Abstract
FAP48 was identified and cloned thanks to its interaction with FK506-binding proteins (FKBPs) such as FKBP52 and FKBP12, which belong to the large family of immunophilins that bind the macrolide immunosuppressant drugs FK506 and rapamycin. We have previously shown that FAP48–FKBP complexes are dissociated by FK506 and rapamycin, suggesting that FAP48 is an endogenous ligand of FKBP. The present work describes the biochemical consequences of FAP48 overexpression, induced by the tetracycline analogue doxycycline, in an established cell line derived from Jurkat T cells. We report that overexpression of FAP48 results in the inhibition of cellular proliferation as does the exposure of Jurkat T cells to FK506. We also show that the expression levels of argininosuccinate synthetase and the Myc antagonist Mxi1 are modified by overexpression of FAP48, suggesting that these proteins could be good candidates to mediate the antiproliferative effect of FAP48. FAP48 affects neither the calcineurin-dependent nuclear factor of activated T cells (NFAT)1 nor JNK/p38-dependent pathways that mediate immunosuppression by FK506. However, contrary to FK506, which blocks IL2 synthesis, we observed that FAP48–FKBP complexes increase IL2 production, thus revealing a previously uncharacterized aspect of the immunosuppressive mechanism of FK506.
The pharmacological actions of immunosuppressant drugs such as FK506 and rapamycin are mediated in the cell by intracellular proteins named FK506-binding proteins (FKBPs) belonging to the immunophilin family (reviewed in refs. 1–3).
The main cytoplasmic FKBP isoform is FKBP12, which, in a complex with FK506, binds to and inhibits the phosphatase calcineurin (4–6). Subsequently, dephosphorylation and nuclear translocation of nuclear factor of activated T cells (NFAT)1 can no longer occur, leading to the inhibition of the transcription of the IL2 gene, IL2 being the major growth factor for T cells (reviewed in ref. 7). In addition, a recent report has demonstrated that immunophilin–FK506 complexes block the JNK and p38 mitogen-activated protein kinases (MAPKs) during T cell activation and also inhibit IL2 synthesis via these pathways (8). The authors showed that direct inhibitors of calcineurin activity had no effect on JNK and p38 activation, suggesting that FK506 leads to immunosuppression via calcineurin-dependent as well as calcineurin-independent pathways.
FKBP52 is another member of the FKBP family that was originally found associated with heat shock protein with a molecular mass of 90 kDa (HSP90) in the heterooligomeric forms of steroid receptor complexes (9, 10). FKBP52 displays a modular organization (11–13) and only the NH2-terminal part of the protein is responsible for the immunophilin character of the whole protein; it binds FK506 and rapamycin and displays a peptidylprolyl isomerase function characteristic of the immunophilin family (14). Although this NH2-terminal part shares high structural and functional homologies with FKBP12, it does not inhibit the phosphatase activity of calcineurin, in contrast with FKBP12 (15–17).
We isolated and cloned FAP48 (FKBP-associated protein with a molecular mass of 48 kDa) from Jurkat T cells, as a specific ligand of FKBP52 as well as of FKBP12 (18). FAP48 does not have any homologies with already known proteins except with its splice variant FAP68 (19).
With the biological role of FAP48 as well as the physiological significance of the FAP48–FKBP interaction remaining to be elucidated, we stably overexpressed FAP48 in Jurkat T cells by using the tet-on system (20) and studied the biochemical consequences of FAP48 overexpression with regard to its interaction with FKBP. We show that FAP48 binds endogenous FKBP52 in Jurkat T cells and that, in contrast with FK506, which inhibits the synthesis of IL2, overexpression of FAP48 results in a significant increase of IL2 secretion. Furthermore, we demonstrate that FAP48, like FK506, inhibits the proliferation of Jurkat T cells and we identify two proteins as molecular targets of FAP48. First, argininosuccinate synthetase (ASS), which functions in the urea cycle to produce arginine, is down-regulated (reviewed in ref. 21), and second, Max interactor 1 (Mxi1), an antagonist of the oncogene Myc (reviewed in ref. 22), which is up-regulated. We discuss the potential implication of these two proteins in the biological effects observed on overexpression of FAP48.
Experimental Procedures
Antibodies and Reagents.
The following antibodies were used. The anti-FAP48 polyclonal antibody (pAb) 766 was generated from a peptide localized at the COOH-terminal part of the coding sequence of human FAP48 (amino acids 400–417) and encompassed six amino acids common for the two isoforms FAP48/FAP68. Anti-FKBP52 mAb EC1 and anti-FKBP52 pAB173 were described (12, 23). Anti-6-His mAb was from CLONTECH, anti-FKBP12 pAb was from Affinity Bioreagents (Golden, CO), anti-calcineurin/PP2Bβ was from Upstate Biotechnology (Lake Placid, NY), anti-ACTIVE p38 pAb and anti-ACTIVE JNK pAb were both from Promega, anti-ASS mAb and anti-Mxi1 mAb were both from Becton Dickinson, anti-HSP70 was from StressGen Biotechnologies (Victoria, BC, Canada), and anti-NFAT pAb 67.1 was generously provided by A. Rao (Harvard Medical School, Boston). Doxycycline was from CLONTECH, FK506 was from Fujisawa Pharmaceutical (Osaka), and rapamycin was from Wyeth Ayerst Laboratories (Marietta, PA). Phorbol 12-myristate 13-acetate (PMA) was purchased from Biomol and ionomycin was purchased from Calbiochem.
