Abstract
ClpC of Bacillus subtilis is an ATP-dependent HSP100/Clp protein involved in general stress survival. A complex of ClpC with the protease ClpP and the adaptor protein MecA also controls competence development by regulated proteolysis of the transcription factor ComK. We investigated the in vitro chaperone activity of ClpC and found that the presence of MecA was crucial for the major chaperone activities of ClpC. In particular, MecA enabled ClpC to solubilize and refold aggregated proteins. Finally, in the presence of ClpP, MecA allowed the ClpC-dependent degradation of unfolded or heat-aggregated proteins. This study demonstrates that adaptor proteins like MecA through interaction with their cognate ClpC proteins can have a dual role in the protein quality-control network by rescuing, or together with ClpP, by degrading, aggregated proteins. MecA can thereby coordinate substrate targeting with ClpC activation, adding another layer to the regulation of HSP100/Clp protein activity.
In eubacteria, the GroEL/GroES chaperone system and the DnaK chaperone with its cochaperones DnaJ and GrpE (KJE) form a functional network, which together with ribosome-associated chaperones assists the folding of newly synthesized proteins (1). Under stress conditions, unfolded and misfolded proteins may be generated, which escape this chaperone network and accumulate as protein aggregates. Such aggregates cannot be efficiently disaggregated and refolded by either the GroEL/GroES or KJE chaperone system. In contrast, ClpB, a member of the HSP100/Clp family (2), in cooperation with the KJE chaperone system, has the capacity to facilitate the efficient disaggregation and refolding of a large variety of protein aggregates in vitro and in vivo (3–8).
HSP100/Clp proteins are ATPases and members of the AAA+ superfamily of proteins, which form oligomeric ring structures (2, 9–11). Unlike ClpB or its yeast Hsp104 homolog, most members of the HSP100/Clp family associate with an oligomeric peptidase to form an ATP-dependent protease. For some HSP100/Clp proteins, like ClpA and ClpX, it was shown that they act as chaperones by promoting the ATP-dependent unfolding (12) and translocation of substrate proteins into the catalytically active centers of the associated ClpP peptidase ring (13, 14). However, these and other HSP100/Clp proteins also have chaperone activities independent of their role in the protease complex (12, 15, 16). The HSP100/Clp proteins cooperate with the other chaperone systems in a combined network that cannot only prevent aggregation and refold misfolded proteins but also disaggregate and refold or degrade previously aggregated proteins (9, 17, 18).
The first gene encoding an HSP100/Clp homolog in Bacillus subtilis, clpC, was discovered together with mecA in a screen for repressors of competence development (19). Further studies revealed that MecA acts as an adaptor protein, which targets the master regulator for competence development, ComK, for regulated proteolysis by the ClpC/ClpP protease (ClpCP) (20–24). In addition, ClpC functions as a general stress protein in B. subtilis. Its expression is increased by heat shock, and a clpC deletion strain is thermosensitive in growth and less thermotolerant than WT cells (25, 26).
Although ClpC is involved in both stress-related and various other regulatory processes in B. subtilis, the biochemical activity of ClpC has been characterized only with respect to specific substrate degradation in regulatory processes, and not in its more general chaperone activities (23, 24, 27–29). In this study, we therefore investigated the chaperone activities of ClpC and the influence of MecA on those activities. We demonstrate that ClpC, only together with MecA, can disaggregate and refold aggregated proteins. Furthermore, in the presence of ClpP, MecA enables ClpC-mediated degradation of unfolded or aggregated proteins.
Materials and Methods
Proteins.
Purifications of DnaK, DnaJ, GrpE, and ClpB were performed according to published procedures (4). Pyruvate kinase was purchased from Sigma, α-casein from Fluka, luciferase from Sigma, and malate dehydrogenase (MDH) from Roche Molecular Biochemicals. Monomer protein concentrations were determined by using the Bio-Rad Bradford assay with BSA as a standard. ClpP, MecA, and the N- and C-terminal domains of MecA were purified as described (22–24). ClpC was expressed as intein fusion protein in Escherichia coli and purification was carried out as described (28, 30).
ATPase Assay.
The ATPase assay was carried out according to procedures described by Liberek et al. (31). The respective HSP100/Clp protein concentration was 400 nM and the final ATP concentration was 2 mM. The resulting specific ATPase rates are indicated (molecules of ATP hydrolyzed per sec per molecule of the HSP100/Clp protein).
Prevention of Aggregation and Refolding with Heat-Denatured Luciferase.
