Abstract
Facilitated translocation of molecules through channels and pores is of fundamental importance for transmembrane transport in biological systems. Several such systems have specific binding sites inside the channel, but a clear understanding of how the interaction between channel and molecules affects the flow is still missing. We present a generic analytical treatment of the problem that relates molecular flow to the first passage time across and the number of particles inside the channel. Both quantities depend in different ways on the channel properties. For the idealized case of noninteracting molecules, we find an increased flow whenever there is a binding site in the channel, despite an increased first passage time. In the more realistic case that molecules may block the channel, we find an increase of flow only up to a certain threshold value of the binding strength and a dependence on the sign of the concentration gradient, i.e., asymmetric transport. The optimal binding strength in that case is analyzed. In all cases the reason for transport facilitation is an increased occupation probability of a particle inside the channel that overcomes any increase in the first passage time because of binding.
Keywords: binding site, first passage time, membrane
Diffusion of molecules through channels and pores of an otherwise impermeable membrane is an important issue in biological transport at the cellular level (1, 2). Early considerations of channel transport have been concerned mainly with the effect of barriers inside channels (3). However, various means of facilitated transport across a membrane have also been considered, e.g., shuttle mechanisms (4). In recent years it has been noted that there are several cases where the molecules transported interact strongly with regions inside the channel (5–13), apparently leading to an increase in transmembrane transport. However, a fundamental understanding and quantitative description of such an increased transport due to in-channel binding sites is still missing.
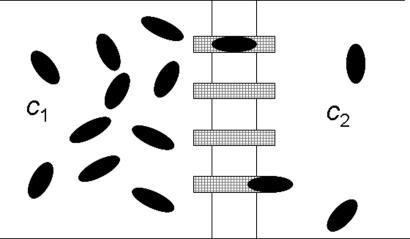
From an intuitive point of view, it is not clear at all why a strong interaction with the channel should facilitate transport. Conversely, one could argue that a strong binding is associated with a longer residence time inside the channel, which reduces flow. Furthermore, molecules bound temporarily inside the channel may hamper transport of other molecules, especially when they are large (13, 14), and block the channel. So, why do traps and/or reaction sites within the channel facilitate molecular flow? These questions have to be addressed in the generic biological setting of a macroscopic concentration gradient across the membrane (see Fig. 1). An appropriate quantitative description should give the flow for a given concentration difference depending on the potential and other parameters describing the molecule–channel interaction. Physical insight can be gained if the flow can be related to other global properties of the system in question.
Fig. 1.
Basic biological situation. A membrane separates two baths with molecular concentrations c1 and c2. The baths are connected by channels (hatched rectangles), allowing only access to a single molecule.
Past efforts (15, 16) to analyze this situation used the concepts of conditional exit (17) or splitting probabilities (18) and conditional mean first passage times (CMFPTs) (19). It was found that in the presence of a potential well the probability to enter the channel at one end and to leave it at the other can be increased (15). However, the crucial condition for this result is that the time-limiting steps are the entrance and exit rates. Conversely, the average lifetime in the channel of such a particle traversing it was found to increase with the depth of the potential well (16). Recently it was conjectured that both dependencies could nevertheless corroborate to give a total increase of the flow across a membrane (20, 21). However, it is not clear at all that the particular quantities analyzed in refs. 15, 16, 20, and 21 have any relationship to the flow arising in the situation given by Fig. 1 or which quantities at all need to be used. For example, CMFPT and regular mean first passage times (MFPTs) (see, e.g., refs. 17 and 18) can differ by orders of magnitude, a phenomenon that is known, although this knowledge does not appear to be very widespread, under the name of the “great leap forward” (22). Moreover, the considerations in refs. 15, 16, 20, and 21 hold only for noninteracting particles. It therefore would be important to analyze the flow in Fig. 1 also by allowing a blocking of the channel.