Expression Vectors.
Construction of pTRE2-His-FAP48: Six His codons were added to the NH2-terminal part of the cDNA encoding full-length FAP48 by PCR with synthetic oligonucleotides as primers. The forward primer was 5′-GCG CCG GAA TTC ATG CAT CAC CAT CAC CAT CAC GCT GTA GAG GAA CTT CAG-3′. The reverse primer was 5′-TGT TTG TAA TGG CTG AAG C-3′. The resulting fragment was inserted into the EcoRI/EcoRV restriction sites of vector pGEM-11Zf, which contained full-length FAP48 and was checked by sequencing. Subsequently, the insert including full-length FAP48 with the six His residues as a tag was subcloned into the EcoRI/NotI restriction sites of pGADGE (gift of J. Camonis, INSERM, Unité 528) and tested for its ability to interact with FKBP52 and FKBP12 in the yeast two-hybrid system. The same insert was then introduced into the BamHI/NotI restriction sites of pTRE2 vector (CLONTECH) to give pTRE2-His-FAP48.
Cell Line and Stable DNA Transfection.
The Jurkat tet-on cell line (CLONTECH) was maintained in RPMI medium 1640 supplemented with 10% Tet System Approved FBS (CLONTECH), 100 μg/ml G418, 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml gentamicin. Transfection of 100 μg of pTRE2-His-FAP48 plasmid and 10 μg of pTK-hygromycin plasmid was carried out by electroporation of 2 × 107 cells with settings at 960 μF and 200 V. Stably transfected cells were selected with 100 μg/ml hygromycin and individually screened for high tetracycline inducibility by Western blotting with the anti-FAP48 pAb 766.
Coimmunoprecipitation.
Cells were washed in PBS and lysed in 50 mM Tris (pH 7.5)/150 mM NaCl/2 mM CaCl2/2 mM MgSO4/10% glycerol/0.1% Brij97 (Sigma)/protease inhibitors/Pefabloc. Protein concentrations were adjusted to 1 mg or 0.5 mg total protein in 200 μl, and coimmunoprecipitations were carried out by adding either 5 μl of anti-His mAb or 1 μg of EC1 for 1 h on ice. FK506 or rapamycin vs. ethanol as control was added during the precipitation. After separation of bound proteins on 10% SDS gels or 4–12% NuPAGE [bis(2-hydroxyethyl)imino]tris(hydroxymethyl)methane (Bistris) gels in Mops/SDS running buffer (NOVEX), proteins were detected with the indicated antibodies and visualized by enhanced chemiluminescence.
NFAT1 Activation Status.
To induce the dephosphorylation of NFAT1, cells were stimulated for 15 min with 1 μg/ml ionomycin at 37°C and were washed in cold PBS containing 400 μM Na3VO4, 10 mM NaF, and 5 mM EDTA and lysed in 50 mM Tris (pH 7.5) containing 1 mM Na3VO4, 10 mM NaF, and 2% SDS. Phosphorylated and dephosphorylated NFAT1 were detected by Western blotting with the anti-NFAT antiserum 67.1 (24). Cell suspensions were preincubated with 100 nM FK506 for 30 min at 37°C.
p38 and JNK Activation Assays.
Daisy cells, either untreated or induced with doxycycline, were stimulated with 50 ng/ml PMA and 1 μg/ml ionomycin for 15 min at 37°C then washed, lysed, and prepared for SDS/PAGE as described previously. p38 and JNK were detected by Western blotting.
IL2 Assays.
Daisy and Jurkat tet-on cells were treated with 0.5 μg/ml doxycycline for the indicated period and then synchronized at the G0–G1 phase of the cell cycle overnight in medium containing 0.5% FCS (25). The following day, they were washed, adjusted to 2 × 106 cells per ml, and stimulated with 50 ng/ml PMA and 1 μg/ml ionomycin for 24 h in medium without doxycycline and hygromycin. In some experiments, rapamycin was added during the stimulation at the indicated concentrations. The supernatants were collected, and IL2 was measured with the Quantikine Human IL2 kit from R & D Systems by following the manufacturer's instructions. Data are given as the percentage of IL2 concentration from doxycycline-treated compared with untreated cells, which were set at 100%.
Determination of Cell Growth.
Cells were untreated or grown with doxycycline, FK506, and rapamycin alone, or in combination (i.e., doxycycline plus FK506 and doxycycline plus rapamycin). Cells were counted in triplicate in a Neubauer chamber, excluding dead cells by trypan blue staining. Alternatively, cellular growth was measured with Cell Proliferation kit I [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)-based] from Roche Molecular Biochemicals by following the manufacturer's protocol.
Fluorescence-Activated Cell Sorter (FACS) Analyses.
For the determination of cell cycle progression and measurement of apoptosis, Daisy cells were induced with doxycycline over 24 h, 48 h, 3 days, 5 days, or 10 days. FACS analyses were carried out at the Cytometry Service of the Hôpital St. Antoine, Paris.
Quantification of Protein Expression Levels.
Daisy cells were grown in the presence of 0.5 μg/ml doxycycline for 7 days. Doxycycline-treated and untreated cells were simultaneously prepared for PowerBlot analysis (Becton Dickinson), which determined in three runs the expression levels of 750 different signal-transducing proteins by immunoblotting with mouse mAbs and computerized processing of the densitometric data (for details, see www.translab.com/TOC.shtml). Protein expression changes were confirmed in our laboratory in at least five independent experiments. After Western blotting, bands were quantified (Molecular Analyst, Bio-Rad) and normalized to HSP70 protein levels. Results are given as percentage of normalized densitometric data of treated-cell samples compared with untreated controls (100%).
