Abstract
After mRNA transcription termination in eukaryotes, the hyperphosphorylated form of RNA polymerase II (pol II0) must be recycled by TFIIF-associating C-terminal domain phosphatase (FCP1), the phosphatase responsible for dephosphorylating the C-terminal domain of the largest polymerase subunit. Transcription factor (TF)-IIF stimulates the activity of FCP1, and the RNA polymerase II-associating protein 74 subunit of TFIIF forms a complex with FCP1 in both human and yeast. Here, we report a cocrystal structure of the winged-helix domain of human RNA polymerase II-associating protein 74 bound to the α-helical C terminus of human FCP1 (residues 944–961). These results illustrate the molecular mechanism by which TFIIF efficiently recruits FCP1 to the pol II transcription machinery for recycling of the polymerase.
Transcription of class II nuclear genes in eukaryotes requires a complicated assembly of proteins composed of RNA polymerase II (pol II) and five general transcription factors (TFs), which are required for preinitiation complex (PIC) formation on promoter DNA and initiation of premessenger RNA transcription (1). In the most general case, PIC assembly begins with the TATA box-binding protein subunit of TFIID recognizing a TATA element, followed by coordinated accretion of TFIIB, pol II plus TFIIF, TFIIE, and TFIIH. Transcription initiation is followed by C-terminal domain (CTD) phosphorylation which, in turn, facilitates promoter clearance and productive elongation. After termination of RNA synthesis, a phosphatase recycles the polymerase to its dephospho-form (pol IIA), making it competent for initiation of another round of transcription.
Biochemical activities responsible for CTD dephosphorylation have been purified from HeLa cells, Saccharomyces cerevisiae (2, 3), and Schizosaccharomyces pombe (4, 5), and detected in Xenopus laevis (6). TFIIF-associating CTD phosphatase (FCP1) was first identified as an essential subunit of human TFIIF-associating CTD phosphatase (7–9). It was subsequently shown to be a component of the pol II holoenzyme in both human and yeast (5, 8) and to catalyze directly dephosphorylation of the CTD (10). The C-terminal region of human RNA polymerase II-associating protein (RAP)74 (residues 436–517) interacts with FCP1 and is required for RAP74-mediated stimulation of CTD phosphatase activity in vitro (8). TFIIF modulation of FCP1 activity underscores the importance of the pol II phosphorylation cycle in eukaryotic transcription initiation.
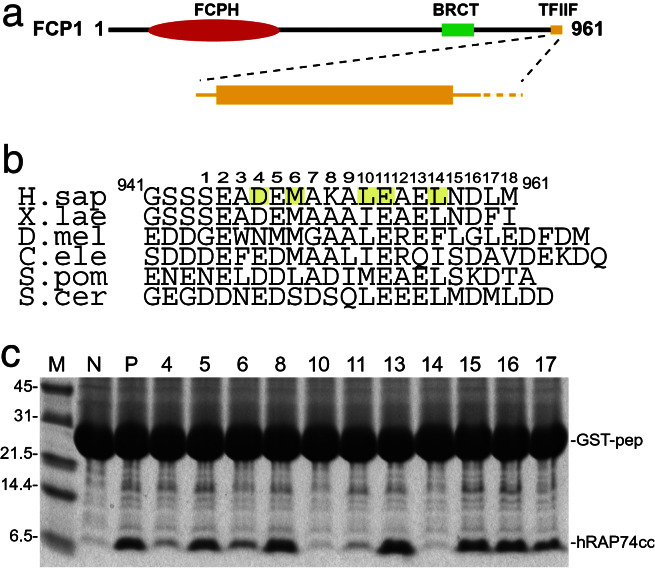
Human FCP1 is a 104-kDa protein consisting of three distinct segments (Fig. 1a): an N-terminal FCPH domain (residues 181–334, italics denotes human FCP1 numbering), a BRCA-1 C-terminal (BRCT) subdomain (residues 629–728), and a C-terminal TFIIF-binding region (7, 9). Although the FCPH domain is catalytic, it cannot act as a CTD phosphatase by itself. BRCT subdomains often support protein–protein interactions as tandem repeats in proteins that contribute to DNA repair, recombination, and cell cycle control (11). The integrity of the single BRCT subdomain found in FCP1 is essential for RNA synthesis and CTD dephosphorylation (12), implying that this polypeptide chain segment mediates protein–protein interactions within the transcription machinery. The C-terminal region of FCP1 and its interactions with TFIIF are the focus of this paper.