Extending an old approach by Hardt (23), we showed recently (24) that in very general situations the flow J of noninteracting particles across some region is given by a macroscopic version of Fick’s law
where τ is the regular MFPT to cross the region (and different from a CMFPT), and n is a measure of the stationary-state particle number in that region. In the next section, we summarize these results and apply them to the case of noninteracting particles in a one-dimensional (1D) channel with a binding site. We show that a binding site always increases the flow, despite an increased first passage time (FPT). The reason is that the stationary-state particle number measure n always dominates over an increase in the MFPT. In the subsequent section, we extend our model to include also the effects of channel blocking. There we will show that blocking limits the flow-increasing effects of a binding site, leading to an optimal value for the binding strength, and induces asymmetric transport. The dependence of that optimal value on the channel parameters as well as the asymmetry effects will be analyzed, and we close with a discussion of our results.
Transport of Noninteracting Particles Through a Channel
We describe the transport of noninteracting molecules through a channel as a 1D diffusion process. The dynamics of the density of the molecules inside the channel, ρ(x, t), is determined by the Smoluchowski equation (17, 18)
where x is the channel coordinate and D(x) is the possibly position-dependent diffusion coefficient; note, however, that we will be using D(x) ≡ D = const in the remainder of this work. F(x) is the force that describes the molecule–channel interaction that can always be derived from a potential function in one dimension, F(x) = −Φ′(x). Taking into account that the length of a diffusing molecule, Lm, may not be negligible compared with the channel length, Lc, the length of the diffusion system in Eq. 2 is L = Lc + Lm. At the ends of the channel, we assume that baths hold the molecular densities constant at ρ(0, t) ≡ c1 and ρ(L, t) ≡ c2, respectively, as shown in Fig. 1. We are mainly interested in the steady state of the system. Setting the time derivative to zero in Eq. 2, the stationary density ρs(x) is given by the condition that the flow J is constant
Let us assume first that c1 > 0 and c2 = 0. This assumption is no restriction because the molecules are assumed to be noninteracting, and, therefore, for c2 > 0 the total flow results simply as a linear superposition of the contributions from {c1 > 0, c2 = 0} and {c1 = 0, c2 > 0}. We will denote quantities related to the first case by the index 1 → 2 and vice versa for the second. It was shown in ref. 24 that under the condition {c1 > 0, c2 = 0} the number of particles in the channel, N1→2 = ∫0Ldxρs,1→2(x), is related to the flow, J, and to the MFPT to get from x = 0 to x = L, denoted by τ1→2, by the simple relation
Note that τ1→2 is the regular MFPT that is derived usually with a reflective boundary condition at x = 0 and an absorptive one at x = L (17, 18, 25), in contrast to CMFPTs (compare refs. 16 and 19). In the 1D setting of Eq. 2 it is given for example by (25)
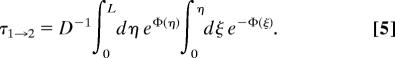 |
As mentioned above, in the general situation the total flow is a superposition of the contributions for {c1 > 0, c2 = 0} and {c1 = 0, c2 > 0}. Taking also into account that the particle number N depends linearly on the concentration c, we can introduce the specific particle numbers n1→2 = N1→2/c1 and n2→1 = N2→1/c2 and have the total flow as
This equation a very general form of a macroscopic Fick’s equation that also takes into account forces across the membrane (see ref. 24). We will assume in the following that there is no potential energy difference between the channel ends, i.e., Φ(0) = Φ(L), so that the flow is purely concentration-gradient driven. Because in this situation J ≡ 0 for c1 = c2, it is seen immediately that n1→2/τ1→2 = n2→1/τ2→1 holds. We can write Eq. 6 therefore as
using the symmetrized quantities n = (n1→2 + n2→1)/2 and τ = (τ1→2 + τ2→1)/2. §
Implications of Particle–Channel Interaction
Taking into account Eqs. 3 and 5 and using the channel average 〈〉 = L−1 ∫0L dx we get the results
for MFPT and specific particle number, where, without loss of generality, have set Φ(0) = Φ(L) = 0. These results have important consequences for any form of the channel potential. By using the Cauchy–Schwarz inequality in the form 〈f〉〈g〉 ≥ 〈 〉2 on Eq. 8, we immediately see that any form of in-channel interaction that is nonconstant leads to an increase in the MFPT, and by this effect hampers particle flow: τ ≥ L2/2D. The physical reason is that potential barriers and potential wells have walls, and the particles have to get over these walls irrespective of whether they belong to wells or barriers. That is reflected also in the invariance of τ upon changing barriers to wells and vice versa by setting Φ(x) → −Φ(x). Conversely, the specific particle number increases only for predominantly attractive potentials, i.e., if the potential wells overcome the effects of barriers in Eq. 9. Both effects conspire to give the total flow as
So, for fully attractive potentials, i.e., Φ(x) ≤ 0, we always find an increase of the flow when compared with Φ(x) = 0. For more complicated forms, i.e., including barriers in addition to wells, a case by case analysis is necessary.