Luciferase was diluted to a final concentration of 100 nM in refolding buffer containing an additional 4 mM ATP supplemented with various chaperones as indicated [0.6 μM ClpC, 0.6 μM MecA, or KJE (1 μM DnaK, 0.2 μM DnaJ, 0.1 μM GrpE)] in refolding buffer (25 mM Hepes/KOH pH 7.6/5 mM MgCl2/50 mM KCl/1 mM DTT/2 mM ATP). The reaction mixture was incubated for 15 min at 43°C. After incubation, either KJE or buffer was added, and luciferase activity was determined at various time points by diluting 1 μl of the reaction mixture into a 124-μl assay buffer (25 mM glycylglycine/5 mM ATP/15 mM MgSO4). after immediate addition of 125 μl of d-luciferin (Sigma) (44 μM, final concentration), light emission at 560 nm was measured over a period of 5 s in a Berthold (Bad Wildbad, Germany) Lumat 9501. To determine the prevention of aggregation abilities, luciferase (100 nM) was heat-denatured in the presence of chaperones (0.6 μM ClpC/0.6 μM MecA/0.6 μM DnaJ in refolding buffer with 50 μl of regeneration mix) for 15 min at 43°C, after which the total luciferase activity was determined as described above. The remaining reaction mixture (30 μl) of each experiment was centrifuged at 16,000 × g for 20 min at 4°C. The supernatant was carefully removed, and the pellet was resuspended in the appropriate amount of refolding buffer. Pellet and supernatant were analyzed by SDS/PAGE followed by a Western blot analysis with antiluciferase Abs and subsequently quantified by using the MACBAS program (Fuji).
Luciferase Disaggregation and Refolding.
The luciferase (200 nM) was heat-denatured in the absence of chaperones for 15 min at 43°C, after which the luciferase was diluted 1:1 with the reaction mixture containing the chaperones [0.6 μM ClPC, 1.8 μM MecA, 0.4 μM ClpB, KJE (1 μM DnaK, 0.2 μM DnaJ, 0.1 μM GrpE)] and 2 mM ATP in a regeneration mixture. The luciferase activity was determined as described previously.
MDH Disaggregation and Refolding.
MDH (2 μM) was denatured at 47°C for 30 min in MDH-refolding buffer [100 mM Tris, pH 7.5/150 mM KCl/20 mM Mg(OAc)2/1 mM DTT] and diluted to 0.72 μM in the presence of chaperones [0.72 μM ClpC, 0.72 μM MecA, 0.72 μM ClpB, KJE (1 μM DnaK, 0.2 μM DnaJ, 0.1 μM GrpE)] and 2 mM ATP in the presence of an ATP-regenerating system (4 mM phosphoenolpyruvate and 20 ng/ml pyruvate kinase). MDH disaggregation was determined by monitoring light scattering. MDH (2 μM) was denatured at 47°C for 30 min in MDH-refolding buffer and diluted to 0.72 μM in the presence or absence of indicated chaperones [0.72 μM ClpC, 0.72 μM or 0.24 μM MecA, 0.72 μM ClpB, KJE (1 μM DnaK, 0.2 μM DnaJ, 0.1 μM GrpE)]. The reaction mixture was incubated for 2 min at 30°C and the reaction was started by the addition of 2 mM ATP in the presence of an ATP-regenerating system. Right-angle light scattering was determined at 550-nm excitation and emission wavelength in a Perkin–Elmer LS55B spectrofluorometer. The activity of MDH was assayed at 30°C in 150 mM potassium phosphate buffer (pH 7.6), 1 mM DTT, 0.5 mM oxaloacetate, and 0.28 mM NADH (Sigma). The time-dependent oxidation of NADH by MDH was monitored at 340 nm in a Shimadzu UV-1601 spectrophotometer. Diluting the reaction mixture 1:70 into the assay buffer started the reaction.
Interaction of Chaperones with MDH Aggregates.
MDH (2 μM) was denatured in MDH-refolding buffer as described and diluted to a final concentration of 0.72 μM supplemented with the indicated chaperones at the same concentration. After the addition of 4.5 mM ATP in the presence of an ATP-regenerating system (where indicated), the reaction mixture was incubated at room temperature for 5 min and subsequently centrifuged at 16,000 × g for 30 min at 4°C. The supernatant was carefully removed and the pellet was resuspended in folding buffer. Supernatant and pellet fractions were analyzed by Coomassie-stained SDS/PAGE.
MDH/Casein Degradation.
MDH (4 μM) was denatured at 47°C for 30 min in MDH-refolding buffer and diluted to 1.44 μM in the reaction mix supplemented with ClpC, ClpP, and MecA (all 0.88 μM). To start the reaction, ATP (5 mM) with the ATP-regenerating system was added to the chaperone mixture. The reaction was stopped at different time points by mixing an aliquot of the reaction mixture with SDS-sample buffer on ice. Casein (5.8 μM) degradation was also carried out in the MDH-refolding buffer with the same concentration of chaperones.
Results
Prevention of Aggregation and Refolding of Misfolded Substrates by ClpC and MecA.
