of consensus acetylation sites in forkhead proteins suggests the possible existence of a common regulatory pathway involving acetylation.
FHA (forkhead-associated domain)
Alignment of a subset of forkhead transcription factors from multiple species led to the identification of a second conserved domain, designated the FHA domain ([52]; see Figure 2). With the cloning of additional forkhead genes, however, it became clear that it is not a common feature for forkhead factors to contain such an FHA domain, and none have been identified outside the FoxK subfamily. Although the FHA domain is a highly conserved structural module, this does not apply to the amino acid sequence, which is highly divergent. It is found in proteins in a wide variety of species, from bacteria to humans, and its presence in Arabidopsis suggests that the FHA domain stems from earlier periods in ancient evolutionary history than the forkhead DNA-binding domain. It specifically recognizes and binds phosphorylated threonine residues on target proteins, thereby mediating protein–protein interactions. FHA domains have been identified in proteins ranging from kinases to ubiquitin ligases, implicated in divergent processes such as protein degradation and vesicular trafficking, but the majority are involved in checkpoint and cell-cycle control [53,54]. An important role for the FHA domain has been postulated in expression timing of a whole set of genes referred to as the Clb2 cluster in S. cerevisiae (reviewed in [55]). Here, the forkhead transcription factor Fkh2 is dependent on its FHA domain for interactions with the phosphorylated Ndd1 cofactor and subsequent transcriptional activation [56,57], providing a very elegant mechanism for tightly controlled transcriptional regulation.
SIDs [Smad (similar to mothers against decapentaplegic)-interaction domains]
Forkhead transcription factors have been implicated in signalling pathways of the TGFβ (transforming growth factor β) superfamily. Activation of TGFβ receptors leads to phosphorylation and formation of Smad protein complexes which subsequently translocate to the nucleus where they mediate transcriptional regulation of downstream target genes [58]. FoxH1 was identified as a cofactor for SMAD complexes, through direct binding of its C-terminal SID [59,60]. Comparison of the FoxH1 SID with other Smad-interacting proteins resulted in the identification of two Smad-interaction motifs, PPNK [SIM (Smad-interaction motif)] and LPTSY or PN{V/A}V{A/M}P{L/P} [FM (FoxH1 motif)] in the C-terminus of FoxH1 (Figure 2), which contact the MH2 (mothers against decapentaplegic homology 2) domain of Smad2 [61,62]. Recently, FoxO factors were shown to interact directly with Smad complexes at the p21Cip1 locus [63]. This connection is achieved through binding of the FoxO DNA-binding domain to the MH1 domain in Smad3 and Smad4. Interestingly, the transcriptional repressor FoxG1 can bind directly to the FoxO–Smad complex through a C-terminal domain, thereby inhibiting the transactivation of p21Cip1 [63]. Similarly, FoxG1 can inhibit TGFβ signalling through comparable interactions with FoxH1 [64]. To our knowledge, no SID nor FM sequences have been identified in forkhead factors other than FoxH1, suggesting the existence of other interaction domains.
Nuclear receptor interaction motifs
Forkhead transcription factors are known to interact with members of nuclear receptor families. Members of the FoxO subfamily in particular are known to associate with multiple nuclear receptors [50], including the androgen receptor [65], the glucocorticoid receptor [66] and the retinoic acid receptor [66]. These interactions are thought to be mediated by a short motif containing the amino acid sequence LXXLL [26], located in the C-terminal region of all FoxO factors (Figure 2). Interestingly, this motif is also found in other forkhead proteins, including Foxm1, Foxk1 and all members of the Foxp subfamily. However, interactions with nuclear receptors have also been demonstrated for other forkheads lacking the LXXLL motif, such as FoxA members and FoxH1 [67,68], suggesting that other structural attributes may be involved. The general consequence of forkhead interactions with nuclear receptors is highly inconsistent, as they can act both to enhance or interfere with each other's function [67,69,70]. Receptor type, nuclear receptor ligand binding, phosphorylation status and cell type all determine the final outcome of a particular interaction.
Homeodomain interactions
As forkhead factors generally recognize and bind similar DNA elements, interactions with members of other transcription factor families could underlie specificity for gene activation of particular downstream targets. This view is supported by observations of direct protein–protein interactions between forkhead factors and homeodomain-containing transcription factors. FOXC1, FoxD3 and, in particular, Foxa2 have been shown to interact with several homeodomain proteins, including PITX2 (paired-like homeo-domain transcription factor 2), Oct-4 (octamer 4), Pdx-1 (pancreas duodenum homeobox 1) and HOXA10 (homeobox A10) and engrailed [71–74]. Similar to nuclear receptor interactions, the result of interactions between these factors can vary, and is dependent on issues such as cell type and the presence of DNA-binding elements for both factors in target gene promoters.
Strikingly, the interaction between forkhead and homeodomain factors appears to be mediated by their DNA-binding domains. As the forkhead domain is the most conserved domain in forkhead factors, this suggests a general mechanism of protein–protein interactions between members of these two transcription factor families. Not surprisingly, Foxa2 is able to bind to most of the aforementioned and other homeodomain proteins [72]. If target specificity was to benefit from this interaction, however, one would expect that some degree of selectivity would be coded for in the forkhead domain. It will therefore be interesting to learn which specific components of this domain are involved in this interaction.
MECHANISMS OF TRANSCRIPTIONAL REGULATION
Despite the expanding list of forkhead genes and knowledge of in vitro and in vivo functions, relatively little is known about the mechanisms of activation or repression of gene expression by forkhead transcription factors. Direct interactions with components of the basic transcriptional machinery [e.g. TBP (TATA-box-binding protein) and TFIIB (transcription factor IIB)] have been described for FOXF2 and FoxO family members [19,75]. As mentioned above, acetylation of FoxO factors attenuates their transactivational properties. In contrast, binding of CBP/p300 to FoxO proteins and its acetylation activity are also required for transcriptional activation by FoxO factors [47,49], suggestive of an additional regulatory role for CPB/p300 as a cofactor of FoxO factors. It is therefore tempting to speculate that CBP/p300 must be recruited by FoxO factors to target genes for histone acetylation, resulting in local changes in chromatin conformation and access to the promoter for general transcription factors, providing a second mechanism by which FoxO members regulate transactivation [50]. Interestingly, FoxO mutants that are unable to bind DNA were shown to still localize to sets of target gene promoters [76], suggesting that, at specific sets of genes, FoxO factors can influence transcription via a mechanism independent of DNA binding. Although the exact mechanism is still unclear, it has been suggested that acetylation could influence the balance between DNA-dependent and -independent transcriptional regulation, thereby switching the function of FoxO transcription factors to specific sets of target genes that do or do not require FoxO binding [50,76].
FoxA1, and possibly other FoxA members, determines nucleosome positioning of enhancer sites in transcriptionally competent cells, as has been established for the albumin enhancer [77]. Similar to linker histones H1 and H5, which both contain a winged-helix domain but lack a second wing [78,79], FoxA factors can bind to nucleosomal core histones, thereby replacing linker histones and relieving chromatin compaction at target enhancer and/or promoter sites. In addition, FoxA binding causes a sharp bend of the DNA helix, resulting in widening of the minor groove [7,16] and increased DNaseI sensitivity [80]. Together, this creates an open chromatin configuration, enabling other transcriptional activators to induce target gene expression. Consistent with this, a recent study on long-range gene regulation by ER (oestrogen receptor) binding revealed the presence of FoxA1 in the proximity of sites where ERs can subsequently bind. This binding of FoxA1 appeared to be essential for gene expression of ER target genes, since the removal of FoxA1 resulted in the inability of ER to associate with its binding elements [81]. Although essential for gene activation, FoxA binding is not necessarily sufficient for nucleosomal positioning at the MMTV (murine mammary tumour virus) LTR (long terminal repeat), depending on hormone-induced glucocorticoid receptor binding for nucleosomal rearrangements. Initial changes in chromatin structure, however, are induced by binding and activity of FoxA in both the absence and presence of glucocorticoid receptors, hence the name pioneer factor [82].
In the case of the transcriptional repressor Foxk1, interactions with the scaffolding protein Sin3b have been described [83]. This large protein is part of a co-repressor complex that also includes HDACs (histone deacetylases) and/or nucleosomal remodelling factors. Since this complex lacks DNA-binding activity, recruitment by Foxk1 could direct a reversal of open chromatin conformation and specific repression of downstream target genes. Similarly, the transcriptional repressor Foxn3 associates with SKIP (Ski-interacting protein) [84], a transcriptional adaptor protein known to interact with repressor complexes that involve Sin3a and HDACs. It is currently not known whether other transcriptional repressors, such as Foxd3 and Foxp1, could operate through a similar mechanism.
FUNCTIONS OF FORKHEAD TRANSCRIPTION FACTORS
Cell-cycle regulation
In yeast, progression through the different stages of the cell cycle is co-ordinated by waves of expression of a large number of genes. The controlled periodicity of G2/M-phase-specific expression of >30 genes collectively known as the Clb2 cluster is controlled by the forkhead transcription factor Fkh2 (reviewed in [55]). In mammalian cells too, expression of multiple G2/M-phase-related genes is regulated by a forkhead transcription factor. Foxm1 gene expression is restricted to proliferating cells, and FoxM1 activity is cell-cycle-regulated [85]. Dividing cells from Foxm1-deficient mice exhibit mitotic defects, resulting from deregulated expression of genes required for mitotic entry and progression, including CCNB (cyclin B), Plk1 (Polo-like kinase 1), cdc25 (cell division cycle 25) and the kinetochore component CENP-F (centromere protein F) [86,87]. In addition, FoxM1 controls progression into S-phase by up-regulating expression of the CDK2 (cyclin-dependent kinase 2)-activating phosphatase Cdc25A, while down-regulating protein levels of the CKIs (CDK inhibitors) p21Cip1 and p27Kip1 [88,89]. These CKIs are targeted by the SCF ubiquitin ligase complex, subunits of which are transcriptionally regulated by Foxm1 [87]. Together, defects in S- and M-phase by loss of FoxM1 result in chromosomal instability and polyploidy [86].
