Abstract
Ecphory occurs when one recollects a past event cued by a trigger, such as a picture, odor, or name. It is a central component of autobiographical memory, which allows us to “travel mentally back in time” and re-experience specific events from our personal past. Using fMRI and focusing on the role of medial temporal lobe (MTL) structures, we investigated the brain bases of autobiographical memory and whether they change with the age of memories. Importantly, we used an ecphory task in which the remote character of the memories was ensured. The results showed that a large bilateral network supports autobiographical memory: temporal lobe, temporo-occipito-parietal junction, dorsal prefrontal cortex, medial frontal cortex, retrosplenial cortex and surrounding areas, and MTL structures. This network, including MTL structures, changed little with the age of the memories.
Keywords: remote memory, episodic memory, semantic memory, consolidation
Introduction
We experience our lives in a stream of events. Autobiographical memory - one of the most complex memory systems - allows us to mentally travel back in time and re-experience these events (Tulving & Markowitsch, 1998). In this study, we used fMRI to uncover the neural substrates of autobiographical memory, and explored whether and how the locus and extent of activation changed with the age of memories. We were particularly interested in whether and how the role of medial temporal lobe (MTL) structures altered. It is well known that MTL structures are engaged in the formation of autobiographical and semantic memories (Corkin, 1984; Gabrieli et al., 1988; Halgren et al., 1985; Milner & Penfield, 1955; Scoville & Milner, 1957). Its role in remote memory, however, is highly debated. The generally accepted view is that MTL structures are essential for the initial storage and retrieval of autobiographical and semantic memories, but that they become unnecessary over time when autobiographical and semantic memory traces are established permanently in the neocortex, due to the process of consolidation (Bayley et al., 2003; Squire & Zola, 1998). A contrasting position is that MTL structures are always needed for autobiographical memory and that only semantic memory becomes independent from them (Bayley et al., 2003; Moscovitch et al., 1999; Nadel et al., 2000).
In the present study, participants reminisced about specific events that had occurred in their personal past. Previous studies showed that highly specific retrieval cues can elicit ecphory, the recognition process that results in vivid episodic recollections of particular situations (Brewer, 1988; Wheeler et al., 1997). We, therefore, provoked ecphory using stimulus sentences, tailored individually for each participant that asked about salient aspects of specific events that had occurred in their personal past. Other studies have used adaptations on the Crovitz & Schiffman (Crovitz & Schiffman, 1974) technique (Conway et al., 1999; Ryan et al., 2001) to elicit memories in a rather vague way or interviewed participants shortly before scanning (Fink et al., 1996; Maguire, 2001; Maguire et al., 2001; Maguire & Mummery, 1999; Maguire et al., 2000). Recent retrieval, however, destroys the remote character of memories because each time one remembers an event one encodes it anew (Buckner et al., 2001; Nyberg et al., 2000). Our study avoided recent retrieval, ensuring the remote character of the memories, by using participant’s diaries and information obtained from interviews with family members and friends to create the stimuli. Avoiding recent retrieval has, to our knowledge, only been accomplished in two other studies {Ryan, 2001 #49; Gilboa, 2004 #509} which, however, included a smaller number of participants (n ≤ 9).
Our results showed a large network of activation that included MTL structures and was specific to autobiographical memory, independent of the age of the memories.
Materials and Methods
Participants
The participants were 16 right-handed healthy volunteers (9 men). Their mean age was 41.4 ± 7.9 years, and mean education was 16.7 ± 2.1 years. Participants had to be at least 28 years old, so that we could obtain a sufficient number of remote autobiographical events, and younger than 60 years old to ensure a vivid character of autobiographical memories, which are likely to become semanticized with age of participants (Levine et al., 2002). All participants were native English speakers. Five additional participants were excluded because of claustrophobia (1), technical scanner problems (1), a low hit rate in the semantic condition (2), and left handedness (1).
Written informed consent was obtained prior to scanning. All procedures were approved by the Massachusetts General Hospital Human Research Committee and by the MIT Committee on the Use of Humans as Experimental Subjects.
Task Design and Procedure
The task consisted of three memory conditions - recent autobiographical, remote autobiographical, and remote semantic – which were presented in random order with a fixation condition (Figure 1). Each memory condition comprised 50 items that were presented in identical trials in which participants were asked to remember a specific event from his or her recent or remote personal past (autobiographical conditions), or to visualize mentally a well known object (remote semantic memory condition). Stimulus sentences were matched for length (number of syllables) and grammatical complexity. In each condition, participants gave two responses. After presentation of the stimulus sentence, they pressed a button as soon as they could recall the personal event (autobiographical conditions) or visualize the well known object or figure (remote semantic memory condition). Reaction times were measured from the time of stimulus onset to the button press. This self-paced “search” period was followed by a 6 s “reminiscence” period during which the participant was asked to remember in as much detail as possible the event, or to visually imagine in as much detail as possible the well-known object. In the autobiographical conditions, participants were instructed to re-experience the event and to orient themselves towards their own past. They were specifically instructed not to wander mentally to other events. In the remote semantic memory condition, participants were told that they could rotate the object mentally if they wished. All of the objects or figures of the remote semantic memory condition were renowned for at least 10 years, but were currently not frequently published in the media. The 6 s “reminiscence” period was followed by a 2 s period in which participants were asked to rate the vividness of the memory. The vividness rating was requested to ensure that participants continued to perform the mental task and did not drift off to other thoughts. If a participant could not remember the event or visualize the well known object (left button press at the end of the self-paced search period), the next trial appeared. During fixation trials, participants focused on a cross on the screen. The task was presented in three runs. Immediately before scanning, participants performed a practice trial to become familiarized with the task. Only successfully retrieved memories were included in the analyses. Participants needed to recall at least 30 items of each condition to be included into the study.
Figure 1.
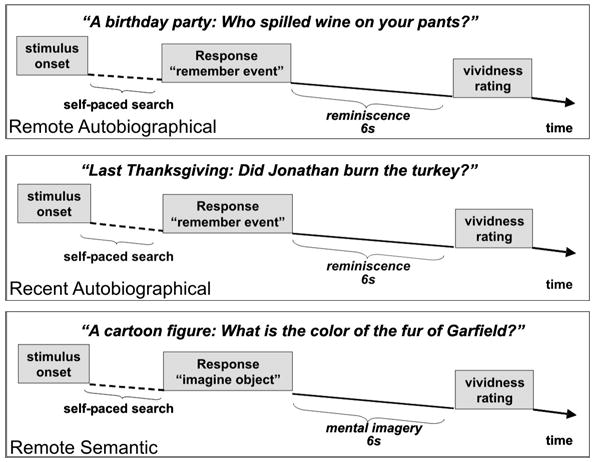
Outline of task design and sample sentences for the three memory conditions
The stimuli for the autobiographical memory condition, tailored individually for each participant, asked about a key aspect of a specific event that lasted not more than several hours. Events for the remote autobiographical condition occurred 10 or more years ago, usually during the participant’s childhood or adolescence, whereas events for the recent autobiographical condition stemmed from the preceding 1 – 2 years. Information about specific events came from diaries that participants (N = 5) had not looked at for the last 8–10 years (for the remote time period only) or from interviews with family members or friends. We selected only diary entries that indicated a particular event specific in time and place, such as “going to a prom” or “picking out a pet” for the creation of stimulus sentences. During the interviews, we asked family members and friends not to give events that were “personal folklore,” memories told over and over again, because such memories may refer to previous recollections rather than to the original event. Family members and friends agreed not to discuss the content of the interview with the participant until after scanning was conducted. Written informed consent was obtained from family members and friends before the interviews.