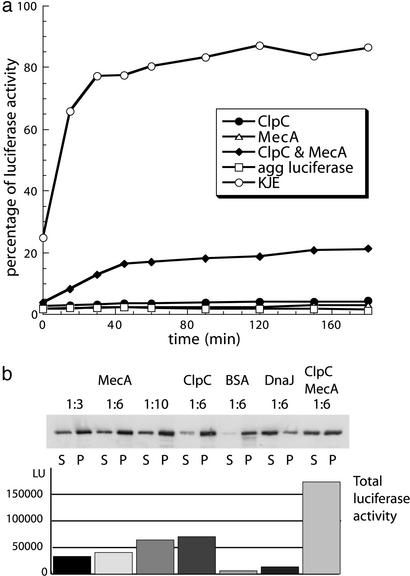
We tested the chaperone activity of ClpC and MecA by heat-denaturing luciferase in the presence or absence of chaperones. We first assayed the prevention of aggregation and reactivation of luciferase by following the luciferase activity with time, subsequent to the heat treatment. Only ClpC, in the presence of MecA and not MecA or ClpC alone, could recover luciferase activity. The addition of KJE during heat denaturation resulted in the recovery of ≈80% of luciferase activity (Fig. 1a). These results were confirmed by using chemically denatured luciferase as model substrate (data not shown).
Figure 1.
Prevention of aggregation and refolding of heat-denatured luciferase. (a) Prevention of aggregation and refolding assays with heat-aggregated luciferase. Luciferase was heat-denatured 15 min at 43°C with ClpC, MecA, ClpC, and MecA, or KJE as shown. Subsequently, luciferase activity was determined at the indicated time points. (b) Luciferase was heat-denatured 15 min at 43°C together with MecA, ClpC, BSA, DnaJ, or ClpC and MecA (in the indicated molar ratios of luciferase to the respective proteins). Subsequently aliquots were centrifuged. The Western blot with anti-luciferase Abs of the resulting supernatant and pellet fraction is depicted. The concomitantly measured total luciferase activities [in light units (LU)] before centrifugation for the different experiments are shown in the graph below the Western blot. S, supernatant; P, pellet.
The high initial luciferase activity (25%) in the presence of the KJE system reflects the prevention of aggregation abilities of this chaperone system. In contrast, the initial luciferase activity was <5% in the presence of ClpC and/or MecA, already indicating that ClpC and MecA either alone or together exhibit only weak activity in preventing protein aggregation (Fig. 1a).
To directly test the ability of ClpC and MecA to prevent protein aggregation during heat shock, we repeated the previous experiment. After a short incubation (5 min at 30°C), however, we measured the luciferase activity only once and then determined the amount of soluble or aggregated luciferase by centrifugation of the sample. The resulting supernatant and pellet fractions were analyzed by SDS/PAGE with subsequent Western blot (Fig. 1b). A quantification of the amount of luciferase, from multiple experiments represented in Fig. 1b, demonstrates that ClpC, together with MecA, was able to maintain 40–50% of the luciferase in the soluble fraction. Interestingly, 30–40% of luciferase remained soluble in the presence of MecA or ClpC alone. As a control, DnaJ, a very efficient holder chaperone, maintained 60–70% of luciferase in the soluble fraction whereas only 10% remained soluble in the presence of BSA. The luciferase activity of these samples revealed that although either MecA or ClpC were able to partially maintain luciferase in a soluble state, only when both proteins were present could significantly higher activity be rescued. Furthermore, as a control, almost no activity was rescued by BSA or DnaJ, although luciferase solubility was greatest in the presence of DnaJ (Fig. 1b). Because both experiments were carried out under the same conditions they were directly comparable.
From these experiments we conclude that ClpC together with MecA exhibit some, albeit weak, ability to prevent luciferase aggregation, and that this level is comparable to that of MecA or ClpC alone. The slightly higher prevention of aggregation ability of ClpC together with MecA (40–50%) compared with MecA or ClpC alone (30–40%) for prevention of aggregation could result from an additive effect of the two components. Nevertheless, the increasing level of luciferase activity (20% after 2 h), rescued by ClpC together with MecA compared with the constant amount of <5% of ClpC or MecA alone (Fig. 1a), cannot be explained solely by the small prevention of aggregation activity that MecA, ClpC, or both together showed and cannot be a simple additive effect of ClpC and MecA. These results favor the testable hypothesis that ClpC together with MecA may act to disaggregate and assist subsequent refolding, instead of preventing aggregation and assisting refolding of luciferase.
ClpC Together with MecA Can Disaggregate and Refold Protein Aggregates.
To dissect further the possible chaperone activities of MecA and/or ClpC, we examined the influence of ClpC and MecA on preformed protein aggregates by using heat-aggregated MDH and luciferase as model substrates.