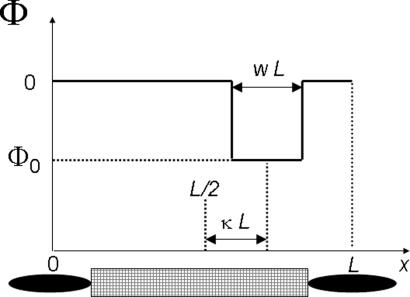
The interplay between specific particle number and FPT is illustrated by the rectangular potential well of depth Φ0 sketched in Fig. 2. The parameter w, 0 < w < 1, denotes the relative width of the potential well, and κ is the relative shift of the center of the well from the middle of the channel L/2, i.e., |κ| ≤ (1 − w)/2, and describes the asymmetry. The symmetrized FPT and relative particle number for this potential are
Fig. 2.
Rectangular-shaped (box-like) attractive potential of molecule–channel interaction. w is the relative width of the potential well, and |Φ0| is its depth. The relative shift of the well from its symmetric position at L/2 is denoted by κ. Note that the interaction length L consists of the length of the channel (hatched rectangular) plus the length of the molecule oriented in the direction of transport.
As mentioned above, these results and, consequently, the flow in Eq. 10 do not depend on κ; i.e., they are invariant upon changing the position of the well within the channel. The FPT increases with increasing potential depth (as well as with increasing height in case of a barrier) and for large values of |Φ0|, an inverse Arrhenius law follows
However, the specific particle number in the channel is increased, and for sufficient depth we also have an exponential increase
These effects conspire to give indeed a total increase in the flow that depends on the width of the well only through
Note that a “barrier” of height |Φ0| would give n ≈ L/2(1 − w); i.e., particles inside the channel reside mainly outside the location of the barrier, resulting in an Arrhenius-like exponential decrease of the flow, J ∼ e−|Φ0|.
Blocking the Channel
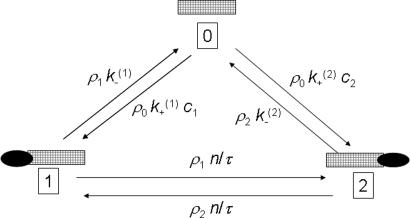
The approach presented above can be readily extended to describe the effect of molecules that block the channel. As an extreme case we assume in the following that only a single molecule can occupy the channel, which is realistic in many cases. Before, the quantity ρ(x, t) was the density of molecules in the channel at position x. We now interpret ρ(x, t) as the “probability density” that a channel contains a particle at x. It is obvious that this density follows the same dynamics as described above in Eq. 2. However, the state variable x does not completely describe all states, but the empty channel has to be added as an additional state, the probability of which we will denote as ρ0. This additional “empty channel state” leads to a cyclic state model (see Fig. 3) because a molecule can enter the empty channel from either side. The corresponding transition rates between the empty channel state and the states in which a molecule is attached to either end ρ1 = ρ(0, t), ρ2 = ρ(L, t) are proportional to the concentration of molecules of the adjacent baths, which we denote again as c1 and c2. In the steady state the equation system
holds, where Eq. 17 derives from Eq. 7, and n and τ are given as above in Eqs. 8 and 9; i.e., we assume that there is no net force across the channel. k±(i), i = 1, 2, are the reaction rate constants describing attachment of molecules to and dissociation from either end of the channel, giving rise to the equilibrium constants of this attachment–dissociation process at the respective ends K(i) = k+(i)/k−(i). We have to assume that K(1) = K(2) ≡ K, else we would get a nonzero net flow across the membrane for zero concentration gradient, i.e., pumping, a case we want disregard in this work. ¶
Fig. 3.