Postscan Testing
Immediately after each imaging session, 15 of the 16 participants rated their memories on a 5-point scale regarding the vividness of the memory during scanning, the emotional impact of the original event, its personal importance, and the numbers of prior rehearsals of the memory.
A critical issue in interpreting the results concerned the depth of encoding during scanning. Participants retrieved and, therefore, encoded the memories anew inside the scanner. It is possible that the memories in the autobiographical conditions were more richly and deeply re-encoded than those in the semantic condition because of self-referential elaboration of the former. Enriched encoding could result in greater activation in the autobiographical conditions, especially in MTL structures. If encoding were enhanced in a particular condition, one would expect a disproportionate decrease in reaction times when that condition was presented a second time. To address this issue, we asked 11 participants to retrieve each memory again after scanning so that reaction time decrease could be compared across conditions.
We further performed Pearson correlation analyses between the quality ratings (vividness, emotional impact of original event, personal importance, and number of rehearsals) and the results of the region of interest analyses (percentage signal change in the left and right amygdala, hippocampus, and parahippocampal region, respectively). These analyses were performed for the search time period only because the ROI analyses of the reminiscence period did not reveal significant effects.
Image Acquisition
Images were acquired using a 3.0-T Trio imager (Siemens Medical Systems, Iselin, NJ). Participants wore foam earplugs for noise protection and laid on a padded scanner table in a dimly lit room. Foam padding minimized head movement. Stimuli were generated by Presentation, Version 0.71 (Neurobehavioral Systems, Albany, CA) on a Sony Vaio PCG-GRS100, and were projected with an LCD projector through a collimating lens onto a plastic screen secured to the back of the scanner. Participants viewed the stimuli through an angled mirror positioned immediately in front of their eyes. Stimuli subtended ~ 10° of visual angle vertically. Two structural scans were collected before the functional scans using a MPRAGE sequence (TR = 2530 ms, TE = 3.31 ms, matrix = 192 x 256, orientation = sagittal, slice thickness = 1.33 mm, band width of 200 Hz/Pix). Functional data were acquired using gradient echo EPI sequences (TR = 2000 ms, TE = 25 ms, 30 slices, orientation = sagittal, slice thickness 5mm, band width of 2232 Hz/Pix). Functional and structural sequence images were acquired in sagittal orientation aligned along the longitudinal gradient of both hippocampi. This orientation maximized the in-plane resolution within the hippocampus.
Event-Related Analyses
Hemodynamic activation maps were estimated for neural activity using FS-FAST with the gamma function, e.g. assuming a specific shape of the hemodynamic response (Burock & Dale, 2000). By this approach, the hemodynamic responses of search and reminiscence could be disentangled, even though both had a fixed temporal relation. This analysis technique takes the overlap of the hemodynamic responses of both time periods into account by modeling the hemodynamic response function for each phase independently by means of the gamma function. For example, the tail of the gamma function for the search phase overlaps the onset of the gamma function for the reminiscence phase. The contribution of each phase during the overlap period is dictated by the relative proportion predicted by the gamma functions. This will result in the correct loading of the regressors for each phase, under the assumption that the gamma function is the correct model. We acknowledge, however, that in case the assumptions of the gamma function are not correct, then model errors can result in misappropriation of regressor weighting.
In addition, we focused on contrasts across memory conditions, which were aided by the fact that reaction times between memory conditions did not differ significantly (Table 1).
Consequently, any overlap of activation from the search and reminiscence periods should be equivalent in the different conditions and thus removed in their comparisons. Only hit trials were included in the analyses (i.e., trials in which participants could remember in the autobiographical memory conditions or imagine in the remote semantic memory condition). Contrasts included: remote autobiographical vs. remote semantic memory, recent autobiographical vs. remote semantic memory and recent autobiographical vs. remote autobiographical memory.
Data from individual runs were first normalized, correcting for signal intensity changes and temporal drift, and then spatially smoothed (1.5 voxel Hanning radius). The normalized data were then averaged in relation to the beginning of each trial type for each participant across runs (Burock et al., 1998). Statistical average event-related activation maps were constructed, using FS-FAST with the gamma-function, based on the averaged event-related responses for each trial type. Structural images were resampled automatically into Talairach and spherical space (see below) to allow averaging of the group data. Group statistic voxelwise maps were created using a t statistic with random effects to contrast the effects of the different conditions. We displayed our data in a spherical space for visualization of the neocortical activation maps, and in Talairach space for visualization of activation in MTL structures that reached a threshold of p ≤ 0.001.
Cortical Surface Analyses
A model for each participant’s cortical surface was created using the two MPRAGE sequences. After segmentation of the cortical white matter and tessellation of the gray/white matter border, the folded surface tessellation patterns were inflated (Fischl et al., 1999), and an automated correction of the topological defects resulted in the manifold (i.e., cortical surface model) (Fischl et al., 2001). The reconstructed brain of each participant was morphed to an average spherical surface image optimally orienting the major sulcal and gyral characteristics of each brain (Fischl et al., 1999; Fischl et al., 1999). The individual spherical surface coordinate transformations were then used to re-sample the selective averages and variances of each participant’s functional data.
ROI Analyses Within MTL structures
ROIs were hand-drawn on coronal and sagittal slices of T1 images of each participant distinguishing the amygdala, hippocampus, and parahippocampal region (entorhinal, perirhinal and parahippocampal cortices), following published guidelines for segmentation of MTL structures (Pruessner et al., 2002; Pruessner et al., 2000). We performed ANOVAs (mixed models) for search and reminiscence periods, respectively, with laterality (left, right), brain area (amygdala, hippocampus, and parahippocampal region), and memory condition (remote autobiographical, recent autobiographical, and remote semantic memory) as fixed factors and subjects as random factors, using motion corrected, intensity-normalized, but not spatially smoothed, functional data.
Results
Behavioral
The hit rates (averaged for each participant; F(1,1.635) = 1.797 p = 0.280) and the reaction times (averaged for each participant; F(1, 1.196) = 3.613, p = 0.076) for successfully retrieved memories did not differ across memory conditions (Table 1). Mean performance time and SD for each of the 3 functional runs was 12.98 ± 3.76 min.
