Abstract
We have identified, cultured, characterized, and propagated adult pluripotent stem cells (PSC) from a subset of human peripheral blood monocytes. These cells, which in appearance resemble fibroblasts, expand in the presence of macrophage colony-stimulating factor and display monocytic and hematopoietic stem cell markers including CD14, CD34, and CD45. We have induced these cells to differentiate into mature macrophages by lipopolysaccharide, T lymphocytes by IL-2, epithelial cells by epidermal growth factor, endothelial cells by vascular endothelial cell growth factor, neuronal cells by nerve growth factor, and liver cells by hepatocyte growth factor. The pluripotent nature of individual PSC was further confirmed by a clonal analysis. The ability to store, expand, and differentiate these PSC from autologous peripheral blood should make them valuable candidates for transplantation therapy.
Pluripotent stem cells (PSC) are a valuable resource for research, drug discovery, and transplantation (1, 2). These cells or their mature progeny can be used to study differentiation processes, identify and test lineage-specific drugs, or replace tissues damaged by a disease. However, the use of PSC from human fetuses, umbilical cords, or embryonic tissues derived from in vitro fertilized eggs raises ethical and legal questions, poses a risk of transmitting infections, and/or may be ineffective because of immune rejection. A way to circumvent these problems is by exploiting autologous stem cells, preferably from an accessible tissue. In this context, it has been reported that bone marrow contains cells that appear to have the ability to transdifferentiate into mature cells belonging to distinct cell lineages (2). A recent study indicated that bone marrow mesenchymal PSC can be expanded in vitro and after transplantation differentiate in vivo into cells belonging to distinct lineages (3). Other studies have, however, raised the possibility that such mature cells may result from fusion of stem cells with mature resident tissue cells (4, 5).
In the present studies, we have described the characterization and expansion in vitro of a yet unidentified subset of human peripheral blood monocytes that behave as PSC. We have shown that these cells can be induced to acquire macrophage, lymphocyte, epithelial, endothelial, neuronal, and hepatocyte phenotypes in the absence of a fusion with preexisting mature tissue cells. The ability to obtain these PSC from an easily accessible source such as peripheral blood and to store them in liquid nitrogen should make them valuable candidates for autologous transplantation.
Materials and Methods
Cell Culture.
Monocytes were obtained from buffy coats (each from 500 ml of peripheral blood) of healthy individuals (LifeSource Blood Services, Glenview, IL) by using a selective attachment procedure as described (6, 7). Fresh mononuclear cells for this procedure and/or after storage in liquid nitrogen in FBS (Harlan Breeders, Indianapolis) containing 10% dimethyl sulfoxide (Sigma) were obtained after Ficoll-Hypaque fractionation and two to three washes with RPMI medium 1640 (Life Technologies, Grand Island, NY). Cells, including those from liquid nitrogen, were incubated at 2–3 × 107 cells per 15-cm dish for 8–12 h at 37°C (8% CO2). After that the floating cells were removed, dishes were rinsed five times with medium, and the cells were detached from the dishes by forceful pipetting with 10 ml of RPMI medium 1640 supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (Life Technologies; growth medium). These preparations contained 90–95% monocytes, as determined by FACScan (Becton Dickinson) flow analysis after immunostaining of the cells with R-phycoerythrin-conjugated mouse anti-human CD14 mAb. In some experiments, the monocytes were further enriched to 99.97% by cell sorting using a 5 detector FACStar PLUS cell sorter (Becton Dickinson). Cells were inoculated at 1 × 105 cells per ml in eight-well Lab-Tek chamber slides (Nunc) at 0.4 ml per well, and half of the medium was replaced every 5–7 d and treated with macrophage colony-stimulating growth factor (M-CSF) (Sigma), phorbol 12-myristate 13-acetate (PMA) (Chemicals for Cancer Research, Eden Prairie, MN), lipopolysaccharide (LPS) (Sigma), human recombinant IL-2, IL-6, epidermal growth factor (EGF), β-nerve growth factor (NGF) (R & D Systems), vascular endothelial growth factor isoform 165 (VEGF165), hepatocyte growth factor (HGF) (Cell Sciences, Norwood, MA), and/or leukemia inhibitory factor (LIF) (Sigma). Cell suspensions were obtained by pipetting after incubation for 5–8 min with 2% lidocaine (Sigma) in PBS as described (8). The individual monocyte-derived cultures used in these experiments were obtained from separate donors at different times.
Colony Formation.