Cell division is influenced by FoxO factors at two separate levels. First, FoxO transcription factors prevent cells from entering the cell cycle. This quiescent state is maintained through regulation of a whole set of cell-cycle mediators. Induction of p27Kip1 and p21Cip1 results in inhibition of cyclin E–CDK2 and cyclin D–CDK4 complexes, involved in entry into S-phase [63,90,91]. Up-regulation of the retinoblastoma protein family member p130 by FoxO factors prevents transcriptional activation of an array of cell-cycle-entry inducers by E2F4 (E2F transcription factor 4) [92]. In addition, stimulation of CCNG2 (cyclin G2) expression, specifically expressed in quiescent cells, and down-regulation of CCND family members, involved in regulation of cell-cycle entry, assist further in preventing cell-cycle entry [76,93,94]. Secondly, FoxO factors seem to have contrasting roles in the regulation of G2/M-phase regulation. During G2-phase, they increase the expression of CCNB and Plk1 genes, which are required for mitotic entry and completion [95]. In contrast, FoxO factors were also found to induce a delay in G2-phase and entry of mitosis, possibly through regulation of GADD45a (growth arrest and DNA-damage-inducible protein 45α), a component of the G2/M-checkpoint system [86,96]. Precisely how FoxO factors regulate the transition from G2- to M-phase clearly has not yet been established, and requires further investigation.
Interestingly, members of the CIP (CDK-interacting protein)/KIP (kinase inhibitor protein) family of CKIs appear to be common target genes for forkhead transcription factors. In addition to FoxO factors and FoxM1, additional forkheads such as FOXA1, Foxg1 and Foxk1 can also act upon p21Cip1 or p27Kip1 expression [63,97,98], although they can differ in their activating or repressive effect.
Cellular survival
A first indication that forkhead transcription factors are implicated in regulation of cell survival came from observations in Caenorhabditis elegans, where the forkhead DAF-16 is an important regulator of longevity [99,100]. Subsequently, lifespan regulation by DAF-16 appeared to be controlled by Akt-mediated insulin-responsive signalling pathways [101]. Interestingly, these pathways are highly conserved in other species, and in mammals the DAF-16 orthologues FoxO1, FoxO3, FoxO4 and FoxO6 are all regulated by PKB/Akt (see above). Mammalian PKB, induced by insulin-like growth signals, actively suppresses apoptosis through inhibition of FoxO transcription factors [35], but cannot prevent apoptosis triggered by expression of PKB-resistant FoxO mutants [102]. In vivo, a wealth of data point to a role for FoxO factors in mediating haematopoietic and neuronal apoptosis [35,103,104], and they have recently been linked to amyloid β toxicity in Alzheimer's disease [105]. Interestingly, other cell types generally respond to FoxO activation by going into cell-cycle arrest [91,92]. Such a quiescent state has a more favourable metabolic rate and can promote cell survival under stressful conditions, analogous to increased stress resistance and longevity during periods of nutrient starvation in C. elegans Dauer formation. This was shown recently to be dependent, at least in part, on interactions with β-catenin, a central mediator of the Wnt signalling pathway [106].
The mechanisms that underlie FoxO-mediated cell-cycle arrest and apoptosis involve transcriptional regulation of pro-apoptotic target genes, such as the FasL and Bim genes [35,103,107]. Alternative pathways that result in a similar cell fate include FoxO regulation of p27Kip1, which prevents cell-cycle entry and induces cell death in certain cell types [90,108]. Exactly why FoxO factors induce cell-cycle arrest in some cell types but cause apoptosis in others is not entirely clear. A major clue could come from studies that have distinguished between DNA-binding-dependent and -independent FoxO target genes, and found that cell-death induction, but not cell-cycle arrest, requires FoxO DNA-binding ability [76].
Longevity (and therefore aging) is inextricably linked to stressful stimuli, mainly in the form of reactive oxidative species. Similar to DAF-16 in C. elegans, FoxO factors provide cells with stress-resistance strategies. Protection of quiescent cells against oxidative stress involves FoxO-mediated up-regulation of MnSOD (manganese superoxide dismutase) and catalase, two enzymes with antioxidant activity [109,110]. Under low-stress conditions, any DNA damage that may result from oxidative stress is overcome by FoxO induction of the GADD45a DNA-repair gene [96]. These stress-response mechanisms suggest that, in mammals too, FoxO factors may have the ability to regulate lifespan. Figure 3 shows the forkhead transcription factors involved in cell cycle and cellular survival.
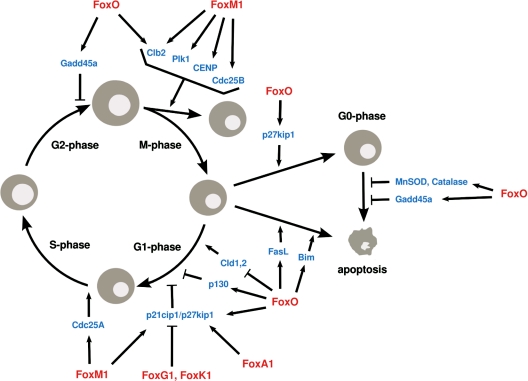
Figure 3. Forkhead transcription factors in cell cycle and cellular survival.
Schematic overview of known roles for forkhead transcription factors (displayed in red) in regulating expression of genes implicated in the process of proliferation, survival and/or apoptosis (blue). Note that particular forkhead factors can have contrasting roles in balancing cell cycle and survival depending on the type (e.g. neuronal or haematopoietic) and status (e.g. cycling or quiescent) of the cell. See text for details and references. CENP, centromere protein; Clb, cyclin B; Cld, cyclin D.
Metabolism
At both the cellular and organismal levels, forkhead transcription factors have been implicated in regulation of metabolic processes. To date, metabolic regulation by forkheads is confined to members of the FoxA, FoxC and FoxO subfamilies. Evidence for involvement of FoxA1 and FoxA3 in metabolic processes comes from findings of hypoglycaemia in Foxa1- and Foxa3-null mutant mice [111–113]. Foxa2-deficient mice are embryonic lethal, but tissue-specific deletion in pancreatic β-cells also results in hypoglycaemia and disorganized islet arrangements [114]. The underlying causes for hypoglycaemia can differ between FoxA family members, however, with glucagon levels affected in Foxa1−/− mice and the GLUT2 glucose transporter down-regulated in Foxa3-deficient mice, whereas subunits of a β-cell-specific potassium channel were identified as Foxa2 target genes. For Foxa2, multiple additional target genes have been identified in pathways controlling insulin secretion and glucose homoeostasis in the pancreas [115,116]. In the liver, Foxa2 has recently been linked to fat metabolism, as it was shown to be responsible for regulation of fatty-acid burning upon fasting [117].
FoxC2 has an important role in regulating energy storage and fat metabolism. Analysis of Foxc2+/− mice (homozygous animals die in utero), in which changes in brown adipose tissue mass were observed, suggests that FoxC2 is involved in regulating energy storage [118]. Upon overexpression of Foxc2 in adipocytes, there is a decrease in total body fat mass, and these transgenic mice are protected against diet-induced insulin resistance [118,119]. FoxC2 in adipocytes therefore seems to influence overall glucose metabolism in transgenic mice, and Foxc2 overexpression seems to prevent diet-induced obesity and insulin resistance.
Members of the FoxO family also occupy important roles in regulation of metabolism (reviewed in [18,120]). A principal feature that links FoxO factors with metabolism is the insulin-dependent regulation by PKB, as described above. Additional links include the fact that FoxO target genes are often implicated in metabolic processes in liver, muscle and pancreas. Examples include G6PC (glucose-6-phosphatase), involved in gluconeogenesis in the liver [121] and PDK4 (pyruvate dehydrogenase kinase 4), implicated in glucose saving in liver and muscle [122]. Related to their function in cell survival, FoxO factors protect β-cells from glucose-induced oxidative stress through up-regulation of transcription factors that induce expression of Ins2 [123]. Other clues come from in vivo models using heterozygous Foxo1-null mice (homozygous Foxo1-null mutants are embryonic lethal [124]), which are protected against diabetes, whereas mice transgenic for a dominant active Foxo1 show opposite effects and develop diabetes [125,126]. Overall, FoxO factors appear to be important in controlling the effects of insulin signalling on gluconeogenesis and other metabolic processes.
Immunoregulation
Multiple forkhead transcription factors are expressed in immune cells, and hence are implicated in regulation of the immune system [127,128]. Foxp3 is essential for specification and function of regulatory (CD4+ CD25+) T-cells, which are important for regulating T-cell reactivity and preventing autoimmunity [129–131]. Similar prevention of autoimmunity additionally requires Foxj1, which is thought to act by transcriptional activation of IκBβ, a potent inhibitor of NF-κB. Since NF-κB can cause T-cell activation, inhibition of this factor results in reduced autoimmunity [132]. Also, Foxj1 is thought to control the release of T-cells into the periphery [133]. The gene responsible for the nude phenotype, Foxn1, is known for its function in the regulation of proliferation and differentiation of epithelial cell populations [134]. The same holds true for the thymus, where Foxn1 is required for development and differentiation of thymic epithelial cells, which are in turn essential for development and selection of T-cells [135]. As described above, FoxO transcription factors are known regulators of the cell cycle. Similar functions have been proposed in thymic cells, where FoxO factors induce T-cell quiescence [136]. Finally, roles for other forkheads such as Foxd2 and Foxp1 have been proposed [137,138], but additional work is required to clarify their functions in immunoregulation.