Figure 1.
FCP1 domain organization, sequence, and RAP74 binding. (a) Domain organization of human FCP1 (red, FCPH catalytic portion; green, BRCT subdomain; orange, TFIIF RAP74 interacting region). (b) Sequence alignment of C-terminal regions of FCP1 from Homo sapiens, X. laevis, Drosophila melanogaster, Caenorhabditis elegans, S. cerevisiae, and S. pombe with α-helical (rectangle), random coil (solid lines), and disordered (dotted line) regions indicated. Amino acids making significant contacts with hRAP74cc are shaded yellow. [Note: residue numbering in this manuscript corresponds to the full-length human sequence, GenBank accession no. AAD42088 (ref. 9).] (c) hRAP74cc binding to GST-hFCP1pep (941–961). Lane designations: M, size markers; N, negative control (GST plus 16 unrelated residues); P, positive control (GST with WT hFCP1pep: GSTpep); 4–17, alanine-scanning mutants with residue numbering keyed to b.
TFIIF, a hetero-oligomer of RAP74 and RAP30 subunits in mammals (13), plays a central role in the pol II transcription cycle by serving as a bridge between pol II and other components of the PIC. TFIIF stabilizes binding of pol II to TFIID and TFIIB at the promoter (14–16) and enables entry of TFIIE and TFIIH. After PIC assembly, TFIIF contributes to open complex formation by the DNA helicase subunit of TFIIH (13, 16, 17), synthesis of the first phosphodiester bond of nascent transcripts (18–20) and elongation (21). After transcription termination, TFIIF is thought to recruit FCP1 for recycling of pol II0 to pol IIA before the next round of transcription initiation (2).
Previously published structural studies of human TFIIF include work on a heterodimer of the N-terminal domains of RAP30 and RAP74 (22) and the CTDs of RAP30 (23) and RAP74 (24). Like the CTD of RAP30, which may be responsible for nonspecific DNA binding, the C terminus of RAP74 possesses a canonical winged-helix fold common to HNF-3γ (25), the linker histones H1 and H5 (26), and a large family of so-called forkhead transcription factors (reviewed in ref. 27). Although detection of a second winged-helix domain within TFIIF does suggest that RAP74 is a DNA-binding protein, surface electrostatic calculations revealed a paucity of basic residues on the putative DNA-binding surface, effectively militating against this inference (24). Moreover, residues on the surface of α-helix H3, the putative DNA recognition helix, are not conserved. Given these findings, we predicted instead that a hydrophobic feature on the surface of the CTD of RAP74 supports interactions with the C terminus of FCP1 (24). We now report identification of a C-terminal segment of FCP1 that interacts with the CTD of human RAP (hRAP)74 and determination of the x-ray structure of a RAP74-FCP1 complex at 2.0-Å resolution.
Materials and Methods
Protein Preparation and Purification.
The winged-helix domain of hRAP74 [residues 449–517: hRAP74cc] was overexpressed in Escherichia coli and purified to homogeneity, as described (24). The human FCP1 C-terminal oligopeptide used for crystallization (residues 944–961: hFCP1pep) was obtained through solid-phase synthesis and purified by reverse-phase HPLC.
FCP1 Alanine-Scanning Mutagenesis and RAP74 Binding.
Thirteen double-stranded DNA fragments encoding human FCP1 (residues 941–961), both WT and single alanine point mutations, were synthesized, sub-cloned into pGEX6-p-1 (Amersham Pharmacia), and confirmed by DNA sequencing. These GST-FCP1 fusions and GST fused to 16 unrelated amino acids were expressed in E. coli and purified with glutathione Sepharose. Immobilized GST fusion proteins, unrelated control, and WT and alanine mutant forms of FCP1 (30 μl of resin volume) were equilibrated with hRAP74cc (30 mg/ml) in 250 μl of buffer (50 mM Tris⋅HCl, pH 7.5/100 mM NaCl), incubated for 30 min at 20°C, and washed three times with the same buffer. Resin-bound proteins were visualized by Tricine SDS/PAGE with Coomassie blue staining.
Crystallization and Structure Determination.