For colony formation, 5-d 50 ng/ml M-CSF-treated cell preparations containing 99.97% monocytes were inoculated into 12 96-well U-bottom tissue culture plates at 0.8 cells per well in 0.1–0.2 ml of growth medium containing 50 ng/ml M-CSF, 1,000 units/ml LIF, and 25% conditioned medium from a 5-d M-CSF-treated monocyte culture. Inspection by light microscopy indicated that ≈70% of the wells contained single cells; the few with more than one cell were excluded. The medium was replaced every 5–7 d. At 20 d there were about two to five colonies per plate of ≈30 cells per colony. At 45–52 d, three of these colonies were manually dispersed and used to determine their susceptibility to differentiation induction.
Phagocytosis and Lipid Staining.
Phagocytosis was determined by the cells' ability to engulf 1.7-μm-diameter Fluoresbrite beads (Polyscience); cells with >20 beads per cell were considered positive (9). For lipid droplet staining, cells were rinsed twice with PBS and fixed for 20 min with PBS containing 4% paraformaldehyde at 20°C. After another PBS rinse and staining for 15 min with Nile red (Sigma), the cells were PBS washed and mounted with phosphate-buffered gelvatol. Fluorescence imaging was performed by using automated excitation and emission filter wheels, a quad-pass cube, and slidebook software (Intelligent Imaging Innovations, Denver).
Immunostaining.
For immunostaining, cells were washed with PBS and fixed with 4% formaldehyde in PBS for 20 min at 20°C. For intracellular proteins, cells were permeabilized with 0.5% Triton X-100 for 5 min at 20°C and incubated for 1 h with primary antibodies diluted with PBS containing 1% BSA to block nonspecific reactivity. The cells were then washed three times with 1% BSA in PBS and incubated for 45 min with FITC-, tetramethylrhodamine B isothiocyanate (TRITC)-, or Cy5-conjugated cross-absorbed donkey secondary antibodies (Jackson ImmunoResearch). Both reactions were performed in a saturating environment at 4°C. The slides were then washed and mounted with phosphate-buffered gelvatol. Fluorescence imaging was performed by using glyceraldehyde 3-phosphate dehydrogenase immunofluorescence (sheep polyclonal antibody, Cortex Biochem, San Leandro, CA) as an internal standard. The fluorescence intensity with isotype-matched IgG antibody was used as background and was designated as 1. Mouse mAbs to IL-1β, IL-6, IL-10, IL-12p70, CD3, CD4, CD8, CD14, CD34, CD40, CD45, HLA-DR, HLA-DQ, CD1a, CD83, von Willebrand factor (vWF), VEGF-R2 (FLK-1), α-fetoprotein (AFP), cytokeratin 7, keratins (Pan Ab-1), microtubule-associated protein-1B (MAP-1B), neurofilament Ab-1 (NF), tumor necrosis factor-α (TNF-α), TNF-α receptor I (TNF-RI), and TNF-RII from were from BD PharMingen (San Diego), Santa Cruz Biotechnology, BioSource International (Camarillo, CA), Accurate Chemical and Scientific (Westbury, NY), and NeoMarkers (Fremont, CA). Mouse IgG1, IgG2A, and IgG2B and goat IgG were from R & D Systems; rat MAb to E-cadherin was from Sigma; rabbit polyclonal antibodies to neuron-specific enolase (NSE), peroxisome proliferator-activated receptor (PPAR)γ2, IL-6, leptin, and VEGF-R3 (FLT-4) were from Affinity BioReagents (Golden, CO), Cortex Biochem, and Santa Cruz Biotechnology; and goat polyclonal antibody to human albumin was from Nordic (Tilburg, The Netherlands).
Cytotoxicity and Lymphocyte Stimulation.
The cytotoxic ability of the macrophages and lymphocytes, obtained after IL-2 treatment, was assayed by a modification of described methods (10, 11). In brief, HL-60 cells, which served as the target cells, were incubated with the effector cells at 37°C in eight-well chamber slides. After 20 h, the target cells were removed and inoculated in a 96-well flat-bottom tissue culture plate. Reduction in HL-60 cell viability (10) and a lymphocyte stimulation assay (12) were performed as described.
Results
Characterization of Two Monocyte Subsets.