Results
Cloning of a Jurkat-Derived T Cell Line Inducibly Overexpressing FAP48.
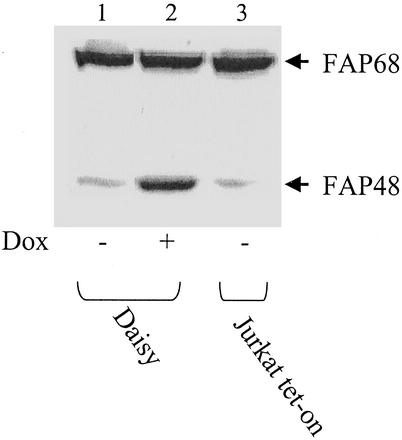
To characterize the biological role of FAP48 in cello, an inducible expression system based on the tetracycline-responsive operon (20) was used to generate a stably transformed cell line. As a cellular host for the inducible expression of FAP48, we chose Jurkat T cells because this cell line has been extensively used as a model system in studies on FK506 and FKBPs. Moreover, FAP48 was originally isolated and cloned from this cell line (18). The commercially available Jurkat tet-on cell line that expressed the reverse tetracycline-controlled transactivator was stably transfected with the pTRE2-His-FAP48 vector. Screening gave one clone named “Daisy,” which under basal conditions expresses a moderate level of endogenous FAP48 compared with that of endogenous FAP68 isoform (Fig. 1, lane 1). However, treatment with doxycycline resulted in a marked increase of recombinant FAP48 protein expression. Fig. 1 shows a representative Western blot of FAP48 induction in Daisy cells, compared with noninduced Daisy and Jurkat tet-on cells. The induction of FAP48 in Daisy cells is about 5-fold, as determined by densitometric analysis.
Figure 1.
Daisy, a Jurkat-derived T cell line, inducibly overexpresses FAP48. Daisy cells were induced by 0.5 μg/ml doxycycline (Dox) to overexpress FAP48 over 7 days. Whole-cell extracts (50 μg each) of noninduced (lane 1) and induced (lane 2) cells and Jurkat tet-on cells (lane 3) were analyzed by Western blotting with the anti-FAP48 antiserum 766.
FAP48 Binds FKBP52 in the Absence of Immunosuppressive Drugs in Daisy T Cells.
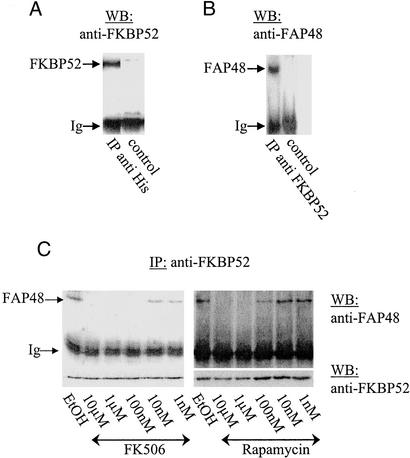
To confirm the specific interactions between FAP48 and FKBP52 or FKBP12 in cello, coimmunoprecipitation assays using lysates of Daisy cells induced by doxycycline were carried out with the anti-6-His mAb that specifically recognizes recombinant FAP48. Fig. 2A shows a Western blot that was revealed by the anti-FKBP52 pAb 173 and indicates that FKBP52 coprecipitates with recombinant FAP48. In parallel, the anti-FKBP52 mAb EC1 coprecipitates FAP48 when detected by the antiserum 766 (Fig. 2B).
Figure 2.
Binding of FAP48 to FKBP52 in Daisy T cells is prevented by FK506 and rapamycin in a dose-dependent manner. (A) Daisy cells were induced with doxycycline over 3 days, lysed, and subjected to immunoprecipitation (IP) with the anti-6-His mAb and a control mAb. Subsequently, immunoprecipitates were analyzed by Western blotting (WB) with the anti-FKBP52 pAb 173. (B) Likewise, cell lysates as in A were incubated with the anti-FKBP52 mAb EC1 or with a control mAb, and the precipitates were analyzed by Western blotting with the anti-FAP48 antiserum 766. (C) Coimmunoprecipitation experiments with the anti-FKBP52 mAb EC1 were performed on Daisy cells overexpressing FAP48. FK506 or rapamycin at the indicated concentrations was included during the incubation with Ab. Controls were performed with ethanol, which was used as vehicle. Equal loading was confirmed by Western blotting with the anti-FKBP52 pAb (Lower). Ig, immunoglobulin light chain.
Because FK506 and rapamycin have been shown to prevent association between FAP48 and FKBP52 (18), we tested whether the interaction between FAP48 and FKBP52 in Daisy cells induced by doxycycline could be blocked by the immunosuppressive drugs. When FK506 or rapamycin were included during precipitation with mAb EC1, no interaction could be detected between FAP48 and FKBP52. As shown in Fig. 2C, the immunosuppressive drugs prevent the binding of FAP48 to FKBP52 in a dose-dependent manner, and 100 nM FK506 or 1 μM rapamycin was sufficient to abolish the binding of FAP48 to FKBP52.