Embryonic development
Similar to most other transcription factor families, forkhead factors are commonly involved in embryonic development, in roles often conserved across different species. There are striking parallels between the number of forkhead genes in different species and their anatomical complexity. Not surprisingly, forkhead transcription factors are often implicated in the regulation of differentiation processes during embryonic development.
Already during early embryonic patterning, forkhead factors are implicated in gastrulation and the formation of midline structures required for establishing the body plan. The severe pheno-type of Foxh1−/− mice reflects the importance of Foxh1 in nodal signalling, as primitive streak, node, notochord and prechordal plate mesoderm are all severely affected in null mutants [139,140]. Foxa2 is also expressed in notochord, floorplate and primitive streak, and, similar to Foxh1, Foxa2-deficient mice do not develop a proper notochord and other midline structures [141,142], and, as a result, mice deficient in these two forkheads display severe secondary defects. Another forkhead, Foxj1, is expressed specifically in ciliated cells, including ciliated cells in the node at presomite stages, and is essential for establishing left–right asymmetry during early development [143].
During the very early stages of organogenesis, forkhead factors are also heavily involved in patterning of the germ layers. In the absence of Foxa2, patterning defects in the definitive endoderm result in malformation of the foregut [141,142], in analogy to the original forkhead gene in Drosophila [1], and similar defects can be observed in Foxh1-deficient mice [139]. In the mesoderm, forkhead factors are involved in specification of developmental fate, in which Foxc factors induce a paraxial mesodermal fate from which somites will subsequently develop [144]. In contrast with Foxc factors, Foxf1 promotes differentiation into lateral plate mesoderm, and subsequently into splanchnic mesoderm, which gives rise to mesenchymal tissues and vasculature. Additionally, Foxf1 is implicated in development of extra-embryonic mesoderm tissues including placenta, amnion and yolk sac [145,146].
During later stages of organogenesis, the cellular role of forkheads in the regulation of differentiation becomes apparent. Many forkheads induce tissue-specific gene expression, and mice mutant for particular forkheads often display differentiation-related developmental defects. Foxa1 and Foxa2, often with complementary activity, are important for regulation of tissue-specific gene-expression programmes during morphogenesis and differentiation of tissues such as prostate, pancreas, lung and liver [147–151]. Foxd1 deficiency leads to impaired branching morphogenesis, nephron patterning and differentiation [152]. Development of the inner ear is severely affected in mice deficient in Foxi1, resulting in hearing and balance impairments [153]. Additionally, Foxi1−/− mice suffer from distal renal tubular acidosis [154], and parallels between cellular defects in inner ear and kidney development suggest a function for Foxi1 in specification of an intercalated cell fate. Foxj1 is a central player in ciliogenesis during development, and Foxj1 deficiency results in the absence of cilia in respiratory, reproductive and central nervous systems [143]. Mice deficient in Foxl2 are characterized by infertility because of premature ovarian failure, and analysis of the mouse mutant suggests that Foxl2 is a key factor in granulosa cell differentiation, which in turn regulates the delicate timing of follicle activation [155,156]. In addition, Foxl2 is a required commitment of an adult ovarian phenotype, while suppressing the testis-determination pathway in granulosa cells in postnatal ovaries [157]. The role of FoxM1 in cell-cycle regulation is well established (see section ‘Cell-cycle regulation’), and Foxm1−/− mice display severe proliferation-related defects. In addition, however, Foxm1 directs hepatoblast differentiation towards a biliary epithelial cell lineage fate [158]. FoxN factors are also implicated in directing differentiation, with Foxn1 regulating differentiation of post-mitotic cells in hair follicles and epithelial cells in thymus, nails and nasal cavity [159,160], whereas Foxn4 controls cell-fate decisions in both retina and spinal cord [161,162]. Homozygous deletion of Foxp1 results in increased proliferation in combination with an aberrant myocardial organization, suggesting a role for Foxp1 in myocyte maturation [163].
In addition to the direct induction of tissue-specific gene expression during development, several forkhead factors regulate differentiation by controlling the decision of cells to commence differentiation. FoxD3, for one, prevents differentiation of embryonic stem cells and trophoblast progenitor cells and maintains them in a pluripotent state [164,165]. Several other forkhead factors, notably Foxe3 in the lens and Foxg1 in the neuroepithelium, prevent differentiation of proliferating cells by stimulating them to keep dividing [166,167].
Are all forkhead transcription factors implicated in regulating differentiation during development? Some factors may serve other roles in addition to their role in differentiation. For example, in addition to its role in differentiation of neural crest cells, FoxD3 also induces neural crest cells to migrate away from the neural tube, and regulates the expression of cell-adhesion molecules such as N-cadherin in migrating neural crest cells [168]. In vitro, FoxO factors have been studied extensively, and, although implicated in differentiation of adipocytes and myoblasts [126,169], they are better known for their role as downstream signal transduction components affecting metabolism, cell-cycle regulation and survival (see above). However, mice mutant for FoxO genes have only recently become available [124,170,171], and future research may eventually disclose some cellular role for FoxO factors in differentiation processes after all. Foxk1 mutant mice that make it to term show a significant growth deficit and muscle atrophy, owing to myogenic progenitor cells having lost the ability to proliferate and provide in novel myocyte populations in repair of muscular injury [172]. However, mice with targeted mutation of Foxk1 only develop in 6% of cases, suggesting that Foxk1 is vital for embryonic development. Not much is known, however, about its function in Foxk1-positive tissues such as somites, myocardium and brain during development.
Mice mutant for other forkhead transcription factors have only been analysed morphologically, and conclusive information on a cellular function is not yet available. Foxb1-deficient mice, for example, display brain defects, growth retardation and muscle weakness after birth [173,174], but the underlying mechanisms remain unclear. Most of what we know about FoxP2 comes from studies in humans, as heterozygous mutations in the FOXP2 gene were identified in patients with a severe speech and language disorder (KE-family), establishing an exciting link between developmental genetics and the human ability to speak [175]. The recent creation of Foxp2-null mutant mice [176] will help in elucidating the function of FoxP2, as well as the molecular cascades involved in FoxP2-mediated development of the neural circuitry that governs speech.
CLINICAL IMPLICATIONS
Several human phenotypes can be attributed to mutation of members of the forkhead family of transcription factors (see also [177]). Often, functional conservation is apparent between species, with many phenotypes of forkhead mutations in mice resembling human anomalies caused by orthologues. Strikingly, a substantial fraction of these mutations are implicated in ocular phenotypes. These include mutations in the FOXC1 gene in cases of congenital glaucoma and Axenfeld–Rieger anomaly [178,179], FOXC2 mutations in distichiasis in addition to lymphoedema [180], FOXE3 mutations in ocular anterior segment anomalies and cataract [181,182] and FOXL2 mutations in BPES (blepharophimosis–ptosis–epicanthus inversus syndrome) [183,184]. Other forkhead-related phenotypes include thyroid agenesis, cleft palate and choanal atresia as a result of mutations in the FOXE1 gene [185] and T-cell immunodeficiency in combination with alopecia and nail dystrophy caused by FOXN1 mutations [186], whereas members of the FOXO subfamily have been implicated in cancers such as rhabdomyosarcoma and leukaemia [187,188]. The identification of mutations in the human FOXP2 gene in a family with a severe speech and language disorder has provided an interesting link between neurodevelopment and development of speech [175]. Finally, mutations in the X-linked FOXP3 gene can result in IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome [189,190]. Additionally, striking similarities have been observed between human anomalies and murine phenotypes caused by mutation or deletion of forkhead genes. Examples include Foxi1−/− mice, which suffer from severely impaired hearing, and developmental defects in the inner ear resemble phenotypes in certain congenital human auditory conditions [153]. However, disease-causing mutations in the human orthologue have not yet been identified. To date, the vast majority of known mutations affect the DNA-binding domain, either by local mutations or as a result of missense mutations N-terminal to the forkhead domain. Although mutations identified so far are mainly homozygous, it remains to be determined whether heterozygous mutations or single nucleotide polymorphisms can cause similar or less severe phenotypes.
Recent studies have provided the first indications that forkhead factors can be exploited therapeutically. Regulatory (CD4+ CD25+) T-cells are indispensable for suppression of immune system activation, thereby maintaining immune system homoeostasis and preventing autoimmunity (see above). As Foxp3 is crucial for development and function of regulatory T-cells [129–131], modulation of Foxp3 expression could be used in immunotherapy in autoimmune diseases. Loser et al. [191] showed that in vitro-generated T-cells transduced with Foxp3 can be used in mice to protect against autoimmune dermatitis. Similarly, such cells were able to reverse disease in a mouse model for autoimmune diabetes [192]. In inflammatory arthritis, overactivated neutrophils often persist in the affected joints. This was recently shown to result from an impairment of transactivation of the apoptosis inducer FasL, due to active suppression by Foxo3a [193], making Foxo3a an attractive target for targeted gene therapy in autoimmune diseases.