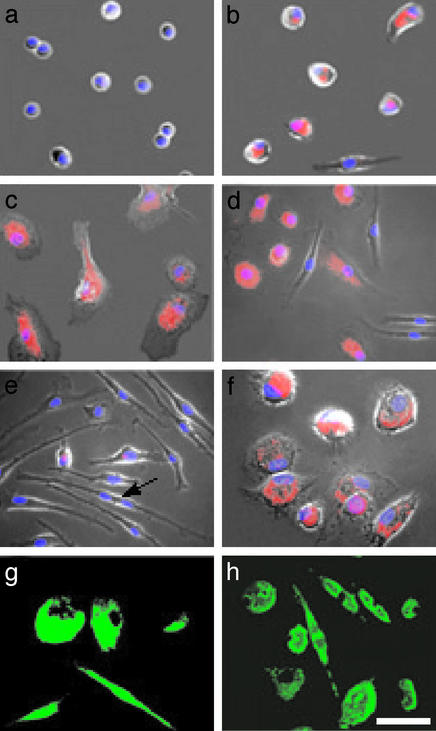
To study macrophage differentiation, we treated seven individual preparations of cultured human peripheral blood cells containing 90–95% monocytes with 50 ng/ml M-CSF and 3 nM phorbol 12-myristate 13-acetate (PMA) (7). After a 5-d incubation, the cultures treated with M-CSF contained two morphologically distinct subsets; one of 65–75% was comprised of standard macrophages (s-MΦ), whereas the other of 25–35% was composed of elongated cells that morphologically resembled fibroblasts, which we termed f-macrophages (f-MΦ) (Fig. 1). Control and PMA-treated cultures also yielded these two populations but with <5% f-MΦ. Both the s-MΦ and f-MΦ attached and spread on culture matrices, engulfed fluorescent beads, and expressed MAC-1 (Fig. 1) and CD14, which are macrophage markers (9, 13). Similar results were obtained from five pairs of untreated and M-CSF-treated monocyte cultures recovered from liquid nitrogen storage.
Figure 1.
Macrophage differentiation of peripheral blood monocytes. (a) Freshly isolated monocytes. (b) Untreated 5-d-old monocyte culture. (c) Five-day PMA-treated culture. (d) Five-day M-CSF-treated culture. (e) Fourteen-day M-CSF-treated culture; the arrow points to a dividing cell. (f) Fourteen-day M-CSF-treated culture incubated for 1 d with LPS. (g) MAC-1 immunostaining of 5-d M-CSF-treated culture. (h) Fluorescence of phagocytized beads in 5-d M-CSF-treated culture. (a–f) Cells visualized by phase-contrast microscopy merged with fluorescence images of lipids stained with Nile red (red) and nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). (Scale bar, 40 μm.)
Three individual monocyte preparations, including one recovered from liquid nitrogen storage, were incubated with 50 ng/ml M-CSF, 1,000 units/ml LIF, or 20 ng/ml IL-6, or a combination of M-CSF and LIF or IL-6. After 5 d, the cultures treated with M-CSF yielded ≈35% f-MΦ, LIF ≈25%, IL-6 ≈20%, and the control ≈5%. Cotreatment with M-CSF and LIF resulted in a nearly additive effect, namely the cultures yielded ≈50% f-MΦ, whereas treatment with M-CSF and IL-6 yielded only ≈25% f-MΦ.
Macrophages are known to function as antigen-presenting cells and as such produce suitable cytokines and display appropriate cell-surface molecules (14, 15). Immunostaining for these proteins indicated that both cell types shared some of these antigen-presenting cell characteristics. Yet, the f-MΦ diverged from s-MΦ in that they exhibited reduced levels of IL-10, TNF-α, TNF-RII, HLA-DR, and HLA-DQ (Table 1). The f-MΦ were also less cytotoxic against human leukemia cells than s-MΦ but more effective in stimulating lymphocyte proliferation (Table 1). An added property that distinguished f-MΦ from s-MΦ was their reduced expression of leptin and PPARγ2 (16, 17), and staining for lipid droplets (Fig. 1d, Table 1), which we have found to be adipocytic indicators of s-MΦ.
Table 1.