The anti-6-His mAb precipitates were also analyzed with an anti-FKBP12 Ab by Western blotting. Although FKBP12 was easily detectable in whole-cell lysates of Daisy cells induced by doxycycline, we could never detect its presence in the precipitates, not even by raising the amount of protein to 2 mg or by precipitating overnight (data not shown).
Effect of FAP48 on IL2 Production.
To date, the mechanism of action of FK506 is mainly explained by the formation of a complex between FKBP12 and FK506, which binds to and inactivates calcineurin, and subsequently impairs NFAT-dependent gene expression (3, 7). Keeping in mind that the absence of FKBP12 in the anti-FAP48 immunoprecipitates (results cited above) does not necessarily exclude the association of these two proteins in the cell, we investigated the influence of FAP48 on the phosphorylation status of NFAT1. Unlike the FK506 effect used as positive control for this assay, the overexpression of FAP48 had no influence on the phosphorylation status of NFAT1 (data not shown).
Besides the calcineurin-dependent mechanism, a calcineurin-independent activation pathway mediated by JNK and p38 is involved in the immunosuppressive effect of FK506 mediated by FKBP (8). We were therefore prompted to study the activation of JNK and p38 in Daisy cells. Under experimental conditions used (doxycycline induction over 7 days for p38 and from 4 to 10 days for JNK), we could never observe a change in the phosphorylation pattern of either JNK or p38 (data not shown).
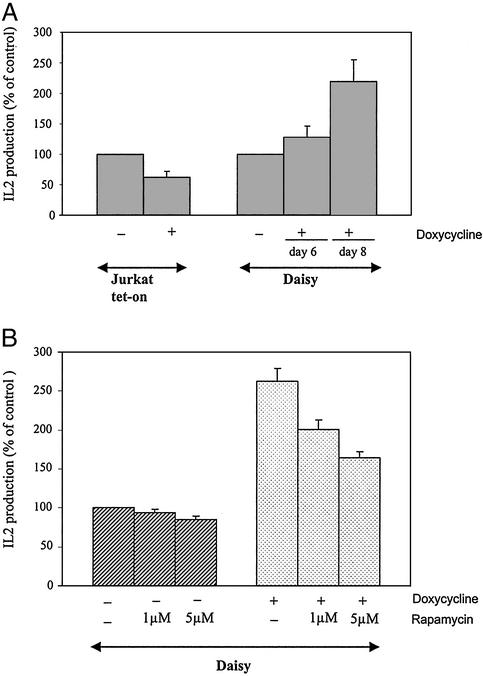
With the inhibition of IL2 production by FK506 being the final outcome of the activation steps blocked by FK506, we checked whether overexpression of FAP48 in activated Jurkat T cells could influence the IL2 production. Daisy cells were induced by doxycycline to overexpress FAP48, synchronized at the G0–G1 phase, and subsequently stimulated with PMA plus ionomycin. Jurkat tet-on cells were treated the same way because tetracyclines have been described as inhibiting IL2 secretion (26). The addition of doxycycline to Jurkat tet-on cultures diminished IL2 secretion to 62 ± 9% compared with untreated control cells, which were set at 100% (Fig. 3A). In contrast, the addition of doxycycline to Daisy cells led to an IL2 production of 128 ± 18% and 220 ± 35% after 6 and 8 days of doxycycline treatment, respectively (IL2 secretion of untreated Daisy cells was at 100%). Because of the immunosuppressive effect of doxycycline, the increasing effect of FAP48 on IL2 production should be considered to be even higher than shown here. Stimulating FAP48-overexpressing Daisy cells in the presence of excess rapamycin (1 and 5 μM), which itself has no effect on IL2 production (27), decreases the amount of secreted IL2 in our system (to 25% and 40%, respectively) (Fig. 3B). This finding indicates that it is the FKBP–FAP48 complex that mediates the increase of IL2 release.
Figure 3.
FAP48 increases IL2 synthesis in activated Daisy T cells. (A) Jurkat tet-on cells were incubated over 4–6 days with doxycycline and Daisy cells for 6 or 8 days. Cells were synchronized overnight and subsequently stimulated with PMA/ionomycin for 24 h. Cell-free supernatants were analyzed for IL2 production by ELISA (n = 3–8). (B) Daisy cells, incubated without or with doxycycline over 8 days, were treated as described for A in the absence or presence of 1 or 5 μM rapamycin during the stimulation. Subsequently, IL2 production was measured (n = 3). Data of IL2 production by drug-treated cells are expressed as mean (±SE) percentage of that from untreated cells (considered as 100%).
Antiproliferative Effect of FAP48.
An antiproliferative effect of FK506 in mature T cells and in EL4 cells lines has been described (28, 29). The antiproliferative effect of rapamycin has also been extensively studied. Rapamycin complexed with FKBP12 interacts with FRAP (30–33) and inhibits or delays G1–S-phase cell cycle progression in mammalian cells. Thus, we investigated the biological consequences of FAP48 overexpression on cellular proliferation and growth arrest.
