Abstract
Despite the use of highly active antiretroviral therapy (HAART), neuronal cell death remains a problem that is frequently found in the brains of HIV-1-infected patients. HAART has successfully prevented many of the former end-stage complications of AIDS, however, with increased survival times, the prevalence of minor HIV-1 associated cognitive impairment appears to be rising among AIDS patients. Further, HIV-1 associated dementia (HAD) is still prevalent in treated patients as well as attenuated forms of HAD and CNS opportunistic disorders. HIV-associated cognitive impairment correlates with the increased presence in the CNS of activated, though not necessarily HIV-1-infected, microglia and CNS macrophages. This suggests that indirect mechanisms of neuronal injury and loss/death occur in HIV/AIDS as a basis for dementia since neurons are not themselves productively infected by HIV-1. In this review, we discussed the symptoms and causes leading to HAD. Outcome from this review will provide new information regarding mechanisms of neuronal loss in AIDS patients.
Definition and causes
Dementia cannot be considered as a disease by itself but it is the term used to describe a set of symptoms resulting from damages and disorders affecting the brain. These symptoms can be caused by a multitude of diseases and depend upon the specific brain regions affected. These symptoms appear as a variety of cognitive, behavioral, affective, motor, and psychiatric disorders. Dementia can be caused by a variety of diseases, known as neurodegenerative diseases resulting from protein aggregation in the brain [1]. These diseases include Alzheimer's, Lewy bodies, Huntington and Parkinson [1]. Infectious diseases affecting the central nervous system (CNS) may lead to dementia. These infections can be caused by different agents such as: abnormal protein in prion diseases (Creutzfeldt-Jakob disease), bacteria in syphilis and borrelia, parasites in toxoplasmosis, cryptococcosis and neurocysticercosis [2], however viral agents are the leading cause of infection related dementia. Among the viruses infecting the brain, human immunodeficiency virus type 1 (HIV-1) is the most common cause of dementia, other CNS viral infection implying herpes simplex virus type I, Varicella zoster virus, cytomegalovirus, Epstein-Barr virus cause encephalitis and severe brain dysfunction. The collection of viral agent infecting the CNS and producing viral encephalitis includes also arboviruses, rabies viruses, polyomaviruses and enteroviruses [3]. Finally, dementia could also be caused by vascular disorders (e.g. multiple-infarct dementia), drug addiction, hydrocephalus, and injury or brain tumors [4,5]. Despite the variability of symptoms with the disease causing dementia there is overlap, potentially because of the involvement of common neural pathways and the nature of the damage. However, the time of appearance, the severity, and type of symptoms allow, in most cases, to help making the distinction between diseases. There are however cases of coexistence of clinical and/or pathological features where more than one disease is manifested in one individual, and which might be due to co-occurrence of common diseases within the individual [6]. In elderly populations, Alzheimer's disease is the most frequent cause of dementia, while neuroAIDS is the major cause of dementia in younger population (less than 60 years old). In the United States, HIV-1 infection is the most common cause of dementia in young adults [7,8]. Since many diseases and viral infection lead to dementia, we focused our review on HIV-1 associated dementia, its symptoms and causes.
Neuropathology of AIDS
HIV-1 is the causative agent of acquired immunodeficiency syndrome (AIDS), which is a multi-system disorder including the CNS. Neurological impairment affects approximately 60% of HIV-infected patients [9]. HIV-1 enters the CNS at the early phase of infection [10], persists in that system for decades and induces multiple symptoms of motor, cognitive dysfunction and behavioral changes. Many factors can contribute to the neuropathology of AIDS, particularly opportunistic brain infections such as cryptococcus, Toxoplasma gondii, JC virus, cytomegalovirus, Epstein-Barr virus, Varicella zoster virus, and human herpes virus type 6 [2]. In the absence of opportunistic infections, major clinical symptoms include impaired short term-memory coupled with reduced ability of mental concentration, leg weakness, slowness of hand movement and gait as well as depression [11,12]. These symptoms are often accompanied by behavioral symptoms such as personality changes, apathy and social withdrawal. The terms AIDS dementia complex (ADC), and HIV-1 associated dementia (HAD), are used to describe these neurological and psychiatric symptoms caused by HIV-1 infection [11,12]. An effective therapy for HIV/AIDS became available in 1995, generally known as highly active antiretroviral therapy (HAART). This therapy consists of a combination of at least three drugs blocking different aspect of viral replication, markedly reverse transcriptase inhibitors and protease inhibitors. HAART has the capability of restoring immune function; suppressing viral replication to nearly undetectable level, consequently ameliorating HIV related symptoms in the CNS and preventing opportunistic conditions. Before the introduction of HAART, nearly 30% of the infected population developed HAD at the late stage of HIV/AIDS. With the use of HAART this rate is reduced to 10% [13]. However, a more subtle form of CNS dysfunction, known as minor cognitive motor disorder (MCMD), has become more common in HIV patients [14]. In this condition, memory loss and the reduction of cognitive and computational functions are much less pronounced. Recently it has been estimated that nearly 30% of adults infected with HIV are affected by MCMD. However, HAD is far from being controlled by HAART; in the setting of HAART the HIV-1 infection become chronic and recent studies show a rise in the incidence of the HAD [3], it is noteworthy that HAART is not designed to target the inflammatory cascade underlying the HAD. In addition some of the HIV-1 infected population develop resistance against HAART and an important fraction of AIDS patients, especially in developing countries, have not access to HAART. In the United States, HIV-1 infection is the most common cause of dementia in young adults [7,8].
The HIV-1 associated neuropathology is characterized by the infiltration of macrophages into the CNS; the formation of microglial nodules; and multinucleated giant cells which result possibly from virus-induced fusion of microglia and/or macrophages in central white and deep gray matter; astrocyte activation and damage; neuronal loss particularly in hippocampus, basal ganglia and caudate nucleus. In addition, a variable degree of white matter pathology with evidence of broad range of myelin damage ranging from pallor to widespread breakdown and loss leading to accumulation of lipid macrophages in extreme cases, with axonal damage in the latter cases, and the presence of HIV-1 in the cerebral spinal fluid (CSF) has been reported [13,15]. These neuropathological consequences of infection are collectively termed HIV-1 associated encephalitis (HIVE).