Signalling pathways involving insulin/PKB, neurotrophin and Wnt have been shown to be important in molecular mechanisms underlying cancer, neurodegenerative diseases and aging, with FoxO factors as important mediators [194,195]. Interestingly, induction of FoxO3a is able to sensitize breast cancer cells to chemotherapy-induced apoptosis [196]. Since much is known about post-translational modifications that influence FoxO function, members of the FoxO subfamily could prove to be useful candidates for the development of gene therapy strategies. In treating metabolic diseases such as diabetes, pharmacological regulation of phosphorylation could theoretically be used to target FoxO factors as well as Foxa2 [197]. Although targeting forkheads in disease treatment sounds promising in theory, this clearly requires additional investigation.
In addition to their possible use in disease therapy, forkhead expression can be useful for the prognosis of the clinical outcome of particular diseases. Consistent with the role of Foxp3 in suppressing autoimmunity, expression levels of Foxp3 appear to be predictive of the clinical outcome of renal transplantation, with higher levels associated with a more favourable outcome [198]. In contrast, increased Foxp3 expression appears to be a warning sign for patients suffering from ovarian carcinoma, whereas decreased expression is associated with a more favourable prognosis [199].
CONCLUDING REMARKS
Forkhead transcription factors are increasingly being recognized for their importance in mammalian development and physiology, acting downstream of multiple conserved signalling pathways, involving phosphoinositide 3-kinase/PKB, Wnt and TGFβ, among others. Still relatively little is known, however, about the mechanisms that underlie target specificity and how forkheads can function in such a large variety of cellular processes.
Existing functional protein domains and interactions are important for the functionality of forkhead factors. However, most of what we know stems from studies on a limited number of family members. As particular regulatory motifs are often absent in most family members, it will be vital to identify structural elements in other forkheads. The increased availability of techniques such as microarrays and MS will help to understand how forkheads are targeted to specific loci, and how binding of forkheads leads to transcriptional activation or repression.
In vitro studies have mainly benefitted research on biochemical properties of forkhead proteins, as functional aspects of forkhead proteins may be highly dependent on cell type and physiological context. Most functional studies on forkhead factors have therefore been limited to regulation of cell cycle, survival and metabolism, which are analysed relatively easily. During development, however, processes such as proliferation, differentiation and migration are tightly regulated and are context-dependent. Useful in vitro models are therefore not commonly available, which is reflected by the low number of studies that have provided evidence for involvement of forkheads in developmental and physiological processes in vivo. However, for the majority of murine forkheads, knockout or transgenic mice are now available and are analysed phenotypically. In the coming years, more elaborate analysis of these and other in vivo models should elucidate a detailed function for each forkhead transcription factor.
Acknowledgments
We apologize to authors whose work could not be discussed or cited. This work was supported by an NWO (Netherlands Organisation for Scientific Research) grant (903-42-190).
References
- 1.Weigel D., Jurgens G., Kuttner F., Seifert E., Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 2.Weigel D., Jackle H. The fork head domain: a novel DNA binding motif of eukaryotic transcription factors? Cell. 1990;63:455–456. doi: 10.1016/0092-8674(90)90439-l. [DOI] [PubMed] [Google Scholar]
- 3.Lai E., Prezioso V. R., Tao W. F., Chen W. S., Darnell J. E., Jr Hepatocyte nuclear factor 3α belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev. 1991;5:416–427. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]
- 4.Kaestner K. H., Knochel W., Martinez D. E. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 5.Carlsson P., Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann E., Knochel W. Five years on the wings of fork head. Mech. Dev. 1996;57:3–20. doi: 10.1016/0925-4773(96)00539-4. [DOI] [PubMed] [Google Scholar]
- 7.Clark K. L., Halay E. D., Lai E., Burley S. K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature (London) 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 8.Gajiwala K. S., Chen H., Cornille F., Roques B. P., Reith W., Mach B., Burley S. K. Structure of the winged-helix protein hRFX1 reveals a new mode of DNA binding. Nature (London) 2000;403:916–921. doi: 10.1038/35002634. [DOI] [PubMed] [Google Scholar]
- 9.Liu P. P., Chen Y. C., Li C., Hsieh Y. H., Chen S. W., Chen S. H., Jeng W. Y., Chuang W. J. Solution structure of the DNA-binding domain of interleukin enhancer binding factor 1 (FOXK1a) Proteins. 2002;49:543–553. doi: 10.1002/prot.10227. [DOI] [PubMed] [Google Scholar]
- 10.Stroud J. C., Wu Y., Bates D. L., Han A., Nowick K., Paabo S., Tong H., Chen L. Structure of the forkhead domain of FOXP2 bound to DNA. Structure. 2006;14:159–166. doi: 10.1016/j.str.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.van Dongen M. J., Cederberg A., Carlsson P., Enerback S., Wikstrom M. Solution structure and dynamics of the DNA-binding domain of the adipocyte-transcription factor FREAC-11. J. Mol. Biol. 2000;296:351–359. doi: 10.1006/jmbi.1999.3476. [DOI] [PubMed] [Google Scholar]
- 12.Weigelt J., Climent I., Dahlman-Wright K., Wikstrom M. Solution structure of the DNA binding domain of the human forkhead transcription factor AFX (FOXO4) Biochemistry. 2001;40:5861–5869. doi: 10.1021/bi001663w. [DOI] [PubMed] [Google Scholar]
- 13.Jin C., Marsden I., Chen X., Liao X. Dynamic DNA contacts observed in the NMR structure of winged helix protein–DNA complex. J. Mol. Biol. 1999;289:683–690. doi: 10.1006/jmbi.1999.2819. [DOI] [PubMed] [Google Scholar]
- 14.Marsden I., Jin C., Liao X. Structural changes in the region directly adjacent to the DNA-binding helix highlight a possible mechanism to explain the observed changes in the sequence-specific binding of winged helix proteins. J. Mol. Biol. 1998;278:293–299. doi: 10.1006/jmbi.1998.1703. [DOI] [PubMed] [Google Scholar]
- 15.Overdier D. G., Porcella A., Costa R. H. The DNA-binding specificity of the hepatocyte nuclear factor 3/forkhead domain is influenced by amino-acid residues adjacent to the recognition helix. Mol. Cell. Biol. 1994;14:2755–2766. doi: 10.1128/mcb.14.4.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierrou S., Hellqvist M., Samuelsson L., Enerback S., Carlsson P. Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. EMBO J. 1994;13:5002–5012. doi: 10.1002/j.1460-2075.1994.tb06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S., Weidenfeld J., Morrisey E. E. Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol. Cell. Biol. 2004;24:809–822. doi: 10.1128/MCB.24.2.809-822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barthel A., Schmoll D., Unterman T. G. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Hellqvist M., Mahlapuu M., Blixt A., Enerback S., Carlsson P. The human forkhead protein FREAC-2 contains two functionally redundant activation domains and interacts with TBP and TFIIB. J. Biol. Chem. 1998;273:23335–23343. doi: 10.1074/jbc.273.36.23335. [DOI] [PubMed] [Google Scholar]
- 20.Mahlapuu M., Pelto-Huikko M., Aitola M., Enerback S., Carlsson P. FREAC-1 contains a cell-type-specific transcriptional activation domain and is expressed in epithelial–mesenchymal interfaces. Dev. Biol. 1998;202:183–195. doi: 10.1006/dbio.1998.9010. [DOI] [PubMed] [Google Scholar]
- 21.Qian X., Costa R. H. Analysis of hepatocyte nuclear factor-3β protein domains required for transcriptional activation and nuclear targeting. Nucleic Acids Res. 1995;23:1184–1191. doi: 10.1093/nar/23.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu W., Yang H., Zhang L., Lu M. M., Morrisey E. E. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J. Biol. Chem. 2001;276:27488–27497. doi: 10.1074/jbc.M100636200. [DOI] [PubMed] [Google Scholar]
- 23.Wang B., Lin D., Li C., Tucker P. Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J. Biol. Chem. 2003;278:24259–24268. doi: 10.1074/jbc.M207174200. [DOI] [PubMed] [Google Scholar]