Cyclic-state model of molecular transport through the channel that allows for blocking. Three states of the channel are depicted: 0 refers to the empty channel, and 1 and 2 are channel states with a single molecule attached either at one or the other end. The respective unidirectional flows between the states are shown above the corresponding arrows.
In the steady state all flows of Eqs. 16–18 are identical. In addition, we have to consider that the total probability is conserved, i.e.,
Note that by omitting this condition and setting ρ0 ≡ 1, we would get only an extended version of the previously discussed model: The channel would be described just by additional on- and off-rates, the resulting flow being simply
The dissociation constant K ≡ e−G, which describes the strength of the attachment of particles at the ends of the channel, acts like an additional sink (for G < 0) or barrier (G > 0). The free energy G can actually be incorporated into the potential by the renormalization Φ(x) → Φ(x) + G, because it affects only the specific particle number and not the FPT. We will therefore set K ≡ 1 in the following and assume that Φ(x) also describes these effects at the channel ends.
Including the normalization condition (Eq. 19) and letting ρ readjust to satisfy it readily describes the effect of self interaction of the molecules within the channel, i.e., blocking. Taking these findings together, we obtain for the flow an expression where n is replaced by a nonlinear function of n (and other parameters)
We will see that this function shows a nonlinear dependence on the concentrations c1 and c2 that exhibits analogies to a Michaelis–Menten-type behavior:
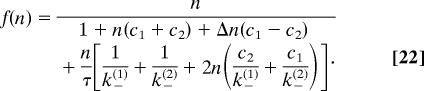 |
The last term in the denominator in Eq. 22 depends mainly on the rate constants at the channel ends. Because we are interested in the case where transport through the channel is the time-limiting step, we can assume τ ≫ 1/k±(i) and omit this term in the following.
The middle term in the denominator of Eq. 22 is proportional to Δn = (n1→2 − n2→1)/2, the asymmetric part of the specific particle number, and it is nonzero only if the underlying potential is asymmetric. Note that this statement is a major difference from the idealized case of noninteracting particles, where asymmetry did not matter for the transport. Here we have a term that depends on the asymmetry of the channel and on the sign of the concentration gradient. This term has the net result that flow is not invariant upon the change of the sign of the concentration gradient. It will be higher in one direction and lower in the other, and we will analyze this effect in more detail below. We note that such a directional behavior has been observed most recently in the channel protein OmpF (26).
The remaining part in the denominator of Eq. 22 depends on the symmetric part of the potential only and leads to a Michaelis–Menten-type dependence on the bath concentrations. If we assume a symmetric channel potential we get
For low bath concentrations, (n(c1 + c2) ≪ 1); i.e., when most channels are empty, one obtains a linear dependence of J on the concentration gradient as before in Eqs. 6 and 20. In that limiting case all our considerations from above about facilitated transport still apply; i.e., a binding site can still increase the flow through the channel.
With increasing bath concentrations all channels are blocked, and flow depends on the channel properties solely via the FPT
Facilitated transport is not possible anymore in that limit. Interestingly, the same happens for large values of n. Hence, strong attachment to the channel and/or a strong binding site also limit the flow to the form given in Eq. 24. Consequently, we expect that there are optimal values for the strength of the binding site. We will analyze this hypothesis in more detail, for example, for the potential well of Fig. 2.