Crystals of the hRAP74cc-hFCP1pep complex were grown by hanging drop vapor diffusion (1:1 molar ratio, 20 mg/ml hRAP74cc) against a reservoir containing 100 mM Mes, pH 6.5, 10 mM zinc sulfate heptahydrate, and 25% (vol/vol) polyethylene glycol monomethyl ether 550. Crystals grew to a typical size of 0.05 mm × 0.05 mm × 1.0 mm and belong to the monoclinic space group C2 (a = 59.5 Å, b = 30.4 Å, c = 47.4 Å, β = 106.4°), with one complex per asymmetric unit. Cryoprotection of crystals was achieved with added ethylene glycol 20% (vol/vol), and diffraction data were collected under standard cryogenic conditions on Beamline X9A (National Synchrotron Light Source, Brookhaven National Laboratory). After data processing with DENZO/SCALEPACK (28), initial phases were obtained with AMORE (29) by using our structure of hRAP74cc as a search model (PDB ID 1I27: correlation coefficient = 47.6%; R factor = 44.4%). Rigid body refinement and Powell minimization with CNS (30) yielded clear electron density for hFCP1pep in (|Fobserved| − |Fcalculated|) difference Fourier syntheses. The final refinement model (hRAP74cc residues 451–517 plus 3 additional N-terminal residues from a cloning artifact, hFCP1pep residues 944–961, 2 sulfate ions, 1 zinc ion, and 56 water molecules) gave an R factor of 20.4% and Rfree = 25.5%, with excellent stereochemistry (overall G value = 0.4; see Table 1 for data collection and refinement statistics; ref. 31).
Table 1.
Statistics of the crystallographic analysis
| Data set | |
|---|---|
| Intensity data processing | |
| Resolution, Å | 30–2.0 |
| Rsym, % | 4.5 |
| Number of measurements | 29,692 |
| Number of unique reflections | 5,657 |
| Completeness, % | 99.8 |
| 〈I/σ(I)〉 | 27.9 |
| Refinement statistics | |
| Resolution, Å | 22.7–2.0 (|Fo| > 2σ|Fo|) |
| Completeness, % | 97.5 |
| Rcryst/Rfree, % | 20.4/25.5 |
| 〈B〉 for hRAP74cc/hFCP1pep, Å2 | 20/37 |
| rmsd bond lengths, Å | 0.005 |
| rmsd bond angles, ° | 1.0 |
Rsym = ∑|I − 〈I〉|/∑I, where I is observed intensity, and 〈I〉 is average intensity obtained from multiple observations of symmetry related reflections. Rcryst = ∑∥Fobserved| − |Fcalculated∥/∑|Fobserved|. Rfree is Rcryst, calculated by using 7% of the data, chosen randomly, and omitted from refinement.
Results and Discussion
FCP1 Sequence Alignments.
The C-terminal region of RAP74 (436–517) binds directly to the C terminus of FCP1(879–961) (8). Alignment of FCP1 sequences corresponding to amino acids 879–961 from human, fission and budding yeasts, frog, fly, and worm demonstrates conservation of a C-terminal 20-residue region (Fig. 1b). Secondary structure predictions (32) also attribute high propensities for α-helix formation within each of these conserved 20-aa segments. Therefore, we posited that hRAP74cc binds to a 20-residue α-helix at the C terminus of FCP1. (In all cases, random coil regions are predicted to be immediately N-terminal to the C-terminal 20-residue segments.)
Alanine-Scanning Mutagenesis for FCP1.
To investigate this hypothesis, a series of alanine substitutions were made within residues 944–961 of human FCP1 (hFCP1pep) fused to GST. These fusion proteins were immobilized on glutathione Sepharose and tested for hRAP74cc binding (see Materials and Methods). As shown in Fig. 1c, substitutions of two acidic amino acids [Asp-4 (residue 947 in human FCP1) and Glu-11(954): Fig. 1c, lanes 4 and 11] and three hydrophobic residues [Met-6(949), Leu-10(953), and Leu-14(957): Fig. 1c lanes 6, 10, and 14] showed significant reductions in hRAP74cc binding as compared with WT hFCP1pep (Fig. 1c, lane P). These findings document that residues 947–957 of FCP1 are critical for stable complex formation with the hRAP74cc domain of TFIIF. Moreover, the i → i + 4 recurrence of hydrophobic side chains suggests that hFCP1pep adopts an α-helical conformation.
Structure of the hRAP74cc-hFCP1pep Complex.