Characterization of f-MΦ and s-MΦ
| Relative fluorescence intensity
|
||
|---|---|---|
| f-MΦ | s-MΦ | |
| Surface antigens | ||
| MAC-1 | 78 ± 15 | 81 ± 12 |
| HLA-DR | 19 ± 5 | 102 ± 43 |
| HLA-DQ | 21 ± 8 | 91 ± 27 |
| CD1a | 1 | 12 ± 4 |
| CD14 | 129 ± 27 | 155 ± 22 |
| CD34 | 78 ± 17 | 18 ± 5 |
| CD40 | 51 ± 24 | 48 ± 19 |
| CD45 | 146 ± 24 | 162 ± 41 |
| CD83 | 1 | 1 |
| Cytokine production | ||
| IL-1β | 84 ± 27 | 83 ± 15 |
| IL-6 | 45 ± 20 | 65 ± 16 |
| IL-10 | 9 ± 6 | 56 ± 9 |
| IL-12 p70 | 52 ± 27 | 54 ± 8 |
| TNF-α | 29 ± 13 | 65 ± 18 |
| TNF-RI | 25 ± 7 | 34 ± 15 |
| TNF-RII | 9 ± 4 | 63 ± 27 |
| Adipocyte markers | ||
| Lipids | 14 ± 11 | 157 ± 12 |
| Leptin production | 23 ± 7 | 88 ± 16 |
| PPARγ2 | 20 ± 6 | 110 ± 32 |
| Functional indicators | ||
| Phagocytosis | 189 ± 21 | 197 ± 23 |
| Lymphocyte stimulation, Abs540* | 0.76 ± 0.05 | 0.15 ± 0.03 |
| Cytotoxicity, % | 10 ± 4 | 70 ± 8 |
Relative fluorescence intensity was examined by quantitative ratio imaging microscopy. Stimulation of lymphocyte proliferation was performed by using a 10:1 macrophages-to-lymphocytes ratio, and cytotoxicity was performed by using a 5:1 macrophages-to-target cell ratio (10, 12).
Abs540, optical absorbance at 540 nm.
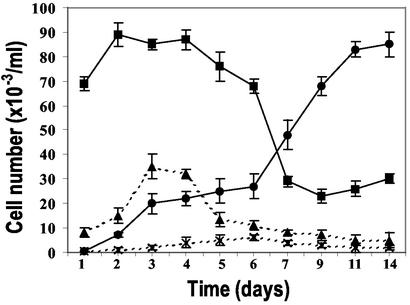
Unlike s-MΦ, the f-MΦ contained dividing cells (Fig. 1e) and displayed elevated levels of the hematopoietic stem cell marker CD34 (ref. 18; Table 1), which raised the possibility that they are replicating progenitors of s-MΦ. For this reason, we postulated that the f-MΦ would with time fully populate the cultures. To test for this premise, we treated five individual preparations of cultured monocytes with 50 ng/ml M-CSF and, by means of their morphology, determined over 14 d the number of f-MΦ and s-MΦ. The results indicated that after 6 d the number of f-MΦ increased, whereas the number of s-MΦ decreased (Fig. 2). Based on their growth kinetics during this time, we estimated that the f-MΦ population replicates about once every 3 d. After the 10th day, the cultures became confluent and were composed of 80–90% f-MΦ (Fig. 2). No such increase was observed in untreated cultures (Fig. 2). Replenishing the cultures with fresh M-CSF on day 5 or 12 had little impact on f-MΦ number or appearance. An additional feature of f-MΦ was their resistance to dispersion by standard trypsin and/or EDTA, or dispase solutions. We were able to disperse them by a nonenzymatic procedure, namely by a short incubation with a lidocaine solution followed by pipetting.
Figure 2.
Replication of M-CSF-treated f-MΦ. f-MΦ in untreated (x-x) and M-CSF-treated (●-●) cultures. s-MΦ in untreated (▴-▴) and M-CSF-treated (■-■) cultures. The results are the mean ± SD of cell counts from four individuals.
Macrophage and T Lymphocyte Cell Differentiation.
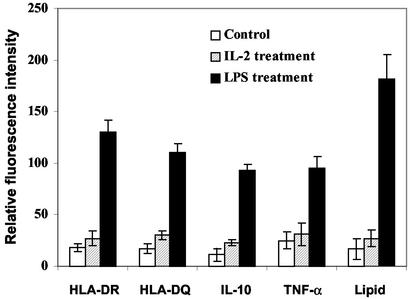
To substantiate their progenitor nature, we incubated four individual preparations of 12- to 14-d-old 50 ng/ml M-CSF-treated monocyte cultures containing 80–90% f-MΦ (f-MΦ cultures) with 1 μg/ml LPS, a macrophage activator (19). This treatment transformed the f-MΦ into s-MΦ, characterized by their morphology, lipid staining (Figs. 1f and 3), increased HLA-DR, HLA-DQ, IL-10, and TNF-α immunostaining (Fig. 3), and cytotoxic ability (Table 1).
Figure 3.
LPS-induced macrophage differentiation of f-MΦ cultures. Fluorescence intensity (mean ± SD of four experiments) is based on 30–50 cells per determination per individual.