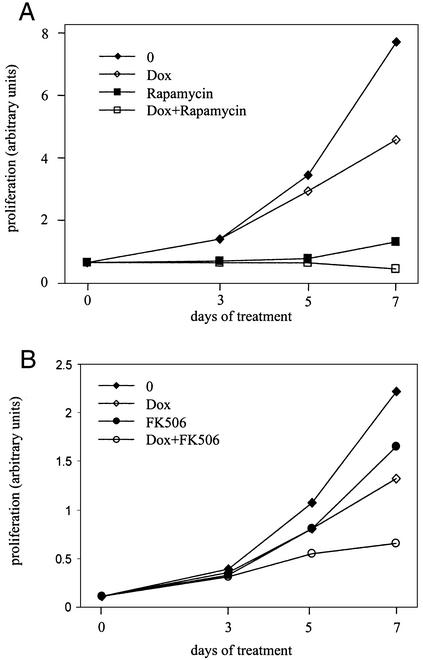
Cellular proliferation was measured by simple counting or by using an MTT assay in Daisy cells induced by doxycycline or not. After 2 days of doxycycline induction, no significant difference in cellular growth between induced and noninduced cells was apparent. However, after 7 days of treatment with 0.5 μg/ml doxycycline, overexpression of FAP48 led to an inhibition of proliferation of ≈40% compared with parental cells (Fig. 4). Long-term proliferation assays of Daisy cells up to 14 days of treatment with doxycycline did not lead to a further decrease of cellular proliferation compared with noninduced cells (data not shown). A possible antiproliferative effect of 0.5 μg/ml doxycycline was assessed in Jurkat T cells and was found nonsignificant (data not shown). Also, a possible cytotoxicity of FAP48 overexpression in Daisy cells was excluded by trypan blue staining (data not shown).
Figure 4.
Influence of FAP48, rapamycin, and FK506 on the proliferation of Daisy cells. Cellular proliferation was measured by MTT tests. (A) Daisy cells were treated with 0.5 μg/ml doxycycline (Dox, ⋄), 10 nM rapamycin (■), or 0.5 μg/ml Dox plus 10 nM rapamycin (□), or were untreated (♦). This is one representative experiment of three. (B) Cells were treated with 0.5 μg/ml Dox (⋄), 100 nM FK506 (●), or with either compound over 7 days (○), or were untreated (♦). This is one representative experiment of five.
In the same way, Daisy cells were incubated with 100 nM FK506 in the presence or absence of doxycycline over 7 days. As shown in Fig. 4B, 100 nM FK506 inhibited the proliferation of Daisy cells in a way similar to that of FAP48 (≈26% inhibition by FK506, ≈40% by FAP48), and a potent additive effect of FK506 was observed in FAP48-overexpressing cells (65% inhibition). Fluorescence-activated cell sorter experiments carried out with Daisy cells overexpressing FAP48 or treated with FK506 or both proved that cell cycle progression was not affected and also excluded a possible apoptotic effect of FAP48 and FK506 in this biological system (data not shown). That FAP48 plus FK506 has an additive antiproliferative effect (Fig. 4B) suggests that FAP48 free from FKBP is the antiproliferative effector.
Next, we tested the effect of rapamycin and FK506 on the growth arrest of Daisy cells. As expected, 10 nM rapamycin inhibited the cellular proliferation but, unlike FAP48, the effect of rapamycin was fast: only 72 h of treatment with 10 nM rapamycin led to a complete growth arrest (Fig. 4A). We examined the phosphorylation status of p70s6 kinase, whose activation is blocked by rapamycin (reviewed in refs. 34 and 35), in Daisy cells induced by doxycycline. In contrast to a rapamycin-treated control, the phosphorylation pattern of p70s6 kinase in FAP48-overexpressing cells was unchanged (data not shown). This result suggests that FAP48 is not involved in the mTOR signaling pathway.
Identification of FAP48 Molecular Targets.
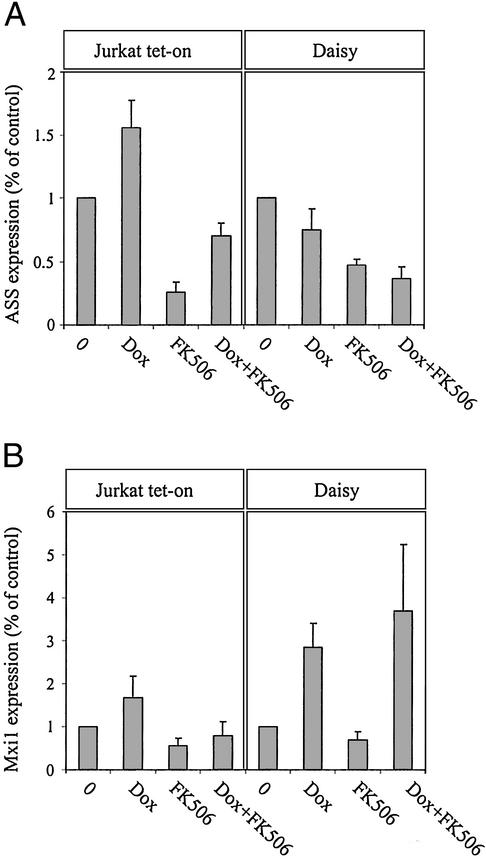
To identify the molecules that mediate the signals initiated by FAP48, protein changes caused by overexpressed FAP48 in Jurkat T cells were investigated by proteomics. Lysates of untreated or doxycycline-treated (for 7 days) Daisy cells were screened by using a large panel of different mAbs. Some major alterations were identified, namely a down-regulation of the expression of ASS, which is involved in the urea cycle to generate arginine (reviewed in ref. 21). Fig. 5A shows that ASS protein expression is down-regulated by overexpressed FAP48 in Daisy cells (75 ± 16.7% expression level compared with that in untreated cells, which were set at 100%). Likewise, FK506 diminished ASS levels to 47 ± 4.9% in Daisy cells and an additive effect was observed in FAP48-overexpressing cells after treatment with FK506 (down-regulation to 36 ± 9.6%). In Jurkat tet-on cells, the expression of ASS was increased by doxycycline to 156 ± 21.6% compared with untreated cells (100%), whereas it was blocked by FK506 at a level comparable to that in Daisy cells (down-regulation to 26 ± 7.8%; down-regulation by doxycycline plus FK506 to 70 ± 9.5%). Because of the up-regulating effect of doxycycline itself on ASS expression levels, the blocking effect of FAP48 can be considered to be even higher than demonstrated here.