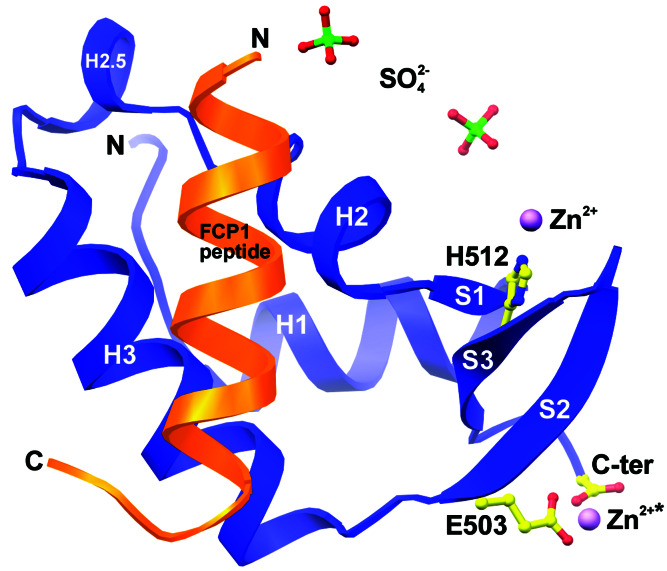
Having identified and partially delineated a TFIIF-binding site within the C terminus of human FCP1, we sought to examine directly how TFIIF stimulates the activity of the CTD phosphatase by determining the x-ray structure of FCP1 bound to RAP74. Cocrystals of an 18-residue synthetic peptide (hFCP1pep, residues 944–961) and the winged-helix domain of hRAP74cc yielded the structure depicted in Fig. 2 (see Materials and Methods and Table 1). The overall structure of hRAP74cc is essentially unchanged by binding of hFCP1pep [rms deviation (rmsd) with PDB ID 1I27 = 0.8 Å, 65 α-carbon pairs]. The N terminus of hRAP74cc (residues 449–451) participates in different sets of lattice-packing interactions in the apo and FCP1 complex crystals. As seen in our structure of hRAP74cc alone, zinc-mediated interactions between neighboring copies of hRAP74cc are present in our cocrystals of the hRAP74cc-hFCP1pep complex. Differences in lattice packing notwithstanding, the relative positions of Zn2+ and the three chemical groups responsible for metal ion chelation (the side chains of Glu-503 and His-512 and the C-terminal carboxylate group) are also present in our cocrystal structure (Fig. 2), imply that hRAP74cc may serve a physiologically relevant Zn2+ or divalent metal-binding role during eukaryotic transcription in vivo. Unlike our apo protein crystals, the hRAP74cc-hFCP1pep cocrystal lattice is stabilized by two sulfate anions (components of the crystallization condition), which bind to several lysine residues found within a highly basic surface feature of hRAP74cc.
Figure 2.
Structure of hRAP74cc-hFCP1pep complex. ribbons (34) drawing of hRAP74cc (blue) bound to hFCP1pep (orange) with labeled N and C termini and secondary structural elements. Zinc (*, crystallographically identical divalent cation), sulfate ions, and zinc-binding residues are shown as color-coded ball-and-stick figures: S, green; C, yellow; O, red; N, blue; and Zn2+, magenta.
RAP74 Recognition of FCP1.
