Abstract
The c-Jun NH2-terminal kinase (JNK) is activated when cells are exposed to environmental stress, including UV radiation. Gene disruption studies demonstrate that JNK is essential for UV-stimulated apoptosis mediated by the mitochondrial pathway by a Bax/Bak-dependent mechanism. Here, we demonstrate that JNK phosphorylates two members of the BH3-only subgroup of Bcl2-related proteins (Bim and Bmf) that are normally sequestered by binding to dynein and myosin V motor complexes. Phosphorylation by JNK causes release from the motor complexes. These proapoptotic BH3-only proteins therefore provide a molecular link between the JNK signal transduction pathway and the Bax/Bak-dependent mitochondrial apoptotic machinery.
The molecular machinery of cell death has been studied intensively, and many of the components have been identified (1). Two pathways of cell death have been elucidated: a pathway that is directly activated by death receptors and a pathway that involves the mitochondria. It is established that signal transduction pathways initiated at the cell surface by death receptors are mediated by adapter complexes that lead to caspase activation (1). However, the signal transduction pathways that activate the mitochondrial apoptotic pathway are poorly understood (1). One class of stimulus that activates the mitochondrial pathway is the exposure of cells to stress. An example is represented by ionizing radiation, which engages the p53 tumor suppressor and leads to the expression of a number of proapoptotic proteins, including members of the BH3-only group of Bcl2-related proteins (e.g., Puma and Noxa), that can trigger the mitochondrial apoptosis pathway (2). However, the mechanism by which other stresses (e.g., UV radiation) activate the mitochondrial cell death pathway has not been established.
The c-Jun NH2-terminal kinase (JNK) is activated when cells are exposed to multiple forms of stress, and this signaling pathway has been implicated as a mediator of stress-induced apoptosis (3). Examples include nerve growth factor withdrawal-induced apoptosis (4–7), excitotoxic stress-induced apoptosis (8), thymocyte apoptosis (9, 10), and apoptosis in response to UV radiation (11, 12). It is therefore likely that JNK contributes to apoptotic signal transduction in response to the exposure of cells to many stresses, including changes in the physical and chemical properties of the environment (3).
Gene disruption studies demonstrate that JNK is required for the release of mitochondrial proapoptotic molecules (including cytochrome c) and apoptosis in response to UV radiation (12). Bax and Bak (members of the proapoptotic group of multidomain Bcl2-related proteins) are essential for the JNK-stimulated release of cytochrome c and apoptosis (13). Furthermore, the activation of the Bax subfamily of Bcl2-related proteins by UV radiation requires JNK (13). Bax activation is thought to be mediated, in part, by sequestration of multidomain antiapoptotic Bcl2 proteins (e.g., Bcl2 and Bcl-Xl) by BH3-only members of the Bcl2 family (14, 15). For example, inhibition of the Akt pathway can lead to activation of the BH3-only protein Bad, sequestration of multidomain antiapoptotic members of the Bcl2 family, and, consequently, Bax/Bak-dependent apoptosis (14–16). However, the mechanism by which JNK might cause the activation of BH3-only proteins is unclear. One potential target of JNK is Bim, a BH3-only protein that is transcriptionally up-regulated in neurons undergoing JNK-dependent apoptosis (17–19).
Three major Bim isoforms are created by alternative splicing: BimS, BimL, and BimEL (20). The short isoform (BimS) potently induces apoptosis and is normally only transiently expressed in cells during apoptosis. The longer isoforms (BimL and BimEL) are expressed in normal cells, but the apoptotic activity of these proteins is suppressed by binding to the dynein motor complex via an interaction with dynein light chain (DLC)1. A short peptide motif (DKSTQTP) present in BimL and BimEL (but absent in BimS) mediates the binding of Bim to DLC1. The absence of this motif from BimS accounts for the extremely potent apoptotic activity of this Bim isoform (21). Interestingly, the Bim-related molecule Bmf contains a similar motif (DKATQTLSP) that binds DLC2, a component of the myosin V motor complex (22). In normal cells, the BH3-only proteins Bim and Bmf are sequestered in motor complexes that interact with the cytoskeleton. Apoptosis induced by these molecules involves the release of Bim and Bmf from these complexes (23). Thus, exposure of cells to UV radiation causes the release of both Bim and Bmf from sites of sequestration by dissociation of Bim from dynein motor complexes (21) and Bmf from myosin V motor complexes (22). The mechanism that accounts for the release of these proteins has not been established.