First, we tested the physical association of ClpC and MecA with heat-aggregated MDH. We incubated heat-aggregated MDH and native MDH with the various chaperones for 5 min and separated the pelletable fraction from the soluble fraction by centrifugation. Only in the presence of ATP could ClpC and MecA be recovered in the pellet fraction (Fig. 2a). In the absence of ATP, the recovery of MecA and ClpC in the pellet was greatly reduced. If MecA was omitted, then ClpC did not associate with the aggregated MDH, and if ClpC was omitted, no association of MecA with the aggregate was visible. This result indicated the formation of a tertiary complex in which ClpC together with MecA associated with the protein aggregate, similar to the interaction observed for ClpC, MecA, and ComK (24). As a control, native MDH was always recovered in the supernatant fraction and heat-denatured MDH in the absence of chaperones always localized to the pellet fraction (data not shown).
Figure 2.
Interaction with aggregated protein. (a) ClpC together with MecA and ATP associates with aggregated protein. Heat-aggregated MDH was incubated for 5 min at room temperature with ClpC in the presence or absence of MecA with or without ATP [with pyruvate kinase (PK) and phosphoenolpyruvate (PEP) as ATP regenerating system], as indicated below the gel. In addition, a control without aggregated MDH (agg MDH) is shown. A subsequent centrifugation for 30 min at 16,000 × g separated supernatant and pellet fractions. Supernatant (S) and pellet (P) fractions were analyzed by Coomassie-stained SDS/PAGE. Positions of the different proteins on the gel are marked. (b) ClpC together with MecA can disaggregate heat-aggregated MDH. Previously heat-aggregated MDH was incubated with the indicated proteins ATP and the PK/PEP regenerating system. The disaggregation reaction was subsequently followed in time by light-scattering measurements. In addition, curve fits of the data points are shown. (c) ClpC together with MecA can disaggregate and refold previously heat-aggregated MDH. Previously heat-aggregated MDH was incubated with the proteins as indicated, and MDH activity was determined. (d) ClpC together with MecA can disaggregate and refold previously heat-aggregated luciferase. Previously heat-aggregated luciferase was incubated with the indicated proteins, and luciferase activity was determined.
Next, we examined the ability of ClpC and MecA to disaggregate aggregated MDH by monitoring the changes in light scattering after addition of the respective chaperones. We could show that only ClpC together with MecA was able to disaggregate MDH aggregates at rates comparable to the ClpB/KJE system (Fig. 2b). Interestingly, neither MecA nor ClpC alone could disaggregate these MDH aggregates. The disaggregation reaction did not proceed in the presence of the noncleavable ATP analog adenosine 5′-[γ-thio]triphosphate (ATP[γ-S]; data not shown).
To determine the fate of the disaggregated MDH we monitored its activity after addition of the chaperones. Only ClpC together with MecA were able to disaggregate and refold heat-aggregated MDH, comparable to the ClpB/KJE control (Fig. 2c). Neither ClpC nor MecA alone was able to recover MDH activity from the protein aggregates. To ensure that these results were not specific for heat-aggregated MDH we also monitored the disaggregation and refolding of heat-aggregated luciferase (Fig. 2d). As with aggregated MDH, only ClpC together with MecA could rescue luciferase activity from the aggregated state.
From these experiments we conclude that ClpC, together with the adaptor protein MecA, forms a chaperone system, which is able to disaggregate and refold aggregated proteins, and, furthermore, MecA is essential for this activity.
MecA Is Necessary for the ClpCP-Mediated Degradation of Unfolded or Aggregated Proteins.
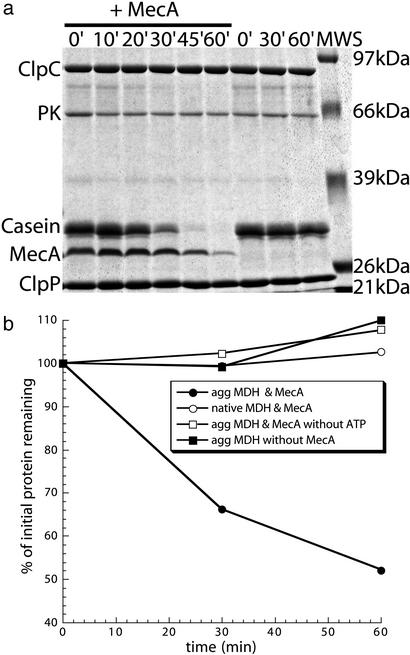
It was shown that ClpCP degrades ComK and ComS but only in the presence of MecA. Interestingly, MecA itself is also degraded by ClpCP in vivo and in vitro but at a much slower rate than the substrates (23).
Because MecA and ClpC can promote the disaggregation and refolding of our aggregated model substrates, we wanted to test whether unfolded or aggregated proteins can get degraded by ClpCP and whether that depends on MecA. Therefore, we first probed the MecA-dependent degradation of α-casein by ClpCP (Fig. 3a). α-Casein is a mostly unstructured protein (32) that alone does not significantly induce the ATPase activity of ClpC (see next paragraph). In the presence of MecA, α-casein is rapidly degraded after which MecA is degraded (Fig. 3a). The degradation of α-casein by ClpCP in the absence of MecA is very slow, with ≈90% remaining after 60 min. This result demonstrates that the presence of MecA is essential for the efficient ClpCP-mediated degradation of α-casein.