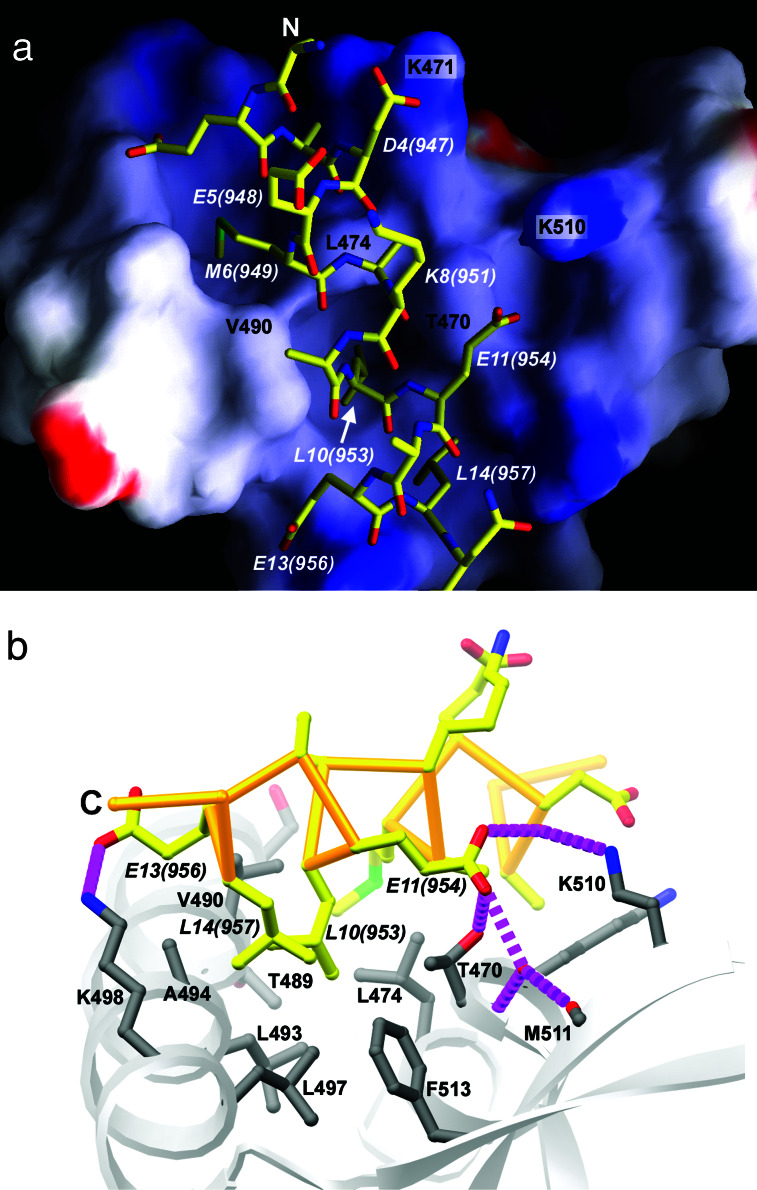
As predicted, hFCP1pep forms an α-helix that binds to a concave feature on the surface of the RAP74 winged-helix domain overlying α-helices H2 and H3 (Fig. 2). This hydrophobic/basic region appears ideally suited to accommodate both hydrophobic and acidic side chains projecting from the hFCP1pep α-helix, making intermolecular van der Waals contacts, salt bridges, and a water-mediated hydrogen bond (Fig. 3). The total solvent-accessible surface area buried upon complex formation is 1,264 Å2.
Figure 3.
FCP1 binding to TFIIF. (a) grasp (35) representation of the molecular surface of hRAP74cc, color coded for electrostatic potential (red <−15kBT, blue >+15kBT, where kB is the Boltzmann constant and T is temperature in Kelvins, calculated in the absence of peptide). hFCP1pep is depicted as a ball-and-stick figure. The view and color coding are as in Fig. 2. (b) hRAP74cc-hFCP1pep complex viewed close to the cylindrical axis of the peptide α-helix (C → N, ≈90o rotation about horizontal from a), showing hRAP74cc surface residues (gray) and hFCP1pep (atomic stick figure, color coded as in a). C terminus is labeled, and hydrogen bonds and salt-bridges are denoted with broken magenta lines.
DNA recognition by canonical winged-helix domains exploits the presence of a highly basic surface region located in the vicinity of α-helix H3 and β-strands S2 and S3, which form one of the eponymous wings (reviewed in ref. 27). In hRAP74cc, however, this region is much less basic than the corresponding portions of bona fide winged-helix transcription factors. There is at least one precedent for winged-helix domains supporting protein–protein interactions. The structure of a Skpl-Cullin-F-box protein ubiquitin ligase complex revealed two winged-helix motifs in the Cul1 subunit that serve as structural modules within the multiprotein complex (33). Unlike hRAP74cc, the Cul1 winged-helix domains do not resemble beads on a string but are tightly associated within the globular portion of the subunit itself. Intra-subunit protein–protein interactions involve α-helices H1 and H2 of the WH-A domain and α-helix H1 of the WH-B domain. We suggest that Cul1 and RAP74 represent illustrative examples of a new class of winged-helix proteins that mediate protein–protein recognition in large macromolecular complexes.