The effect of UV radiation to cause JNK activation (3) and the release of Bim and Bmf (23) suggests that these processes may represent related steps in an apoptotic signal transduction pathway. Indeed, studies of JNK-deficient and Bim-deficient mice indicate similar defects in thymocyte apoptosis (23, 24). The purpose of this study was to examine the role of Bim-related BH3-only proteins in JNK-dependent apoptosis. We report that JNK phosphorylates Bim and Bmf within the conserved DLC binding motif. Phosphorylation by JNK disrupts the function of the DLC binding motif and consequently may cause the release of Bim from sequestration by dynein motor complexes and the engagement of the mitochondrial apoptotic pathway. These BH3-only proteins therefore represent direct targets of JNK that can mediate apoptotic signaling. The significance of this finding is that it provides an important missing link that connects the JNK signal transduction pathway to the apoptotic machinery.
Methods
Plasmid Construction.
Human BimL, Bmf, DLC1, DIC1, and mouse DLC2 cDNA were isolated by PCR and subcloned in the HindIII and EcoRI sites of the mammalian expression vector pCDNA3 (Invitrogen) with an NH2-terminal Flag or T7 epitope tag. Bacterial expression vectors were constructed by using the GST-fusion protein vector pGSTag by subcloning PCR fragments in the BamHI and XhoI restriction sites. The GST-fusion proteins were expressed in BL21 codon-plus cells (Stratagene) and were purified by affinity chromatography using glutathione-Sepharose. Point mutations were constructed by overlapping PCR. JNK expression vectors have been described (13, 25).
Cell Culture.
Kidney cells (293T, American Type Culture Collection) were cultured in Dulbecco's modified Eagle's medium supplemented with 5% FCS (Invitrogen). Transfection assays were performed by using Lipofectamine (Invitrogen) and following the supplier's recommendations.
Biochemical Assays.
Cells were lysed in Triton lysis buffer containing 20 mM Tris (pH 8.0), 1% Triton X-100, 10% glycerol, 137 mM NaCl, 2 mM EDTA, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 10 μg/ml aprotinin and leupeptin. Extracts (100 μg of protein) and immunoprecipitates prepared from the cell extracts were examined by protein immunoblot analysis by probing with antibodies to JNK (PharMingen), T7 (Novagen), Flag M2 (Sigma), and Bim (Calbiochem). Immunocomplexes were detected by enhanced chemiluminescence (NEN). Phosphoamino acid analysis was performed by partial acid hydrolysis and thin-layer chromatography (26). Mitogen-activated protein kinase (MAPK) activity was measured by in vitro kinase assays (27). Cell viability was examined by using a luciferase reporter assay (13). Apoptosis was examined by analysis of DNA fragmentation by using the Cell Death Detection ELISAPlus kit (Roche) and by flow cytometry by staining with phycoerythrin-conjugated annexin V and 7-aminoactinomycin D (PharMingen).
Results
The expression of Bim has been reported to be regulated by JNK (17–19). We therefore investigated the expression of Bim in wild-type and JNK-deficient fibroblasts; no marked difference in Bim expression was detected. It is therefore unlikely that altered Bim expression accounts for the apoptotic defect of JNK-deficient fibroblasts (12). However, Bim-deficient cells (18, 19, 28, 29) have apoptotic defects that share some similarities with JNK-deficient cells (3, 12). Thus, although JNK may not be essential for Bim expression in fibroblasts, JNK might alter Bim function. To test this hypothesis, we examined the possibility that JNK might phosphorylate and regulate Bim.
Bim Is Phosphorylated by JNK in Vitro.