- 24.Attisano L., Wrana J. L. Smads as transcriptional co-modulators. Curr. Opin. Cell Biol. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- 25.Brownawell A. M., Kops G. J., Macara I. G., Burgering B. M. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell. Biol. 2001;21:3534–3546. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Der Heide L. P., Hoekman M. F., Smidt M. P. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem. J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X., Gan L., Pan H., Kan D., Majeski M., Adam S. A., Unterman T. G. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem. J. 2004;378:839–849. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry F. B., Saleem R. A., Walter M. A. FOXC1 transcriptional regulation is mediated by N- and C-terminal activation domains and contains a phosphorylated transcriptional inhibitory domain. J. Biol. Chem. 2002;277:10292–10297. doi: 10.1074/jbc.M110266200. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Ferreria M. A., Rey-Campos J. Functional domains of FOXJ2. J. Mol. Biol. 2003;329:631–644. doi: 10.1016/s0022-2836(03)00524-2. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs F. M., van der Heide L. P., Wijchers P. J., Burbach J. P., Hoekman M. F., Smidt M. P. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J. Biol. Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- 31.Rena G., Prescott A. R., Guo S., Cohen P., Unterman T. G. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targeting. Biochem. J. 2001;354:605–612. doi: 10.1042/0264-6021:3540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunet A., Park J., Tran H., Hu L. S., Hemmings B. A., Greenberg M. E. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol. Cell. Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rena G., Woods Y. L., Prescott A. R., Peggie M., Unterman T. G., Williams M. R., Cohen P. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J. 2002;21:2263–2271. doi: 10.1093/emboj/21.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods Y. L., Rena G., Morrice N., Barthel A., Becker W., Guo S., Unterman T. G., Cohen P. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem. J. 2001;355:597–607. doi: 10.1042/bj3550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 36.Kops G. J., de Ruiter N. D., De Vries-Smits A. M., Powell D. R., Bos J. L., Burgering B. M. Direct control of the forkhead transcription factor AFX by protein kinase B. Nature (London) 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 37.Biggs W. H., III, Meisenhelder J., Hunter T., Cavenee W. K., Arden K. C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu M. C., Lee D. F., Xia W., Golfman L. S., Ou-Yang F., Yang J. Y., Zou Y., Bao S., Hanada N., Saso H., et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 39.Huang H., Regan K. M., Wang F., Wang D., Smith D. I., van Deursen J. M., Tindall D. J. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzaki H., Daitoku H., Hatta M., Tanaka K., Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plas D. R., Thompson C. B. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J. Biol. Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 42.Wolfrum C., Besser D., Luca E., Stoffel M. Insulin regulates the activity of forkhead transcription factor Hnf-3β/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11624–11629. doi: 10.1073/pnas.1931483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alessi D. R., Caudwell F. B., Andjelkovic M., Hemmings B. A., Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 44.De Ruiter N. D., Burgering B. M., Bos J. L. Regulation of the Forkhead transcription factor AFX by Ral-dependent phosphorylation of threonines 447 and 451. Mol. Cell. Biol. 2001;21:8225–8235. doi: 10.1128/MCB.21.23.8225-8235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Essers M. A., Weijzen S., de Vries-Smits A. M., Saarloos I., de Ruiter N. D., Bos J. L., Burgering B. M. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukuoka M., Daitoku H., Hatta M., Matsuzaki H., Umemura S., Fukamizu A. Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int. J. Mol. Med. 2003;12:503–508. [PubMed] [Google Scholar]
- 47.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 48.van der Horst A., Tertoolen L. G., de Vries-Smits L. M., Frye R. A., Medema R. H., Burgering B. M. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J. Biol. Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 49.Motta M. C., Divecha N., Lemieux M., Kamel C., Chen D., Gu W., Bultsma Y., McBurney M., Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 50.van der Heide L. P., Smidt M. P. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem. Sci. 2005;30:81–86. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Matsuzaki H., Daitoku H., Hatta M., Aoyama H., Yoshimochi K., Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hofmann K., Bucher P. The FHA domain: a putative nuclear signalling domain found in protein kinases and transcription factors. Trends Biochem. Sci. 1995;20:347–349. doi: 10.1016/s0968-0004(00)89072-6. [DOI] [PubMed] [Google Scholar]
- 53.Durocher D., Jackson S. P. The FHA domain. FEBS Lett. 2002;513:58–66. doi: 10.1016/s0014-5793(01)03294-x. [DOI] [PubMed] [Google Scholar]
- 54.Hammet A., Pike B. L., McNees C. J., Conlan L. A., Tenis N., Heierhorst J. Fha domains as phospho-threonine binding modules in cell signaling. IUBMB Life. 2003;55:23–27. doi: 10.1080/1521654031000070636. [DOI] [PubMed] [Google Scholar]
- 55.Wittenberg C., Reed S. I. Cell cycle-dependent transcription in yeast: promoters, transcription factors, and transcriptomes. Oncogene. 2005;24:2746–2755. doi: 10.1038/sj.onc.1208606. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds D., Shi B. J., McLean C., Katsis F., Kemp B., Dalton S. Recruitment of Thr319-phosphorylated Ndd1p to the FHA domain of Fkh2p requires Clb kinase activity: a mechanism for CLB cluster gene activation. Genes Dev. 2003;17:1789–1802. doi: 10.1101/gad.1074103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darieva Z., Pic-Taylor A., Boros J., Spanos A., Geymonat M., Reece R. J., Sedgwick S. G., Sharrocks A. D., Morgan B. A. Cell cycle-regulated transcription through the FHA domain of Fkh2p and the coactivator Ndd1p. Curr. Biol. 2003;13:1740–1745. doi: 10.1016/j.cub.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 58.ten Dijke P., Hill C. S. New insights into TGF-β–Smad signalling. Trends Biochem. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Chen X., Rubock M. J., Whitman M. A transcriptional partner for mad proteins in TGF-β signalling. Nature (London) 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- 60.Chen X., Weisberg E., Fridmacher V., Watanabe M., Naco G., Whitman M. Smad4 and FAST-1 in the assembly of activin responsive factor. Nature (London) 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- 61.Germain S., Howell M., Esslemont G. M., Hill C. S. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes Dev. 2000;14:435–451. [PMC free article] [PubMed] [Google Scholar]
- 62.Randall R. A., Howell M., Page C. S., Daly A., Bates P. A., Hill C. S. Recognition of phosphorylated-Smad2-containing complexes by a novel Smad interaction motif. Mol. Cell. Biol. 2004;24:1106–1121. doi: 10.1128/MCB.24.3.1106-1121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seoane J., Le H. V., Shen L., Anderson S. A., Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 64.Dou C., Lee J., Liu B., Liu F., Massague J., Xuan S., Lai E. BF-1 interferes with transforming growth factor β signaling by associating with Smad partners. Mol. Cell. Biol. 2000;20:6201–6211. doi: 10.1128/mcb.20.17.6201-6211.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li P., Lee H., Guo S., Unterman T. G., Jenster G., Bai W. Akt-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol. Cell. Biol. 2003;23:104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao H. H., Herrera R. E., Coronado-Heinsohn E., Yang M. C., Ludes-Meyers J. H., Seybold-Tilson K. J., Nawaz Z., Yee D., Barr F. G., Diab S. G., et al. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J. Biol. Chem. 2001;276:27907–27912. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- 67.Chen G., Nomura M., Morinaga H., Matsubara E., Okabe T., Goto K., Yanase T., Zheng H., Lu J., Nawata H. Modulation of androgen receptor transactivation by FoxH1: a newly identified androgen receptor corepressor. J. Biol. Chem. 2005;280:36355–36363. doi: 10.1074/jbc.M506147200. [DOI] [PubMed] [Google Scholar]
- 68.Yu X., Gupta A., Wang Y., Suzuki K., Mirosevich J., Orgebin-Crist M. C., Matusik R. J. Foxa1 and foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Ann. N. Y. Acad. Sci. 2005;1061:77–93. doi: 10.1196/annals.1336.009. [DOI] [PubMed] [Google Scholar]
- 69.Kodama S., Koike C., Negishi M., Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schuur E. R., Loktev A. V., Sharma M., Sun Z., Roth R. A., Weigel R. J. Ligand-dependent interaction of estrogen receptor-α with members of the forkhead transcription factor family. J. Biol. Chem. 2001;276:33554–33560. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- 71.Marshak S., Benshushan E., Shoshkes M., Havin L., Cerasi E., Melloul D. Functional conservation of regulatory elements in the pdx-1 gene: PDX-1 and hepatocyte nuclear factor 3β transcription factors mediate β-cell-specific expression. Mol. Cell. Biol. 2000;20:7583–7590. doi: 10.1128/mcb.20.20.7583-7590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foucher I., Montesinos M. L., Volovitch M., Prochiantz A., Trembleau A. Joint regulation of the MAP1B promoter by HNF3β/Foxa2 and engrailed is the result of a highly conserved mechanism for direct interaction of homeoproteins and Fox transcription factors. Development. 2003;130:1867–1876. doi: 10.1242/dev.00414. [DOI] [PubMed] [Google Scholar]
- 73.Guo Y., Costa R., Ramsey H., Starnes T., Vance G., Robertson K., Kelley M., Reinbold R., Scholer H., Hromas R. The embryonic stem cell transcription factors Oct-4 and FoxD3 interact to regulate endodermal-specific promoter expression. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3663–3667. doi: 10.1073/pnas.062041099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berry F. B., Lines M. A., Oas J. M., Footz T., Underhill D. A., Gage P. J., Walter M. A. Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld–Rieger syndrome and anterior segment dysgenesis. Hum. Mol. Genet. 2006;15:905–919. doi: 10.1093/hmg/ddl008. [DOI] [PubMed] [Google Scholar]
- 75.So C. W., Cleary M. L. Common mechanism for oncogenic activation of MLL by forkhead family proteins. Blood. 2003;101:633–639. doi: 10.1182/blood-2002-06-1785. [DOI] [PubMed] [Google Scholar]
- 76.Ramaswamy S., Nakamura N., Sansal I., Bergeron L., Sellers W. R. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 77.Shim E. Y., Woodcock C., Zaret K. S. Nucleosome positioning by the winged helix transcription factor HNF3. Genes Dev. 1998;12:5–10. doi: 10.1101/gad.12.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cerf C., Lippens G., Ramakrishnan V., Muyldermans S., Segers A., Wyns L., Wodak S. J., Hallenga K. Homo- and heteronuclear two-dimensional NMR studies of the globular domain of histone H1: full assignment, tertiary structure, and comparison with the globular domain of histone H5. Biochemistry. 1994;33:11079–11086. doi: 10.1021/bi00203a004. [DOI] [PubMed] [Google Scholar]