Figure 5.
The expression levels of ASS and Mxi1 are modulated by overexpressed FAP48. (A) Densitometric data of Western blots are shown as ASS/HSP70 (A) or Mxi1/HSP70 (B) ratios. Cells were incubated with 0.5 μg/ml doxycycline, 100 nM FK506, or both compounds over 7 days. Total cell lysates were analyzed by Western blotting with mAb against ASS or Mxi1. Values are given as mean ± SE (n = 5 or 6).
The other protein whose expression is modulated by the overexpression of FAP48 is Mxi1, a member of the family of transcriptional repressors of Myc (reviewed in ref. 22). Fig. 5B shows that in Daisy cells, Mxi1 expression levels were increased approximately 3-fold by overexpressed FAP48, although they were not significantly affected by FK506. Doxycycline itself has no significant influence on Mxi1 protein levels as determined in Jurkat tet-on cells.
Discussion
In the present study, we provide evidence for an implication of FAP48 in the activation of T lymphocytes and for its antiproliferative properties. The involvement of target molecules in the signaling pathway of FAP48 is discussed.
The Complex FKBP52–FAP48 Is Present in Jurkat T Cells.
We confirm by coimmunoprecipitation studies that FAP48 interacts with endogenous FKBP52 in Jurkat T cells. This interaction is highly specific because it can be disrupted by the immunosuppressive drugs FK506 and rapamycin in a dose-dependent manner (Fig. 2). Surprisingly, we could not detect a complex of FAP48 and FKBP12 by coimmunoprecipitation experiments. This result could, of course, reflect the incapacity of FAP48 to associate with FKBP12 in Jurkat T cells. However, other possibilities for explaining the apparent discrepancy between the present results and the experiments carried out in the yeast two-hybrid system or with classical in vitro biochemical assays should be considered. (i) Experiments in the yeast two-hybrid system, which we used to demonstrate the interaction between FAP48 and FKBP12 (18), are highly sensitive and able to detect low-affinity protein–protein interactions. (ii) Alternatively, FAP48 may have a higher affinity for FKBP52 than for FKBP12, leading to a low amount of FAP48–FKBP12 complexes that are not detectable in coimmunoprecipitation assays. (iii) The lack of coprecipitated FKBP12 protein might also be due to the use of inappropriate antibodies, or to the fact that the antibodies used during the coimmunoprecipitation assays could mask or modify the binding site of FAP48 for FKBP12.
Regulation of IL2 Synthesis by FAP48.
As already mentioned, the transcription of the IL2 gene in activated Jurkat T cells is inhibited by FK506. In this study, we found that FAP48, unlike FK506, does not affect the calcineurin-dependent NFAT signaling pathway and that FAP48 is not implicated in the activation of JNK and p38, as is FK506 (results not shown). In contrast, we show that the overexpression of FAP48 significantly augments the secretion of IL2 by PMA/ionomycin-activated T cells (Fig. 3A).
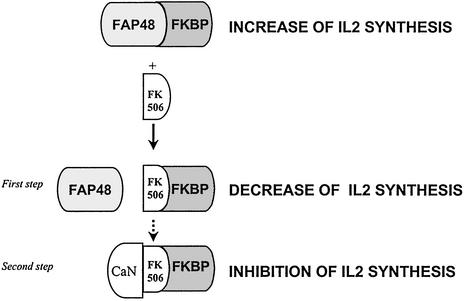
Additionally, in our system, we show that rapamycin decreases the production of IL2 in FAP48-overexpressing cells (Fig. 3B), indicating that it is not FKBP-free FAP48 but rather the FAP48–FKBP complexes that are responsible for this effect. This result suggests that the FAP48–FKBP complexes are involved in the control of IL2 synthesis in activated T cells. The administration of immunosuppressive drugs breaks up the complexes and the elevated production of IL2 is disturbed. This would seem to be a novel aspect of the mechanism of action of immunosuppression by FK506. The apparent antagonistic effect of FK506 on the function of FAP48 would therefore include (i) disruption of the increased production of IL2 in response to antigen and (ii) complete inhibition of IL2 synthesis by means of inhibition of the calcineurin/NFAT pathway (Fig. 6).
Figure 6.
An aspect of the mechanism of immunosuppression by FK506 revealed by the present experiments. The FAP48–FKBP complex up-regulates IL2 production in activated Jurkat T cells. First step: The addition of FK506 breaks up the FAP48–FKBP complex, leading to an IL2 production decrease. Second step: The FKBP–FK506 interacts with calcineurin (CaN), therefore blocking IL2 synthesis.
From these results we hypothesize that FAP48, when complexed to FKBP, could prevent associations between FKBP and other proteins that are nonproductive for the cell. It has been reported that in the absence of exogenous ligand, FKBP12 interacts with and modulates calcineurin activity, and that the immunosuppressant FK506 enhances preexisting immunophilin–calcineurin complexes (36). In this context, we can imagine that FAP48 could interfere in the formation of the complex between calcineurin and FKBP12 to prevent their association, thus leading to an optimum immune response of T cells.