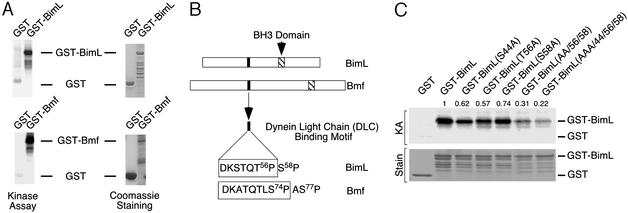
We found that recombinant BimL was phosphorylated by JNK in vitro (Fig. 1A). Interestingly, the BimL phosphorylated by JNK was found to undergo an electrophoretic mobility shift during SDS/PAGE. It is known that JNK phosphorylates Ser/Thr-Pro motifs in target proteins (3) and that BimL contains three of these motifs: S44P, T56P, and S58P (Fig. 1B). In vitro kinase assays demonstrated reduced phosphorylation if any of the three potential phosphorylation sites was replaced with Ala, and phosphorylation was markedly reduced if the three predicted sites were simultaneously replaced with Ala (Fig. 1C). These data indicate that all three of the potential phosphorylation sites are substrates for JNK in vitro.
Figure 1.
The BH3-only proteins Bim and Bmf are substrates of JNK. (A) Bacterially expressed GST, GST-BimL, and GST-Bmf were incubated in vitro with activated JNK and [γ-32P]ATP. The products of the phosphorylation reaction were examined by SDS/PAGE, staining with Coomassie blue (Right), and autoradiography (Left). (B) The structures of BimL and Bmf are illustrated schematically. The BH3 domain and the DLC binding motif are indicated. (C) Mutational analysis of BimL phosphorylation by JNK. In vitro protein kinase assays (KA) were performed by using recombinant Bim with point mutations at Ser-44, Thr-56, and Ser-58 (replaced with Ala). A double point mutation (Ala-56 Ala-58) and a triple point mutation (Ala-44 Ala-56 Ala-58) were also examined. The products of the phosphorylation reaction were examined by SDS/PAGE, staining with Coomassie blue (Lower), and autoradiography (Upper). The numbers above the autoradiograph of the kinase assay represent relative phosphorylation measured by PhosphorImager analysis.
Bim Is Phosphorylated by JNK in Vivo.
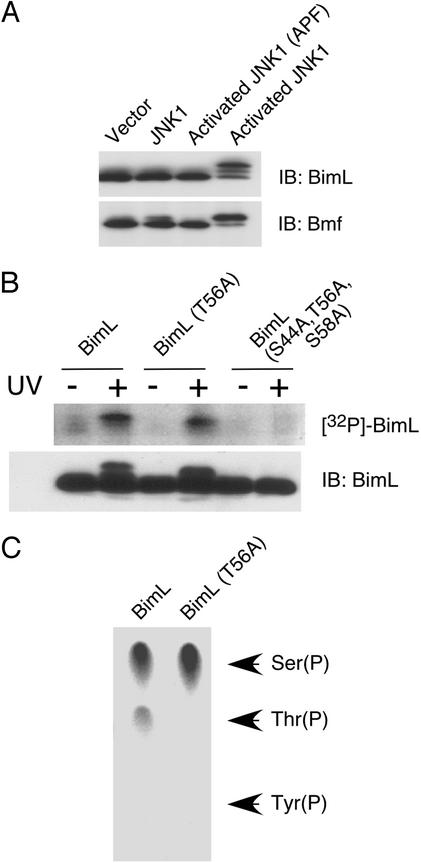
To test whether Bim was a JNK substrate in vivo, we initially examined the effect of activated JNK1 in cotransfection assays with BimL. Phosphorylation was monitored by measurement of the electrophoretic mobility of BimL by immunoblot analysis. Expression of activated JNK1 caused a marked decrease in the electrophoretic mobility of BimL (Fig. 2A). In contrast, mutation of the sites of activating phosphorylation of JNK1 (T180A, Y182F) eliminated the changes in the electrophoretic mobility of BimL. These data suggest that BimL is a substrate of JNK in vivo.
Figure 2.