Postscan Questionnaire and Reaction Times
An ANOVA for repeated measures of the reaction times (averaged for each participant) during and after scanning indicated that this measure decreased significantly in all memory conditions (F(1,1) = 32.99, p ≤ 0.001) with no significant interaction effect between memory condition and time of retrieval (during/after scanning; F(1,1.612 ) = 0.098, p = 0.868; Figure 2). An ANOVA for repeated measures disclosed significant difference across memory conditions for the ratings of the number of rehearsals (averaged for each participant; F(1, 1.804) = 26.550, p ≤ 0.001). Paired t-tests revealed that participants indicated that they rehearsed recent (t(15) = 7.277, p ≤ 0.001) and remote (t(15) = 4.733, p ≤ 0.001) autobiographical memories significantly more often than semantic memories, and recent autobiographical memories significantly more often than remote autobiographical memories (t(15) = 2.887, p ≤ 0.001; Figure 2). An ANOVA for repeated measures showed a significant difference across memory conditions for vividness ratings memories, (averaged for each participant; F(1, 1.931) = 7.940 p ≤ 0.01), and for the ratings of emotional impact of the original event/object, (averaged for each participant; F(1, 1.943) = 67.005, p ≤ 0.001). Paired t-tests indicated that participants rated the vividness of recent autobiographical memories significantly higher than of remote autobiographical (t(15) = 2.528), p ≤ 0.05) and remote semantic memories (t(15) = 3.719, p ≤ 0.005) and the emotional impact of recent (t(15) = 8.826, p ≤ 0.001) and remote autobiographical memories (t(15) = 11.819, p ≤ 0.001) significantly higher than that for remote semantic memories (Figure 2). A repeated measures ANOVA (averaged for each participant; F(1, 1.720) = 104.707, p ≤ 0.001) also showed a significant difference across memory conditions for the personal importance of memories. Paired t-tests revealed that participants rated the personal importance of recent (t(15) = 10.932, p ≤ 0.001) and remote (t(15) = 16.938 , p ≤ 0.001) autobiographical conditions significantly higher than the remote semantic memories (Figure 2).
Figure 2.
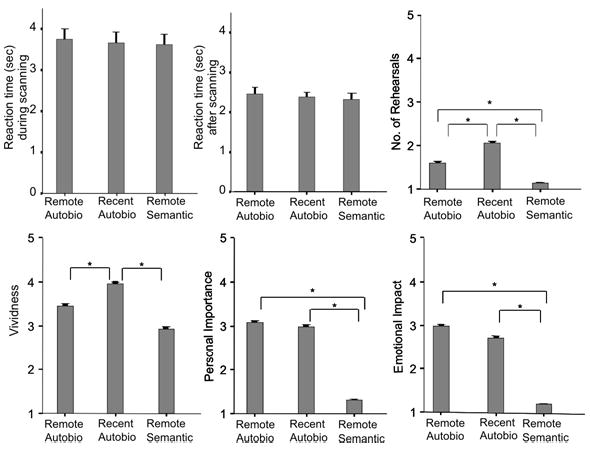
Mean and SEM of reaction times (difference during and after scanning) and post-scan ratings of vividness, number of rehearsals, personal importance of the memory, and emotional impact of the original event or of the well-known object. An asterix (*) indicates a significant difference between memory conditions, p ≤ 0.05.
For five of the 16 participants diaries were used to create remote autobiographical stimuli. The reaction times (averaged for each participant) for successfully recalled remote autobiographical stimuli of those 5 participants were not significantly different from the reaction times of participants whose family members/friends had been interviewed (t(14) = −1.141, p ≥ 0.05), nor were their hit rate (t(14) = −0.820, p ≥ 0.05). They rated the vividness (t(14) = −2.237, p≤ 0.05) of these memories significantly lower than participants whose family members/friends had been interviewed, but did not differ on any other quality ratings of the memories (all p > 0.05).
Pearson correlation analyses between the quality ratings of the memories and the results of the region of interest analyses of the search period did not find any significant correlations. Results are shown in supplementary Table 1 to 3.
Brain Activation for Remote Autobiographical Versus Remote Semantic Memory
Search period
We observed no activation specific for remote semantic memory, but did find a widespread bilateral network of activation specific for remote autobiographical memory (Figure 3). Laterally, it comprised the anterior and posterior part of the middle temporal gyrus spreading into the superior temporal sulcus, temporo-occipito-parietal junction, middle and superior frontal gyri, and left inferior orbital frontal gyrus pars orbitalis. Medially, the activation network included the retrosplenial cortex spreading to calcerine sulcus, cuneus, parieto-occipital sulcus, and precuneus. It also included an area in the superior frontal gyrus, spreading into the anterior paracingulate and cingulate gyri. In the MTL structures, we observed small activation spots along the collateral sulcus and in the right parahippocampal gyrus.
Figure 3.
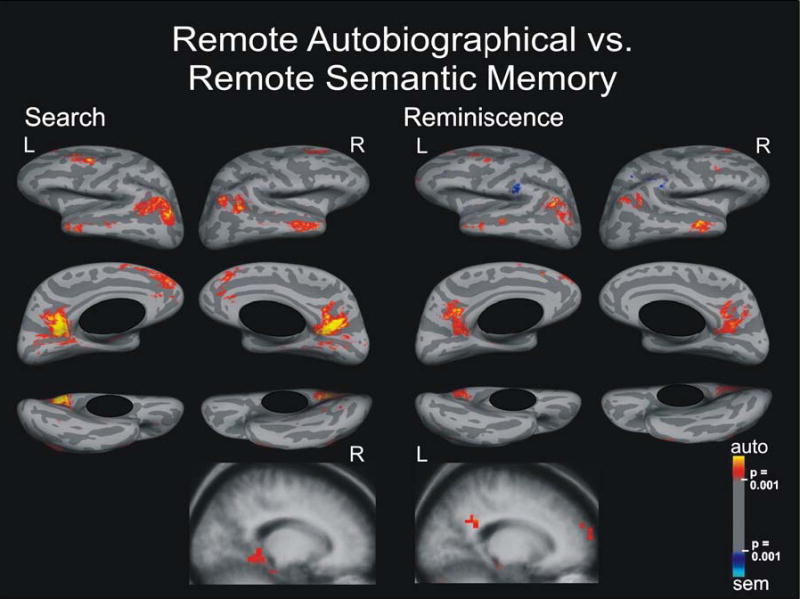
Search and Reminiscence activation maps displayed on spherical surfaces and in MTL structures in Talairach space for the contrast between remote autobiographical and remote semantic memory, p ≤ 0.001.
Reminiscence period
We found a similar bilateral neocortical network of activation specific to remote autobiographical memory that was less extensive with smaller signal strength than for the search period. For this time period, the parahippocampal activation was left-sided. Activation areas specific for remote semantic memory appeared bilaterally in supramarginal gyri and in left inferior frontal sulcus.
Brain Activation for Recent Autobiographical Versus Remote Semantic Memory
Search period
We observed a network of activation specific for recent autobiographical memory, in the same areas described above, in the contrast between remote autobiographical memory and remote semantic memory (Figure 4). We, however, did not observe activation in the left inferior frontal gyrus, and we found additional activation in the right hippocampus. Activation specific to remote semantic memory occurred in the left supramarginal gyrus.
Figure 4.