The most intimate hRAP74cc-hFCP1pep contacts occur in a concavity that is lined by phylogenetically conserved hydrophobic side chains. Three bulky hydrophobic hFCP1pep residues [Met-6(949), Leu-10(953), and Leu-14(957)] were identified as important contributors to intermolecular interactions by means of alanine-scanning mutagenesis (Fig. 1c). The side chain of Met-6(949) overlies a small depression created by Leu-474, Thr-489, and Val-490 (Fig. 3). Leu-10(953) and Leu-14(957) occupy a second depression on the surface of hRAP74cc that is surrounded by hydrophobic residues, making multiple van der Waals contacts (Fig. 3). Leu-10(953), which projects into a hydrophobic pocket created by three hydrophobic residues (Leu-474, Val-490, and Leu-493), provides a striking example of shape and chemical complementarity in the protein–protein interface. Finally, Ala-7(950) makes a close contact with Leu-474, which explains why small side chains are conserved at this position among FCP1 family members.
Observed polar interactions also explain the results of our alanine-scanning mutagenesis exercise. Asp-4(947) forms a salt bridge with Lys-471 (OD1—NZ = 3.0 Å, italics denote hFCP1pep atoms; Fig. 3a). Glu-11(954) makes a series of water-mediated hydrogen bonds with Lys-510 and Met-511 and a direct hydrogen bond with the side chain of Thr-470 (Fig. 3b). These acidic hFCP1pep amino acids are also conserved among the organisms enumerated in Fig. 1b. To our surprise, the alanine mutant of Glu-13(956) actually increases hFCP1pep binding (Fig. 1c, lane 13). We presume that the observed salt bridge between Glu-13(956) and Lys-498 (OE1—NZ = 3.7 Å), which involves kinked side-chain conformations (Fig. 3), is less favorable than a van der Waals contact between an alanine at position 13(956) of hFCP1pep and the aliphatic portions of Lys-498 and Ala-494. hFCP1pep also exhibits an intramolecular salt bridge, between Glu-5(948) and Lys-8(951) (OE1—NZ = 2.8 Å), which may stabilize the structure of its hRAP74cc-bound α-helical conformation. Mutations of either charged residue to Ala do not, however, abolish RAP74 binding in our qualitative pull-down assays (Fig. 1c), and these residues are not invariant within the FCP1 family.
Although we believe that our work has revealed most of the interactions stabilizing the RAP74-FCP1 complex, other residues within FCP1 may make additional contacts with RAP74. The face of the winged-helix domain opposite to the hFCP1pep-binding site is highly basic and makes a sulfate-mediated lattice contact with a neighbor in the crystal. This basic feature represents a good candidate for interactions with a cluster of acidic residues located immediately N-terminal to hFCP1pep (omitted from Fig. 1b). Alternatively, this basic region in RAP74 could make contacts with promoter DNA.
Acknowledgments
We thank Dr. K. R. Rajashankar for help with x-ray measurements and Drs. J. B. Bonanno and F. Hanaoka for assistance and advice with x-ray crystallography. S.K.B. is a Howard Hughes Medical Institute Investigator.
Abbreviations
- pol II
RNA polymerase II
- PIC
preinitiation complex
- TF
transcription factor
- CTD
C-terminal domain of RNA polymerase II
- FCP1
TFIIF-associating CTD phosphatase
- RAP
RNA polymerase II-associating protein
- hRAP
human RAP
Footnotes
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1J2X).