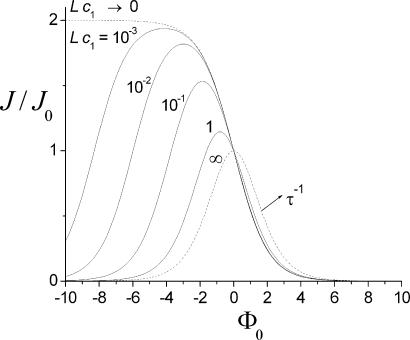
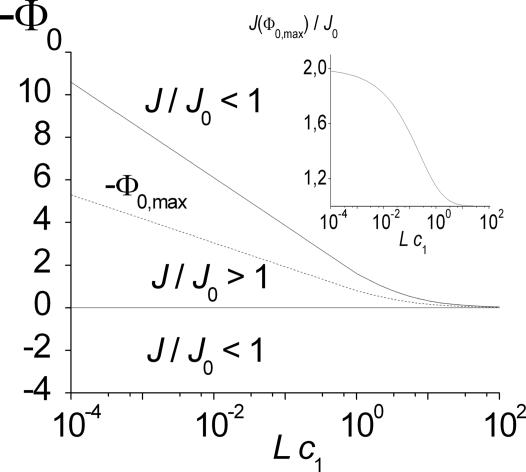
Fig. 4shows the relative increase of the flow, J/J0, for a particular set of parameters of a symmetric channel potential. The increase and subsequent decrease of the flow with increasing binding strength (negative Φ0) can be seen clearly. Note that maximal flow is limited by Eq. 15, i.e., Jmax/J0 ≤ 1/(1 − w), and occurs at the potential depth
Fig. 4.
Effect of the molecular channel interaction on flow. J0 refers to vanishing interaction. The potential is box-shaped (see Fig. 2) and assumed to be centered (κ = 0) with relative width w = 1/2. Φ0 > 0 denotes a barrier, and Φ0 < 0 is an attractive potential, i.e., a potential well. Unidirectional flow is considered, i.e., c2 is set to zero, and the concentration c1 is varied. The limiting cases of vanishing and very high concentration are also considered (dotted lines). Note that for the latter, flow is proportional to the inverse FPT τ.
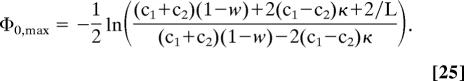 |
Setting c2 = 0, we obtain for low concentrations (c1L ≪ 1) a direct proportionality between optimal depth and the logarithm of the concentration, i.e.,
This behavior is demonstrated in Fig. 5. In addition, it can be seen that the range of values for the potential depth for which flow increase upon binding occurs lies between Φ0 = 0 and Φ0 = 2Φ0,max. The latter feature can already be concluded from the behavior of the J/J0 curves in Fig. 4.
Fig. 5.
Contour plot showing the range of enhanced flow. Parameters are those of Fig. 4. The solid lines separate the areas of enhanced and reduced flow (with respect to flow in the absence of any interaction, Φ(x) ≡ 0). The potential depth Φ0,max of maximum flow is denoted by the dotted line (Eq. 25). Inset shows the corresponding flow as a function of the bath concentration.
We will now discuss asymmetric transport through the channel. First, we will look at the more general properties that arise when asymmetry is introduced. Let Jasym be the current that arises in the asymmetric case and compare it with the case that Δn = 0 but with the same value for n. Then
Note that |Δn| ≤ n, so the expression 27 always stays finite. For Δn(c1 − c2) < 0, flow is increased in comparison with the symmetric case and vice versa. For the case of our rectangular well (Fig. 2), Δn assumes the form
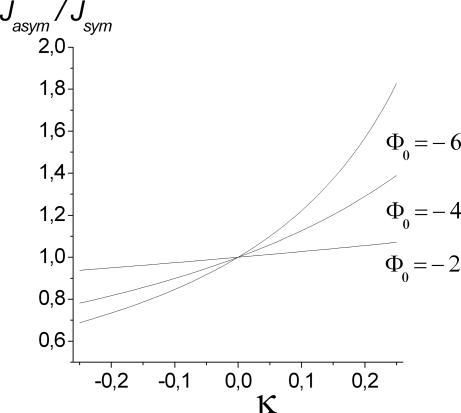
For a potential well (Φ0 < 0) Δn is positive if κ < 0, i.e., if the binding site is located at the left side of the channel, and negative otherwise. Note that the concentration on the left side is c1. Upon inspecting Eq. 27, we see that flow is decreased if the binding site is close to the larger concentration, whereas the flow is increased otherwise. This behavior is illustrated in Fig. 6 for a particular set of parameters. Intuitively, flow increase due to the binding site being in the trans position, i.e., away from the higher concentration, can be viewed as the binding site “pulling” the molecules across the channel. Because in the blocking situation only one molecule is in the channel, exiting to the lower concentration side is faster than diffusing all of the way back.