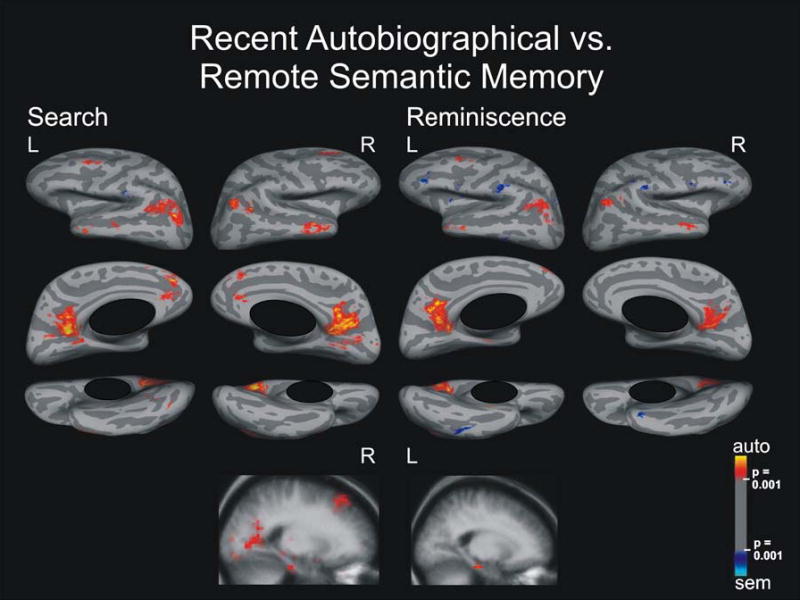
Search and Reminiscence activation maps displayed on spherical surfaces and in MTL structures in Talairach space for the contrast between recent autobiographical and remote semantic memory, p ≤ 0.001.
Reminiscence period
This contrast revealed a network of activation specific for recent autobiographical memory that was less extensive with smaller signal strength in the same areas as during the search period (and as for remote autobiographical memory compared to remote semantic memory). For reminiscence, activation in the parahippocampal gyrus was left-sided and no hippocampal activation was observed. For remote semantic memory, we found bilateral activation in the inferior temporal gyrus, supramarginal gyrus, anterior inferior frontal sulcus, inferior precentral sulcus (bordering the inferior frontal gyrus pars orbitalis), and left circular insular sulcus.
Brain Activation for Recent Autobiographical Versus Remote Autobiographical Memory
Search period
Only a tiny area in the central sulcus was activated specifically to remote autobiographical memory (Figure 5). Surprisingly, no other activation areas were observed at a statistical threshold of p ≤ 0.001. Lowering the threshold to p ≤ 0.01 revealed spotty activation bilaterally that was specific to remote autobiographical memory only in the inferior frontal lobes, spreading into orbital areas in the right hemisphere and into prefrontal areas in the left hemisphere (Figure 6). Activation spots also occurred around the supramarginal gyri, spreading into the postcentral sulcus and central sulcus. Additional small spotty activation was revealed around the inferior temporal and fusiform gyri bilaterally, and in the left cingulate gyrus. Importantly, no activation differences appeared in MTL structures, even after lowering the statistical threshold to p ≤ 0.05 (supplementary Figure 1).
Figure 5.
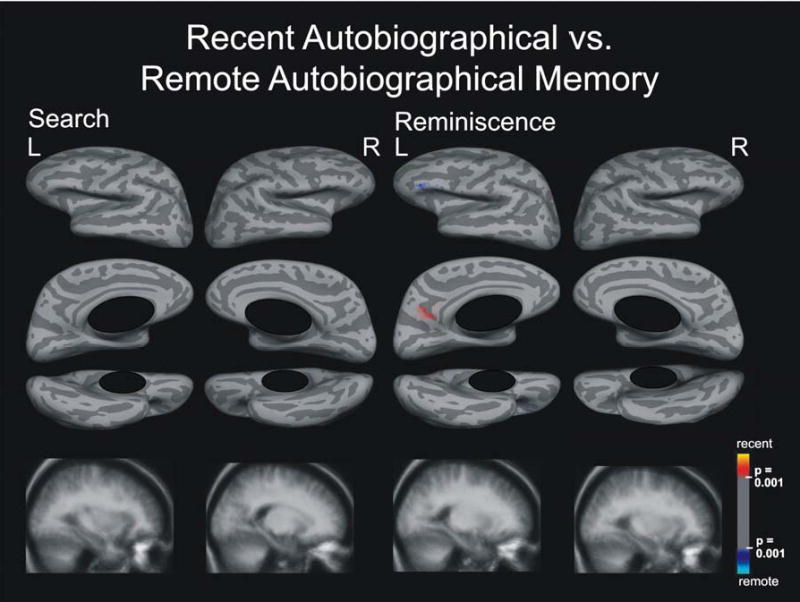
Search and Reminiscence activation maps displayed on spherical surfaces and in MTL structures in Talairach space for the contrast between recent and remote autobiographical memory, p ≤ 0.001.
Figure 6.
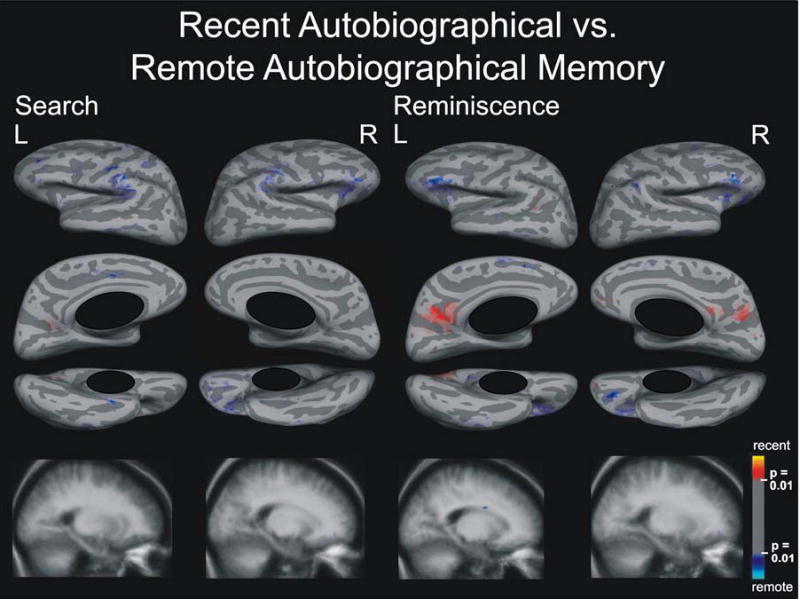
Search and Reminiscence activation maps displayed on spherical surfaces and in MTL structures in Talairach space for the contrast between recent and remote autobiographical memory, p ≤ 0.01.