JNK phosphorylates BimL in vivo. (A) Activated JNK causes BimL and Bmf phosphorylation in vivo. Epitope-tagged BimL or Bmf (0.3 μg) were coexpressed together with 0.05 μg of a Bcl2 expression vector. The effect of coexpression (0.3 μg) of an empty vector or expression vectors for JNK1 or activated JNK1 was examined. Control studies were performed by replacing the sites of Thr and Tyr phosphorylation in activated JNK1 with Ala and Phe, respectively. The electrophoretic mobility of BimL and Bmf was examined by immunoblot (IB) analysis. (B) Epitope-tagged BimL (0.3 μg) was coexpressed together with 0.05 μg of a Bcl2 expression vector in cells labeled with [32P]phosphate. The BimL was isolated by immunoprecipitation and examined by SDS/PAGE, electrotransfer onto a poly(vinylidene difluoride) membrane, autoradiography (Upper), and immunoblot analysis (Lower). The effect of mutation of the JNK phosphorylation sites and exposure to UV radiation (60 J/m2) were examined. (C) The phosphorylated wild-type and T56A BimL were examined by phosphoamino acid analysis performed by partial acid hydrolysis and thin-layer electrophoresis. The migration of phosphoserine, phosphothreonine, and phosphotyrosine standards is indicated. [32P]Phosphothreonine was detected in wild-type BimL but was not detected if Thr-56 was replaced with Ala.
To confirm the conclusion that BimL is phosphorylated in vivo, we examined the phosphorylation of BimL in cells labeled with [32P]phosphate (Fig. 2B). Exposure to UV caused a marked increase in BimL phosphorylation. This increased phosphorylation was eliminated if the three sites of phosphorylation by JNK (Ser-44, Thr-56, and Ser-58) were replaced with Ala and was partially suppressed when Thr-56 alone was replaced with Ala. Phosphoamino acid analysis demonstrated that wild-type Bim in cells exposed to UV radiation contained phosphothreonine and phosphoserine (Fig. 2C). In contrast, only phosphoserine was detected if Thr-56 was replaced with Ala. These data demonstrate that BimL is phosphorylated in vivo on Thr-56 and that JNK also phosphorylates BimL on at least one serine residue (Ser-44 and/or Ser-58).
The Sites of Bim Phosphorylation Are Conserved in the Related Protein Bmf.
Bim is a proapoptotic BH3-only member of the Bcl2-related family that is sequestered in normal cells by binding to dynein motor complexes (21, 23, 30). This interaction is mediated by a small region of Bim (Fig. 1B) that binds DLC1. Interestingly, one of the JNK phosphorylation sites on Bim (Thr-56) is located within this region, and a second phosphorylation site (Ser-58) is located adjacent to this region (Fig. 1B). Importantly, this binding motif and these phosphorylation sites are conserved in the related BH3-only protein Bmf, which binds DLC2 (22). In vitro protein kinase assays demonstrated that Bmf, like Bim, is a JNK substrate in vitro (Fig. 1A). Furthermore, cotransfection assays demonstrated that activated JNK (Fig. 2A) caused decreased Bmf electrophoretic mobility, consistent with phosphorylation of Bmf by JNK in vivo. Together, these data indicate that phosphorylation by JNK within the DLC binding motif is a conserved property of both Bim and Bmf.
Phosphorylation of Bim on Thr-56 Inhibits Binding to DLC1.
The observation that JNK phosphorylates BimL within the motif that binds DLC1 suggests that JNK might regulate the interaction of BimL with DLC1. To test this hypothesis, we examined the effect of activated JNK on the properties of BimL in vivo. Immunoblot analysis demonstrated that activated JNK caused a marked decrease in the electrophoretic mobility of BimL (Fig. 3A). Consistent with the presence of multiple phosphorylation sites, two major forms of BimL with decreased electrophoretic mobility were detected. No change in the electrophoretic mobility of DLC1 was detected (Fig. 3A), and DLC1 was not found to be a JNK substrate in vitro (data not shown). Coimmunoprecipitation analysis demonstrated that activated JNK caused decreased binding of BimL to DLC1 (Fig. 3 A and B). The observed dissociation of BimL from DLC1 requires the use of phosphatase inhibitors in the cell lysis buffer to prevent dephosphorylation and reassociation with DLC1. Replacement of the JNK phosphorylation site Ser-44 or Ser-58 with Ala to eliminate phosphorylation did not alter the coimmunoprecipitation of Bim with DLC1 (Fig. 3B). These data indicate that Ser-44 and Ser-58 are not critical for regulation by JNK of the interaction between Bim and DLC1. In contrast, substitution of Thr-56 with Ala prevented the JNK-stimulated release of Bim from DLC1 (Fig. 3B). These data indicate that the phosphorylation of Bim on Thr-56 within the DLC binding motif may cause the release of Bim from DLC1. Strong support for this conclusion was obtained from the observation that the replacement of Thr-56 with an acidic residue (Asp) to mimic phosphorylation completely disrupted the interaction of Bim with DLC1 (Fig. 4A). Interestingly, the substitution of Ser-44 or Ser-58 with Asp did not inhibit the binding of Bim to DLC1, but these mutant Bim proteins were resistant to the effects of JNK, suggesting that the regulatory phosphorylation site Thr-56 may be influenced by phosphorylation of Bim on Ser-44 and Ser-58.