Clinical observations, using MRI, confirm that HIV infection is associated with progressive cortical atrophy within the gray and white matter in the brain, particularly in the later stage of the disease [16-19]. These studies report a correlation between the deterioration of cognitive function and the reduction in volume of certain brain structures including the basal ganglia and caudate nucleus. Volumetric MRI analysis has shown that cortical atrophy associated with HIV infection might be caused by neuronal loss and demyelination. The degree of atrophy is correlated to the degree of cognitive motor dysfunction in both cross-sectional and longitudinal cohorts [16,19,20]. Quantitative MRI shows a correlation between cerebral atrophy and neuropsychological performance. Over time, the correlation persists between an increase in atrophy and worsening in certain cognitive functions [16].
Viral entry and replication
HIV-1 targets the lymphoid and nervous systems by infecting cells containing major HIV-1 receptors, CD4 and CD8, and various chemokine receptors considered as HIV-1 co-receptors. These receptors help the attachment of the virus to the cell and the fusion of their membrane resulting in the entry of the virus into the cell [21]. HIV-1-specific CD4+ helper T lymphocytes and CD8+ cytotoxic T lymphocytes have been detected within 4-6 weeks after HIV-1 inoculation [22]. Infected CD4+ T cells and monocytes, which circulate in the blood, are the potential source of CNS infection [14]. The mechanisms of entry of these cells into the CNS are discussed in the next section. Among the chemokine receptors expressed on human cells, CXCR4 appears to be the most important for HIV-1 entry into lymphocytes and CCR5 for monocytes, macrophages and microglia [23]. Because of the variability of HIV-1 phenotypes, these strains of virus are defined by their usage of the CCR5 or CXCR4 co-receptors, and designated as R5- and X4- viruses respectively [23]. Following entry into the cell, the virus undergoes reverse transcription of its RNA genome to form a double-stranded DNA, a pre-integration complex of viral DNA with integrase and other viral protein including Vpr and matrix protein is transported to the nucleus. The pre-integration complex facilitates the integration of the HIV-1 DNA genome into host chromatin. The integration of viral DNA into the host cell genome generates the provirus that allows the production of HIV-1. In addition, high levels of viral DNA remain non-integrated in the nucleus and are capable of directing expression of viral transcript [24-26]. The generation of infectious virus particles involves the production of viral transcripts and proteins and viral assembly, release and maturation. During the production phase, first the vial regulatory factors Nef, Tat and Rev are generated, and viral structural proteins and the RNA genome are produced in a later phase. In the assembly phase, Gag and Gag-Pol Polyproteins, envelope proteins and viral RNA genomes are assembled into immature virus particles at the cell membrane and released from the host cell. The cleavage of Gag and Gag-Pol Polyproteins by the HIV-1 protease results in the production of mature virus [27-29].
The intracellular environment plays a major role in HIV-1 virus replication [30]. HIV-1 infected cells are classified as highly active producers and low or non-producers of viruses, known as "productive" and "restricted" infection, respectively. Both types of infections occur in the CNS. Productively infected cells support productive viral replication and participate in the transmission of the infection and the rapid evolution of viral genome in the human host and die ultimately. Restricted infection is only detectable by highly sensitive methods showing the presence of HIV-1 DNA or RNA. However, in the absence of structural viral protein expression, it has been reported that accessory/regulatory protein such as Rev and Nef have been expressed [31,32]. Restrictedly infected cells are permissive to infection by HIV-1 strains but are refractory to efficient virus expression, they restrict the HIV-1 replication and survive as virus reservoir in which replication-competent viral genome persists. The restricted infection implies that efficient HIV-1 replication might be blocked at different stage of virus life cycle, including virus entry, reverse transcription, nucleo-cytoplasmic HIV-1 RNA transport, translation of viral DNA, and maturation of progeny virion. Studies of different astrocytes cell lines, which are known to be non-productively infected, demonstrated a cytoplasmic presence of Rev up to seven time more elevated than in productively infected cells [33,34]. These observations lead to the hypothesis that restricted HIV-1 production in astrocyte may be partly due to a cell determined block in nucleo-cytoplasmic Rev shuttling causing the nuclear retention of Rev-dependent HIV-1 mRNA classes where they are degraded [35,32]. Changes in cell environment, like the elevation in the level of cytokines such as TNF-α and IL-1β, might reactivate virus production [10,36].
Neuroinvasion of HIV-1
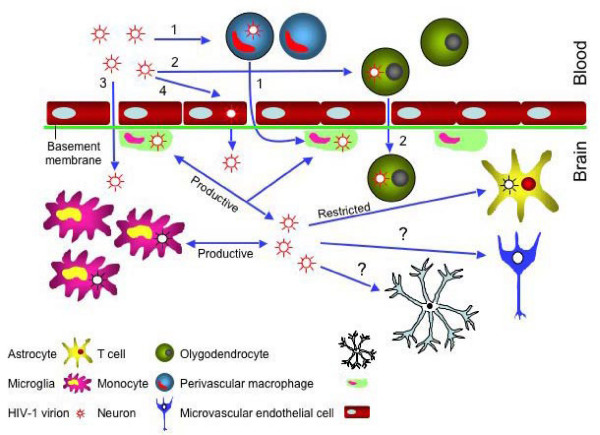
The role of blood-brain barrier (BBB), which is a continuous cellular layer of tightly linked brain microvascular endothelial cells, is to separate the CNS from the periphery (Figure 1). The BBB is selectively permeable and regulates the trafficking of cells and substances between the brain parenchyma and the bloodstream [14,15]. The CSF is also separated from the periphery by the blood-CSF barrier of the choroids-plexus epithelium. In order to enter the brain, HIV-1 must cross the BBB using mechanisms that remain unclear. Numerous studies have used animal models and in vitro experimentation to understand the mechanisms of HIV-1 introduction into the CNS through BBB [14]. The generally accepted model, with most compelling evidence, is the "Trojan Horse hypothesis" [37,38]. According to this model, HIV-1 and other lentiviruses enter the CNS as a passenger in cells trafficking to the brain (Figure 1). Many CD4+ cells, such as T cells and monocytes are infected by HIV-1, these cells circulate in the blood and can cross the BBB and propagate the infection within the CNS [37]. This model was confirmed by in situ hybridization and immunohistochemical analysis that brought evidence of virus accumulation in perivascular regions [39-41]. Though BBB abnormalities due to HIV-1 infection have been observed, however, the mechanisms of endothelial cells infection and the expression of conventional HIV receptors in these cells remain a controversial issue. Although some studies suggest that human brain microvascular endothelial cells lack CD4 receptors [42], other studies have found that CD4 was expressed in isolated endothelial cells and microvessels of HIV-1 infected children's brains [43,44], moreover the expression of HIV-1 co-receptors such as CCR5 and CXCR4 have also being reported on isolated primary human brain's microvascular endothelial cells [45]. An alternative hypothesis of HIV-1 neuro-invasion proposes the entry of free HIV-1 by migration between or, transcytosis of endothelial cells [10,14,46,47]. Theoretically all the main cell types of the CNS, astrocytes, oligodendrocytes, neurons, perivascular macrophage and microglia, can be infected by HIV-1 since they possess the receptors and/or co-receptors for HIV-1 entry, but only the latter two are the most commonly infected cells by HIV-1 [14].