- 79.Ramakrishnan V., Finch J. T., Graziano V., Lee P. L., Sweet R. M. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature (London) 1993;362:219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- 80.Cirillo L. A., McPherson C. E., Bossard P., Stevens K., Cherian S., Shim E. Y., Clark K. L., Burley S. K., Zaret K. S. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 1998;17:244–254. doi: 10.1093/emboj/17.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carroll J. S., Liu X. S., Brodsky A. S., Li W., Meyer C. A., Szary A. J., Eeckhoute J., Shao W., Hestermann E. V., Geistlinger T. R., et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Holmqvist P. H., Belikov S., Zaret K. S., Wrange O. FoxA1 binding to the MMTV LTR modulates chromatin structure and transcription. Exp. Cell Res. 2005;304:593–603. doi: 10.1016/j.yexcr.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Yang Q., Kong Y., Rothermel B., Garry D. J., Bassel-Duby R., Williams R. S. The winged-helix/forkhead protein myocyte nuclear factor β (MNF-β) forms a co-repressor complex with mammalian sin3B. Biochem. J. 2000;345:335–343. [PMC free article] [PubMed] [Google Scholar]
- 84.Scott K. L., Plon S. E. CHES1/FOXN3 interacts with Ski-interacting protein and acts as a transcriptional repressor. Gene. 2005;359:119–126. doi: 10.1016/j.gene.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 85.Korver W., Roose J., Heinen K., Weghuis D. O., de Bruijn D., van Kessel A. G., Clevers H. The human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and promoter characterization. Genomics. 1997;46:435–442. doi: 10.1006/geno.1997.5065. [DOI] [PubMed] [Google Scholar]
- 86.Laoukili J., Kooistra M. R., Bras A., Kauw J., Kerkhoven R. M., Morrison A., Clevers H., Medema R. H. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 87.Wang I. C., Chen Y. J., Hughes D., Petrovic V., Major M. L., Park H. J., Tan Y., Ackerson T., Costa R. H. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell. Biol. 2005;25:10875–10894. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye H., Holterman A. X., Yoo K. W., Franks R. R., Costa R. H. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol. Cell. Biol. 1999;19:8570–8580. doi: 10.1128/mcb.19.12.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X., Hung N. J., Costa R. H. Earlier expression of the transcription factor HFH-11B diminishes induction of p21CIP1/WAF1 levels and accelerates mouse hepatocyte entry into S-phase following carbon tetrachloride liver injury. Hepatology. 2001;33:1404–1414. doi: 10.1053/jhep.2001.24666. [DOI] [PubMed] [Google Scholar]
- 90.Medema R. H., Kops G. J., Bos J. L., Burgering B. M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature (London) 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 91.Nakamura N., Ramaswamy S., Vazquez F., Signoretti S., Loda M., Sellers W. R. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kops G. J., Medema R. H., Glassford J., Essers M. A., Dijkers P. F., Coffer P. J., Lam E. W., Burgering B. M. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol. Cell. Biol. 2002;22:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Gac L., Marques M., Garcia Z., Campanero M. R., Carrera A. C. Control of cyclin G2 mRNA expression by forkhead transcription factors: novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol. Cell. Biol. 2004;24:2181–2189. doi: 10.1128/MCB.24.5.2181-2189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt M., Fernandez de Mattos S., van der Horst A., Klompmaker R., Kops G. J., Lam E. W., Burgering B. M., Medema R. H. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol. Cell. Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alvarez B., Martinez-A C., Burgering B. M., Carrera A. C. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature (London) 2001;413:744–747. doi: 10.1038/35099574. [DOI] [PubMed] [Google Scholar]
- 96.Tran H., Brunet A., Grenier J. M., Datta S. R., Fornace A. J., Jr, DiStefano P. S., Chiang L. W., Greenberg M. E. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 97.Hawke T. J., Jiang N., Garry D. J. Absence of p21CIP rescues myogenic progenitor cell proliferative and regenerative capacity in Foxk1 null mice. J. Biol. Chem. 2003;278:4015–4020. doi: 10.1074/jbc.M209200200. [DOI] [PubMed] [Google Scholar]
- 98.Williamson E. A., Wolf I., O'Kelly J., Bose S., Tanosaki S., Koeffler H. P. BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle-dependent kinase inhibitor p27Kip1. Oncogene. 2006;25:1391–1399. doi: 10.1038/sj.onc.1209170. [DOI] [PubMed] [Google Scholar]
- 99.Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., Tissenbaum H. A., Ruvkun G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature (London) 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 100.Lin K., Dorman J. B., Rodan A., Kenyon C. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 101.Paradis S., Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang E. D., Nunez G., Barr F. G., Guan K. L. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 103.Gilley J., Coffer P. J., Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dijkers P. F., Birkenkamp K. U., Lam E. W., Thomas N. S., Lammers J. W., Koenderman L., Coffer P. J. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith W. W., Norton D. D., Gorospe M., Jiang H., Nemoto S., Holbrook N. J., Finkel T., Kusiak J. W. Phosphorylation of p66Shc and forkhead proteins mediates Aβ toxicity. J. Cell Biol. 2005;169:331–339. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Essers M. A., de Vries-Smits L. M., Barker N., Polderman P. E., Burgering B. M., Korswagen H. C. Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 107.Dijkers P. F., Medema R. H., Lammers J. W., Koenderman L., Coffer P. J. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 108.Dijkers P. F., Medema R. H., Pals C., Banerji L., Thomas N. S., Lam E. W., Burgering B. M., Raaijmakers J. A., Lammers J. W., Koenderman L., Coffer P. J. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27KIP1. Mol. Cell. Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kops G. J., Dansen T. B., Polderman P. E., Saarloos I., Wirtz K. W., Coffer P. J., Huang T. T., Bos J. L., Medema R. H., Burgering B. M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature (London) 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 110.Nemoto S., Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 111.Shih D. Q., Navas M. A., Kuwajima S., Duncan S. A., Stoffel M. Impaired glucose homeostasis and neonatal mortality in hepatocyte nuclear factor 3α-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10152–10157. doi: 10.1073/pnas.96.18.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaestner K. H., Katz J., Liu Y., Drucker D. J., Schutz G. Inactivation of the winged helix transcription factor HNF3α affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev. 1999;13:495–504. doi: 10.1101/gad.13.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen W., Scearce L. M., Brestelli J. E., Sund N. J., Kaestner K. H. Foxa3 (hepatocyte nuclear factor 3γ) is required for the regulation of hepatic GLUT2 expression and the maintenance of glucose homeostasis during a prolonged fast. J. Biol. Chem. 2001;276:42812–42817. doi: 10.1074/jbc.M106344200. [DOI] [PubMed] [Google Scholar]
- 114.Sund N. J., Vatamaniuk M. Z., Casey M., Ang S. L., Magnuson M. A., Stoffers D. A., Matschinsky F. M., Kaestner K. H. Tissue-specific deletion of Foxa2 in pancreatic β cells results in hyperinsulinemic hypoglycemia. Genes Dev. 2001;15:1706–1715. doi: 10.1101/gad.901601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang H., Gauthier B. R., Hagenfeldt-Johansson K. A., Iezzi M., Wollheim C. B. Foxa2 (HNF3β) controls multiple genes implicated in metabolism-secretion coupling of glucose-induced insulin release. J. Biol. Chem. 2002;277:17564–17570. doi: 10.1074/jbc.M111037200. [DOI] [PubMed] [Google Scholar]
- 116.Lantz K. A., Vatamaniuk M. Z., Brestelli J. E., Friedman J. R., Matschinsky F. M., Kaestner K. H. Foxa2 regulates multiple pathways of insulin secretion. J. Clin. Invest. 2004;114:512–520. doi: 10.1172/JCI21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolfrum C., Asilmaz E., Luca E., Friedman J. M., Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature (London) 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 118.Cederberg A., Gronning L. M., Ahren B., Tasken K., Carlsson P., Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 119.Kim J. K., Kim H. J., Park S. Y., Cederberg A., Westergren R., Nilsson D., Higashimori T., Cho Y. R., Liu Z. X., Dong J., et al. Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes. 2005;54:1657–1663. doi: 10.2337/diabetes.54.6.1657. [DOI] [PubMed] [Google Scholar]
- 120.Accili D., Arden K. C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 121.Schmoll D., Walker K. S., Alessi D. R., Grempler R., Burchell A., Guo S., Walther R., Unterman T. G. Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the forkhead transcription factor FKHR: evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J. Biol. Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 122.Furuyama T., Kitayama K., Yamashita H., Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem. J. 2003;375:365–371. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kitamura Y. I., Kitamura T., Kruse J. P., Raum J. C., Stein R., Gu W., Accili D. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 124.Hosaka T., Biggs W. H., III, Tieu D., Boyer A. D., Varki N. M., Cavenee W. K., Arden K. C. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakae J., Biggs W. H., III, Kitamura T., Cavenee W. K., Wright C. V., Arden K. C., Accili D. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor foxo1. Nat. Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 126.Nakae J., Kitamura T., Kitamura Y., Biggs W. H., III, Arden K. C., Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 127.Coffer P. J., Burgering B. M. Forkhead-box transcription factors and their role in the immune system. Nat. Rev. Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 128.Jonsson H., Peng S. L. Forkhead transcription factors in immunology. Cell Mol. Life Sci. 2005;62:397–409. doi: 10.1007/s00018-004-4365-8. [DOI] [PubMed] [Google Scholar]