To determine whether the f-MΦ could also be induced to mature along another blood lineage, we tested the ability of IL-2, a T lymphocyte differentiation inducer (20), to evoke such a differentiation. Treatment of four f-MΦ cultures with 1,200 units/ml IL-2 for 4 d induced the cells to acquire a round morphology. This treatment also caused ≈90% of the cells to express CD3, an indicator of mature T lymphocytes (13); ≈75% of CD3-positive cells also displayed CD8, which characterizes cytotoxic/suppressor T lymphocytes (21, 22). Control cultures contained 3–4% cells that immunostained for CD3 and CD8. Less than 3% of control or IL-2-treated cells exhibited CD4, a helper T lymphocyte marker (13). The IL-2-induced cells also acquired an increased ability to kill target cells, a functional indicator of cytotoxic/suppressor T lymphocytes. When a 5:1 effector-to-target cell ratio was used, the IL-2-treated lymphocytes lysed 35 ± 7% of the target cells compared with 12 ± 3% by control cells.
Epithelial Differentiation.
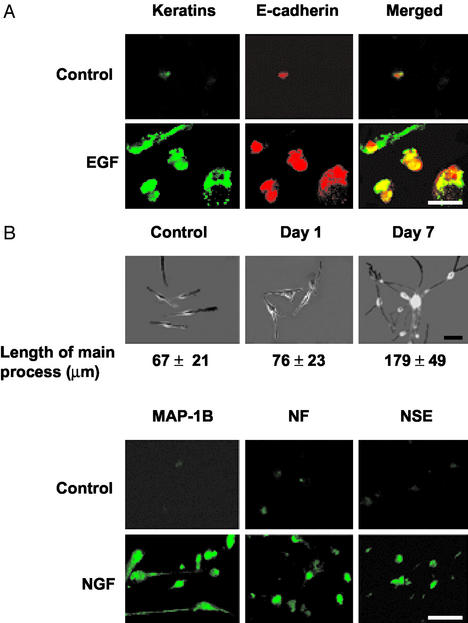
To determine whether f-MΦ could differentiate into lineages other than those of the blood, we initially tested their ability to mature into epithelial cells. Treatment of four individual f-MΦ cultures for 4 d with 100 ng/ml EGF, a promoter of epithelial cell growth and differentiation (23), induced ≈70% of the f-MΦ to display an epithelial cell morphology. This treatment also caused 71 ± 4% of the cells to display immunostaining for pan-keratins and 68 ± 5% for E-cadherin, which are epithelial cell markers (24, 25). Only 4 ± 1% of control cells stained for keratins and 3 ± 2% stained for E-cadherin. Cells that stained positive for E-cadherin consistently stained for the keratins (Fig. 4A).
Figure 4.
Epithelial and neuronal cell differentiation of f-MΦ. (A) EGF-induced epithelial differentiation was assessed by double immunostaining for keratins (green) and E-cadherin (red). Each field contains four to five cells. The control panel was selected to include a positive cell. (B) NGF-induced neuronal differentiation was assessed by length of the main processes (mean ± SD) of 50 randomly selected cells by using SLIDEBOOK software (Upper) and by immunostaining for neuron-specific antigens (Lower). Each immunostained field contains 10–15 cells with the control panel selected to contain positive cells. (Scale bar, 50 μm.)
Neuronal and Endothelial Cell Differentiation.
To examine the ability of f-MΦ to mature along another cell lineage, we tested the effect of NGF, an inducer of neuronal differentiation (26). Treatment of four individual f-MΦ cultures with 200 ng/ml NGF caused ≈90% of f-MΦ to display a neuronal morphology (Fig. 4B). These cells had a smaller cell body and displayed neurite- and axon-like processes (27). After 5–8 d, these processes, some of which were exceedingly long, formed cell–cell contacts and created the appearance of a network (Fig. 4B). These mature cells were further characterized by immunostaining for NSE, NF, and MAP-1B, which are common neuronal markers (28, 29). After 3 d of treatment, 25% of the cells displayed a robust immunostaining for these three proteins, and after 5–8 d this staining was detected in ≈90% of the cells and was also observed in their processes, especially with regards to MAP-1B (Fig. 4B). After a 5- to 8-d incubation, <9% of control cells displayed elongated processes, and those stained only weakly for the neuron-specific antigens (Fig. 4B). Little to no neuronal differentiation was observed when cultured monocytes were treated with NGF for 7 or 20 d.
Next, we treated f-MΦ cultures with 50 ng/ml VEGF for 5–7 d. This treatment induced ≈70% of the cells to display endothelial cell morphology, with a fraction of these forming chains of cobblestone-like formations, which were parallel or crossed each other. VEGF-treatment also caused 74 ± 3% of the cells to immunostain for VEGF-R2, VEGF–R3, and vWF, which are commonly used endothelial cell markers (30, 31). The endothelial appearing cells consistently displayed these markers. In the absence of VEGF, only 5 ± 1% of the cells stained for VEGF-R2, VEGF–R3, and vWF.