Figure 1.
HIV-1 neuroinvasion. 1) According to the "Trojan Horse hypothesis" entry of HIV-1 into the brain takes place by the migration of infected monocytes which differentiate into perivascular macrophage. 2) The passage of infected CD4+ T cells can be another source of infection in the brain. Other probable causes of CNS infection might be: 3) the direct entrance of the virus or 4) entrance of HIV-1 by transcytosis of brain microvascular endothelial cells. Once the virus is in the brain it infects productively macrophages and microglia. Astrocyte infection is known to be restricted. The infection of oligodendrocytes and specially neurons is questionable.
Macrophage and microglia
Perivascular macrophage, microglia, and astrocytes are the cells coming into direct contact with infected cells in perivascular region. The two first types of cells are the resident immunocompetent cells of the brain and their major role is to respond to all types of insults. Peripheral macrophage population is replenished through the lifespan with a relatively fast turnover, probably because of its proximity to the interface with the periphery. This replenishment that takes place by the migration of monocytes into the CNS has the side effect of opening the door to the intracellular pathogen. As the monocytes take residency in the CNS they differentiate into macrophages. Microglia and monocyte-derived macrophage are considered to be the main sources of productive HIV-1 infection in the brain [48,49]. One of the characteristics of HIVE is the presence of multinucleated giant cells expressing CD4. These cells are assumed to be infected monocytes differentiated into macrophage after entering the brain or arising from the fusion of infected microglia [50]. It has been shown that in the primate Simian Immunodeficiency Virus (SIV) model the spread of the virus from perivascular cells to the parenchymal microglia does not occur [51], however this issue remains controversial and has not been confirmed for SIV and HIV-1. In contrast, many studies suggest the opposite for HIV-1. Immunostaining has revealed HIV-1 infection of parenchymal microglia, in some cases the infection is widespread, but in other cases it is restricted to the perivascular compartment [52]. It is not clear whether the HIV-1 immunopositive microglia consists of an influx of infected cells from the blood or results from long-term infection in the CNS. In-vitro studies have demonstrated that HIV-1 replication takes place in primary microglia isolated from adults [53,54], infants [55], and fetal brain [56,57]. HIV-1 infection in Microglia can be associated with cytopathology, including the formation of syncytia [54]. The study of the course of HIV-1 infection in purified primary cultures of human microglia shows that productive infection was more readily established by R5-tropic strains of HIV-1 than by an X4-tropic strain [55]. Microglial cells similar to macrophage express, CD4/CCR5, major receptors/co-receptors used by HIV-1 [58-60]. Other chemokine receptors, e.g. CCR3, CCR2b, CCR8, CXCR6, and CX3CR1, are also expressed by these cells but less efficiently used by HIV-1 [60,61]. In vitro studies have shown that long-lived mixed microglial cultures isolated from human brain, when infected with R5 HIV-1, retain replication competent viruses for up to 2.5 months with low level virus replication, providing an activating condition can result in productive virus replication [62].
Astrocytes
Astrocytes do not have the CD4 receptor, which plays an important role in the infection of immune system cells, but they express CXCR4 and possibly other HIV-1 co-receptors including CCR5 [32]. However, several studies have reported the infection of astrocytes by HIV-1 although the mechanisms of viral attachment to astrocytes remain unclear. Immunopositivity of astrocytes for HIV-1 structural proteins has occasionally been reported [35]. However, in situ hybridization, or in situ PCR have revealed the presence of HIV-1-specific nucleic acids in astrocytes [40,63,64]. Other studies reported the presence of the viral DNA and HIV-1 Nef protein in astrocytes [65].
HIV-1 infection was studied using primary human fetal astrocytes and tumor derived cell lines, several HIV-1 isolates, namely X4-using T-cell line adapted (NL4-3, 1 MB, SF2), R5-using, macrophage tropic (JR-FL, SF162) strains and primary isolates from blood [32,66,67]. The participation of astrocytes in productive infection has not been reported, though virus production in persistently infected cells can be transiently activated by the treatment with inflammatory cytokines [32,66,67].
Oligodendrocytes
In vivo, Oligodendrocytes infection by HIV-1 remains controversial. While some studies have detected viral nucleic acids by in situ PCR [63,64], other studies have reported the absence of HIV-1 markers in oligodendrocytes [33]. In vitro studies, using human oligodendrocytes indicates restricted infection by R5 and X4 strains of the virus [68]. Some studies have reported a reduced expression of specific oligodendrocyte markers, such as MBP and CNPase, in mice expressing HIV-1 Nef [69]. Oligodendrocytes do not possess CD4 receptors and the mechanisms of their potential infection remain unclear.
Neurons
Most studies have indicated an absence of in vivo infection in neurons, however a few studies have reported the presence of HIV-1 DNA and proteins in neurons [63,64]. It has been suggested that the detection of infected neurons in the brain might be complicated by the loss of the infected neuronal populations [14]. In vitro studies have reported restricted infection of primary neurons [70], and neuronal cell lines by X5 and R4 viruses [71,72].