Antiproliferative Effect of FAP48.
In addition to the involvement of FAP48 in the activation of Jurkat T cells, we show that its overexpression in Daisy T cells causes a marked inhibition of cellular proliferation (Fig. 4). In addition, we observe that 100 nM FK506 inhibits the proliferation of Daisy T cells at a level similar to that of the inhibition by FAP48. This effect is increased by FAP48 overexpression (Fig. 4B), suggesting that it is not the FAP48–FKBP complex that mediates this effect but rather FAP48 dissociated from FKBP by FK506. These antiproliferative effects cannot be explained by changes in the cell cycle because the antiproliferative effect is slow, and no modulation of the expression level or the phosphorylation status of several key players in the control of eukaryotic cell cycle, such as p27/kip1 or pRB, was observed in our system (U.K., unpublished results).
The possibility that FAP48 acts in a signal transduction pathway targeted by rapamycin can be ruled out, because the activation of p70s6 kinase, which is blocked by FKBP12–rapamycin through mTOR, is not modulated by the overexpression of FAP48 in Daisy cells (results not shown). Interestingly, it has been reported that FAP68, unlike FAP48, stimulates p70s6 kinase (19). This observation suggests that these two FAP targets of FKBPs might be involved in different cellular signaling pathways.
Molecular Mechanisms Implied in the Biological Effects of FAP48.
In search of a molecular mechanism mediating the biological effects of overexpressed FAP48 in Daisy T cells, we found that at least two proteins are involved in the FAP48 signaling pathway. In the present study, we show that Mxi1 protein expression levels are up-regulated by overexpressed FAP48, whereas FK506 apparently had no such effect (Fig. 5B). A role of Mxi1 in the control of proliferation in a transfected glioblastoma cell line and in lymphocytes has been described, leading to Mxi1 being considered as a tumor suppressor molecule (37, 38). However, the additive effect of the combination of overexpressed FAP48 plus FK506 on cellular proliferation could not be explained by Mxi1 alone, because FK506 does not seem to influence Mxi1 protein expression levels. ASS is another candidate mediating the biological effects of FAP48. ASS synthesizes the amino acid arginine in the urea cycle. Here we show that both overexpressed FAP48 and FK506 inhibit the expression level of ASS (Fig. 5A) and we observe a good correlation between expression levels of ASS and proliferation inhibition with FAP48 and/or FK506. Therefore, we suggest that in unstimulated T cells, FAP48 and FK506 may exert their antiproliferative effects partly by means of a decrease of ASS protein levels leading to a decreased amount of the arginine for the synthesis of newly required proteins.
Conclusion
To sum up, we provide evidence that the FKBP endogenous ligand FAP48 exerts different biological effects in T cells. In unstimulated T cells, free FAP48 regulates cellular proliferation possibly by down-regulating ASS with a resulting lack of arginine for protein synthesis and by up-regulating the tumor-suppressor molecule Mxi1. In activated T cells, the FAP48–FKBP complexes up-regulate IL2 synthesis. Additional studies are required to identify which FKBP when complexed with FAP48 is involved in the increase of IL2 secretion and to assess the possible role of ASS and Mxi1 as mediators of the biological effects of FAP48. The results presented here suggest that FAP48 could be implicated in the control of the immune response and exert an antigrowth function.
Acknowledgments
We thank Dr. Anjana Rao for the generous gift of the anti-NFAT1 Ab, Dr. Jan Mester (Institut National de la Santé et de la Recherche Médicale, Unité 482, Hôpital St. Antoine, Paris) for his help with the fluorescence-activated cell sorter analyses, Dr. K. Murato (Fujisawa Pharmaceuticals) for the gift of FK506, and Drs. Y. Akwa and K. Rajkowski (Institut National de la Santé et de la Recherche Médicale, Unité 488) for critical reading of the manuscript. This work was funded in part by the Association pour la Recherche sur le Cancer (Contract 5826) and the Collège de France. U.K. was supported by a grant from the French Ministry of Foreign Affairs, from the Société de Secours des Amis des Sciences, Paris, and from the Artémis-Fondation Nationale de Gérontologie.