Figure 3.
MecA enables ClpCP to recognize and degrade unfolded or aggregated protein. (a) Degradation of α-casein by ClpCP depends on the presence of MecA. ClpCP with ATP and pyruvate kinase and phosphoenolpyruvate (PK/PEP) regenerating system was incubated with α-casein with or without MecA. Samples were taken at indicated time points and analyzed by Coomassie-stained SDS/PAGE. Sizes of the molecular weight standard (MWS) are depicted on the right and the proteins are marked on the left side of the gel. (b) Previously heat-aggregated MDH is degraded by ClpCP only when MecA is present. Heat-aggregated (agg MDH) or native MDH was incubated with ClpCP with the indicated components. Samples were taken at indicated time points and analyzed by Coomassie-stained SDS/PAGE. The gels were scanned, and the relative amount of MDH was determined by image analysis and plotted.
We next examined the ClpCP-mediated degradation of native or heat-aggregated MDH. As a control we showed that native MDH is stable in the presence of ClpCP and MecA. In contrast the ClpCP-mediated degradation of aggregated MDH depends both on the presence of MecA and ATP (Fig. 3b). This degradation, however, is much slower and less efficient than that of α-casein, as 50% of the MDH remained after 60 min (Fig. 3b). It is interesting to note that in the presence of an appropriate substrate (e.g., aggregated MDH), MecA degradation was reduced when compared with native MDH (data not shown; Fig. 3a).
The ATPase Stimulating Activity of MecA Is Necessary but Not Sufficient to Facilitate the Chaperone Activity of ClpC.
To determine further the mechanism by which MecA activates ClpC, we determined the basal ATPase rate of ClpC (0.004 s−1) and compared it to the basal rates of ClpA (0.54 s−1) and ClpB (0.02 s−1). The basal ClpC ATPase rate is ≈100-fold lower than the basal rate of ClpA. The addition of MecA to ClpC induced the ATPase activity of ClpC to a level similar and higher compared with the ClpA basal rate (Fig. 4). In contrast, the ATPase activity of ClpC was not significantly induced by the addition of different substrates such as heat-aggregated MDH (0.004 s−1), luciferase (0.004 s−1), or α-casein (0.007 s−1). Furthermore, there was no significant additional induction of the ClpC ATPase by these substrates in the presence of MecA (data not shown). MecA had no influence on the ATPase rate of either ClpA or ClpB (data not shown).
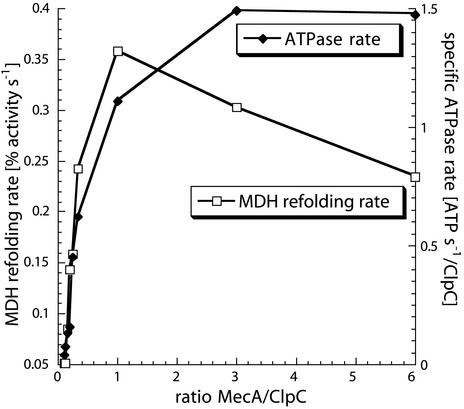
Figure 4.
Comparison of the MDH refolding rate with the ATPase rate. MDH refolding rates of the disaggregation and refolding of heat-aggregated MDH (agg MDH) assay (left y axis) and specific ATPase rates (right y axis) were determined at different ratios of MecA to ClpC monomers (depicted on x axis) and plotted as indicated.
These experiments are consistent with the hypothesis that MecA is needed for ClpC function, because it induces the ATPase and thereby the general activity of ClpC, rather than as a molecule that only targets substrate to ClpCP. Previous experiments demonstrated that MecA consists of two domains connected by a flexible loop. The N-terminal domain interacts with ComK and ComS, whereas the C-terminal domain interacts with ClpC and is responsible for the induction of the ClpC ATPase reaction (22). With these fragments of MecA we tested whether the stimulation of the ClpC ATPase by MecA was sufficient to activate the disaggregation and refolding capacity of ClpC. The experiments were carried out with luciferase and MDH as aggregated protein substrates. Neither the addition of the N- or the C-terminal domains of MecA nor the simultaneous addition of both domains to ClpC resulted in detectable disaggregation and refolding activity (data not shown). Nevertheless, the C-terminal MecA domain by itself induced the ClpC ATPase to the same extent as full-length MecA (data not shown). Consistently, addition of the C-terminal MecA domain alone was unable to mediate degradation of α-casein or aggregated MDH by ClpCP (data not shown). We conclude that the induction of the ClpC ATPase is not sufficient for the disaggregation and refolding activity of ClpC together with MecA or the degradation by ClpCP.