Mechanisms of neurodegeneration in HIV-associated dementia
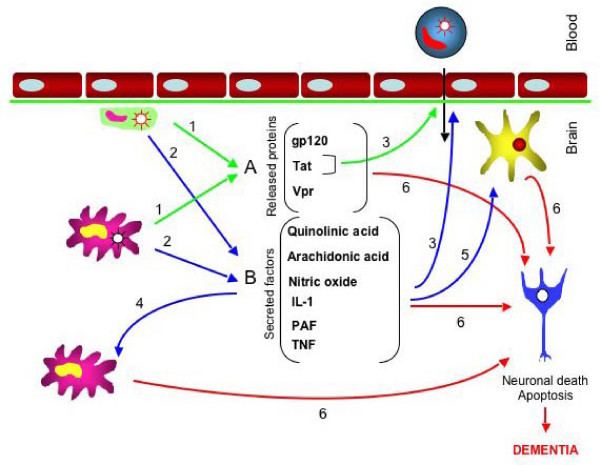
The absence of significant neuronal infection by HIV-1 contrasts with the extensive neuropathological damage observed in HAD, therefore different mechanisms involving the HIV-1 infection of perivascular macrophages, microglia, and possibly astrocytes might play the principal role in neuronal injury and the disruption of normal neurological function. The neuronal injury can result from a direct mechanism by interaction with viral proteins, such as gp120, Tat (Transcriptional transactivator) and Vpr (viral protein R) produced by infected cells, or by an indirect effect resulting from the inflammatory process involving activated monocytes, macrophages and astrocytes (Figure 2).
Figure 2.
Mechanism of neuropathogenesis. Two components of this mechanism are: A) the direct effect of the HIV-1 infection, including HIV-1 proteins and B) the indirect consequence of infection comprising the secretion of cytokines and neurotoxins. The infected macrophages and microglia participate actively in the neurodegeneration by: 1) shedding viral proteins and 2) releasing significant amount of cytokines and neurotoxins into the CNS. 3) Tat and TNF-α contribute to the disruption of the blood brain barrier, which in turn become more permeable to infected monocytes and cytokines present in the periphery. The secreted pro-inflammatory cytokines activates 4) microglia and 5) astrocytes which in turn secrete neurotoxins, moreover the alteration of astrocytes function results in an increase in the level of neurotoxicity in the brain. 6) Multifactorial neuronal injury: neurotoxins released from several sources, as the direct and indirect consequences of HIV-1 infection, lead to neuronal injury.
HIV-1 Tat
The viral protein Tat, which is mainly active in the nucleus, was shown to be secreted at high-level in vitro. Secreted Tat can cause direct or indirect injury to neurons, therefore it has been suggested that Tat contributes to HAD neuropathogenesis [73]. The neurotoxicity of Tat involves prolonged increase in intracellular calcium followed by an increase of reactive oxygen species and caspase activation of apoptotic pathway [73,74], in addition it has been shown that the up-regulation of caspase-8 by HIV-1 Tat expression in CD4 T cell lines may contribute to the increased apoptosis and sensitivity to apoptotic signals [75]. Tat is shown to alter the expression distribution of tight junction proteins, claudin-1 and claudin-5 in cerebral microvascular endothelial cells [76]. By affecting endothelial permeability, Tat contributes to the disruption of the BBB that leads to infiltration of inflammatory cells into the CNS [76,77]. Further, Tat participates in the HAD associated inflammatory cascade by promoting TNF-α and interleukin IL-1 production by monocytes and macrophages, and stimulates the production of several cytokines and chemokines, including IL-8, RANTES, MCP-1 and TNF-α in astrocytes, which leads to neurotoxicity [73].
HIV-1 Vpr
The regulatory protein Vpr might also be a player in the direct mechanism of neuronal damage (reviewed in [78]). Vpr has been found in the CSF of HAD patients [79]. Vpr induces cell cycle arrest at G2/M phase, which leads to cell death [80], a recent model of Vpr mediated induction of apoptosis, in CD4+ cells, proposes that Vpr expression activates cancer-associated protein BRCA1 and up-regulates the expression of DNA damage-45 protein α (GADD45α) [81]. It has been reported that Vpr also alters mitochondrial permeability, which can cause cytochrome c release and eventually lead to apoptosis [82], however this issue needs to be confirmed. Furthermore, another study of Vpr-mitochondria interaction has shown that Vpr targets HAX-1, an antiapoptotic mitochondrial protein, Vpr associates physically to that protein and Vpr over-expression leads to dislocation of HAX-1 from its normal mitochondrial residence and causes mitochondrial instability and apoptosis [83]. Recent studies have demonstrated that both intracellular and extra cellular Vpr can induce apoptosis of human neuronal-precursor cells and mature, differentiated neurons by increasing the activation of caspase-8 [84]. Finally, Tat and Vpr mediated-apoptosis could increase significantly by co-exposure of cells to ethanol [84,85].
HIV-1 gp120
HIV-1 envelope glycoprotein gp160 is shown to have neurotoxic effect. This protein can be cleaved into two products that remain non-covalently associated: gp120 and gp41. The soluble viral envelope protein gp120, which is released in large quantities by HIV infected cells, might be involved in neuronal injury. The toxic effect of gp120 on neuronal population was demonstrated by many studies [86,87], dopaminergic neurons might be more susceptible to gp120 neurotoxicity [88]. It has been shown that transgenic mice overexpressing gp120 had neuropathological features similar to abnormalities in brains of HAD patients [89]. Neurodegeneration induced by gp120 can be direct through interaction with NMDA (N-Methyl-D-Aspartate) receptor or indirect by interaction with chemokine receptors [90,91]. Further, it has been shown that the presence of p53 is essential for gp120-induced neuronal apoptosis [92]. Furthermore, both gp120 and Tat have been shown to disrupt neuronal calcium homeostasis by perturbing calcium-regulating systems in the plasma membrane and endoplasmic reticulum, which leads to neuronal death [93]. Recently, it has been described that SDF-1α and gp120 induced a similar level of neuronal apoptosis, but by activating different intracellular pathways. SDF-1α enhanced NMDA activity indirectly via Src phosphorylation, whereas gp120 probably activated the NMDA receptor directly and phosphorylated JNK [94]. These results are in accord with other studies, where gp120 was shown to induce neuronal dysfunction and death through actions at p38 mitogen-activated protein kinase, while Tat kills neurons through actions that are independent of p38 or c-jun-N-terminal kinase mitogen-activated protein kinase, or through the concurrent activation of multiple pro-apoptotic pathways [95].