Abbreviations
- FKBP
FK506-binding protein
- ASS
argininosuccinate synthetase
- pAb
polyclonal Ab
- PMA
phorbol 12-myristate 13-acetate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NFAT
nuclear factor of activated T cells
References
- 1.Marks A R. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 2.Gothel S F, Marahiel M A. Cell Mol Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont F J. Curr Med Chem. 2000;7:731–748. doi: 10.2174/0929867003374723. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, Schreiber S L. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 5.Mckeon F. Cell. 1991;66:823–826. doi: 10.1016/0092-8674(91)90426-y. [DOI] [PubMed] [Google Scholar]
- 6.Fruman D A, Klee C B, Bierer B E, Burakoff S J. Proc Natl Acad Sci USA. 1992;89:3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiber S L. Cell. 1992;70:365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda S, Shibasaki F, Takehana K, Mori H, Nishida E, Koyasu S. EMBO Rep. 2000;1:428–434. doi: 10.1093/embo-reports/kvd090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai P K, Maeda Y, Nakao K, Wakim N G, Duhring J L, Faber L E. Biochemistry. 1986;25:5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- 10.Renoir J M, Radanyi C, Faber L E, Baulieu E E. J Biol Chem. 1990;265:10740–10745. [PubMed] [Google Scholar]
- 11.Callebaut I, Renoir J M, Lebeau M C, Massol N, Burny A, Baulieu E E, Mornon J P. Proc Natl Acad Sci USA. 1992;89:6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebeau M C, Massol N, Herrick J, Faber L E, Renoir J M, Radanyi C, Baulieu E E. J Biol Chem. 1992;267:4281–4284. [PubMed] [Google Scholar]
- 13.Peattie D A, Harding M W, Fleming M A, DeCenzo M T, Lippke J A, Livingston D J, Benasutti M. Proc Natl Acad Sci USA. 1992;89:10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambraud B, Rouviere-Fourmy N, Radanyi C, Hsiao K, Peattie D A, Livingston D J, Baulieu E E. Biochem Biophys Res Commun. 1993;196:160–166. doi: 10.1006/bbrc.1993.2229. [DOI] [PubMed] [Google Scholar]
- 15.Wiederrecht G, Hung S, Chan H K, Marcy A, Martin M, Calaycay J, Boulton D, Sigal N, Kincaid R L, Siekierka J J. J Biol Chem. 1992;267:21753–21760. [PubMed] [Google Scholar]
- 16.Yem A W, Reardon I M, Leone J W, Heinrikson R L, Deibel M R., Jr Biochemistry. 1993;32:12571–12576. doi: 10.1021/bi00210a004. [DOI] [PubMed] [Google Scholar]
- 17.Lebeau M C, Myagkikh I, Rouviere-Fourmy N, Baulieu E E, Klee C B. Biochem Biophys Res Commun. 1994;203:750–755. doi: 10.1006/bbrc.1994.2246. [DOI] [PubMed] [Google Scholar]
- 18.Chambraud B, Radanyi C, Camonis J H, Shazand K, Rajkowski K, Baulieu E E. J Biol Chem. 1996;271:32923–32929. doi: 10.1074/jbc.271.51.32923. [DOI] [PubMed] [Google Scholar]
- 19.Grisendi S, Chambraud B, Gout I, Comoglio P M, Crepaldi T. J Biol Chem. 2001;276:46632–46638. doi: 10.1074/jbc.M104323200. [DOI] [PubMed] [Google Scholar]
- 20.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori M, Gotoh T. Biochem Biophys Res Commun. 2000;275:715–719. doi: 10.1006/bbrc.2000.3169. [DOI] [PubMed] [Google Scholar]
- 22.Foley K P, Eisenman R N. Biochim Biophys Acta. 1999;1423:M37–M47. doi: 10.1016/s0304-419x(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 23.Nakao K, Myers J E, Faber L E. Can J Biochem Cell Biol. 1985;63:33–40. doi: 10.1139/o85-005. [DOI] [PubMed] [Google Scholar]
- 24.Ho A M, Jain J, Rao A, Hogan P G. J Biol Chem. 1994;269:28181–28186. [PubMed] [Google Scholar]
- 25.Baksh S, DeCaprio J A, Burakoff S J. Oncogene. 2000;19:2820–2827. doi: 10.1038/sj.onc.1203585. [DOI] [PubMed] [Google Scholar]
- 26.Kloppenburg M, Verweij C L, Miltenburg A M, Verhoeven A J, Daha M R, Dijkmans B A, Breedveld F C. Clin Exp Immunol. 1995;102:635–641. doi: 10.1111/j.1365-2249.1995.tb03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bierer B E, Mattila P S, Standaert R F, Herzenberg L A, Burakoff S J, Crabtree G, Schreiber S L. Proc Natl Acad Sci USA. 1990;87:9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawada S, Suzuki G, Kawase Y, Takaku F. J Immunol. 1987;139:1797–1803. [PubMed] [Google Scholar]
- 29.Yamamoto-Yamaguchi Y, Okabe-kado J, Kasukabe T, Honma Y. Exp Hematol. 2001;21:582–588. doi: 10.1016/s0301-472x(01)00626-9. [DOI] [PubMed] [Google Scholar]
- 30.Heitman J, Movva N R, Hall M N. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 31.Sabers C J, Martin M M, Brunn G J, Williams J M, Dumont F J, Wiederrecht G, Abraham R T. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 32.Sabatini D M, Erdjument-Bromage H, Lui M, Tempst P, Snyder S H. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 33.Brown E J, Albers M W, Shin T B, Ichikawa K, Keith C T, Lane W S, Schreiber S L. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 34.Brown E J, Schreiber S L. Cell. 1996;86:517–520. doi: 10.1016/s0092-8674(00)80125-7. [DOI] [PubMed] [Google Scholar]
- 35.Peterson R T, Beal P A, Comb M J, Schreiber S L. J Biol Chem. 2000;275:7416–7423. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]
- 36.Cardenas M E, Hemenway C, Muir R S, Ye R, Fiorentino D, Heitman J. EMBO J. 1994;13:5944–5957. doi: 10.1002/j.1460-2075.1994.tb06940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wechsler D S, Shelly C A, Petroff C A, Dang C V. Cancer Res. 1997;57:4905–4912. [PubMed] [Google Scholar]
- 38.Schreiber-Agus N, Meng Y, Hoang T, Hou H, Jr, Chen K, Greenberg R, Cordon-Cardo C, Lee H W, DePinho R A. Nature. 1998;393:483–487. doi: 10.1038/31008. [DOI] [PubMed] [Google Scholar]