To get some insight into the correlation of the ATPase and the disaggregation and refolding activity, we followed both the ClpC ATPase rates and MDH refolding rates at several different ratios of MecA to ClpC (Fig. 4). A clear correlation between the MecA concentration and the yields and rates of refolding and ClpC ATPase rates was apparent. With increasing amounts of MecA up to a protomer ratio of 1:1 (MecA/ClpC) both the ATPase rate and the refolding rate correlate (Fig. 4). Above the 1:1 ratio, the refolding reaction of ClpC/MecA is inhibited, whereas the ClpC ATPase rate continues to increase. This finding could represent a competitive inhibition by MecA, because MecA also acts as a substrate for ClpC. Similar experiments with heat-aggregated luciferase instead of MDH gave essentially the same results (data not shown).
These results suggest that the concomitant targeting of substrate by MecA together with the ATPase induction of ClpC is necessary for the disaggregation and refolding activity of ClpC with MecA.
Discussion
The presented results demonstrate that the adaptor protein MecA is necessary for the in vitro disaggregation and refolding of protein aggregates by ClpC and for the in vitro proteolysis of unfolded or aggregated proteins by ClpCP. MecA enables the recognition and targeting of substrate proteins to ClpC and simultaneously induces the ATPase activity of ClpC.
MecA was considered a specific regulatory adaptor for the recognition of ComK and ComS (22, 23). However, as this study demonstrates, MecA is also able to target unfolded and aggregated proteins to ClpC.
Adaptor proteins, like MecA, bound to their cognate HSP100/Clp proteins, could enable, diversify, and expand substrate recognition and thereby raise the overall affinity for complex substrates like aggregated proteins. Studies on E. coli ClpX showed that the simultaneous multiple recognition of substrates like λO is an important prerequisite for the disaggregation and refolding activity by HSP100/Clp proteins (33, 34). But MecA is not only important for the recognition and disaggregation of protein aggregates, it is also necessary for efficient recognition of unfolded proteins, like α-casein, which are recognized and degraded by other AAA+ proteins (e.g., ClpAP or Lon) without any adaptor protein.
Interestingly, in B. subtilis a paralog of MecA exists (YpbH). This protein, however, is not involved in the targeting and degradation of ComK in competence development (35). Preliminary studies with purified YpbH demonstrate that it also induces the ATPase activity of ClpC and enables ClpCP to degrade α-casein (T.S. and K.T., unpublished data). This finding indicates that MecA and YpbH may have a general and complementary function in protein quality control. MecA homologs are found in all sequenced genomes of low GC Gram-positive bacteria, which also always encode ClpC homologs but not necessarily ComK homologs. Other known ClpCP substrates from B. subtilis, like Spx (28) or CtsR (29), need MecA or YpbH for the in vitro degradation by ClpCP (data not shown; ref. 28). Although SpoIIAB is degraded in vivo depending on ClpCP, a possible adaptor enabling the in vitro degradation of SpoIIAB by ClpCP has yet to be identified (27).
Taken together, these findings strongly suggest that, in contrast to E. coli ClpA, ClpC alone cannot select and recognize substrates and that an adaptor protein like MecA is necessary for all of the functions of ClpC-like HSP100/Clp proteins in low GC Gram-positive bacteria.
In E. coli several HSP100/Clp adaptor-like cofactors such as RssB, SspB, and ClpS are known. They are very specialized, and unlike MecA, are not necessary for all of the functions of their cognate partner ATPase (18, 36–38).
The MecA-dependent ATPase of ClpC is significantly induced by ComK and ComS (24) but for yet unknown reasons not significantly induced by aggregated proteins or α-casein, distinguishing these substrate classes. Little is known about the substrate specificity of MecA, besides the interaction of ComK and ComS with the N-terminal domain of MecA (22). The N-terminal domain of MecA could as well be necessary for the recognition of heat-aggregated proteins. The recognition sequences located in ComK and ComS may be similar to those recognized by MecA in unfolded or aggregated proteins. Consistent with this idea, NMR studies suggest for ComS an unfolded state (J. Cavanagh, personal communication).
Under normal growth conditions the ComK turnover can be handled by a fraction of the available MecA and ClpCP (23). ComK is not synthesized in heat-shocked cells (30), and competence develops only under very specific conditions where protein aggregation does not play a role. This indicates that a direct competition between ComK and misfolded or aggregated proteins should not pose a problem in vivo.
B. subtilis ClpC, ClpX, and ClpP associate in vivo with protein aggregates and inclusion bodies. In addition, their involvement in the in vivo degradation of misfolded proteins has been demonstrated (39, 40). The B. subtilis genome does not encode an ortholog of ClpB of E. coli. ClpC and MecA could fulfill this disaggregation function in B. subtilis, as suggested by the presented in vitro results. These results also suggest that MecA may be involved in vivo in the degradation of misfolded proteins by ClpCP. It has been shown that HSP100/Clp proteins and also ClpC can have dual functions as a chaperone and as part of a protease complex (15, 16, 23, 24). The involvement of the possibly redundant functions of ClpE, ClpX, the MecA paralog YpbH, and their functional interplay with other chaperone systems, like KJE and GroEL/ES of B. subtilis, in disaggregation and refolding or degradation remains to be elucidated.