Figure 3.
Phosphorylated BimL is released from DLC1. (A and B) Cells were cotransfected with 0.3 μg of epitope-tagged BimL and DLC1 expression vectors together with 0.05 μg of a Bcl2 expression vector. The effect of coexpression of activated JNK1 was investigated. The presence of BimL and DLC1 in the cell lysate was examined by immunoblot (IB) analysis. Coimmunoprecipitation analysis was performed by immunoblot analysis of DLC1 immunoprecipitates (IP) with an antibody to Bim. The results of mutational analysis of the Bim phosphorylation sites Ser-44, Thr-56, and Ser-58 replaced with Asp (A) or with Ala (B) are shown. (C) DLC1 interacts with nonphosphorylated BimL. Cells were cotransfected with 0.3 μg of BimL, 0.05 μg of Bcl2, 0.3 μg of activated JNK1, and 0.5 μg of DLC1 expression vectors. The amount of BimL and DLC1 in cell lysates and in DLC1 immunoprecipitates was examined by immunoblot analysis. Phosphorylated BimL with reduced electrophoretic mobility was not coimmunoprecipitated with DLC1.
Figure 4.
Mutational analysis of the JNK phosphorylation site Thr-56 on BimL-stimulated apoptosis. (A and B) Cells were cotransfected with 0.4 μg of an empty vector or with an expression vector for BimL. The effect of replacement of the JNK phosphorylation site Thr-56 with Ala and Asp was examined. In addition, the effect of mutational disruption of the BH3 domain (Leu-92 and Ile-95 replaced with Asp and Glu, respectively) was investigated. The cells were harvested 40 h after transfection. Cell viability (A) and DNA fragmentation (B) were measured. The data presented are the mean ± SD (n = 3). (C) Cells were cotransfected with pEGFP (CLONTECH) together with a BimL expression vector. The cells expressing EGFP were examined 16 h after transfection by flow cytometry after staining with phycoerythrin-conjugated annexin V and 7-aminoactinomycin D. Cells of the annexin V-positive population of 7-aminoactinomycin D-negative cells (Upper Left) are apoptotic.
To test whether phosphorylated Bim fails to interact with DLC1, we performed coimmunoprecipitation analysis to examine the subpopulation of Bim molecules that remain bound to DLC1 after JNK activation (Fig. 3C). Bim phosphorylation was assessed by measurement of electrophoretic mobility during SDS/PAGE. Bim with reduced electrophoretic mobility failed to interact with DLC1. In contrast, nonphosphorylated Bim was found to coimmunoprecipitate with DLC1. These data confirm the conclusion that the phosphorylation of Bim by JNK causes the release of Bim from DLC1.
JNK Phosphorylation of Bim on Thr-56 Increases Apoptotic Activity.
Exposure of cells to UV radiation causes the release of both Bim and Bmf from motor complexes and induction of the mitochondrial cell death pathway (21, 22). This apoptotic activity is mediated by the engagement of the Bax/Bak-dependent pathway (14, 15, 23, 30). This mechanism indicates that BimL phosphorylated by JNK on Thr-56, which does not bind DLC1, may have increased apoptotic activity. To test this hypothesis, we examined the survival and apoptosis of cultured cells transfected with BimL. A similar level of survival (Fig. 4A) and apoptosis (Fig. 4B) was detected when cells were transfected with BimL or [Ala-56]BimL. In contrast, increased apoptosis was detected in experiments using BimL with an acidic amino acid (Asp) replacing Thr-56 to mimic phosphorylation (Fig. 4 A and B). These data were confirmed by analysis of apoptosis by flow cytometry (Fig. 4C). Mutational analysis demonstrated that the increased apoptosis caused by [Asp-56]BimL was dependent on the BH3 domain because mutational inactivation of this domain eliminated the proapoptotic effects of both wild-type and [Asp-56]BimL (Fig. 4 A and B). Interestingly, [Asp-56]BimL-stimulated apoptosis was also observed in JNK-deficient cells (data not shown), indicating that whereas JNK does phosphorylate Bim, JNK is not essential for subsequent biochemical steps that lead to apoptosis. Together, these data indicate that the JNK-stimulated phosphorylation of BimL on Thr-56 may be sufficient to induce Bax-dependent apoptosis.