Some chemokine receptors are considered to act as a direct conduit for gp120 neurotoxicity, whereas others can have neuro-protective effects [49,87]. The role of CXCR4 in the gp120 mediated neurotoxicity can be direct, through the activation of neuronal receptors by gp120, or indirect through the stimulation of glial cells leading to release of neurotoxic factors. Several studies have shown that T tropic (X4) and dual tropic (X4/R5) gp120 induce apoptosis in primary neurons and in neuronal cell lines [96,97]. In contrast to the neuroprotective role of RANTES/CCL5 and MIP-1β against gp120, in mixed neurons/glial cultures, it has been shown that SDF-1α/CXCL2 not only failed to provide neuro-protection from gp120, but induced apoptosis in its absence [49]. Beside its direct neurotoxic effect, the viral protein gp120 has a significant role in the indirect mechanisms of neurodegenertion by acting on macrophages, microglia or astrocytes [87,96]. Gp120 interaction with astrocytes stimulates the inducible form of nitric oxide synthase and increases the release of arachidonic acid from astrocytes, which leads to the inhibition of glutamate uptake by astrocytes and neurons [98]. As a result the extracellular concentration of glutamate increases and could lead to neurotoxicity via activation of excitatory amino acid receptors on neurons [73]. By acting on monocytes and macrophages gp120 induces the production of TNF-α, IL-1 and arachidonic acid metabolites which are implicated in HIV-1 neuropathogenesis.
HIV-1 associated chemokines
The chemokines and their receptors are considered to be involved in the pathogenesis of a number of neurological diseases including HAD, multiple sclerosis, Alzheimer's disease, and prion infection. The over-expression of some chemokines in specific brain areas might contribute to the pathological condition. The chemokines and their receptors are the gate of entrance of HIV into the CNS [99]. Because of the alterations and abnormalities in the expression of chemokines and their receptors in the HIV infected CNS cells, and the role of chemokines in several neurodegenerative diseases, they have been the focus of attention in studies of HAD pathogenesis [100]. All members of the CXCR family are expressed, mainly by neurons, in the brains of individuals affected by HAD [101]. Semiquantitative immunohistochemical analysis of the brain of HIV-1 infected individual, investigating the expression of four HIV-1 co-receptors CCR2, CCR3, CCR5 and CXCR4 has shown that the hippocampal neurons were positive for CCR2, CCR3, and CXCR4 [102]. In other regions of the brain, neurons, as well as glial cells were positive for CCR2, CCR3, and CXCR4, whereas only primary microglial cells were positive for CCR5. The areas of highest expression seem to be subcortical regions and the limbic system. The role of limbic system in memory and other cognitive functions, and the presence of CXCR4 on a subpopulation of neuron from this system might explain cognitive and memory dysfunction in HAD. The presence of chemokines and chemokine receptors increases in the brain tissues of HIVE patients, particularly in areas of neuroglial reaction, where they might be involved in the recruitment of inflammatory infiltrates and formation of microglial nodules. The levels of expression of CCR1, CCR3, CCR5 and CXCR4 are especially elevated in the microglial nodules [59,103]. Moreover, CCR3 and CXCR4 are highly expressed in the pyramidal neurons of hippocampus, and in the enthorinal cortex for CCR3. Compared to AIDS patients without HAD, the brain tissue of patients with HAD shows an over-expression of CX3C chemokine, fractalkine/CX3CL1 [104,105]. The upregulation of fractalkine/CX3CL1 was found in neurons in brains of pediatric patients [104]. In contrast, fractalkine/CX3CL1 was found to be over-expressed in astrocytes in adult patients [105]. The level of chemokines in the CSF of HIV-infected patients with and without HAD has been determined in several studies. The results show that CSF chemokine concentration of MCP-1/CCL2, MIP-1α/CCL3, MIP-1β/CCL4, RANTES/CCL5, IL-8/CXCL8 and fractalkine/CX3CL1 is positively correlated with the severity of dementia and the viral load, indicating HIV induced brain damage. The role of CCR5, which is expressed by neurons, microglia and astrocytes in the brain, seems more controversial in the pathogenesis of HAD. The activation of CCR5 by RANTES or MIP-1α/β, in in-vitro studies, is shown to offer neuro-protection against gp120 induced apoptosis [87,106]. However, in vitro observations indicate that neuro-virulent strains of HIV are essentially M-tropic with increased affinity for CCR5 [107]. It has also been shown that CCR5 activation via its specific ligand induced apoptosis in neuroblastoma but not in fibroblast cell lines [108]. Therefore, it can be assumed that CCR5 might act as a death receptor in neurons and participate in HIV-1 induced neuropathology.
In brief, cognitive, motor decline and behavioral disorders in HAD can be explained by significant neuronal cell death that has been reported as a consequence of HIV-1 infection in the brain [109,110]. However, very few trace of infection has been found in neurons of HAD patients' brains. Therefore the neuronal loss might be caused by the release of neurotoxic factors by HIV infected microglia and astrocytes and/or by neurotoxic HIV-1 proteins.
The inflammatory cascade
The indirect mechanisms of AIDS neuropathogenesis also include the effect of the inflammation resulting from the modification of extracellular secretory functions of microglia and brain macrophages and inflammatory cytokine production in the CNS (Figure 2). Following entry to the brain, monocytes, lymphocytes, activated macrophage, microglia and astrocytes release cytokines, reactive oxygen species, and other neurotoxins that disrupt normal cellular functioning, modify neurotransmitter action, and may lead to leukoencephalopathy and ultimately neuronal apoptosis [111,112]. Some of these neurotoxins include TNF-α, arachidonic acid, platelet activating factors (PAF), nitric oxide (NO), and quinolinic acid (QUIN). NO is synthesized by endothelial cells, macrophages and neurons and might be associated with the NMDA type glutamate associated neurotoxicity. A high level of inducible NO synthase has been found in the brain of HAD patients [113]. In HIV-1 patients who also are/were drug addicted (e.g. cocaine, heroine), a 40-fold increase in expression of NO synthase in neurons of temporal lobes was reported [114]. TNF-α is released by HIV-1 infected macrophage microglia and particularly affects oligodendrocytes [115]. It has been shown that TNF-α mRNA level in the subcortical regions of HAD patients' CNS are higher than in AIDS patients without neurological symptoms [116]. In addition, TNF-α can damage the BBB, as shown in an in-vivo model, which could facilitate entry into the brain of HIV-1 protein(s) and cytokines secreted in the periphery [117]. Not only the level of pro-inflammatory cytokines, such as TNF-α, IL-1 and IFN-γ, anti-inflammatory cytokines including TGF-β and IL-6, and soluble cytokine receptors is elevated in AIDS patients, but the cytokine production is correlated with the gravity of the neuropathology [118,119].