A mecA ypbH double-mutant strain is not defective in thermosensitivity, as measured by growth on plates at high temperature. However, when additional MecA is supplied on a plasmid in trans (22), B. subtilis cells are rendered more thermosensitive (K.T. and T.S., unpublished data), suggesting that MecA may function in the protein quality control system in vivo. Nevertheless, because of the involvement of MecA and ClpC in multiple regulatory processes, considerably more effort will be required to dissect these functions in vivo.
Unlike the synthesis of clpC or clpP mRNA, neither mecA nor ypbH transcription is heat shock-regulated (41–43). However, it was already observed in vivo and in vitro that MecA is also a ClpCP substrate (23, 44). We observed in vitro that the presence of our model substrates also stabilize MecA (data not shown; Fig. 3a). Therefore, it is tempting to speculate that the availability of MecA substrates could regulate the cellular level of MecA, as proposed by Msadek et al. (44) based on their in vivo observations.
Adaptor proteins like MecA enable the recognition and targeting of misfolded/aggregated proteins or specific proteins like ComK to ClpC and concurrently activate the ATPase activity of ClpC. Therefore the adaptor protein coordinates substrate availability with the activation of ClpC, adding a new layer of regulation of HSP100/Clp proteins and enabling the involvement of ClpC in a wide variety of different aspects of the B. subtilis cellular physiology.
Acknowledgments
The kind gift of the clpC-intein-plasmid from Michiko Nakano (OGI School of Science and Engineering, Beaverton, OR) is greatly appreciated. K.T. thanks David Dubnau, Jeanette Hahn, and Marian Persuh for discussion, strains, and reagents. K.T., A.M., and B.B. were supported by the Deutsche Forschungsgemeinschaft.
Abbreviation
- MDH
malate dehydrogenase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hartl F U, Hayer-Hartl M. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 2.Schirmer E C, Glover J R, Singer M A, Lindquist S. Trends Biochem Sci. 1996;21:289–296. [PubMed] [Google Scholar]
- 3.Parsell D A, Kowal A S, Singer M A, Lindquist S. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 4.Goloubinoff P, Mogk A, Zvi A P, Tomoyasu T, Bukau B. Proc Natl Acad Sci USA. 1999;96:13732–13737. doi: 10.1073/pnas.96.24.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glover J R, Lindquist S. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 6.Mogk A, Tomoyasu T, Goloubinoff P, Rüdiger S, Röder D, Langen H, Bukau B. EMBO J. 1999;18:6934–6949. doi: 10.1093/emboj/18.24.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motohashi K, Watanabe Y, Yohda M, Yoshida M. Proc Natl Acad Sci USA. 1999;96:7184–7189. doi: 10.1073/pnas.96.13.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zolkiewski M. J Biol Chem. 1999;274:28083–28086. doi: 10.1074/jbc.274.40.28083. [DOI] [PubMed] [Google Scholar]
- 9.Horwich A L, Weber-Ban E U, Finley D. Proc Natl Acad Sci USA. 1999;96:11033–11040. doi: 10.1073/pnas.96.20.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuwald A F, Aravind L, Spouge J L, Koonin E V. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 11.Ogura T, Wilkinson A J. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 12.Weber-Ban E U, Reid B G, Miranker A D, Horwich A L. Nature. 1999;401:90–93. doi: 10.1038/43481. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y I, Burton R E, Burton B M, Sauer R T, Baker T A. Mol Cell. 2000;5:639–648. doi: 10.1016/s1097-2765(00)80243-9. [DOI] [PubMed] [Google Scholar]
- 14.Reid B G, Fenton W A, Horwich A L, Weber-Ban E U. Proc Natl Acad Sci USA. 2001;98:3768–3772. doi: 10.1073/pnas.071043698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickner S, Gottesman S, Skowyra D, Hoskins J, McKenney K, Maurizi M R. Proc Natl Acad Sci USA. 1994;91:12218–12222. doi: 10.1073/pnas.91.25.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton B M, Williams T L, Baker T A. Mol Cell. 2001;8:449–454. doi: 10.1016/s1097-2765(01)00307-0. [DOI] [PubMed] [Google Scholar]