Discussion
JNK Is Not Essential for Bim Expression in Fibroblasts.
Previous studies of neuronal apoptosis caused by nerve growth factor withdrawal have demonstrated an important role for Bim, a BH3-only protein that is transcriptionally up-regulated in neurons undergoing JNK-dependent apoptosis (17–19). Thus, the proapoptotic role of JNK may be to increase the expression of Bim. However, studies of JNK-deficient cells demonstrate that JNK is not essential for Bim expression in fibroblasts. This difference between neurons and fibroblasts may reflect complex regulation of Bim gene expression. For example, if JNK is critical for Bim expression in neurons, other signaling pathways may act as the primary regulator of Bim expression in other cell types. Consistent with this hypothesis, studies of other cell types have uncovered important roles for Akt and forkhead family transcription factors (31–33), the phosphatidylinositol 3-kinase/TOR pathway (34), and the Ras/ERK MAPK pathway (34) in the expression of Bim. The critical role of JNK for Bim expression may be selectively required only for certain cell types, including neurons.
Studies of JNK-dependent apoptosis in fibroblasts demonstrate that new gene expression is not required for UV-stimulated apoptosis (12). This observation suggests that the target of JNK that mediates apoptotic signaling is a protein that preexists in normal cells before apoptosis. These considerations suggest that Bim may be regulated by a posttranslational mechanism. Indeed, it is established that Bim is regulated by sequestration in the cytoplasm by binding to DLC1, a component of the dynein motor complex (21). Furthermore, the Bim-related protein Bmf binds DLC2, a component of the myosin V motor complex (22). Exposure to UV radiation causes the release of both Bim and Bmf from these motor complexes (21, 22). However, the mechanism that accounts for the release of Bim and Bmf was not established by previous studies. One possible mechanism is phosphorylation. Indeed, Bim has been demonstrated to be a phosphoprotein (34). Here, we demonstrate that JNK phosphorylates and inactivates the DLC binding motif located in Bim. This phosphorylation provides a mechanism for the release of Bim and Bmf from sequestration in the cytoplasm.
Together, these data indicate that JNK can engage the Bim apoptotic pathway by at least two different mechanisms. First, in some cell types (e.g., neurons), JNK plays an important role in the transcriptional induction of Bim expression. Second, JNK phosphorylates Bim and releases Bim from sequestration in the cytoplasm. The relative importance of these two mechanisms may differ between cell types. For example, if JNK causes the expression of BimS (which lacks the DLC1 binding motif and is not sequestered in the cytoplasm), JNK may cause Bim-dependent apoptosis by a mechanism that does not require Bim phosphorylation. In contrast, in other cell types (e.g., fibroblasts), BimEL or BimL is expressed in a latent form bound to dynein motor complexes, and increased Bim expression may not be required for apoptosis if JNK is able to activate the latent Bim proteins by phosphorylation. It is likely that these two scenarios represent the two extreme forms of the different requirements for JNK-dependent Bim expression and phosphorylation in various cell types within the body.
The Repertoire of Bim Isoform Expression May Contribute to the Regulation of Apoptotic Responses.