Reminiscence period
Again, at a statistical threshold of p ≤ 0.001, we observed activation in small brain areas: one specific for remote autobiographical memory in the left inferior frontal gyrus pars triangularis, and another specific for recent autobiographical memory in left retrosplenial cortex. Decreasing the statistical threshold to p ≤ 0.01 revealed bilateral spotty activation specific to remote autobiographical memory in the inferior frontal lobes spreading orbitally, and in the right supramarginal, left paracingulate, and inferior temporal gyri (Figure 6). We saw mottled activation specific to recent autobiographical memory bilaterally in retrosplenial cortex spreading to cuneus, parieto-occipital sulcus, precuneus and subparietal sulcus. Activation specific for recent autobiographical memory occurred in the left angular sulcus and right inferior frontal gyrus spreading into the circular sulcus of insula. We found no activation differences in MTL structures, which was also true after lowering the statistical threshold to p ≤ 0.05 (supplementary Figure 1).
ROI Analyses Within MTL Structures
For the search time period, an mixed ANOVA with laterality, brain area, and memory condition as fixed factors, and subjects as random factor, detected significant main effects for laterality (F(1, 259) = 4.924, p ≤ 0.05), brain area (F = (2, 259) = 10.390 p ≤ 0.001), and memory condition (F (2, 259) = 9.378, p ≤ 0.001). The main effects of laterality and brain area are likely to reflect variables that are irrelevant to the current study and will not be discussed further. Results from post-hoc paired t-tests are shown in Figure 7. The percentage signal change was greater for recent (t(96) = 3.091, p ≤ 0.001) and remote (t(96) = 3.134, p ≤ 0.001) autobiographical than for remote semantic memory. The ANOVA further revealed an interaction effect between brain area and memory condition (F (4, 259.001) = 9.079, p ≤ 0.001). Significant results of the post-hoc paired t tests of the interaction effect are shown in Table 2 (Bonferroni corrections for multiple comparisons, p ≤ 0.001). While hippocampal and parahippocampal activations were significantly larger for autobiographical than semantic memory, the amygdala showed the opposite tendency. No significant differences were found between remote and recent autobiographical memories in any structure. A mixed model ANOVA examining the reminiscence period with laterality, memory condition, and brain area as fixed factors and subjects as random factor did not reveal any significant effects (all p > 0.05).
Figure 7.
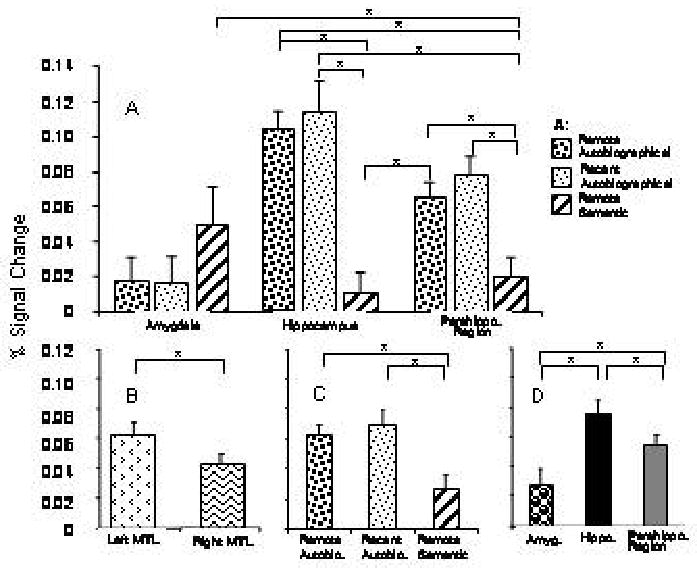
ROI analysis of MTL structures for the search period. Percentage signal change (A) within different MTL structures specific to each of the three memory conditions; (B) for each of the three memory conditions. Horizontal bars indicate significant differences between conditions, p ≤ 0.05.
Discussion
Autobiographical Memory
Our results revealed a strong bilateral network of activation, left larger than right, specific to autobiographical memory compared to remote semantic memory. The activation network was similar for recent and remote memory during both retrieval periods. The extent of activation and the signal strength seemed greater for remote than for recent autobiographical memory. Also, for both autobiographical conditions, activation during reminiscence appeared less extensive with less signal strength than during search. The large network supporting autobiographical memory encompassed the middle temporal gyrus spreading into the superior temporal sulcus; temporo-occipito-parietal junction; middle and superior frontal gyri spreading into the anterior cingulate and paracingulate gyri and frontal pole; inferior frontal gyrus pars orbitalis; and retrosplenial cortex extending to calcerine sulcus, cuneus, parieto-occipital sulcus, and precuneus; and MTL structures (Table 3 and 4). This network of activation is similar to activation patterns observed in other neuroimaging studies of autobiographical memory (Addis et al., 2004; Andreasen et al., 1995; Andreasen et al., 1999; Cabeza et al., 2004; Fink et al., 1996; Gilboa et al., 2004; Levine et al., 2004; Maguire, 2001; Markowitsch et al., 2003; Piefke et al., 2003; Piolino et al., 2003; Piolino et al., 2004; Tulving, 1989).
Role of MTL structures in autobiographical memory
MTL structures have been strongly associated with episodic memory, that is, the memory of autobiographical events: “what” happened “where”, and “when” (Tulving, 2002). The recollection of an event’s rich spatio-temporal context distinguishes autobiographical memory from semantic memory and from recognition memory based on familiarity (Burgess et al., 2002). The Standard Model of Consolidation assumes that memory representations are initially established as short-lived modifications in reciprocal connections between neocortex and MTL structures. Repeated reactivations of neocortical representations via MTL structures lead eventually to strong interconnections among cortical sites, which support memory independently of MTL structures (Bayley et al., 2003; Squire & Alvarez, 1995). An alternative view - the Multiple Trace Theory - is that autobiographical memory is always supported by reciprocal connections between neocortex and MTL structures independent of its age, and that only semantic memory (factual knowledge that is independent of a specific time and place) becomes independent of the MTL (Moscovitch et al., 1999; Nadel et al., 2000). Thus, while the Standard Model of Consolidation predicts no activation in MTL structures specific to remote autobiographical memory, the Multiple Trace Theory does. We tested these predictions by comparing (a) remote autobiographical memory with remote semantic memory, and (b) recent autobiographical memory with remote autobiographical memory. The Standard Model of Consolidation predicts no differential activation of MTL structures in comparison (a), but more activation for recent than for remote autobiographical memory in (b). In contrast, the Multiple Trace Theory predicts more activation of MTL structures specific to remote autobiographical memory in (a), but no activation differences within MTL structures in comparison (b). Our results demonstrated activation of MTL structures specific to remote autobiographical memory compared to remote semantic memory (a), but no activation differences within MTL structures comparing recent with remote autobiographical memory (b), even after lowering the statistical threshold to p ≤ 0.01 and p ≤ 0.01 (see supplementary data). Thus, our results are consistent with the Multiple Trace Theory but inconsistent with the Standard Model of Consolidation. This conclusion is strengthened by the fact that we observed activation in MTL structures at the single subject level (p ≤ 0.05): During search, 13 of 16 participants activated left and 14 right MTL structures (12 bilaterally), while during reminiscence 14 of 16 participants activated left and 12 right MTL structures (11 bilaterally).