- 129.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 130.Khattri R., Cox T., Yasayko S. A., Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 131.Fontenot J. D., Gavin M. A., Rudensky A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 132.Lin L., Spoor M. S., Gerth A. J., Brody S. L., Peng S. L. Modulation of Th1 activation and inflammation by the NF-κB repressor Foxj1. Science. 2004;303:1017–1020. doi: 10.1126/science.1093889. [DOI] [PubMed] [Google Scholar]
- 133.Srivatsan S., Peng S. L. Cutting edge: Foxj1 protects against autoimmunity and inhibits thymocyte egress. J. Immunol. 2005;175:7805–7809. doi: 10.4049/jimmunol.175.12.7805. [DOI] [PubMed] [Google Scholar]
- 134.Brissette J. L., Li J., Kamimura J., Lee D., Dotto G. P. The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev. 1996;10:2212–2221. doi: 10.1101/gad.10.17.2212. [DOI] [PubMed] [Google Scholar]
- 135.Nehls M., Pfeifer D., Schorpp M., Hedrich H., Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature (London) 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 136.Yusuf I., Fruman D. A. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 137.Johansson C. C., Dahle M. K., Blomqvist S. R., Gronning L. M., Aandahl E. M., Enerback S., Tasken K. A winged helix forkhead (FOXD2) tunes sensitivity to cAMP in T lymphocytes through regulation of cAMP-dependent protein kinase RIα. J. Biol. Chem. 2003;278:17573–17579. doi: 10.1074/jbc.M300311200. [DOI] [PubMed] [Google Scholar]
- 138.Shi C., Zhang X., Chen Z., Sulaiman K., Feinberg M. W., Ballantyne C. M., Jain M. K., Simon D. I. Integrin engagement regulates monocyte differentiation through the forkhead transcription factor Foxp1. J. Clin. Invest. 2004;114:408–418. doi: 10.1172/JCI21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoodless P. A., Pye M., Chazaud C., Labbe E., Attisano L., Rossant J., Wrana J. L. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 2001;15:1257–1271. doi: 10.1101/gad.881501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yamamoto M., Meno C., Sakai Y., Shiratori H., Mochida K., Ikawa Y., Saijoh Y., Hamada H. The transcription factor FoxH1 (FAST) mediates nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev. 2001;15:1242–1256. doi: 10.1101/gad.883901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weinstein D. C., Ruiz i Altaba A., Chen W. S., Hoodless P., Prezioso V. R., Jessell T. M., Darnell J. E., Jr The winged-helix transcription factor HNF-3β is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 142.Ang S. L., Rossant J. HNF-3β is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 143.Chen J., Knowles H. J., Hebert J. L., Hackett B. P. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left–right asymmetry. J. Clin. Invest. 1998;102:1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilm B., James R. G., Schultheiss T. M., Hogan B. L. The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev. Biol. 2004;271:176–189. doi: 10.1016/j.ydbio.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 145.Kalinichenko V. V., Lim L., Stolz D. B., Shin B., Rausa F. M., Clark J., Whitsett J. A., Watkins S. C., Costa R. H. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box F1 transcription factor. Dev. Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 146.Mahlapuu M., Enerback S., Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- 147.Wan H., Dingle S., Xu Y., Besnard V., Kaestner K. H., Ang S. L., Wert S., Stahlman M. T., Whitsett J. A. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J. Biol. Chem. 2005;280:13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- 148.Costa R. H., Kalinichenko V. V., Lim L. Transcription factors in mouse lung development and function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L823–L838. doi: 10.1152/ajplung.2001.280.5.L823. [DOI] [PubMed] [Google Scholar]
- 149.Lee C. S., Sund N. J., Behr R., Herrera P. L., Kaestner K. H. Foxa2 is required for the differentiation of pancreatic α-cells. Dev. Biol. 2005;278:484–495. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 150.Gao N., Ishii K., Mirosevich J., Kuwajima S., Oppenheimer S. R., Roberts R. L., Jiang M., Yu X., Shappell S. B., Caprioli R. M., et al. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132:3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 151.Lantz K. A., Kaestner K. H. Winged-helix transcription factors and pancreatic development. Clin. Sci. 2005;108:195–204. doi: 10.1042/CS20040309. [DOI] [PubMed] [Google Scholar]
- 152.Levinson R. S., Batourina E., Choi C., Vorontchikhina M., Kitajewski J., Mendelsohn C. L. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 153.Hulander M., Wurst W., Carlsson P., Enerback S. The winged helix transcription factor Fkh10 is required for normal development of the inner ear. Nat. Genet. 1998;20:374–376. doi: 10.1038/3850. [DOI] [PubMed] [Google Scholar]
- 154.Blomqvist S. R., Vidarsson H., Fitzgerald S., Johansson B. R., Ollerstam A., Brown R., Persson A. E., Bergstrom G., Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J. Clin. Invest. 2004;113:1560–1570. doi: 10.1172/JCI20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Uda M., Ottolenghi C., Crisponi L., Garcia J. E., Deiana M., Kimber W., Forabosco A., Cao A., Schlessinger D., Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- 156.Schmidt D., Ovitt C. E., Anlag K., Fehsenfeld S., Gredsted L., Treier A. C., Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- 157.Ottolenghi C., Omari S., Garcia-Ortiz J. E., Uda M., Crisponi L., Forabosco A., Pilia G., Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- 158.Krupczak-Hollis K., Wang X., Kalinichenko V. V., Gusarova G. A., Wang I. C., Dennewitz M. B., Yoder H. M., Kiyokawa H., Kaestner K. H., Costa R. H. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev. Biol. 2004;276:74–88. doi: 10.1016/j.ydbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 159.Lee D., Prowse D. M., Brissette J. L. Association between mouse nude gene expression and the initiation of epithelial terminal differentiation. Dev. Biol. 1999;208:362–374. doi: 10.1006/dbio.1999.9221. [DOI] [PubMed] [Google Scholar]
- 160.Baxter R. M., Brissette J. L. Role of the nude gene in epithelial terminal differentiation. J. Invest. Dermatol. 2002;118:303–309. doi: 10.1046/j.0022-202x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- 161.Li S., Weidenfeld J., Morrisey E. E. Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol. Cell. Biol. 2004;24:809–822. doi: 10.1128/MCB.24.2.809-822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li S., Misra K., Matise M. P., Xiang M. Foxn4 acts synergistically with Mash1 to specify subtype identity of V2 interneurons in the spinal cord. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10688–10693. doi: 10.1073/pnas.0504799102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang B., Weidenfeld J., Lu M. M., Maika S., Kuziel W. A., Morrisey E. E., Tucker P. W. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–4487. doi: 10.1242/dev.01287. [DOI] [PubMed] [Google Scholar]
- 164.Hanna L. A., Foreman R. K., Tarasenko I. A., Kessler D. S., Labosky P. A. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tompers D. M., Foreman R. K., Wang Q., Kumanova M., Labosky P. A. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Dev. Biol. 2005;285:126–137. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 166.Blixt A., Mahlapuu M., Aitola M., Pelto-Huikko M., Enerback S., Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- 167.Hanashima C., Shen L., Li S. C., Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J. Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cheung M., Chaboissier M. C., Mynett A., Hirst E., Schedl A., Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 169.Hribal M. L., Nakae J., Kitamura T., Shutter J. R., Accili D. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J. Cell Biol. 2003;162:535–541. doi: 10.1083/jcb.200212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Furuyama T., Kitayama K., Shimoda Y., Ogawa M., Sone K., Yoshida-Araki K., Hisatsune H., Nishikawa S., Nakayama K., Nakayama K., et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J. Biol. Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 171.Castrillon D. H., Miao L., Kollipara R., Horner J. W., DePinho R. A. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 172.Garry D. J., Meeson A., Elterman J., Zhao Y., Yang P., Bassel-Duby R., Williams R. S. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5416–5421. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Labosky P. A., Winnier G. E., Jetton T. L., Hargett L., Ryan A. K., Rosenfeld M. G., Parlow A. F., Hogan B. L. The winged helix gene, Mf3, is required for normal development of the diencephalon and midbrain, postnatal growth and the milk-ejection reflex. Development. 1997;124:1263–1274. doi: 10.1242/dev.124.7.1263. [DOI] [PubMed] [Google Scholar]
- 174.Wehr R., Mansouri A., de Maeyer T., Gruss P. Fkh5-deficient mice show dysgenesis in the caudal midbrain and hypothalamic mammillary body. Development. 1997;124:4447–4456. doi: 10.1242/dev.124.22.4447. [DOI] [PubMed] [Google Scholar]