VEGF treatment also induced 31 ± 4% of the cells to acquire immunostaining for the neuronal markers NSE, NF, and MAP-1B compared with 7 ± 4% in the absence of VEGF. Nearly all of the NSE-, NF-, and MAP-1B-stained cells exhibited a neuronal morphology. A small percentage of cells, which displayed an intermediate morphology between endothelial and neuronal cells, stained for both the endothelial and neuronal markers.
Hepatocyte Differentiation.
To determine whether f-MΦ can also differentiate into hepatocytes, we tested the effect of HGF, an inducer of liver cell growth and differentiation (32). Treatment of three individual f-MΦ cultures for 5–7 d with 100 ng/ml HGF caused 75–80% of the cells to acquire a round or oval-like flattened morphology. It also caused 75 ± 7% of the cells to display immunostaining for albumin and 81 ± 7% for AFP, which typify hepatocytes (33), and 33 ± 4% for cytokeratin 7, which marks bile duct epithelium (34). Only 8 ± 5% of control cells immunostained for albumin, 6 ± 5% for AFP, and 7 ± 3% for cytokeratin 7.
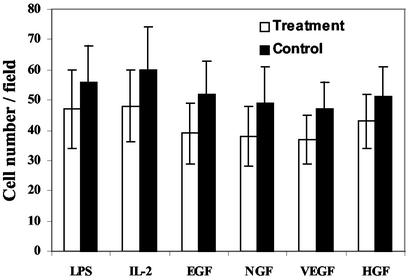
The induction of lymphocyte, epithelial, neuronal, endothelial, or hepatocyte differentiation, which was associated with a somewhat reduced cell number (Fig. 5), was coupled with a marked decrease in MAC-1 display.
Figure 5.
Relative cell number in f-MΦ cultures treated with or without differentiation inducers. The results are the mean ± SD of five randomly selected microscopic fields each from four different experiments for each treatment.
Sorted Cells and Clonal Analysis.
In addition to regular f-MΦ cultures, we also tested three individual flow-sorted preparations of blood cells enriched to contain 99.97% monocytes. Incubation of these cells for 5 and 14 d with 50 ng/ml M-CSF yielded about 40% and 90% f-MΦ, respectively, which expressed CD14, CD34, CD45, and MAC-1. As with f-MΦ cultures, treatment of these 14-d M-CSF-treated cells with 200 ng/ml NGF or 100 ng/ml HGF caused >80% of the f-MΦ to acquire a neuronal and hepatocyte phenotype, respectively.
To further determine the pluripotent nature of individual f-MΦ, we obtained colonies from single cells derived from M-CSF-treated cultures, enriched to contain 99.97% monocytes. Most cells in these colonies exhibited f-MΦ morphology. Analysis of a number of these colonies indicated that their cells also displayed immunostaining for CD14, CD34, and CD45. Cells from three such colonies were dispersed and inoculated into 96-well plates. These wells were incubated with IL-2, EGF, NGF, VEGF, or HGF, and 7 d later the cells were tested for lineage-specific markers: CD3 for T lymphocytes, pan-keratin for epithelial cells, vWF for endothelial cells, MAP1-B for neuronal cells, and AFP for hepatocytes. The results indicated that the differentiation inducers caused 70–90% of the treated cells to display maturation markers that epitomize the specific differentiated state (Table 2) and to display morphologies consistent with the expected lineages. These observations indicate that progeny of single f-MΦ have the ability to be induced to differentiate into distinct cell lineages, thus further substantiating the pluripotent nature of the f-MΦ.
Table 2.
Induction of differentiation markers in progeny derived from single f-MΦ
| Inducer | Lineage | Marker | Treatment | Immunostained cells, %
|
||
|---|---|---|---|---|---|---|
| Clone 1 | Clone 2 | Clone 3 | ||||
| IL-2 | Lymphocyte | CD3 | − | 6 | 3 | 4 |
| + | 75 | 81 | 83 | |||
| EGF | Epithelial | Keratins | − | 7 | 5 | 9 |
| + | 89 | 76 | 82 | |||
| NGF | Neuronal | MAP1-B | − | 3 | 4 | 5 |
| + | 83 | 80 | 76 | |||
| VEGF | Endothelial | vWF | − | 8 | 5 | 9 |
| + | 80 | 87 | 71 | |||
| HGF | Hepatocyte | AFP | − | 7 | 2 | 5 |
| + | 88 | 75 | 76 | |||
The cells were treated with 1,200 units/ml IL-2, 100 ng/ml EGF, 200 ng/ml NGF, 50 ng/ml VEGF, or 50 ng/ml HGF. The percentage of cells immunostained for the lineage-specific markers was determined 7 d after initiating the treatment.