References
- 1.Roeder R G. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 2.Chambers R S, Wang B Q, Burton Z F, Dahmus M E. J Biol Chem. 1995;270:14962–14969. doi: 10.1074/jbc.270.25.14962. [DOI] [PubMed] [Google Scholar]
- 3.Chambers R S, Kane C M. J Biol Chem. 1996;271:24498–24504. doi: 10.1074/jbc.271.40.24498. [DOI] [PubMed] [Google Scholar]
- 4.Hausmann S, Shuman S. J Biol Chem. 2002;277:21213–21220. doi: 10.1074/jbc.M202056200. [DOI] [PubMed] [Google Scholar]
- 5.Kimura M, Suzuki H, Ishihama A. Mol Cell Biol. 2002;22:1577–1588. doi: 10.1128/mcb.22.5.1577-1588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palancade B, Dubois M F, Dahmus M E, Bensaude O. Mol Cell Biol. 2001;21:6359–6368. doi: 10.1128/MCB.21.19.6359-6368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archambault J, Chambers R S, Kobor M S, Ho Y, Cartier M, Bolotin D, Andrews B, Kane C M, Greenblatt J. Proc Natl Acad Sci USA. 1997;94:14300–14305. doi: 10.1073/pnas.94.26.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archambault J, Pan G, Dahmus G K, Cartier M, Marshall N, Zhang S, Dahmus M E, Greenblatt J. J Biol Chem. 1998;273:27593–27601. doi: 10.1074/jbc.273.42.27593. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Kim T K, Mancebo H, Lane W S, Flores O, Reinberg D. Genes Dev. 1999;13:1540–1552. doi: 10.1101/gad.13.12.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobor M S, Archambault J, Lester W, Holstege F C, Gileadi O, Jansma D B, Jennings E G, Kouyoumdjian F, Davidson A R, Young R A, Greenblatt J. Mol Cell. 1999;4:55–62. doi: 10.1016/s1097-2765(00)80187-2. [DOI] [PubMed] [Google Scholar]
- 11.Derbyshire D J, Basu B P, Serpell L C, Joo W S, Date T, Iwabuchi K, Doherty A J. EMBO J. 2002;21:3863–3872. doi: 10.1093/emboj/cdf383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobor M S, Simon L D, Omichinski J, Zhong G, Archambault J, Greenblatt J. Mol Cell Biol. 2000;20:7438–7449. doi: 10.1128/mcb.20.20.7438-7449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conaway R C, Conaway J W. Annu Rev Biochem. 1993;62:161–190. doi: 10.1146/annurev.bi.62.070193.001113. [DOI] [PubMed] [Google Scholar]
- 14.Conaway R C, Garrett K P, Hanley J P, Conaway J W. Proc Natl Acad Sci USA. 1991;88:6205–6209. doi: 10.1073/pnas.88.14.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buratowski S, Sopta M, Greenblatt J, Sharp P A. Proc Natl Acad Sci USA. 1991;88:7509–7513. doi: 10.1073/pnas.88.17.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores O, Lu H, Reinberg D. J Biol Chem. 1992;267:2786–2793. [PubMed] [Google Scholar]
- 17.Conaway R C, Conaway J W. J Biol Chem. 1990;265:7559–7563. [PubMed] [Google Scholar]
- 18.Tirode F, Busso D, Coin F, Egly J M. Mol Cell. 1999;3:87–95. doi: 10.1016/s1097-2765(00)80177-x. [DOI] [PubMed] [Google Scholar]
- 19.Pan G, Greenblatt J. J Biol Chem. 1994;269:30101–30104. [PubMed] [Google Scholar]
- 20.Parvin J D, Sharp P A. Cell. 1993;73:533–540. doi: 10.1016/0092-8674(93)90140-l. [DOI] [PubMed] [Google Scholar]
- 21.Conaway J W, Shilatifard A, Dvir A, Conaway R C. Trends Biochem Sci. 2000;25:375–380. doi: 10.1016/s0968-0004(00)01615-7. [DOI] [PubMed] [Google Scholar]
- 22.Gaiser F, Tan S, Richmond T J. J Mol Biol. 2000;302:1119–1127. doi: 10.1006/jmbi.2000.4110. [DOI] [PubMed] [Google Scholar]
- 23.Groft C M, Uljon S N, Wang R, Werner M H. Proc Natl Acad Sci USA. 1998;95:9117–9122. doi: 10.1073/pnas.95.16.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamada K, De Angelis J, Roeder R G, Burley S K. Proc Natl Acad Sci USA. 2001;98:3115–3120. doi: 10.1073/pnas.051631098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark K L, Halay E D, Lai E, Burley S K. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan V, Finch J, Graziano V, Sweet R. Nature. 1993;362:219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- 27.Gajiwala K S, Burley S K. Curr Opin Struct Biol. 2000;10:110–116. doi: 10.1016/s0959-440x(99)00057-3. [DOI] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 29.Navaza J. Acta Crystallogr A. 1994;50:157–163. [Google Scholar]
- 30.Brünger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jiang J S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 31.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 32.Rost B. Methods Enzymol. 1996;266:525–539. doi: 10.1016/s0076-6879(96)66033-9. [DOI] [PubMed] [Google Scholar]
- 33.Zheng N, Schulman B A, Song L, Miller J J, Jeffrey P D, Wang P, Chu C, Koepp D M, Elledge S J, Pagano M, et al. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 34.Carson M. In: Methods in Enzymology. Carter C W Jr, Sweet R M, editors. Vol. 277. New York: Academic; 1997. pp. 493–505. [PubMed] [Google Scholar]
- 35.Nicholls A, Sharp K A, Honig B. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]