Fig. 6.
Dependence of flow Jasym on the position of the binding site, κ (see Fig. 2 and Eqs. 27 and 28). Jsym is the flow for the symmetric potential (κ= 0). The parameters used are Lc1 = 0.1, c2 = 0, and w = 1/2; i.e., κ can vary from −1/4 to 1/4. The flow increases when the binding site moves from the cis to the trans position with respect to the larger concentration.
For a more quantitative analysis, we look into the effect of interchanging the bath concentrations
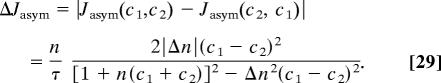 |
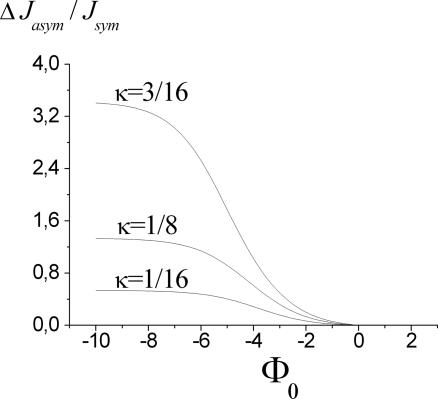
Note that this quantity depends quadratically on the concentration difference, i.e., for small concentration gradients transport may still appear to be symmetric, whereas for higher concentration gradients asymmetry dominates. As Fig. 7 demonstrates, for sufficiently strong binding sites, Φ0 ≪ 0, the effect becomes independent of Φ0, and one obtains the asymptotic relation
Fig. 7.
Asymmetry of flow related to position κ (see Fig. 2) and strength Φ0 of the potential well. Asymmetry is quantified by the difference of flows upon interchanging bath concentrations (see Eq. 29). Parameters are those of Fig. 6. Note that for strong binding, the asymmetric effect becomes independent of the binding strength (see also Eq. 30).
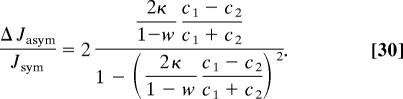 |
We note again that asymmetric transport behavior has been observed most recently in the channel protein OmpF (26).
Summary and Discussion
We presented an analytical approach to describe molecular transport through a membrane channel in the biological setting of a macroscopic concentration gradient across the membrane as depicted in Fig. 1. The goal was, in particular, to understand whether and why binding sites in a channel can facilitate transport and to understand the effect of channel blocking.
By using the macroscopic version of Fick’s equation (Eq. 6), we could demonstrate that for fully attractive channel potentials a transport increase always occurs. The reason is that, although the FPT increases, this increase is overcome by a concomitant increase of the specific particle number, a quantity that measures the number of particles/the probability to be in the channel. Allowing for the situation that a transported molecule may block the channel for other particles, it was seen that the effect of transport facilitation occurs only below a certain threshold strength of the binding site. As an interesting aside, we could show that asymmetric transport arises for asymmetric channel potentials in the blocking situation.
On a more technical side, our results also resolve the question of whether regular MFPTs or CMFPTs (that arise in flow-splitting situations) are the relevant quantities to describe channel flow in the setting of Fig. 1. Although there may be experimental situations where CMFPTs can be measured directly, the quantity to describe and understand gradient flow is certainly the MFPT. The intuitive physical explanation for that is actually quite simple: If there is a bath of constant concentration at one end, under stationary conditions for every particle that moves into the bath there is one that leaves it. This situation is equivalent to the reflective boundary condition used for deriving a MFPT (25).