One might argue that the activation of MTL structures occurred because memories were newly encoded when retrieved in the scanner. Activation due to new encoding, however, should be canceled out in our results because comparisons were made between memory conditions. One might further argue that encoding during retrieval would occur to a greater depth for autobiographical than for semantic memories because autobiographical memories are more vivid and personally more important. We estimated the effect of new encoding during scanning by examining reaction time decreases at the second presentation of the same cue (Buckner et al., 2001). If more robust encoding for autobiographical memories occurred, we should have seen a greater decrease in reaction times for autobiographical than for semantic memories. The results, however, showed an equivalent change in reaction times across memory tasks (Figure 2). While we cannot exclude that the decrease in reaction times had been influenced by priming effects, we rule out major encoding differences between memory conditions.
An alternative approach to maintain the remote character of memories is to interview amnesic patients about events from their pre-amnesic life soon before scanning, because such participants will not remember the actual interview when the memories are probed during scanning (Maguire et al., 2005; Maguire et al., 2001). It is unclear, however, to which degree this approach accesses detailed truly episodic memories, because deficits in remote autobiographical memories in amnesic patients have been observed (Moscovitch et al., 1999; Spiers et al., 2001; Steinvorth et al., 2005).
ROI analyses for the search period confirmed the role of MTL structures in autobiographical memory independent of its age. Within the MTL, the hippocampus and parahippocampal region responded particularly to autobiographical memory, but the amygdala did not. The absence of activation in the amygdala was surprising because autobiographical memories were rated to have more emotional impact than semantic memories (Figure 2). Other studies, including one that specifically investigated the emotional tone of autobiographical memories, however, also found no activation of the amygdala (Addis et al., 2004; Piefke et al., 2003). ROI analyses for the reminiscence period revealed no significant effects, and the activations within MTL structures in the voxel based analyses (as well as in neocortical areas) were overall less extensive with less signal strength during reminiscence than during search. This finding suggests that MTL structures may be recruited more for retrieval of recollected information than for the recollection experience.
According to the Multiple Trace Theory each re-activation of a memory trace leads to the creation of a newly encoded hippocampal trace, so that over time older memories compared to younger are associated with a larger number of memory traces because they have a greater probability of repeated rehearsal. The net effect is that older memories will, on average, involve an ever greater proportion of the hippocampal complex (Nadel & Moscovitch, 1998; Nadel & Moscovitch, 1997). While this prediction is not easy to translate into terms of fMRI results, one way it has been interpreted is to expect greater activation and/or a different distribution of activation within MTL structures for remote than for recent autobiographical memories (Gilboa et al., 2004). Our results, however, did not show significant activation differences within MTL structures at the voxel level between recent and remote autobiographical memories, even after lowering the statistical threshold to p ≤ 0.05 (see supplementary Figure 1). Furthermore, no overall difference was found between recent and remote autobiographical memories for any MTL structure or for the MTL as a whole in the ROI analysis. Thus, our data do not confirm this possible prediction of the MTT model. Our data are, nevertheless, in accordance with the main claim of the Multiple Trace Theory that MTL structures are engaged during the retrieval of remote autobiographical memory.
Other neuroimaging studies of remote autobiographical memory also reported activation of MTL structures. In most of these studies, however, participants had retrieved the autobiographical memories shortly before scanning thereby destroying the remote character of the memories (Fink et al., 1996; Maguire, 2001; Piefke et al., 2003). Two studies (Gilboa et al., 2004; Ryan et al., 2001), which ensured that memories had not been retrieved recently, detected hippocampal activation for recent and remote autobiographical memories. Both studies found no activation differences between recent and remote autobiographical memory, which included an ROI analysis of the hippocampus in the Gilboa et al. study (Gilboa et al., 2004). Another recent neuroimaging study by Piolino et al. (Piolino et al., 2004) also used autobiographical memories that had not been retrieved newly. Using an ROI approach, the authors found greater hippocampal activation for remote than for recent autobiographical memories and relatively greater activation in the right than left hippocampus, regardless of the age of the memories. While these results are consistent with our conclusion highlighting the role of MTL structures in remote autobiographical memory, the present study found similar activation for remote and recent autobiographical memory and more robust activation in the left hippocampal than in the right during search. The Piolino et al. (Piolino et al., 2004) study has crucial differences, however: Each memory condition included only eight stimulus sentences, and the number of participants was small (N=7). In contrast, the present study included 50 sentences in each condition, and enrolled 16 participants. Thus, the results described here represent more reliable findings.
Two of these studies found a relationship between the activation in MTL structures, and quality ratings of the memories, while we did not find significant correlations between any quality ratings and the results from the ROI analyzes (Addis et al., 2004; Gilboa et al., 2004). One of these two studies observed an effect of detailedness and personal significance on bilateral hippocampal activation (Addis et al., 2004), and another found vividness to be crucial for hippocampal activation (Gilboa et al., 2004). It is possible that our results differ from those because one (and possibly both) of these studies included non-successfully retrieved memories into the group of low vivid memories (Gilboa et al., 2004), while we only included successfully retrieved memories. Further, the fact that the memories were retrieved immediately before scanning in one of those studies might have influence the quality rating or the observed activation patterns (Addis et al., 2004). Another study that controlled for the quality of memories, however, did also not find an association between emotional valence or intensity and activation patterns for autobiographical memories (Maguire & Frith, 2003).
In contrast to other neuroimaging studies of autobiographical memory, we distinguished between search and reminiscence, the time periods when one seeks for the memory and when one recollects the memory. While we did not formally compare activations between time periods, the similarity of the activation patterns between both time periods is apparent. Several models of autobiographical memory suggest that retrieval starts with processes that guide search. These processes include search initiation, generation and maintenance of cues and evaluation of the outcome. Other aspects of organizing the retrieval effort may include suppression of external input, attention to internally generated images, and maintenance of a set both clues and partial results. In these models, these processes are believed to be supported by anterior areas, especially prefrontal and anterior cingulate cortices, as well as the temporal poles and temporo-frontal junctions (Conway et al., 2001; Moscovitch & Winocur, 2002). Posterior cortical and MTL structures, on the other hand, would become important in memory retrieval only after initial access to the memory has been obtained (Conway et al., 2001; Moscovitch & Winocur, 2002). Although we did observe prefrontal and anterior cingulate activations during search, activation was also present in MTL, temporo-occipito-parietal and retrosplenial regions. These posterior activations may reflect the progressive retrieval of details in search-evaluate-elaborate cycles that may repeat several times before a memory is completely recovered. The presentation of salient information of the original event as cues, as in our task, might have greatly reduced the initial search period (Conway et al., 2001; Moscovitch & Winocur, 2002), rapidly initiating ecphory, thus giving rise to recollection and activation of posterior areas. A role for the MTL in the retrieval set is also suggested by the ‘dreamy state,’ déjà vu and vivid recollections, that can be evoked by MTL hyperactivation (Bancaud et al., 1994; Bartolomei et al., 2004; Halgren et al., 1978). Moreover, the continuation of activation of anterior areas during reminiscence might be grounded in our instructions to participants to conjure up as many details as possible during this period, thereby requiring further cycles of search, evaluation and elaboration.