This review is a summary of some of the current data supporting both the direct and indirect mechanisms by which neuronal death may occur during infection with HIV-1. HAD is a complex phenomenon, which could be the result of several mechanisms caused by players using different pathways. Some of these players, mechanisms, and pathways were mentioned in this review and some of them are either un-identified or left out e.g. MCP-1, cellular proteins involved in the regulation of HIV-1 gene expression, Ca++ induction, HIV-1 activated apoptotic programs (reviewed in [120]). Finally, more strategies are needed for treating or preventing HAD by targeting specific neurotoxic mechanisms used by the above-mentioned viral proteins.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
MG wrote the manuscript, SA and KK shared ideas and discussion, BES conceived of the plan for the manuscript and coordinated its preparation. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank past and present members of the Center for Neurovirology for their insightful discussions and sharing of ideas.
This review was made possible by Grants awarded by NIH to B.E.S.
Contributor Information
Mohammad Ghafouri, Email: ghafouri@temple.edu.
Shohreh Amini, Email: shohreh.amini@temple.edu.
Kamel Khalili, Email: kamel.khalili@temple.edu.
Bassel E Sawaya, Email: sawaya@temple.edu.
References
- Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager NT. Dementia associated with infectious diseases. Int Psychogeriatr. 2005;17:S65–S77. doi: 10.1017/S104161020500195X. [DOI] [PubMed] [Google Scholar]
- Wang T, Rumbaugh JA, Nath A. Viruses and the brain from inflammation to dementia. Clin Sci (Lond) 2006;110:393–407. doi: 10.1042/CS20050278. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R. Dementia after traumatic brain injury. Int Psychogeriatr. 2005;17:S93–S107. doi: 10.1017/S1041610205001973. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Lautenschlager NT, Tait RT, Almeida OP. Dementia associated with alcohol and other drug use. Int Psychogeriatr. 2005;17:S109–S127. doi: 10.1017/S1041610205001985. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Lantos PL, Cairns NJ. Overlap between neurodegenerative disorders. Neuropathology. 2005;25:111–124. doi: 10.1111/j.1440-1789.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- Janssen RS. Epidemiology of human immunodeficiency virus infection and the neurologic complications of the infection. Semin Neurol. 1992;12:10–17. doi: 10.1055/s-2008-1041152. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–150. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7:1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42:1472–1476. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8:410–424. doi: 10.1017/S1355617702813212. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Major EO. HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 2002;4:301–308. doi: 10.1016/S1286-4579(02)01542-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI, Nottet HS. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Hall M, Whaley R, Robertson K, Hamby S, Wilkins J, Hall C. The correlation between neuropsychological and neuroanatomic changes over time in asymptomatic and symptomatic HIV-1-infected individuals. Neurology. 1996;46:1697–1702. doi: 10.1212/wnl.46.6.1697. [DOI] [PubMed] [Google Scholar]
- Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, Kumar AJ, Mellits ED, McArthur JC. Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology. 1992;42:2125–2130. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Tucker KA, Robertson KR, Lin W, Smith JK, An H, Chen Y, Aylward SR, Hall CD. Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol. 2004;157:153–162. doi: 10.1016/j.jneuroim.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Zaitseva M, Peden K, Golding H. HIV coreceptors: role of structure, posttranslational modifications, and internalization in viral-cell fusion and as targets for entry inhibitors. Biochim Biophys Acta. 2003;1614:51–61. doi: 10.1016/S0005-2736(03)00162-7. [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Yu XG, Le Gall S, Altfeld M. Immunodominance of HIV-1-specific CD8(+) T-cell responses in acute HIV-1 infection: at the crossroads of viral and host genetics. Trends Immunol. 2005;26:166–171. doi: 10.1016/j.it.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors-central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- Wu Y, Marsh JW. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science. 2001;293:1503–1506. doi: 10.1126/science.1061548. [DOI] [PubMed] [Google Scholar]
- Wu Y, Marsh JW. Gene transcription in HIV infection. Microbes Infect. 2003;5:1023–1027. doi: 10.1016/S1286-4579(03)00187-4. [DOI] [PubMed] [Google Scholar]
- Kilzer JM, Stracker T, Beitzel B, Meek K, Weitzman M, Bushman FD. Roles of host cell factors in circularization of retroviral DNA. Virology. 2003;314:460–467. doi: 10.1016/S0042-6822(03)00455-0. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya AG. HIV-1 assembly and maturation. Arch Virol. 2004;149:1067–1082. doi: 10.1007/s00705-003-0281-8. [DOI] [PubMed] [Google Scholar]
- Nielsen MH, Pedersen FS, Kjems J. Molecular strategies to inhibit HIV-1 replication. Retrovirology. 2005;2:10–15. doi: 10.1186/1742-4690-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelamgari A, Maddukuri A, Berro R, de la Fuente C, Kehn K, Deng L, Dadgar S, Bottazzi ME, Ghedin E, Pumfery A, Kashanchi F. Role of viral regulatory and accessory proteins in HIV-1 replication. Front Biosci. 2004;9:2388–2413. doi: 10.2741/1403. [DOI] [PubMed] [Google Scholar]
- Trkola A. HIV-host interactions: vital to the virus and key to its inhibition. Curr Opin Microbiol. 2004;7:555–559. doi: 10.1016/j.mib.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Neumann M, Afonina E, Ceccherini-Silberstein F, Schlicht S, Erfle V, Pavlakis GN, Brack-Werner R. Nucleocytoplasmic transport in human astrocytes: decreased nuclear uptake of the HIV Rev shuttle protein. J Cell Sci. 2001;114:1717–1729. doi: 10.1242/jcs.114.9.1717. [DOI] [PubMed] [Google Scholar]
- Ludwig E, Silberstein FC, van Empel J, Erfle V, Neumann M, Brack-Werner R. Diminished rev-mediated stimulation of human immunodeficiency virus type 1 protein synthesis is a hallmark of human astrocytes. J Virol. 1999;73:8279–8289. doi: 10.1128/jvi.73.10.8279-8289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neurovirol. 1998;4:377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- Haase AT. Pathogenesis of lentivirus infections. Nature. 1986;322:130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, Rappaport J. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol. 2004;164:2089–2099. doi: 10.1016/S0002-9440(10)63767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Cash KS. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–666. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- Stins MF, Shen Y, Huang SH, Gilles F, Kalra VK, Kim KS. Gp120 activates children's brain endothelial cells via CD4. J Neurovirol. 2001;7:125–134. doi: 10.1080/13550280152058780. [DOI] [PubMed] [Google Scholar]