- 175.Lai C. S., Fisher S. E., Hurst J. A., Vargha-Khadem F., Monaco A. P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature (London) 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 176.Shu W., Cho J. Y., Jiang Y., Zhang M., Weisz D., Elder G. A., Schmeidler J., De Gasperi R., Sosa M. A., Rabidou D., et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lehmann O. J., Sowden J. C., Carlsson P., Jordan T., Bhattacharya S. S. Fox's in development and disease. Trends Genet. 2003;19:339–344. doi: 10.1016/S0168-9525(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 178.Lehmann O. J., Ebenezer N. D., Jordan T., Fox M., Ocaka L., Payne A., Leroy B. P., Clark B. J., Hitchings R. A., Povey S., et al. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am. J. Hum. Genet. 2000;67:1129–1135. doi: 10.1016/s0002-9297(07)62943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mears A. J., Jordan T., Mirzayans F., Dubois S., Kume T., Parlee M., Ritch R., Koop B., Kuo W. L., Collins C., et al. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld–Rieger anomaly. Am. J. Hum. Genet. 1998;63:1316–1328. doi: 10.1086/302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fang J., Dagenais S. L., Erickson R. P., Arlt M. F., Glynn M. W., Gorski J. L., Seaver L. H., Glover T. W. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am. J. Hum. Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Semina E. V., Brownell I., Mintz-Hittner H. A., Murray J. C., Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum. Mol. Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- 182.Ormestad M., Blixt A., Churchill A., Martinsson T., Enerback S., Carlsson P. Foxe3 haploinsufficiency in mice: a model for Peters' anomaly. Invest. Ophthalmol. Visual Sci. 2002;43:1350–1357. [PubMed] [Google Scholar]
- 183.De Baere E., Dixon M. J., Small K. W., Jabs E. W., Leroy B. P., Devriendt K., Gillerot Y., Mortier G., Meire F., Van Maldergem L., et al. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype–phenotype correlation. Hum. Mol. Genet. 2001;10:1591–1600. doi: 10.1093/hmg/10.15.1591. [DOI] [PubMed] [Google Scholar]
- 184.Crisponi L., Deiana M., Loi A., Chiappe F., Uda M., Amati P., Bisceglia L., Zelante L., Nagaraja R., Porcu S., et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- 185.Clifton-Bligh R. J., Wentworth J. M., Heinz P., Crisp M. S., John R., Lazarus J. H., Ludgate M., Chatterjee V. K. Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat. Genet. 1998;19:399–401. doi: 10.1038/1294. [DOI] [PubMed] [Google Scholar]
- 186.Frank J., Pignata C., Panteleyev A. A., Prowse D. M., Baden H., Weiner L., Gaetaniello L., Ahmad W., Pozzi N., Cserhalmi-Friedman P. B., et al. Exposing the human nude phenotype. Nature (London) 1999;398:473–474. doi: 10.1038/18997. [DOI] [PubMed] [Google Scholar]
- 187.Borkhardt A., Repp R., Haas O. A., Leis T., Harbott J., Kreuder J., Hammermann J., Henn T., Lampert F. Cloning and characterization of AFX, the gene that fuses to MLL in acute leukemias with a t(X;11)(q13;q23) Oncogene. 1997;14:195–202. doi: 10.1038/sj.onc.1200814. [DOI] [PubMed] [Google Scholar]
- 188.Galili N., Davis R. J., Fredericks W. J., Mukhopadhyay S., Rauscher F. J., III, Emanuel B. S., Rovera G., Barr F. G. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat. Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 189.Bennett C. L., Christie J., Ramsdell F., Brunkow M. E., Ferguson P. J., Whitesell L., Kelly T. E., Saulsbury F. T., Chance P. F., Ochs H. D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 190.Wildin R. S., Ramsdell F., Peake J., Faravelli F., Casanova J. L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L., et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 191.Loser K., Hansen W., Apelt J., Balkow S., Buer J., Beissert S. In vitro-generated regulatory T cells induced by Foxp3-retrovirus infection control murine contact allergy and systemic autoimmunity. Gene Ther. 2005;12:1294–1304. doi: 10.1038/sj.gt.3302567. [DOI] [PubMed] [Google Scholar]
- 192.Jaeckel E., von Boehmer H., Manns M. P. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 193.Jonsson H., Allen P., Peng S. L. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat. Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 194.Fukunaga K., Ishigami T., Kawano T. Transcriptional regulation of neuronal genes and its effect on neural functions: expression and function of forkhead transcription factors in neurons. J. Pharmacol. Sci. 2005;98:205–211. doi: 10.1254/jphs.fmj05001x3. [DOI] [PubMed] [Google Scholar]
- 195.Greer E. L., Brunet A. Foxo transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 196.Real P. J., Benito A., Cuevas J., Berciano M. T., de Juan A., Coffer P., Gomez-Roman J., Lafarga M., Lopez-Vega J. M., Fernandez-Luna J. L. Blockade of epidermal growth factor receptors chemosensitizes breast cancer cells through up-regulation of Bnip3L. Cancer Res. 2005;65:8151–8157. doi: 10.1158/0008-5472.CAN-05-1134. [DOI] [PubMed] [Google Scholar]
- 197.Wang H., Wollheim C. B. Does chasing selected ‘Fox’ to the nucleus prevent diabetes? Trends Mol. Med. 2005;11:262–265. doi: 10.1016/j.molmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 198.Muthukumar T., Dadhania D., Ding R., Snopkowski C., Naqvi R., Lee J. B., Hartono C., Li B., Sharma V. K., Seshan S. V., et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N. Engl. J. Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 199.Wolf D., Wolf A. M., Rumpold H., Fiegl H., Zeimet A. G., Muller-Holzner E., Deibl M., Gastl G., Gunsilius E., Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin. Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 200.Liu Y., Shen W., Brubaker P. L., Kaestner K. H., Drucker D. J. Foxa3 (HNF-3γ) binds to and activates the rat proglucagon gene promoter but is not essential for proglucagon gene expression. Biochem. J. 2002;366:633–641. doi: 10.1042/BJ20020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Raney A. K., Zhang P., McLachlan A. Regulation of transcription from the hepatitis B virus large surface antigen promoter by hepatocyte nuclear factor 3. J. Virol. 1995;69:3265–3272. doi: 10.1128/jvi.69.6.3265-3272.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Freyaldenhoven B. S., Freyaldenhoven M. P., Iacovoni J. S., Vogt P. K. Avian winged helix proteins CWH-1, CWH-2 and CWH-3 repress transcription from Qin binding sites. Oncogene. 1997;15:483–488. doi: 10.1038/sj.onc.1201189. [DOI] [PubMed] [Google Scholar]
- 203.Dahle M. K., Gronning L. M., Cederberg A., Blomhoff H. K., Miura N., Enerback S., Tasken K. A., Tasken K. Mechanisms of FOXC2- and FOXD1-mediated regulation of the RIα subunit of cAMP-dependent protein kinase include release of transcriptional repression and activation by protein kinase Bα and cAMP. J. Biol. Chem. 2002;277:22902–22908. doi: 10.1074/jbc.M200131200. [DOI] [PubMed] [Google Scholar]
- 204.Zhang H., Palmer R., Gao X., Kreidberg J., Gerald W., Hsiao L., Jensen R. V., Gullans S. R., Haber D. A. Transcriptional activation of placental growth factor by the forkhead/winged helix transcription factor FoxD1. Curr. Biol. 2003;13:1625–1629. doi: 10.1016/j.cub.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 205.Sutton J., Costa R., Klug M., Field L., Xu D., Largaespada D. A., Fletcher C. F., Jenkins N. A., Copeland N. G., Klemsz M., Hromas R. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J. Biol. Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- 206.Perrone L., Pasca diMagliano M., Zannini M., Di Lauro R. The thyroid transcription factor 2 (TTF-2) is a promoter-specific DNA-binding independent transcriptional repressor. Biochem. Biophys. Res. Commun. 2000;275:203–208. doi: 10.1006/bbrc.2000.3232. [DOI] [PubMed] [Google Scholar]
- 207.Li J., Chang H. W., Lai E., Parker E. J., Vogt P. K. The oncogene qin codes for a transcriptional repressor. Cancer Res. 1995;55:5540–5544. [PubMed] [Google Scholar]
- 208.Zhou S., Zawel L., Lengauer C., Kinzler K. W., Vogelstein B. Characterization of human FAST-1, a TGFβ and activin signal transducer. Mol. Cell. 1998;2:121–127. doi: 10.1016/s1097-2765(00)80120-3. [DOI] [PubMed] [Google Scholar]
- 209.Overdier D. G., Ye H., Peterson R. S., Clevidence D. E., Costa R. H. The winged helix transcriptional activator HFH-3 is expressed in the distal tubules of embryonic and adult mouse kidney. J. Biol. Chem. 1997;272:13725–13730. doi: 10.1074/jbc.272.21.13725. [DOI] [PubMed] [Google Scholar]
- 210.Lim L., Zhou H., Costa R. H. The winged helix transcription factor HFH-4 is expressed during choroid plexus epithelial development in the mouse embryo. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3094–3099. doi: 10.1073/pnas.94.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang X. L., Matsuura H., Fu Y., Sugiyama T., Miura N. MFH-1 is required for bone morphogenetic protein-2-induced osteoblastic differentiation of C2C12 myoblasts. FEBS Lett. 2000;470:29–34. doi: 10.1016/s0014-5793(00)01285-0. [DOI] [PubMed] [Google Scholar]
- 212.Nirula A., Moore D. J., Gaynor R. B. Constitutive binding of the transcription factor interleukin-2 (IL-2) enhancer binding factor to the IL-2 promoter. J. Biol. Chem. 1997;272:7736–7745. doi: 10.1074/jbc.272.12.7736. [DOI] [PubMed] [Google Scholar]
- 213.Pisarska M. D., Bae J., Klein C., Hsueh A. J. Forkhead L2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology. 2004;145:3424–3433. doi: 10.1210/en.2003-1141. [DOI] [PubMed] [Google Scholar]
- 214.Schuddekopf K., Schorpp M., Boehm T. The whn transcription factor encoded by the nude locus contains an evolutionarily conserved and functionally indispensable activation domain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9661–9664. doi: 10.1073/pnas.93.18.9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.So C. W., Cleary M. L. MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function. Mol. Cell. Biol. 2002;22:6542–6552. doi: 10.1128/MCB.22.18.6542-6552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kitamura T., Nakae J., Kitamura Y., Kido Y., Biggs W. H., III, Wright C. V., White M. F., Arden K. C., Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J. Clin. Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schubert L. A., Jeffery E., Zhang Y., Ramsdell F., Ziegler S. F. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 218.Hoggatt A. M., Kriegel A. M., Smith A. F., Herring B. P. Hepatocyte nuclear factor-3 homologue 1 (HFH-1) represses transcription of smooth muscle-specific genes. J. Biol. Chem. 2000;275:31162–31170. doi: 10.1074/jbc.M005595200. [DOI] [PubMed] [Google Scholar]