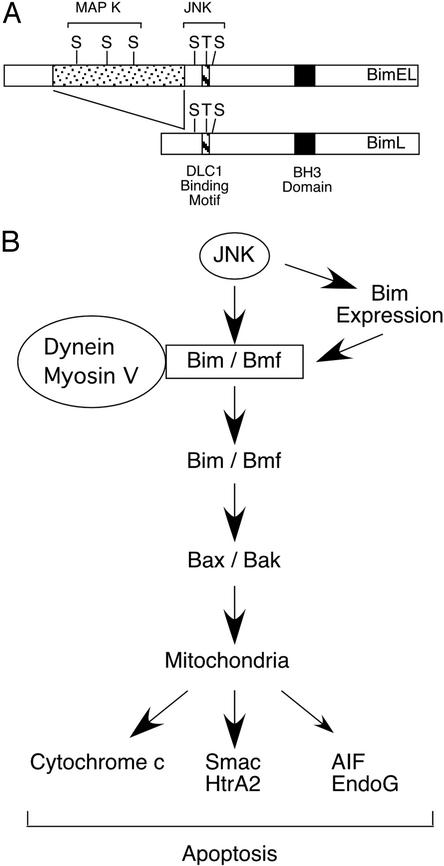
The three major Bim isoforms are differentially phosphorylated by MAPK. For example, BimS lacks the DLC1 binding motif that is phosphorylated by JNK, but both BimL and BimEL are phosphorylated by JNK. However, BimEL contains an NH2-terminal insertion that contains three additional MAPK phosphorylation sites that are phosphorylated by ERKs (Fig. 5). These differences may cause alterations in the apoptotic response to JNK activation. Recently, six additional alternatively spliced Bim isoforms have been described (35–37). The function of these isoforms has not been established, but two of the isoforms contain the BH3 domain and can cause apoptosis in transfection assays. Bim may act by binding to antiapoptotic members of the Bcl2 family (e.g., Bcl2 and Bcl-Xl) (23), but Bim may also directly bind and activate Bax and Bak (37, 38). These data indicate that the role of Bim as an effector of the JNK signaling pathway may be altered by the repertoire of Bim isoform expression in individual cells. Furthermore, because Bim isoforms differ in phosphorylation, the role of phosphorylation may be different in different cell types. For example, the MAPK phosphorylation sites located in the NH2-terminal insertion of BimEL may also contribute to functional regulation and apoptotic responses. It is also possible that other protein kinases may contribute to the regulation of Bim phosphorylation on apoptotic regulatory sites. Indeed, the p38 MAPK can also phosphorylate Bim (unpublished observation).
Figure 5.
A JNK-dependent apoptosis signaling pathway. (A) Schematic illustration of MAPK phosphorylation of BimL and BimEL. Two groups of MAPK sites were identified. Three Ser-Pro sites are phosphorylated by the ERK group of MAPK within the BimEL-specific insert region. In addition, three JNK phosphorylation sites are located within the common region of BimL and BimEL that is absent from BimS. Phosphorylation of Bim on Thr-56 causes dissociation from DLC1. (B) Schematic illustration of a JNK-dependent apoptosis signaling pathway. This signaling pathway is initiated by the phosphorylation of Bim and Bmf by JNK. In some cells, JNK may also contribute to Bim expression. The phosphorylation by JNK is proposed to cause the release of Bim and Bmf from dynein and myosin V motor complexes. The activated Bim and Bmf may directly activate Bax and Bak or may indirectly activate Bax and Bak by binding antiapoptotic Bcl2 family proteins (e.g., Bcl2 and Bcl-Xl). These mechanisms can lead to the engagement of the Bax/Bak-dependent mitochondrial pathway of apoptosis. Smac, second mitochondria-derived activator of caspase; HtrA2, second homology of the bacterial HtrA endoprotease; AIF, apoptosis-inducing factor; and EndoG, endonuclease G.
Conclusions
The identification of BH3-only proteins as targets of the JNK signaling pathway provides a molecular link between JNK and the engagement of the mitochondrial cell death pathway in cells exposed to UV radiation. It is known that UV radiation causes the release of Bim and Bmf from dynein and myosin V motor complexes (21–23, 30) and that these proteins cause Bax/Bak-dependent apoptosis (14, 15, 23, 30). The results of this study demonstrate that JNK can engage this apoptotic pathway by phosphorylation of BH3-only proteins, including Bim and Bmf. This phosphorylation may also account for the apoptotic activity of other protein kinases; for example, Bim and Bmf are substrates of p38 MAPK. There may be additional proapoptotic substrates of JNK that contribute to cell death, for example Mcl-1 (39) and Bad (40). Nevertheless, this study establishes the molecular framework for a proapoptotic signal transduction pathway that can be used by JNK to cause cell death (Fig. 5).