- Stins MF, Pearce D, Di Cello F, Erdreich-Epstein A, Pardo CA, Sik Kim K. Induction of intercellular adhesion molecule-1 on human brain endothelial cells by HIV-1 gp120: role of CD4 and chemokine coreceptors. Lab Invest. 2003;83:1787–1798. doi: 10.1097/01.LAB.0000107008.13321.C8. [DOI] [PubMed] [Google Scholar]
- Mukhtar M, Harley S, Chen P, BouHamdan M, Patel C, Acheampong E, Pomerantz RJ. Primary isolated human brain microvascular endothelial cells express diverse HIV/SIV-associated chemokine coreceptors and DC-SIGN and L-SIGN. Virology. 2002;297:78–88. doi: 10.1006/viro.2002.1376. [DOI] [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Banks WA, Freed EO, Wolf KM, Robinson SM, Franko M, Kumar VB. Transport of human immunodeficiency virus type 1 pseudoviruses across the blood-brain barrier: role of envelope proteins and adsorptive endocytosis. J Virol. 2001;75:4681–4691. doi: 10.1128/JVI.75.10.4681-4691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31:S43–S54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Multinucleated giant cells in acquired immunodeficiency syndrome encephalopathy. Origin from endogenous microglia? Arch Pathol Lab Med. 1986;110:967–968. [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A, Marsden M, Halcrow K, Hughes ES, Brettle RP, Bell JE, Simmonds P. Mosaic structure of the human immunodeficiency virus type 1 genome infecting lymphoid cells and the brain: evidence for frequent in vivo recombination events in the evolution of regional populations. J Virol. 1999;73:8720–8731. doi: 10.1128/jvi.73.10.8720-8731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright AV, Shieh JT, O'Connor MJ, Gonzalez-Scarano F. Characterization of cultured microglia that can be infected by HIV-1. J Neurovirol. 2000;6:S53–S60. [PubMed] [Google Scholar]
- Watkins BA, Dorn HH, Kelly WB, Armstrong RC, Potts BJ, Michaels F, Kufta CV, Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Reichlin S, Skolnik PR. Long-term productive human immunodeficiency virus-1 infection in human infant microglia. Am J Pathol. 1995;147:1200–1206. [PMC free article] [PubMed] [Google Scholar]
- McCarthy M, He J, Wood C. HIV-1 strain-associated variability in infection of primary neuroglia. J Neurovirol. 1998;4:80–89. doi: 10.3109/13550289809113484. [DOI] [PubMed] [Google Scholar]
- Sundar KS, Kamaraju LS, Dingfelder J, McMahon J, Gollapudi S, Wilson WH, Kong LY, Hong JS, Weiss JM, Lee JE. beta-Endorphin enhances the replication of neurotropic human immunodeficiency virus in fetal perivascular microglia. J Neuroimmunol. 1995;61:97–104. doi: 10.1016/0165-5728(95)00089-K. [DOI] [PubMed] [Google Scholar]
- Jordan CA, Watkins BA, Kufta C, Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991;65:736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat AV, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith TW, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O'Connor MJ, Doms RW, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Garcia J, Kolson DL, Gonzalez-Scarano F. Chemokine receptors in the brain: their role in HIV infection and pathogenesis. AIDS. 2002;16:1709–1730. doi: 10.1097/00002030-200209060-00003. [DOI] [PubMed] [Google Scholar]
- Albright AV, Vos RM, Gonzalez-Scarano F. Low-level HIV replication in mixed glial cultures is associated with alterations in the processing of p55(Gag) Virology. 2004;325:328–339. doi: 10.1016/j.virol.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ, Becker J, Burk MW, Margiotta M, Fuhrer J, Steigbigel RT. In situ detection of PCR-amplified HIV-1 nucleic acids in lymph nodes and peripheral blood in patients with asymptomatic HIV-1 infection and advanced-stage AIDS. J Acquir Immune Defic Syndr. 1994;7:916–923. [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco MC, Verani A, Fiore JR, Lusso P, Major E, Chiodi F, Scarlatti G. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology. 1999;264:370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, Fisher PB, Volsky DJ. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10:25–32. doi: 10.1080/13550280490270851. [DOI] [PubMed] [Google Scholar]
- Albright AV, Strizki J, Harouse JM, Lavi E, O'Connor M, Gonzalez-Scarano F. HIV-1 infection of cultured human adult oligodendrocytes. Virology. 1996;217:211–219. doi: 10.1006/viro.1996.0108. [DOI] [PubMed] [Google Scholar]
- Radja F, Kay DG, Albrecht S, Jolicoeur P. Oligodendrocyte-specific expression of human immunodeficiency virus type 1 Nef in transgenic mice leads to vacuolar myelopathy and alters oligodendrocyte phenotype in vitro. J Virol. 2003;77:11745–11753. doi: 10.1128/JVI.77.21.11745-11753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli F, Cafaro A, Fiorelli V, Vannelli B, Ensoli B, Thiele CJ. HIV-1 infection of primary human neuroblasts. Virology. 1995;210:221–225. doi: 10.1006/viro.1995.1336. [DOI] [PubMed] [Google Scholar]
- Obregon E, Punzon C, Fernandez-Cruz E, Fresno M, Munoz-Fernandez MA. HIV-1 infection induces differentiation of immature neural cells through autocrine tumor necrosis factor and nitric oxide production. Virology. 1999;261:193–204. doi: 10.1006/viro.1999.9848. [DOI] [PubMed] [Google Scholar]
- Mizrachi Y, Rodriguez I, Sweetnam PM, Rubinstein A, Volsky DJ. HIV type 1 infection of human cortical neuronal cells: enhancement by select neuronal growth factors. AIDS Res Hum Retroviruses. 1994;10:1593–1596. doi: 10.1089/aid.1994.10.1593. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186:S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol. 2003;9:399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- Bartz SR, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–265. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- Le Rouzic E, Benichou S. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology. 2005;2:11. doi: 10.1186/1742-4690-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DN, Refaeli Y, Weiner DB. The vpr regulatory gene of HIV. Curr Top Microbiol Immunol. 1995;193:209–336. doi: 10.1007/978-3-642-78929-8_11. [DOI] [PubMed] [Google Scholar]