When interpreting the absence of differential MTL activation for recent versus remote autobiographical memories, it is important to bear in mind that hemodynamic activation reveals one aspect of the engagement of a structure. In contrast, the behavioural effects of MTL lesions identify the tasks where the MTL makes an essential contribution. A number of studies in MTL amnesic patients demonstrate a deficit in remote autobiographical memory, (Moscovitch et al., 1999; Spiers et al., 2001; Steinvorth et al., 2005), while others did not find such a deficit (Bayley et al., 2003; Reed & Squire, 1998). It is possible, that methodological differences lead to the discrepancies in these studies and that deficits in remote autobiographical memory in MTL amnesic patients can only be detected when using a technique that not only distinguishes between non-episodic and episodic aspects of autobiographical memory narratives, but also uses a standardized prompting method providing access to unique specific events and elaborating their details, rather than remaining at a more generalized level of personal episodes.
The Role of the Age of Autobiographical Memories for Neocortical Activations
The left inferior frontal gyrus pars triangularis (BA 44/45) activated specifically to remote autobiographical compared to recent autobiographical memory during “reminiscence,” and during both time periods bilaterally after lowering the statistical threshold to p ≤ 0.01. This area has been related to controlled retrieval effort, when a task cannot be mastered through automated stimulus-response mapping (Buckner et al., 1998; Dobbins et al., 2002; Shiffrin & Schneider, 1984) and similarly to conflict resolution due to increased interference between familiar information during retrieval (Jonides et al., 2002). Retrieval of a memory of a personal event that occurred 10 or more years ago probably demands greater control processes and probably elicits greater interference than retrieval of a personal event that occurred during the last 1–2 years. This interpretation is supported by participants’ post-scan ratings indicating that they retrieved recent autobiographical memories significantly more often than remote ones. The reaction times, however, did not differ significantly between conditions.
The ventrolateral prefrontal cortex has been reported to be parametrically responsive to the age of autobiographical memories in a study by Maguire et al. (Maguire et al., 2001). They observed the opposite pattern to our study with most activity, particularly on the right side, during recent memories and decreasing the more remote the memories became. The region of the Maguire et al. (Maguire et al., 2001) study, however, differs slightly from the area in which we observed activation differences. While Maguire et al. (Maguire et al., 2001) observed activation differences in BA region 47, the differences between recent and remote memories in our study occurred in BA 44/45. While area 44/45 has been associated with retrieval effort and conflict resolution due to increased interference (Jonides et al., 2002), area 47 seems to have a role in cue specification when using distinctive retrieval cues to specify information that needs to be recovered from long-term memory (Fletcher & Henson, 2001; Wagner, 1999).
The spotty activation areas in the supramarginal and inferior temporal gyri uncovered after lowering the statistical threshold could be related to the need for greater effort in the remote autobiographical condition to retrieve object representations from long-term memory (Ganis et al., 2004; Ishai et al., 2002). The orbitofrontal activation could be related to greater effort when re-creating a remote social situation (Stone et al., 1998).
The area around retrosplenial cortex was activated specifically by the recent autobiographical condition during reminiscence. Retrosplenial activation has been reported in many neuroimaging studies of autobiographical memory, many of which also observed greater retrosplenial activation for recent than for remote autobiographical memories (Addis et al., 2004; Andreasen et al., 1995; Burgess et al., 2001; Gilboa et al., 2004; Maguire, 2001; Maguire & Mummery, 1999; Maguire et al., 2000; Piefke et al., 2003). Further, it has been associated with person familiarity (Shah et al., 2001), synaesthesia for familiar names (Weiss et al., 2001), emotional processing (Maddock, 1999), “theory of mind” (Calarge et al., 2003), analysis of long-term associations of highly contextual objects (Bar & Aminoff, 2003), and in particular, with spatial and episodic memory (Aggleton & Pearce, 2001; Aggleton et al., 2000; Maguire, 2001; Maguire, 2001; Wiggs et al., 1999). Lesions to the retrosplenial cortex can lead to memory deficits and amnesia, stressing its importance in episodic retrieval (Valenstein et al., 1987). Invoking imaginable representations of spatial context and mentally navigating through an imagined space constitutes an essential aspect of autobiographical recollections. Associated details (e.g., objects and familiar persons) are evoked and “loaded” into this spatial context while emotional facets of the memory are analyzed. Further, in analogy to a “theory of mind,” putting oneself into other’s and one’s own past position is an essential aspect of autobiographical memory. Thus, the retrosplenial region is related to episodic retrieval and recollection, when one remembers in vivid detail (Buckner & Wheeler, 2001; Burgess et al., 2001; Dobbins et al., 2002; Eldridge et al., 2000; Haist et al., 2001). Consistent with this finding, our participants rated recent autobiographical memories as being more vivid than remote ones. We do not believe, however, that remote memories had become fully semanticized because participants indicated that they had rehearsed recent memories more often than remote ones.
Remote Semantic Memory
Areas that responded specifically to remote semantic memory included the supramarginal and inferior frontal cortices bilaterally, the left insular cortex, and inferior temporal gyrus. Activation in these areas has been observed in studies of visual imagery of objects or faces (Ganis et al., 2004; Ishai et al., 2002). Investigators have suggested that these frontal and parietal regions mediate the retrieval of object representations from long-term memory and maintain them in working memory, while temporal regions store the content of the imagery.
Conclusions
A bilateral network of brain areas encompassing the temporal gyrus, the temporo-occipito-parietal junction, the middle and superior frontal gyri, inferior frontal gyrus, retrosplenial region, and MTL structures supports autobiographical memory. This network, including MTL structures, seems to change little with the age of the memories.
Supplementary Figure 1.
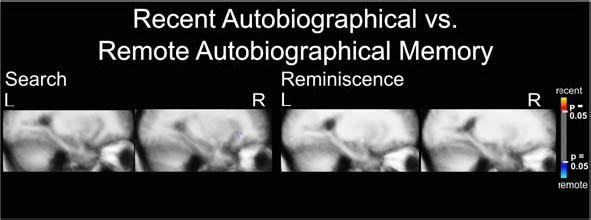
Search and Reminiscence activation maps displayed in MTL structures in Talairach space for the contrast between recent and remote autobiographical memory, p ≤ 0.05.