Acknowledgments
We thank Kathy Gemme for expert administrative assistance. This study was supported, in part, by a grant from the National Cancer Institute. R.J.D. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- JNK
c-Jun NH2-terminal kinase
- DLC
dynein light chain
- MAPK
mitogen-activated protein kinase
References
- 1.Strasser A, O'Connor L, Dixit V M. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Vousden K H. Biochim Biophys Acta. 2002;1602:47–59. doi: 10.1016/s0304-419x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 3.Davis R J. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 4.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 5.Dickens M, Rogers J S, Cavanagh J, Raitano A, Xia Z, Halpern J R, Greenberg M E, Sawyers C L, Davis R J. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 6.Eilers A, Whitfield J, Babij C, Rubin L L, Ham J. J Neurosci. 1998;18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park D S, Stefanis L, Yan C Y, Farinelli S E, Greene L A. J Biol Chem. 1996;271:21898–21905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- 8.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 9.Rincon M, Whitmarsh A, Yang D D, Weiss L, Derijard B, Jayaraj P, Davis R J, Flavell R A. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David J P, Jochum W, Wagner E F, Karin M. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 11.Tournier C, Dong C, Turner T K, Jones S N, Flavell R A, Davis R J. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 13.Lei K, Nimnual A, Zong W X, Kennedy N J, Flavell R A, Thompson C B, Bar-Sagi D, Davis R J. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong W X, Lindsten T, Ross A J, MacGregor G R, Thompson C B. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng E H, Wei M C, Weiler S, Flavell R A, Mak T W, Lindsten T, Korsmeyer S J. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 16.Datta S R, Brunet A, Greenberg M E. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 17.Harris C A, Johnson E M., Jr J Biol Chem. 2001;276:37754–37760. doi: 10.1074/jbc.M104073200. [DOI] [PubMed] [Google Scholar]
- 18.Putcha G V, Moulder K L, Golden J P, Bouillet P, Adams J A, Strasser A, Johnson E M. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 19.Whitfield J, Neame S J, Paquet L, Bernard O, Ham J. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 20.O'Reilly L A, Cullen L, Visvader J, Lindeman G J, Print C, Bath M L, Huang D C, Strasser A. Am J Pathol. 2000;157:449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puthalakath H, Huang D C, O'Reilly L A, King S M, Strasser A. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 22.Puthalakath H, Villunger A, O'Reilly L A, Beaumont J G, Coultas L, Cheney R E, Huang D C, Strasser A. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 23.Puthalakath H, Strasser A. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 24.Rincon M, Flavell R A, Davis R J. Oncogene. 2001;20:2490–2497. doi: 10.1038/sj.onc.1204382. [DOI] [PubMed] [Google Scholar]
- 25.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 27.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 28.Bouillet P, Metcalf D, Huang D C, Tarlinton D M, Kay T W, Kontgen F, Adams J M, Strasser A. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 29.Bouillet P, Purton J F, Godfrey D I, Zhang L C, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams J M, Strasser A. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 30.Bouillet P, Strasser A. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 31.Dijkers P F, Medema R H, Lammers J W, Koenderman L, Coffer P J. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 32.Dijkers P F, Birkenkamp K U, Lam E W, Thomas N S, Lammers J W, Koenderman L, Coffer P J. J Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl M, Dijkers P F, Kops G J, Lens S M, Coffer P J, Burgering B M, Medema R H. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 34.Shinjyo T, Kuribara R, Inukai T, Hosoi H, Kinoshita T, Miyajima A, Houghton P J, Look A T, Ozawa K, Inaba T. Mol Cell Biol. 2001;21:854–864. doi: 10.1128/MCB.21.3.854-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U M, Miyashita T, Shikama Y, Tadokoro K, Yamada M. FEBS Lett. 2001;509:135–141. doi: 10.1016/s0014-5793(01)03145-3. [DOI] [PubMed] [Google Scholar]
- 36.Liu J W, Chandra D, Tang S H, Chopra D, Tang D G. Cancer Res. 2002;62:2976–2981. [PubMed] [Google Scholar]
- 37.Marani M, Tenev T, Hancock D, Downward J, Lemoine N R. Mol Cell Biol. 2002;22:3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Letai A, Bassik M C, Walensky L D, Sorcinelli M D, Weiler S, Korsmeyer S J. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 39.Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, Umezawa A, Ichijo H. J Biol Chem. 2002;277:43730–43734. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 40.Donovan N, Becker E B, Konishi Y, Bonni A. J Biol Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]