- Stewart SA, Poon B, Song JY, Chen IS. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–3111. doi: 10.1128/JVI.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, Zimmerman ES, DeHart JL, Murala S, Ardon O, Blackett J, Chen J, Planelles V. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 2005;12:326–334. doi: 10.1038/sj.cdd.4401565. [DOI] [PubMed] [Google Scholar]
- Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand JP, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedavalli VS, Shih HM, Chiang YP, Lu CY, Chang LY, Chen MY, Chuang CY, Dayton AI, Jeang KT, Huang LM. Human immunodeficiency virus type 1 Vpr interacts with antiapoptotic mitochondrial protein HAX-1. J Virol. 2005;79:13735–13746. doi: 10.1128/JVI.79.21.13735-13746.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz RJ. Effects of HIV-1 Vpr on neuroinvasion and neuropathogenesis. DNA Cell Biol. 2004;23:227–238. doi: 10.1089/104454904773819815. [DOI] [PubMed] [Google Scholar]
- Acheampong E, Mukhtar M, Parveen Z, Ngoubilly N, Ahmad N, Patel C, Pomerantz RJ. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology. 2002;304:222–234. doi: 10.1006/viro.2002.1666. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BA, Rusyniak DE, Hollingsworth CK. HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res. 1995;705:168–176. doi: 10.1016/0006-8993(95)01166-8. [DOI] [PubMed] [Google Scholar]
- Cioni C, Annunziata P. Circulating gp120 alters the blood-brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci Lett. 2002;330:299–301. doi: 10.1016/S0304-3940(02)00814-5. [DOI] [PubMed] [Google Scholar]
- Barks JD, Liu XH, Sun R, Silverstein FS. gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience. 1997;76:397–409. doi: 10.1016/S0306-4522(96)00373-9. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Strongoli MC, Piccirilli S, Nistico R, Costa A, Bilotta A, Turano P, Finazzi-Agro A, Bagetta G. Apoptosis induced by gp120 in the neocortex of rat involves enhanced expression of cyclooxygenase type 2 and is prevented by NMDA receptor antagonists and by the 21-aminosteroid U-74389G. Biochem Biophys Res Commun. 2000;274:664–669. doi: 10.1006/bbrc.2000.3160. [DOI] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002. pp. S55–S61. [DOI] [PubMed]
- Geeraerts T, Deiva K, M'sika I, Salim H, Hery C, Tardieu M. Effects of SDF-1alpha and gp120(IIIB) on apoptotic pathways in SK-N-SH neuroblastoma cells. Neurosci Lett. 2006 doi: 10.1016/j.neulet.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Singh IN, El-Hage N, Campbell ME, Lutz SE, Knapp PE, Nath A, Hauser KF. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–790. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Brenneman DE, Silverstein FS, Masliah E, Mucke L. gp120 and neurotoxicity in vivo. Trends Pharmacol Sci. 1995;16:122–130. doi: 10.1016/S0165-6147(00)88998-1. [DOI] [PubMed] [Google Scholar]
- Pandey V, Bolsover SR. Immediate and neurotoxic effects of HIV protein gp120 act through CXCR4 receptor. Biochem Biophys Res Commun. 2000;274:212–215. doi: 10.1006/bbrc.2000.3113. [DOI] [PubMed] [Google Scholar]
- Lipton SA. AIDS-related dementia and calcium homeostasis. Ann N Y Acad Sci. 1994;747:205–224. doi: 10.1111/j.1749-6632.1994.tb44411.x. [DOI] [PubMed] [Google Scholar]
- Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8:119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- Dou H, Kingsley JD, Mosley RL, Gelbard HA, Gendelman HE. Neuroprotective strategies for HIV-1 associated dementia. Neurotox Res. 2004;6:503–521. doi: 10.1007/BF03033447. [DOI] [PubMed] [Google Scholar]
- Brandimarti R, Khan MZ, Fatatis A, Meucci O. Regulation of cell cycle proteins by chemokine receptors: A novel pathway in human immunodeficiency virus neuropathogenesis? J Neurovirol. 2004;10:108–112. doi: 10.1080/13550280490268287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. doi: 10.1097/00002030-199809000-00008. [DOI] [PubMed] [Google Scholar]
- Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, Bal H, Kinnear SA, Fine S, Epstein LG, Dairaghi D, Schall TJ, Gendelman HE, Dewhurst S, Sharer LR, Gelbard HA. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000;164:1333–1339. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- Pereira CF, Middel J, Jansen G, Verhoef J, Nottet HS. Enhanced expression of fractalkine in HIV-1 associated dementia. J Neuroimmunol. 2001;115:168–175. doi: 10.1016/S0165-5728(01)00262-4. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, McArthur JC, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson RT, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Effect of postmortem interval on in situ end-labeling of DNA oligonucleosomes. J Neuropathol Exp Neurol. 1995;54:761–765. doi: 10.1097/00005072-199511000-00002. [DOI] [PubMed] [Google Scholar]
- Boven LA, van der Bruggen T, Sweder van Asbeck B, Marx JJ, Nottet HS. Potential role of CCR5 polymorphism in the development of AIDS dementia complex. FEMS Immunol Med Microbiol. 1999;26:243–247. doi: 10.1111/j.1574-695X.1999.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Panek RB, Benveniste EN. Class II MHC gene expression in microglia. Regulation by the cytokines IFN-gamma, TNF-alpha, and TGF-beta. J Immunol. 1995;154:2846–2854. [PubMed] [Google Scholar]
- Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/S0022-510X(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Wilt SG, Milward E, Zhou JM, Nagasato K, Patton H, Rusten R, Griffin DE, O'Connor M, Dubois-Dalcq M. In vitro evidence for a dual role of tumor necrosis factor-alpha in human immunodeficiency virus type 1 encephalopathy. Ann Neurol. 1995;37:381–394. doi: 10.1002/ana.410370315. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74:1–8. doi: 10.1016/S0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Fiala M, Rhodes RH, Shapshak P, Nagano I, Martinez-Maza O, Diagne A, Baldwin G, Graves M. Regulation of HIV-1 infection in astrocytes: expression of Nef, TNF-alpha and IL-6 is enhanced in coculture of astrocytes with macrophages. J Neurovirol. 1996;2:158–166. doi: 10.3109/13550289609146878. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Bradley WG, Shapshak P, Nagano I, Stewart RV, Xin KQ, Srivastava A, Nakamura S. Role of immune activation and cytokine expression in HIV-1-associated neurologic diseases. Adv Neuroimmunol. 1995;5:335–358. doi: 10.1016/0960-5428(95)00012-Q. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Cytokines in the brain during viral infection: clues to HIV-associated dementia. J Clin Invest. 1997;100:2948–2951. doi: 10.1172/JCI119847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. doi: 10.1038/nri1087. [DOI] [PubMed] [Google Scholar]