Discussion
In the present study we have identified, characterized, cultured, and propagated a previously unknown subset of human peripheral blood monocytes that act as PSC. These cells, which display a fibroblast-like morphology and exhibit monocyte and hematopoietic stem cell markers including CD14, CD34, and CD45, can be induced to differentiate at 70–90% into macrophages, T lymphocytes, epithelial cells, endothelial cells, neuronal cells, and hepatocytes. The pluripotent nature of these adult PSC was deduced on the basis that the combined absolute number of mature cells belonging to the different induced lineages far exceeded the available starting cells in the f-MΦ cultures. Furthermore, the progeny of single f-MΦ were induced to express differentiated traits belonging to five distinct cell lineages. Other investigators, using a mouse model and engrafting a single CD34-positive bone marrow cell per animal, concluded that these cells could also differentiate into distinct cell lineages (35). Recent studies have, however, questioned the existence of such a transdifferentiation and raised the possibility that the emerging mature cells resulted from fusion of stem cells with preexisting mature tissue cells (4, 5). In our experiments, the induced cells with the mature T lymphocyte, epithelial, neuronal, endothelial, or liver cell phenotypes generated from the progeny of single cells could not have derived from such a fusion.
A number of investigators have described the culture and propagation of mesenchymal PSC from human peripheral blood or bone marrow (3, 36). These cells differ from our f-MΦ in a number of ways including their failure to express CD34 and/or CD45. Also, unlike the mesenchymal stem cells (3, 36), the f-MΦ cultures grow firmly attached to the tissue culture matrices and could not be readily removed and dispersed by standard digestion with trypsin, trypsin–EDTA, or dispase solutions.
The physiological function of the f-MΦ or their parental cells, which are peripheral blood monocytes and as such are dispersed throughout our bodies, is unknown. Perhaps their function is to facilitate tissue repair by replacing lost or damaged specialized resident progenitor cells. In this context, a recent study with transplantation patients that underwent chemotherapy or radiation treatment indicated that blood preparations enriched for CD34-positive cells were able to populate different tissues and differentiate into cells belonging to distinct lineages (37). These preparations probably contained f-MΦ parental cells, which express CD34.
The ability to store, expand in vitro, and differentiate f-MΦ from an easily accessible source such as peripheral blood should make them valuable candidates for transplantation therapy; for example, they can be used to replenish immune cells that have been eradicated by cancer therapy or to replace neuronal tissue damaged during spinal cord injury, stroke, dementia (including Alzheimer's syndrome), or Parkinson's disease. The ability to expand in vitro autologous f-MΦ before transplantation should yield a high number of stem cells for this procedure, which ought to be more effective and versatile than the current transplantation procedures, which do not include such an expansion.
Acknowledgments
This work was supported by National Institutes of Health Grant CA80826.
Abbreviations
- AFP
α-fetoprotein
- EGF
epidermal growth factor
- f-MΦ
f-macrophages
- HGF
hepatocyte growth factor
- HLA
human leukocyte antigen
- LPS
lipopolysaccharide
- MAP-1B
microtubule-associated protein-1B
- M-CSF
macrophage colony-stimulating growth factor
- NF
neurofilament
- NGF
β-nerve growth factor
- NSE
neuron-specific enolase
- PSC
pluripotent stem cells
- s-MΦ
standard macrophages
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
- LIF
leukemia inhibitory factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wagers A J, Christensen J L, Weissman I L. Gene Ther. 2002;9:606–612. doi: 10.1038/sj.gt.3301717. [DOI] [PubMed] [Google Scholar]
- 2.Griffith L G, Naughton G. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Jahgirdar B N, Reinhadt R L, Schwartz R E, Keene C D, Ortiz-Gonzales X R, Reyes M, Lenvik T, Blackstad M, Du J, et al. Nature. 2002;418:1–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz D M, Nakano Y, Meyer E M, Morel L, Petersen B E, Scott E W. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 5.Ying Q-L, Nichols J, Evans E P, Smith A G. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 6.Hoklland M, Jorgensen H, Hoklland P. In: Cell Biology: A Laboratory Handbook. Celis J E, editor. Vol. 1. New York: Academic; 1994. pp. 179–181. [Google Scholar]