This contribution is basically an investigation of generic properties of channel transport, transport facilitation, and the effects of channel blocking. The potential well we use to illustrate our results, Fig. 2, is rather crude. Nevertheless it serves well to gain an understanding of the physical and biological situation. Naturally, application to actual channel proteins should take a more realistic potential into account. Also, the cyclic state model that we introduce to describe the effect of channel blocking, Fig. 3, is an extreme case because it allows only a single molecule in the channel. Note, however, that single-file transport can be described by such an approach, too (27), although channel inhomogeneities are disregarded in that reference. Again, we believe that this model serves well to gain insight into the physical and biological situation.
What came as a surprise to us is that already at this level we were able to describe asymmetric transport. This ability is of particular importance in the light of recent experimental findings (26, 28). Kosztin and Schulten (28) recently also noted that for noninteracting particles, channel transport is symmetric despite an asymmetric channel potential. They propose a ratchet-like mechanism for asymmetric transport, based on nonequilibrium fluctuations. We have shown here that, because of the effect of blocking, already the very basic situation of an asymmetric potential is sufficient to allow for asymmetric transport. The intuitive physical picture explaining qualitatively the asymmetry and its direction is actually quite simple again: Once the single molecule is bound by the binding site, its escape is faster to the closest side.
Finally, we would like to point out that our treatment is not limited to the characterization of channel transport. It can be used whenever the situation of 1D gradient-driven diffusion arises. Another possible application is the description of enzymatic catalysis. Here the potential Φ(x) corresponds to the Helmholtz/Gibbs free energy as a function of the reaction coordinate x that replaces the channel coordinate. Like for channel transport, a local minimum of the free energy, either a minimum of the energy or an increase of entropy, could facilitate enzymatic conversion.
Acknowledgments
We thank one of the referees for drawing our attention to ref. 27. W.N. thanks P. Grassberger and M. Paczuski for stimulating discussions. This work was supported in part by National Science Foundation Grant CHE-0313618 and Deutsche Forschungsgemeinschaft Grant SFB 688.
Abbreviations
- FPT
first passage time
- MFPT
mean FPT
- CMFPT
conditional MFPT.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 11431.
We use here the simple to prove, but apparently not very well known, fact that if a/b = c/d, then a/b = (ma + nc)/(mb + nd) holds with arbitrary m, n (as long as mb + nd ≠ 0).It is important to note already at this point that for noninteracting particles, asymmetries in the channel potential are not important, because only the symmetrized part of n and τ contribute to the flow. The macroscopic Fick’s diffusion equation (Eq. 7) now explicitly relates steady-state flow to the concentration gradient, which acts as the thermodynamic driving force, and to the conductivity n/τ. As already mentioned above, the relevant FPT characterizing steady-state flow is the one considering reflecting boundary conditions at its starting point and not a conditional FPT that arises in exit splitting situations (see ref. 19). The only other relevant quantity is the number of molecules trapped by the channel, which is measured by the specific particle number n. The effect of the molecule–channel interaction on the flow depends on how this interaction affects the FPT and the number of molecules trapped. This effect is analyzed below.
In all our considerations, we assume no potential difference between the baths. However, our model can be readily adapted to a situation when Φ(0) = Φ1 ≠ Φ(L) = Φ2. Flow vanishes if the chemical potentials of the baths μi = Φi + ln(ci) are equal. This result implies equivalence of the diffusive conductivities, e−Φ1n1→2/τ1→2 = e−Φ2n2→1/τ2→1. Defining ñi→j = e−Φini→j as the potential corrected specific particle numbers, we can generalize Fick’s diffusion law in Eq. 7 to J = (ñ/τ)(eμ1 − eμ2)) with ñ = (ñ1→2 + ñ2→1)/2. Also, all other results of our work, in particular those for self-interacting particles, can be generalized to this situation by replacing specific particle numbers n and Δn by the corresponding potential corrected parameters, and concentrations ci by the activities eμi.