- 17.Wickner S, Maurizi M R, Gottesman S. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- 18.Dougan D A, Reid B G, Horwich A L, Bukau B. Mol Cell. 2002;9:673–683. doi: 10.1016/s1097-2765(02)00485-9. [DOI] [PubMed] [Google Scholar]
- 19.Dubnau D, Roggiani M. J Bacteriol. 1990;172:4048–4055. doi: 10.1128/jb.172.7.4048-4055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubnau D, Turgay K. In: Bacterial Stress Responses. Storz G, Hengge-Aronis R, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 249–260. [Google Scholar]
- 21.Dubnau D. Annu Rev Microbiol. 1999;53:217–244. doi: 10.1146/annurev.micro.53.1.217. [DOI] [PubMed] [Google Scholar]
- 22.Persuh M, Turgay K, Mandic-Mulec I, Dubnau D. Mol Microbiol. 1999;33:886–894. doi: 10.1046/j.1365-2958.1999.01544.x. [DOI] [PubMed] [Google Scholar]
- 23.Turgay K, Hahn J, Burghoorn J, Dubnau D. EMBO J. 1998;17:6730–6738. doi: 10.1093/emboj/17.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turgay K, Hamoen L W, Venema G, Dubnau D. Genes Dev. 1997;11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- 25.Krüger E, Völker U, Hecker M. J Bacteriol. 1994;176:3360–3367. doi: 10.1128/jb.176.11.3360-3367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Msadek T, Kunst F, Rapoport G. Proc Natl Acad Sci USA. 1994;91:5788–5792. doi: 10.1073/pnas.91.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Q, Garsin D A, Losick R. Mol Cell. 2001;8:873–883. doi: 10.1016/s1097-2765(01)00362-8. [DOI] [PubMed] [Google Scholar]
- 28.Nakano S, Zheng G, Nakano M M, Zuber P. J Bacteriol. 2002;184:3664–3670. doi: 10.1128/JB.184.13.3664-3670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krüger E, Zühlke D, Witt E, Ludwig H, Hecker M. EMBO J. 2001;20:852–863. doi: 10.1093/emboj/20.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turgay K, Persuh M, Hahn J, Dubnau D. Mol Microbiol. 2001;42:717–727. doi: 10.1046/j.1365-2958.2001.02623.x. [DOI] [PubMed] [Google Scholar]
- 31.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Proc Natl Acad Sci USA. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herskovits T T. Biochemistry. 1966;5:1018–1026. doi: 10.1021/bi00867a030. [DOI] [PubMed] [Google Scholar]
- 33.Wawrzynow A, Wojtkowiak D, Marszalek J, Banecki B, Jonsen M, Graves B, Georgopoulos C, Zylicz M. EMBO J. 1995;14:1867–1877. doi: 10.1002/j.1460-2075.1995.tb07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonciarz-Swiatek M, Wawrzynow A, Um S J, Learn B A, McMacken R, Kelley W L, Georgopoulos C, Sliekers O, Zylicz M. J Biol Chem. 1999;274:13999–14005. doi: 10.1074/jbc.274.20.13999. [DOI] [PubMed] [Google Scholar]
- 35.Persuh M, Mandic-Mulec I, Dubnau D. J Bacteriol. 2002;184:2310–2313. doi: 10.1128/JB.184.8.2310-2313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker G, Klauck E, Hengge-Aronis R. Proc Natl Acad Sci USA. 1999;96:6439–6444. doi: 10.1073/pnas.96.11.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Gottesman S, Hoskins J R, Maurizi M R, Wickner S. Genes Dev. 2001;15:627–637. doi: 10.1101/gad.864401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levchenko I, Seidel M, Sauer R T, Baker T A. Science. 2000;289:2354–2356. doi: 10.1126/science.289.5488.2354. [DOI] [PubMed] [Google Scholar]
- 39.Jürgen B, Hanschke R, Sarvas M, Hecker M, Schweder T. Appl Microbiol Biotechnol. 2001;55:326–332. doi: 10.1007/s002530000531. [DOI] [PubMed] [Google Scholar]
- 40.Krüger E, Witt E, Ohlmeier S, Hanschke R, Hecker M. J Bacteriol. 2000;182:3259–3265. doi: 10.1128/jb.182.11.3259-3265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersohn A, Brigulla M, Haas S, Hoheisel J D, Völker U, Hecker M. J Bacteriol. 2001;183:5617–5631. doi: 10.1128/JB.183.19.5617-5631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price C W, Fawcett P, Ceremonie H, Su N, Murphy C K, Youngman P. Mol Microbiol. 2001;41:757–774. doi: 10.1046/j.1365-2958.2001.02534.x. [DOI] [PubMed] [Google Scholar]
- 43.Helmann J D, Wu M F, Kobel P A, Gamo F J, Wilson M, Morshedi M M, Navre M, Paddon C. J Bacteriol. 2001;183:7318–7328. doi: 10.1128/JB.183.24.7318-7328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Msadek T, Dartois V, Kunst F, Herbaud M L, Denizot F, Rapoport G. Mol Microbiol. 1998;27:899–914. doi: 10.1046/j.1365-2958.1998.00735.x. [DOI] [PubMed] [Google Scholar]