- 7.Semizarov D, Glesne D, Laouar A, Schiebel K, Huberman E. Proc Natl Acad Sci USA. 1998;95:15412–15417. doi: 10.1073/pnas.95.26.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinovitch M, De Stefano M J. J Exp Med. 1976;143:290–304. doi: 10.1084/jem.143.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laouar A, Collart F, Huberman E. In: Cell Biology: A Laboratory Handbook. Celis J E, editor. Vol. 1. New York: Academic; 1997. pp. 233–238. [Google Scholar]
- 10.Nakabo Y, Pabst M J. J Leukocyte Biol. 1996;60:328–336. doi: 10.1002/jlb.60.3.328. [DOI] [PubMed] [Google Scholar]
- 11.Vitale A, Guarini A, Latagliata R, Cignetti A, Foa R. Br J Haematol. 1998;101:150–157. doi: 10.1046/j.1365-2141.1998.00645.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L-J, Tedder T F. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlossman S F, Boumsell L, Gilks W, Harlan J, Kishimoto T, Morimoto T, Ritz J, Shaw S, Silverstein R, Springer T, Tedder T, Todd R, editors. Leukocyte Typing V: White Cell Differentiation Antigens. New York: Oxford Univ. Press; 1995. pp. 301–303. and 778–788. [Google Scholar]
- 14.Martinez-Pomares L, Platt N, McKnight A J, da Silva R P, Gordon S. Immunobiology. 1996;195:407–416. doi: 10.1016/S0171-2985(96)80012-X. [DOI] [PubMed] [Google Scholar]
- 15.Grage-Griebenow E, Flad H D, Ernst M. J Leukocyte Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 16.Mukherjee R, Jow L, Croston G E, Paterniti J R., Jr J Biol Chem. 1997;272:8071–8076. doi: 10.1074/jbc.272.12.8071. [DOI] [PubMed] [Google Scholar]
- 17.Tontonoz P, Nagy L, Alvarez J G, Thomazy V A, Evans R M. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 18.Randall T D, Weissman I L. Stem Cells. 1998;16:38–48. doi: 10.1002/stem.160038. [DOI] [PubMed] [Google Scholar]
- 19.Vadiveloo P K. J Leukocyte Biol. 1999;66:579–582. doi: 10.1002/jlb.66.4.579. [DOI] [PubMed] [Google Scholar]
- 20.Nelson B H, Willerford D M. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 21.Ryffel B, Henning C B, Huberman E. Proc Natl Acad Sci USA. 1982;79:7336–7340. doi: 10.1073/pnas.79.23.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lederman S, Suciu-Foca N. Hum Immunol. 1999;60:533–561. doi: 10.1016/s0198-8859(99)00045-2. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter G. Curr Opin Cell Biol. 1993;5:261–264. doi: 10.1016/0955-0674(93)90113-5. [DOI] [PubMed] [Google Scholar]
- 24.Tseng S C, Jarvinen M J, Nelson W G, Huang J W, Woodcock-Mitchell J, Sun T T. Cell. 1982;30:361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- 25.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 26.McAllister A K. Cell Mol Life Sci. 2001;58:1054–1060. doi: 10.1007/PL00000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacovina A T, Zhong F, Khazanova E, Lev E, Deora A B, Hajjar K A. J Biol Chem. 2001;276:49350–49358. doi: 10.1074/jbc.M106289200. [DOI] [PubMed] [Google Scholar]
- 28.Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella J X. J Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 29.Studahl M, Rosengren L, Gunther G, Hagberg L. J Neurol. 2000;247:636–642. doi: 10.1007/s004150070134. [DOI] [PubMed] [Google Scholar]
- 30.Karkkainen M J, Makinen T, Alitalo K. Nat Cell Biol. 2002;4:E2–E5. doi: 10.1038/ncb0102-e2. [DOI] [PubMed] [Google Scholar]
- 31.Hatzopoulos A K, Folkman J, Vasile E, Eiselen G K, Rosenberg R D. Development (Cambridge, UK) 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 32.Michalopoulus G K, DeFrances M C. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 33.Hamazaki T, Iiboshi Y, Oka M, Papst P J, Meacham A M, Zon L I, Terada N. FEBS Lett. 2001;497:15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- 34.Ruck P, Xiao J C, Pietsch T, Von Schweinitz D, Kaiserling E. Histopathology. 1997;31:324–329. doi: 10.1046/j.1365-2559.1997.2750870.x. [DOI] [PubMed] [Google Scholar]
- 35.Krause D S, Theise N D, Collector M I, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis S J. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 36.Toma C, Pittenger M F, Cahill K S, Byrne B J, Kessler P D. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 37.Korbling M, Katz R L, Khanna A, Ruifrok A C, Rondon G, Albitar M, Champlin R E, Estrov Z. N Engl J Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]