Acknowledgments
This research was supported by NS18741, NS44623, NCRR, P41RR14075 and the MIND Institute. We thank Karen Keller, Philip Janowicz, and Julie Koo for assistance in recruiting participants, stimulus creation, scanning, and analysis of the behavioral data and Doug Greve for discussion of analysis methods. We are grateful for the extraordinary contribution of the participants.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–62. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Pearce JM. Neural systems underlying episodic memory: insights from animal research. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1467–82. doi: 10.1098/rstb.2001.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Oswald CJ, Good M. Identifying cortical inputs to the rat hippocampus that subserve allocentric spatial processes: a simple problem with a complex answer. Hippocampus. 2000;10(4):466–74. doi: 10.1002/1098-1063(2000)10:4<466::AID-HIPO13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152(11):1576–85. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Paradiso S, Cizadlo T, Arndt S, Watkins GL, Ponto LL, Hichwa RD. The cerebellum plays a role in conscious episodic memory retrieval. Hum Brain Mapp. 1999;8(4):226–34. doi: 10.1002/(SICI)1097-0193(1999)8:4<226::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of deja vu and vivid 'memories' in human temporal lobe epilepsy. Brain. 1994;117 ( Pt 1):71–90. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38(2):347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Barbeau E, Gavaret M, Guye M, McGonigal A, Regis J, Chauvel P. Cortical stimulation study of the role of rhinal cortex in deja vu and reminiscence of memories. Neurology. 2004;63(5):858–64. doi: 10.1212/01.wnl.0000137037.56916.3f. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38(1):135–44. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Brewer, W. F. (1988). Memory for randomly sampled autobiographical events. In Remembering Reconsidered. Ecological and Traditional Approaches to the Study of Memory., eds. U. Neisser & W. Winograd, Cambridge University Press. New York, pp. 21–90.
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. Neuroimage. 1998;7(3):151–62. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2(9):624–34. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. Journal of Cognitive Neuroscience. 2001;13(3):406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Burgess N, Becker S, King JA, O'Keefe J. Memory for events and their spatial context: models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1493–503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9(16):3735–9. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11(4):249–60. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16(9):1583–94. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Calarge C, Andreasen NC, O'Leary DS. Visualizing how one brain understands another: a PET study of theory of mind. Am J Psychiatry. 2003;160(11):1954–64. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE. The Neuroanatomy of Autobiographical Memory: A Slow Cortical Potential Study of Autobiographical Memory Retrieval. Journal of Memory and Language. 2001;45:493–524. [Google Scholar]
- Conway MA, Turk DJ, Miller SL, Logan J, Nebes RD, Meltzer CC, Becker JT. A positron emission tomography (PET) study of autobiographical memory retrieval. Memory. 1999;7(5–6):679–702. doi: 10.1080/096582199387805. [DOI] [PubMed] [Google Scholar]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in H.M. Semin Neurol. 1984;4:249–59. [Google Scholar]
- Crovitz HFHS. Frequency of episodic memories as a function of their age. Bulletin of the Psychonomic Society. 1974;4:517–518. [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35(5):989–96. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3(11):1149–52. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one's own past: neural networks involved in autobiographical memory. J Neurosci. 1996;16(13):4275–82. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–81. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Cohen NJ, Corkin S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain Cogn. 1988;7(2):157–77. doi: 10.1016/0278-2626(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res. 2004;20(2):226–41. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14(11):1214–25. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4(11):1139–45. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Halgren E, Walter RD, Cherlow DG, Crandall PH. Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain. 1978;101(1):83–117. doi: 10.1093/brain/101.1.83. [DOI] [PubMed] [Google Scholar]
- Halgren E, Wilson CL, Stapleton JM. Human medial temporal-lobe stimulation disrupts both formation and retrieval of recent memories. Brain Cogn. 1985;4(3):287–95. doi: 10.1016/0278-2626(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Ishai A, Haxby JV, Ungerleider LG. Visual imagery of famous faces: effects of memory and attention revealed by fMRI. Neuroimage. 2002;17(4):1729–1741. doi: 10.1006/nimg.2002.1330. [DOI] [PubMed] [Google Scholar]
- Jonides, J., Badre, D., Curtis, C., Thompson-Schill, S. & Smith, E. (2002). Mechanism of Conflict Resolution in Prefrontal Cortex. In Principles of Frontal Lobe Function., eds. D. T. Stuss & R. T. Knight, Oxford University Press. New York, pp. 233–245.
- Levine B, Svoboda E, Hay J, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17(6):677–689. [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. J Cogn Neurosci. 2004;16(9):1633–46. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1441–51. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001;42(3):225–38. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23(12):5302–7. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, E. A., Frith, C. D., Rudge, P. & Cipolotti, L. (2005). The effect of adult-acquired hippocampal damage on memory retrieval: An fMRI study. Neuroimage. [DOI] [PubMed]
- Maguire EA, Henson RN, Mummery CJ, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12(3):441–4. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Henson RNA, Mummery CJ, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Cognitive Neuroscience and Neuropsychology. 2001;12(3):441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9(1):54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Buchel C. Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus. 2000;10(4):475–82. doi: 10.1002/1098-1063(2000)10:4<475::AID-HIPO14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124(Pt 6):1156–70. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhovel MM, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39(4–5):643–65. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- Milner, B. & Penfield, W. (1955). The effect of hippocampal lesions on recent memory. Trans Am Neurol Assoc(80th Meeting), 42–8. [PubMed]
- Moscovitch, M. & Winocur, G. (2002). The Frontal Cortex and Working with Memory. In Principles of Frontal Lobe Function., eds. D. Stuss & R. T. Knight, Oxford University Press. New York, pp. 188–209.
- Moscovitch, M., Yaschyshyn, T., Ziegler, M. & Nadel, L. (1999). Remote Episodic Memory and Retrograde Amnesia: Was Endel Tulving Right All Along? In Memory, Consciousness, and the Brain: The Tallinn Conference, ed. E. Tulving, Psychology Press. New York, pp. 331–345.
- Nadel L, Moscovitch M. Hippocampal contributions to cortical plasticity. Neuropharmacology. 1998;37(4–5):431–9. doi: 10.1016/s0028-3908(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7(2):217–27. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10(4):352–68. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proc Natl Acad Sci U S A. 2000;97(20):11120–4. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–68. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszewski V, Lalevee C, De la Sayette V, Eustache F. Autobiographical memory and autonoetic consciousness: triple dissociation in neurodegenerative diseases. Brain. 2003;126(Pt 10):2203–19. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- Piolino P, Giffard-Quillon G, Desgranges B, Chetelat G, Baron JC, Eustache F. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. Neuroimage. 2004;22(3):1371–83. doi: 10.1016/j.neuroimage.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb Cortex. 2002;12(12):1342–53. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10(4):433–42. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: findings from four new cases. J Neurosci. 1998;18(10):3943–54. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Nadel L, Keil K, Putnam K, Schnyer D, Trouard T, Moscovitch M. Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11(6):707–14. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurochem. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124(Pt 4):804–15. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Automatic and controlled processing revisited. Psychol Rev. 1984;91(2):269–76. [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7(5):357–82. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5(2):169–77. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8(3):205–11. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia. 2005;43(4):479–96. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10(5):640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory: Performance, Knowledge, and Experience. European Journal of Cognitive Pszchology. 1989;1:3–26. [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8(3):198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110 ( Pt 6):1631–46. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Wagner AD. Working memory contributions to human learning and remembering. Neuron. 1999;22(1):19–22. doi: 10.1016/s0896-6273(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Shah NJ, Toni I, Zilles K, Fink GR. Associating colours with people: a case of chromatic-lexical synaesthesia. Cortex. 2001;37(5):750–3. doi: 10.1016/s0010-9452(08)70631-2. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121(3):331–54. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1999;37(1):103–18. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]