References
- 1.Cooper K. E., Jakobsson E., Wolynes P. Prog. Biophys. Mol. Biol. 1985;46:51–96. doi: 10.1016/0079-6107(85)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Meller A. J. Phys. Condens. Matter. 2003;15:R581–R607. [Google Scholar]
- 3.Cooper K. E., Gates P. Y., Eisenberg R. S. Q. Rev. Biophys. 1988;21:331–364. doi: 10.1017/s0033583500004480. [DOI] [PubMed] [Google Scholar]
- 4.Ebel W. J. Math. Biol. 1985;21:243–271. doi: 10.1007/BF00276225. [DOI] [PubMed] [Google Scholar]
- 5.Jensen M. O., Park S., Tajkorshid E., Schulten K. Proc. Natl. Acad. Sci. USA. 2002;99:6731–6736. doi: 10.1073/pnas.102649299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayson P., Tajkorshid E., Schulten K. Biophys. J. 2003;85:36–48. doi: 10.1016/S0006-3495(03)74452-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luckey M., Nikaido H. Proc. Natl. Acad. Sci. USA. 1980;77:167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benz R., Schmid A., Vos-Scheperkeuter G. H. J. Membr. Biol. 1987;100:21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- 9.Bezrukov S. M., Kullman L., Winterhalter M. FEBS Lett. 2000;476:224–228. doi: 10.1016/s0014-5793(00)01753-1. [DOI] [PubMed] [Google Scholar]
- 10.Hilty C., Winterhalter M. Phys. Rev. Lett. 2001;86:5624–5627. doi: 10.1103/PhysRevLett.86.5624. [DOI] [PubMed] [Google Scholar]
- 11.Kullman L., Winterhalter M., Bezrukov S. M. Biophys. J. 2002;82:803–812. doi: 10.1016/S0006-3495(02)75442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarz G., Danelon C., Winterhalter M. Biophys. J. 2003;84:2990–2998. doi: 10.1016/S0006-3495(03)70025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestorovich E. M., Danelon C., Winterhalter M., Bezrukov S. M. Proc. Natl. Acad. Sci. USA. 2002;15:9789–9794. doi: 10.1073/pnas.152206799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berezhovskii A. M., Gopich I. V. Biophys. J. 2003;84:787–793. doi: 10.1016/S0006-3495(03)74898-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berezhovskii A. M, Pustovolt M. A., Bezrukov S. M. J. Chem. Phys. 2002;116:9952–9955. [Google Scholar]
- 16.Berezhovskii A. M, Pustovolt M. A., Bezrukov S. M. J. Chem. Phys. 2003;119:3943–3951. [Google Scholar]
- 17.Gardiner C. W. Handbook of Stochastic Methods for Physics, Chemistry and the Natural Sciences. 3rd Ed. Berlin: Springer; 2004. p. 142. Chap. 5.2.8, [Google Scholar]
- 18.van Kampen N. G. Stochastic Processes in Physics and Chemistry. Amsterdam: North–Holland; 2001. pp. 292–325. [Google Scholar]
- 19.Agmon N. J. Chem. Phys. 1985;82:2056–2060. [Google Scholar]
- 20.Berezhovskii A. M., Bezrukov S. M. Biophys J. 2005;104:L17–L19. doi: 10.1529/biophysj.104.057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berezhovskii A. M., Bezrukov S. M. Chem. Phys. 2005;319:342–349. [Google Scholar]
- 22.Ludwig D. In: Nonlinear Phenomena in Physics and Biology. Enns R. H., Jones B. L., Miura R. M., editors. New York: Plenum; 1981. pp. 549–566. [Google Scholar]
- 23.Hardt S. Bull. Math. Biol. 1981;43:89–99. [Google Scholar]
- 24.Bauer W. R., Nadler W. J. Chem. Phys. 2005;122:244904. doi: 10.1063/1.1940056. [DOI] [PubMed] [Google Scholar]
- 25.Schulten K., Schulten Z., Szabo A. J. Chem. Phys. 1981;74:4426–4432. [Google Scholar]
- 26.Alcaraz A., Nestorovich E. M., Aguilella-Arzo M., Aguilella V. M., Bezrukov S. M. Biophys. J. 2004;87:943–957. doi: 10.1529/biophysj.104/043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson P. H. J. Chem. Phys. 2002;117:11396–11403. [Google Scholar]
- 28.Kosztin I., Schulten K. Phys. Rev. Lett. 2004;93:238102. doi: 10.1103/PhysRevLett.93.238102. [DOI] [PubMed] [Google Scholar]